Abstract
Objective:
In this study, we sought to assess likelihood of living donor kidney transplantation (LDKT) within a single-center kidney transplant waitlist, by race and gender, following implementation of an incompatible program.
Summary Background Data:
Disparities in access to LDKT exist among minority women and may be partially explained by antigen sensitization secondary to prior pregnancies, transplants or blood transfusions, creating difficulty finding compatible matches. To address these and other obstacles, an incompatible LDKT program, incorporating desensitization and kidney paired donation, was created at our institution.
Methods:
A retrospective cohort study was performed among our kidney transplant waitlist candidates (n=8895). Multivariable Cox regression was utilized, comparing likelihood of LDKT before (Era 1: 01/2007–01/2013) and after (Era 2: 01/2013–11/2018) implementation of the incompatible program. Candidates were stratified by race (white vs. minority (non-white)), sex, and breadth of sensitization.
Results:
Program implementation resulted in the nation’s longest single-center kidney chain, and likelihood of LDKT increased by 70% for whites (aHR 1.70; 95%CI: 1.46–1.99) and more than 100% for minorities (aHR 2.05; 95%CI: 1.60–2.62). Improvement in access to LDKT was greatest among sensitized minority women (cPRA 11–49%: aHR 4.79; 95% CI: 2.27–10.11; cPRA 50–100%: aHR 4.09; 95% CI: 1.89–8.82).
Conclusions:
Implementation of an incompatible program, and the resulting nation’s longest single-center kidney chain, mitigated disparities in access to LDKT among minorities, specifically sensitized women. Extrapolation of this success on a national level may further serve these vulnerable populations.
MINI-ABSTRACT:
Disparities in access to living donor kidney transplantation (LDKT) exist among minority women. Through implementation of an incompatible LDKT program at a single-institution, we sought to mitigate racial and gender disparities in access to transplantation. Herein, we report a 4-fold increased likelihood of LDKT among sensitized minority women post-implementation.
INTRODUCTION
Although kidney transplantation is the standard of care and preferred treatment modality for end-stage-renal disease, access to kidney transplantation is limited.1 Based on Organ Procurement and Transplantation Network (OPTN) data as of March 3, 2019, there were over 94,000 kidney transplant waitlist candidates in the United States, yet only 21,000 kidney transplants were performed in the previous year. Moreover, despite superior outcomes compared with deceased donor kidney transplantation, live donor kidney transplantation (LDKT) accounts for only one-third of all kidney transplants.2,3 Importantly, racial minorities and women have the least access to LDKT.4–11
Barriers to LDKT include blood group (ABO) and tissue type (human leukocyte antigen (HLA)) incompatibilities between living donor candidates and their intended recipients, which affect greater than 45% of living donor-recipient pairs.12 HLA incompatibility between donor-recipient pairs typically occurs due to prior antigen exposure through a sensitizing event, such as previous transplant, prior pregnancy or blood transfusions.13,14 Not surprisingly, pregnancy-induced HLA sensitization drives gender disparity in access to LDKT, as pregnancy is confined to the female sex. In fact, there appears to be a relationship between parity and degree of sensitization, such that multiparous women tend to have the highest levels of HLA sensitization. Moreover, multiparous women have been shown to be less likely to identify compatible donors, particularly when their potential living donor is a spouse or child to whom they have been sensitized through pregnancy.11 This is particularly problematic for minority women as they are more likely to be multiparous.15,16
In order to overcome these barriers to LDKT, incompatible kidney transplant programs have been developed, incorporating one or more strategies to achieve transplantation. The first option is kidney paired exchange (KPE), allowing for one incompatible donor-recipient pair to “swap” kidneys with another incompatible donor-recipient pair, thus achieving two compatible LDKTs. Another option includes pretreatment or desensitization, which allows for removal of antibodies to permit transplantation across ABO or HLA barriers. Finally, desensitization can be integrated into KPE programs as well, maximizing the number of transplants that can be achieved, especially among highly sensitized waitlist candidates.17 Prior simulation work by Segev and colleagues demonstrated that more widespread utilization of KPE would facilitate LDKT for a large proportion of waitlist candidates, and specifically racial minority candidates.18 Similarly, results of another study by Bromberger et. al suggested that KPE programs might diminish gender disparities in access to LDKT.11 To date, however, no study has examined the impact of implementing a comprehensive incompatible kidney transplant program, incorporating both KPE and desensitization, in mitigating disparities in access to LDKT among minority women.
Therefore, we hypothesized that development and implementation of an incompatible kidney transplant program, incorporating both KPE and desensitization, at an institution with a high prevalence of minority women waitlist candidates would mitigate existing racial and gender disparities in LDKT. To this end, in 2013 we implemented an incompatible kidney transplant program at our institution, resulting in the longest single-center kidney chain in the United States. Herein, we report access to LDKT among our kidney transplant waitlist candidates pre and post-implementation of the incompatible program, and demonstrate improved access to LDKT among minority women.
METHODS
Study Design
Study Population
A retrospective cohort study was performed among all kidney-only transplant candidates added to our single-center waitlist from January 1, 2007 through November 2, 2018 (n=8895). Data from our transplant registry was linked with and supplemented by data submitted by our institution to the OPTN. The study also used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data, submitted by members of the OPTN, on all donors, waitlisted candidates, and transplant recipients in the United States. The Health Resources and Services Administration of the US Department of Health and Human Services provides the oversight to the activities of the OPTN and SRTR contractors. This study was approved by the institutional review board at University of Alabama at Birmingham.
Outcome Ascertainment
The primary outcome of interest was access to LDKT, defined as likelihood of achieving LDKT. Candidates were followed until the earliest of kidney transplantation, death, waitlist removal, or administrative end of study (11/2/2018). Candidates who received deceased donor kidney transplants were censored at time of transplant. In order to assess likelihood of LDKT following implementation of the incompatible program, we compared access to LDKT pre-implementation (Era 1: 1/1/2007–12/31/2012) and post-implementation (Era 2: 1/1/2013– 11/2/2018).
Statistical Analyses
Access to Living Donor Kidney Transplantation by Race
In order to assess likelihood of LDKT by race, our cohort was stratified into the following groups: white candidates (n= 3495) and minority (non-white candidates) (n=4500). Bivariate analyses were performed in order to compare the two groups on the basis of baseline demographics, utilizing chi-squared tests for categorical variables and Wilcoxon rank sums for non-parametric continuous variables. Cox regression was utilized to assess access to LDKT. Candidate demographic and health factors associated with access to transplantation were selected a priori and included in the multivariable models, including: candidate age at waitlist addition, sex, and ABO blood group. Status on the waitlist (active or inactive) and calculated panel reactive antibody (cPRA) were also included in the models as time-varying covariates. cPRA was assessed as a categorical variable based on the distribution among our cohort, with the following cut-points created: 0–10%, 11–49%, 50–100%. Era was also included in the multivariate models in order to compare the likelihood of LDKT pre (Era 1) and post-implementation (Era 2) of the incompatible program. For candidates who were on the waitlist in both eras, a second record was created for the second era with wait times attributed to the appropriate era, such that candidates were compared to other candidates with similar wait-times, irrespective of era.
Access to Living Donor Kidney Transplantation by Race, Sex, and Sensitization
To assess likelihood of LDKT on the basis of race, sex, and degree of sensitization, we then subdivided the waitlist candidates within the entire cohort into the following groups: white women with cPRA 0–10%, 11–49%, 50–100%; white men with cPRA 0–10%, 11–49%, 50–100%; minority women with cPRA 0–10%, 11–49%, 50–100%; minority men with cPRA 0–10%, 11–49%, 50–100%. In order to analyze the association between era (implementation of the incompatible program) and access to LDKT within each group, we compared likelihood of LDKT in Era 2 (post-implementation) with Era 1 (pre-implementation).
Subgroup Analysis- Description of Incompatible Program Kidney Transplant Recipients
In order to further characterize the incompatible program participants, we identified all LDKT recipients who achieved transplantation through participation in the incompatible program at our institution (n=154), including recipients of LDKT through the KPE program and/or those who underwent desensitization followed by ABO incompatible or HLA incompatible transplantation. The incompatible program participants were then further assessed including history of sensitizing events and relationship of the recipient with the original intended donor, including: significant others, offspring), parents, extended family, and unrelated donors. Within our KPE program, non-directed donors are also incorporated and paired with waitlist candidates in order to enter the paired exchange, and these relationships were categorized as non-directed donors.
We also identified the reason that the donor-recipient pair entered the incompatible program, such as HLA incompatibility (positive crossmatch), ABO incompatibility, non-directed donors, compatible pair, and size/age mismatch. Several incompatible donor-recipient pairs entered the program and the recipients underwent desensitization prior to incompatible transplantation, either as part of the paired exchange program or independent of the KPE. Among those, incompatible transplants were performed after desensitization for ABO incompatible transplants with predetermined titer levels or for HLA incompatible transplants, defined by flow positive, cytotoxic T and B cell negative crossmatches.
All analyses were performed in SAS 9.4 (Cary, NC).
RESULTS
Cohort Characteristics
Within the study period, there were 8895 kidney-only waitlist candidates at our institution. Of those 3495 were white candidates and 5400 were minority candidates. White candidates were older with a median age of 52 years, compared with a median age of 48 years among minority candidates (p<0.0001) (Table 1). Minority candidates were more commonly female (44.4% compared with 39.7% of white candidates, p<0.0001). The median body mass index was higher among minorities as well (29.2 kg/m2 for minority candidates and 28.3 kg/m2 among white candidates, p<0.0001). The prevalence of diabetes was higher among minority candidates compared with white candidates (45.6 vs. 40.9%, p<0.0001). When assessing blood type, blood group B was more common among minority candidates (21.6% compared with 9.5% of white candidates, p<0.0001), and blood group O was the most prevalent among both minority (50.9%) and white (48.8%) candidates (Table 1).
Table 1.
Cohort demographics among candidates on the kidney transplant waitlist at our institution from 1/1/07–11/2/18, by race (n=8895)
| Characteristics | White Candidates N=3495 | Minority Candidates N=5400 | P value |
|---|---|---|---|
| Age at Listing (years) Median (IQR) | 52 (42–60) | 48 (39–57) | <0.0001 |
| Female Sex % (N) | 39.7 (1389) | 44.4 (2395) | <0.0001 |
| Body Mass Index at Listing (kg/m2) Median (IQR) | 28.32 (24.49–32.76) | 29.21 (25.00–33.86) | <0.0001 |
| History of Diabetes % (N) | 40.9 (1428) | 45.6 (2462) | <0.0001 |
| Blood Type % (N) | <0.0001 | ||
| A | 38.5 (1344) | 23.6 (1273) | |
| AB | 3.3 (115) | 3.9 (210) | |
| B | 9.5 (332) | 21.6 (1166) | |
| O | 48.8 (1704) | 50.9 (2751) | |
| Calculated Panel Reactive Antibody % (N) | <0.0001 | ||
| 0–10 | 80.2 (2739) | 74.3 (4004) | |
| 10–49 | 7.8 (265) | 8.8 (476) | |
| 50–100 | 12.1 (413) | 16.9 (908) | |
| Active Status at Listing | 71.4 (2493) | 63.4 (3422) | <0.0001 |
Access to LDKT by Race
Upon adjustment and stratification by race, we found that white candidates in Era 2 had 70% increased likelihood of LDKT compared with white candidates in Era 1 (aHR 1.70; 95% CI: 1.46–1.99) (Table 2). Minority candidates also had greater than 2-fold increased likelihood of LDKT in Era 2 (post-implementation), compared with Era 1 (pre-implementation) (aHR 2.05; 95% CI: 1.60–2.62). In the adjusted models, we also found that increasing age at listing was associated with a decreased likelihood of LDKT among white candidates (aHR 0.99; 95% CI: 0.98–0.99) and minority candidates (aHR 0.99; 95% CI: 0.98–1.00). Female sex was associated with increased likelihood of LDKT among white candidates (aHR 1.17; 95% CI: 1.00–1.36) and minority candidates (aHR 1.39; 95% CI: 1.11–1.75). Diabetes was also found to be associated with decreased LDKT, irrespective of race (aHR 0.52; 95% CI: 0.43–0.62 among white candidates and aHR 0.68; 0.54–0.86 for minority candidates). Inactive waitlist status was also associated with decreased LDKT among white and minority candidates (Table 2). Irrespective of race, compared with blood group O waitlist candidates, blood group A candidates had improved LDKT access (Table 2). Compared with white candidates with low levels of sensitization (cPRA 0–10%), sensitized white candidates less commonly achieved LDKT (aHR 0.74; 95% CI: 0.58–0.95 for cPRA 11–49%, aHR 0.38; 95% CI: 0.29–0.49 for cPRA 50–100%). Likewise, minority candidates with cPRA 50–100% had decreased access to LDKT, compared with their unsensitized counterparts (aHR 0.41; 95% CI: 1.60–2.62).
Table 2.
Multivariable model for receipt of LDKT by race (n=8895)
| White Candidates | Minority Candidates | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Era 2 | 1.70 (1.46–1.99) | 2.05 (1.60–2.62) |
| Age at Listing | 0.99 (0.98–0.99) | 0.99 (0.98–1.00) |
| Female sex | 1.17 (1.00–1.36) | 1.39 (1.11–1.75) |
| Diabetes | 0.52 (0.43–0.62) | 0.68 (0.54–0.86) |
| Inactive Status | 0.20 (0.16–0.26) | 0.06 (0.03–0.10) |
| ABO | ||
| A | 1.23 (1.06–1.46) | 1.48 (1.13–1.95) |
| AB | 1.17 (0.75–1.81) | 1.66 (0.99–2.81) |
| B | 0.94 (0.70–1.26) | 1.18 (0.88–1.58) |
| O | REF | REF |
| cPRA | ||
| 0–10 | REF | REF |
| 11–49 | 0.74 (0.58–0.95) | 1.16 (0.85–1.59) |
| 50–100 | 0.38 (0.29–0.49) | 0.41 (0.29–0.59) |
Access to Living Donor Kidney Transplantation by Race, Sex, and Sensitization
White Candidates
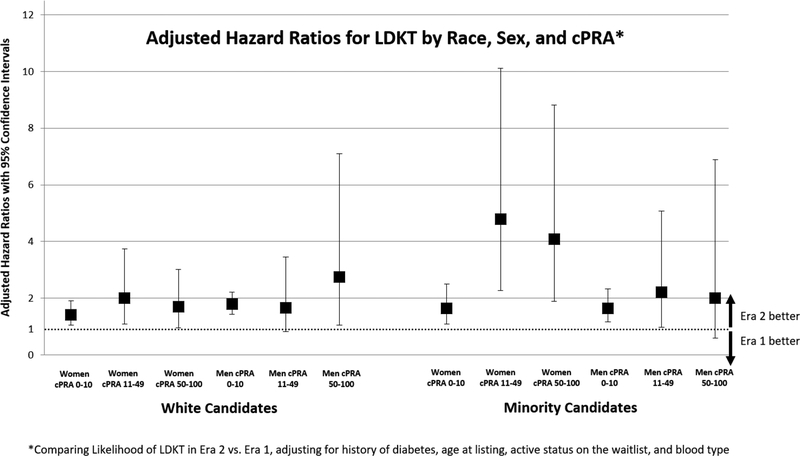
When adjusting for age, diabetes, and active status on the waitlist, we compared access to LDKT in Era 2 with Era 1, by race, sex, and measures of sensitization. Among white women candidates, there was increased likelihood of LDKT in Era 2 compared with Era 1 among candidates with cPRA 0–10% (aHR 1.42; 95% CI: 1.06–1.91) and those with cPRA 11–49% (aHR 2.01; 95% CI: 1.08–3.74). White women candidates with cPRA 50–100% did not have statistically significant change in likelihood of LDKT in Era 2 compared with Era 1 (aHR 1.70; 95% CI: 0.96–3.01). White male candidates with cPRA 0–10% and cPRA 50–100% had increased likelihood of LDKT in Era 2 compared with Era 1 (aHR 1.79; 95% CI: 1.44–2.21 and aHR 2.75; 95% CI: 1.06–7.10 respectively). White men with cPRA 11–49% had no significant change in access to LDKT following implementation of the incompatible program (aHR 1.67; 95% CI: 0.82–3.45).
Minority Candidates
Minority women had increased likelihood of LDKT in Era 2 compared with Era 1 in all cPRA categories. Minority women candidates with cPRA 0–10% were found to have 65% increased likelihood of achieving LDKT in Era 2 compared with Era 1 (aHR 1.65; 95% CI: 1.08–2.51). However, among sensitized women there was a greater than 4-fold likelihood of LDKT in Era 2 compared with Era 1 (aHR 4.79; 95% CI: 2.27–10.11 among women with cPRA 11–49% and aHR 4.09; 95% CI: 1.89–8.82 among women with cPRA 50–100%). For minority men, there was increased access to LDKT in Era 2 among less sensitized men (aHR 1.65; 95% CI: 1.17–2.33) compared with their counterparts in Era 1. There were no statistically significant differences in access to LDKT in Era 2 compared with Era 1 among minority men with cPRA 11–49% (aHR 2.22; 95% CI: 0.97–5.08) or cPRA 50–100% (aHR 2.01; 95% CI: 0.59–6.89).
Subgroup Analysis- Description of Incompatible Program Participants
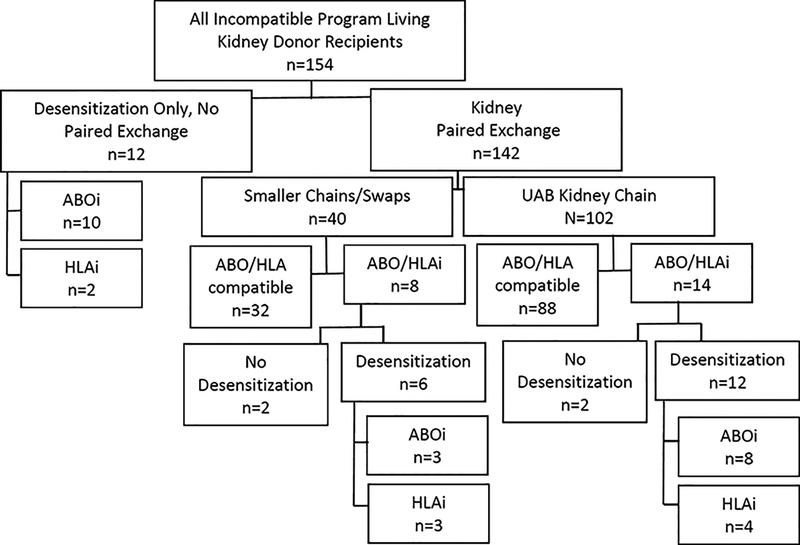
Among the participants who achieved LDKT in the incompatible program, 12 LDKT recipients underwent desensitization only and were not part of a kidney paired exchange (Figure 2). Of those, 10 donor-recipient pairs were ABOi, while 2 were HLAi. Of the LDKT recipients who were part of the kidney paired exchange (n=142), 40 donor-recipient pairs were part of smaller kidney paired exchange swaps or chains, with 32 ABO and HLA compatible LDKT performed. Within the smaller chains or swaps, there were 3 HLAi and 3 ABOi LDKT performed requiring desensitization and 2 incompatible transplants that did not require desensitization (A2 incompatible or low titers). 102 donor-recipient pairs achieved transplantation through the nation’s longest single-center chain, and of those 12 recipients required desensitization due to ABO (n=8) or HLA(n=4) incompatibility.
Figure 2.
Flow diagram for LDKT recipients within the incompatible program. Recipients either underwent desensitization for ABO incompatibility (ABOi) or HLA incompatibility (HLAi) between donor-recipient pairs, or they entered the paired exchange program, or a combination of the two strategies.
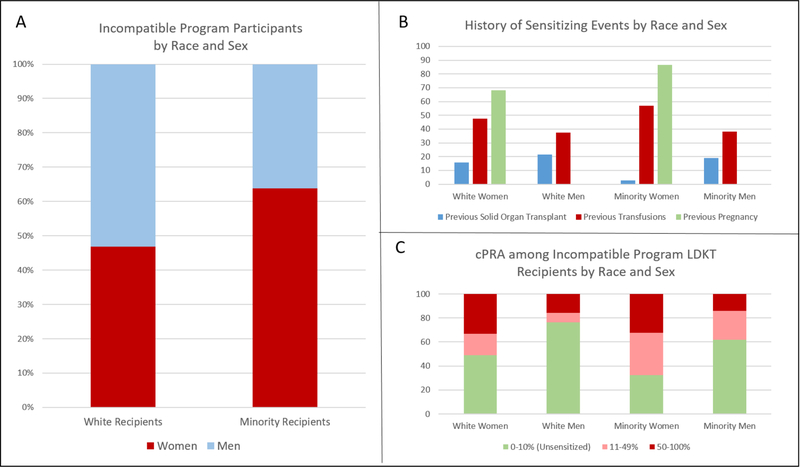
Among minority transplant recipients in the incompatible program, 63.8% (n=37) were female, compared with 46.9% (n=45) of white transplant recipients in the program (Figure 3A). Many of the transplant recipients in the incompatible program had history of sensitizing events including 16.2% (n=25) who had prior solid organ transplant, 44.8% (n=69) who had previous transfusion(s), and 76.4% (n=63) of women had a prior pregnancy. Among white women, 15.6% (n=9) had previous solid organ transplant, 46.7% (n=21) had prior transfusion(s), and 68.9% (n=31) had previous pregnancy (Figure 3B). Among white men, 21.6% (n=11) had undergone previous transplantation, while 37.3% (n=19) had transfusion history. Only one minority woman had prior transplant (2.7%), but 56.8% (n=21) had transfusion history and 86.5% (n=32) had previous pregnancies. 19.1% (n=4) of minority men had history of prior transplant, and 38.1% (n=8) had transfusion history. Importantly, prior pregnancy was the most frequently reported sensitizing event and was most common among minority women. Regarding measures of sensitization, 48.9% (n=22) of white women had cPRA 0–10%, while 76.5% (n=39) of white men had cPRA 0–10% (Figure 3C). Among minority recipients, only 32.4% (n=12) of women were unsensitized, with a cPRA 0–10%, compared with 61.9% (n=13) of men (Figure 3C).
Figure 3.
Panel A: Incompatible program participants by race and sex; Panel B: Proportion of LDKT recipients in the incompatible LDKT program with history of sensitizing events, by race and sex; Panel C: Calculated panel reactive antibody (cPRA) distribution among incompatible program LDKT recipients by race and sex.
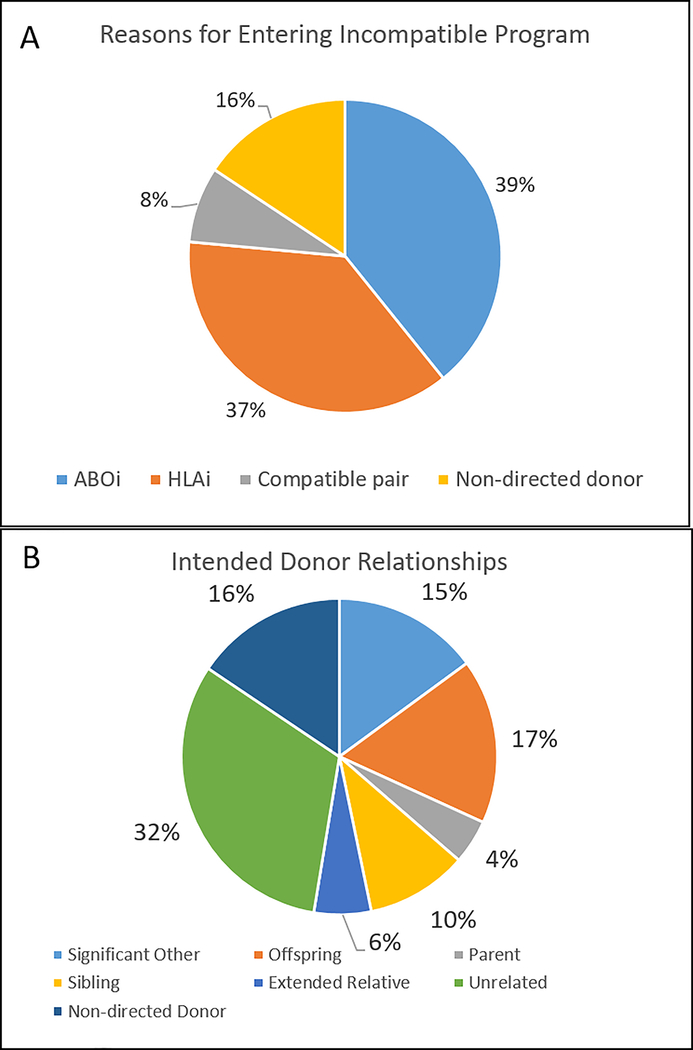
The majority of donor-recipient pairs entered the incompatible program due to either HLA (n=57, 37.0%) or ABO incompatibility (n=60, 39.0%) (Figure 4A). However, 7.8% (n=12) of the donor-recipient pairs were compatible pairs who entered the program, although several of those donor-recipient pairs had either size (n=1) or age mismatch (n=2). The remaining 15.6% (n=24) of donor-recipient pairs were the result of non-directed donors who were paired with waitlist candidates in order to enter the paired exchange program. When assessing relationship of the donor to the intended recipient, 14.9% (n=23) were significant others, while 16.9% (n=26) were offspring, 4.6% (n=7) were parents, 10.4% (n=16) were siblings, and 5.8% (n=9) were extended relatives (Figure 4B). The other donor-recipient relationships included those who were unrelated (n=49, 31.8%) and donation from non-directed donors paired with waitlist candidates (n=24, 15.6%).
Figure 4.
Panel A: Incompatible LDKT recipient reasons for entering the incompatible program; Panel B: Intended donor relationships. ABOi- ABO incompatible, HLAi- HLA incompatible
DISCUSSION
This study of nearly 9,000 kidney transplant waitlist candidates at a single institution sought to assess the significance of expanding LDKT through incompatible transplantation and KPE in order to improve access to transplantation, particularly among minority populations. Most of the transplant recipients entered our incompatible program due to HLA or ABO incompatibility with their original intended donors. When assessing prior sensitizing events, over 75% of women transplant recipients in the incompatible program had a previous pregnancy, and nearly one-half of all incompatible program transplant recipients had prior blood transfusions. Importantly, after implementation of an incompatible program at our institution, incorporating the nation’s longest single-center chain, there was a 70% increase in likelihood of LDKT among white candidates and greater than 100% increase in likelihood of LDKT among minority waitlist candidates. Furthermore, the waitlist candidates who achieved the greatest improvement in likelihood of LDKT post-implementation compared with pre-implementation were sensitized minority women with a greater than four-fold increased likelihood of LDKT.
While this study is the first, to our knowledge, to assess the role for incompatible programs to improve LDKT likelihood among minority women, previous work has demonstrated a potential for KPE to improve LDKT access for disenfranchised patient populations. One of the first studies that assessed the underlying cause of gender disparities in LDKT was a single-center retrospective cohort study by Bromberger et. al, in which the authors demonstrated that disparate LDKT access among women occurred at the time of histocompatibility testing.11 The authors found that women were less likely to identify compatible donors, and were more likely to have history of sensitizing event. Similar to our findings, they found that inclusion in KPE program diminished the gender disparity in access to LDKT.11 Likewise, Melancon and colleagues found that among LDKT recipients served by a combined KPE and desensitization program, ethnic minority candidates were well-represented, leading the authors to suggest that incompatible programs may be associated with improved access to transplantation among minority patient populations with traditionally poor kidney transplantation rates despite high burdens of end-stage renal disease.19
Because of the increased access to LDKT offered by incompatible programs among all patient populations, and particularly underserved populations, the expansion of these programs should be encouraged. The importance of this strategy aimed at ameliorating the organ shortage has been appreciated in the transplant community over the past several years. In fact, recent data suggests that the utilization of KPE has increased 24-fold from 2005 to 2016.20 However, there remains a need to continue the trend of KPE program development, as LDKT overall in the United States has been on the decline.20 Moreover, through a national paired exchange program, greater access to kidney transplantation could be achieved, due to an increase in the pool of potential donor-recipient matches. Modeling by Gentry and colleagues demonstrated that a national registry including compatible pairs would facilitate improved match rates for incompatible donor-recipient pairs.21 The authors also demonstrated that even at the center-level, inclusion of compatible donor-recipient pairs, such as those who participated in our program, would serve to improve the probability of identifying compatible matches by nearly 2-fold.21
This study has several strengths, namely the diverse patient population served by our transplant center which allowed us to assess trends in access to transplantation by race and sex. Also the population was large for a single center study. However, the population may not be representative of other transplant waiting list populations, and as such the results may not be generalizable to all other transplant centers. Additionally, we were unable to identify patients who had entered the incompatible program and did not achieve LDKT, as the database was updated continuously and did not track donor-recipient pairs who left the program for reasons other than achieving transplantation in the program. Also, it is possible that unmeasured confounding may have contributed to the observed era effect aside from implementation of the incompatible program. In assessing donor-recipient relationship and reasons for entering the incompatible program, we only included the reason or relationship for the approved incompatible donor who entered the program. There were likely other donor candidates screened for these participants, and those were not captured or included in this study. Finally, as the focus of our study was LDKT, we did not assess trends in deceased donor kidney transplantation among sensitized waitlist candidates.
The results of this large single-center cohort study demonstrated that implementation of an incompatible LDKT program was associated with improved likelihood of LDKT among both white and minority waitlist candidates. However, the population that appreciated the greatest improvement in access to LKDT in the post-implementation era was sensitized minority women. Therefore, more widespread utilization of incompatible LDKT, particularly through KPE, should be considered in order to alleviate disparities in access to LDKT. Furthermore, improved utilization of a single national KPE program would mitigate costs associated with pursuing incompatible transplantation at the transplant center level, and may allow for improved access to LDKT for vulnerable populations, including minority women.
Figure 1.
Adjusted Hazard Ratios for living donor kidney transplantation in Era 2 vs. Era 1: by sex, race, and calculated panel reactive antibody (cPRA)
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH)- National Research Service Award, through Grant Award Number T32 DK007545 (PI Mustian, mentored), and the National Institute of Diabetes and Digestive and Kidney Diseases – R01 DK113980 (PI Locke) and K23 DK103918 (PI Locke, mentored). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Footnotes
To be presented on April 12, 2019 at the American Surgical Association in Dallas, TX.
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine 1999;341:1725–30. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System. 2018 USRDS annual data report: Epidemiology of kidney disease in the United States National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2018. [Google Scholar]
- 3.Lee LY, Pham TA, Melcher ML. Living Kidney Donation: Strategies to Increase the Donor Pool. The Surgical clinics of North America 2019;99:37–47. [DOI] [PubMed] [Google Scholar]
- 4.Ladin K, Rodrigue JR, Hanto DW. Framing disparities along the continuum of care from chronic kidney disease to transplantation: barriers and interventions. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2009;9:669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gore JL, Danovitch GM, Litwin MS, Pham PT, Singer JS. Disparities in the utilization of live donor renal transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2009;9:1124–33. [DOI] [PubMed] [Google Scholar]
- 6.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clinical journal of the American Society of Nephrology : CJASN 2010;5:2276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purnell TS, Hall YN, Boulware LE. Understanding and overcoming barriers to living kidney donation among racial and ethnic minorities in the United States. Advances in chronic kidney disease 2012;19:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waterman AD, Rodrigue JR, Purnell TS, Ladin K, Boulware LE. Addressing racial and ethnic disparities in live donor kidney transplantation: priorities for research and intervention. Seminars in nephrology 2010;30:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purnell TS, Xu P, Leca N, Hall YN. Racial differences in determinants of live donor kidney transplantation in the United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013;13:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purnell TS, Luo X, Cooper LA, et al. Association of Race and Ethnicity With Live Donor Kidney Transplantation in the United States From 1995 to 2014. Jama 2018;319:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bromberger B, Spragan D, Hashmi S, et al. Pregnancy-Induced Sensitization Promotes Sex Disparity in Living Donor Kidney Transplantation. J Am Soc Nephrol 2017;28:3025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montgomery RA, Locke JE, King KE, et al. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation 2009;87:1246–55. [DOI] [PubMed] [Google Scholar]
- 13.Hyun J, Park KD, Yoo Y, et al. Effects of different sensitization events on HLA alloimmunization in solid organ transplantation patients. Transplantation proceedings 2012;44:222–5. [DOI] [PubMed] [Google Scholar]
- 14.Lopes D, Barra T, Malheiro J, et al. Effect of Different Sensitization Events on HLA Alloimmunization in Kidney Transplantation Candidates. Transplantation proceedings 2015;47:894–7. [DOI] [PubMed] [Google Scholar]
- 15.Ursin G, Bernstein L, Wang Y, et al. Reproductive factors and risk of breast carcinoma in a study of white and African-American women. Cancer 2004;101:353–62. [DOI] [PubMed] [Google Scholar]
- 16.Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System 2012;60:1–21. [PubMed] [Google Scholar]
- 17.Pham TA, Lee JI, Melcher ML. Kidney paired exchange and desensitization: Strategies to transplant the difficult to match kidney patients with living donors. Transplantation reviews (Orlando, Fla) 2017;31:29–34. [DOI] [PubMed] [Google Scholar]
- 18.Segev DL, Gentry SE, Melancon JK, Montgomery RA. Characterization of waiting times in a simulation of kidney paired donation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2005;5:2448–55. [DOI] [PubMed] [Google Scholar]
- 19.Melancon JK, Cummings LS, Graham J, et al. Paired kidney donor exchanges and antibody reduction therapy: novel methods to ameliorate disparate access to living donor kidney transplantation in ethnic minorities. Journal of the American College of Surgeons 2011;212:740–5; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 20.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 Annual Data Report: Kidney. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2018;18 Suppl 1:18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gentry SE, Segev DL, Simmerling M, Montgomery RA. Expanding kidney paired donation through participation by compatible pairs. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2007;7:2361–70. [DOI] [PubMed] [Google Scholar]