Abstract
A poly(7-oxanorbornene-2-carboxylate) polymer containing pendent triethyleneglycol (TEG) chains of 2.8 MDa (“2.8M TEG”) was synthesized and evaluated for long-term lubrication and wear reduction of ex vivo bovine cartilage as well as for synovitis in rats and dogs after intra-articular administration. Bovine cartilage surfaces were tested under torsional friction for 10,080 rotations while immersed in either saline, bovine synovial fluid (BSF), or 2.8M TEG. For each solution, coefficient of friction (μ), changes in surface roughness, and lost cartilage glycosaminoglycan were compared. To directly compare 2.8M TEG and BSF, additional samples were tested sequentially in BSF, BSF, 2.8M TEG, and then BSF. Finally, another set of samples were tested twice in saline to induce surface roughness and then tested in BSF, Synvisc, or 2.8M TEG to determine each treatment’s effect on worn cartilage. Next, male Lewis rats were injected in one knee with 2.8M TEG or saline and evaluated for effects on gait, and female beagles were injected with either 2.8M TEG or saline in one knee, and their synovial tissues analyzed for inflammation by H&E staining. Treatment with 2.8M TEG lowers μ, lessens surface roughness, and minimizes glycosaminoglycan loss compared to saline. The 2.8M TEG also reduces μ compared to BSF in pairwise testing and on worn cartilage surfaces. Injection of 2.8M TEG in rat or beagle knees gives comparable effects to treatment with saline, and does not cause significant synovitis.
Keywords: biolubricant, synovial fluid, viscosupplement, chondroprotection, osteoarthritis, CT imaging
Graphical Abstract
INTRODUCTION
In healthy diarthrodial joints, articular cartilage and synovial fluid (SF) maintain a nearly frictionless surface that supports and dissipates applied loads. Specifically, interstitial fluid exudation from cartilage surfaces enables fluid-enhanced lubrication during locomotion by creating a fluid film between apposing cartilage surfaces in the articulating joint. Further, the SF constituents hyaluronic acid (HA) and lubricin maintain low friction by preventing direct contact between the apposing cartilage surfaces. During early osteoarthritis (OA) the hydraulic permeability of cartilage increases due to loss of glycosaminoglycans (GAGs)1–3 resulting in more rapid fluid excretion during joint movements and diminished fluid-film lubrication. Therefore, more of the friction is born by the extracellular matrix surface. This occurs simultaneously with decreases in the concentrations of lubricin4 and HA5, affording early surface damage to the collagen network. This damage decreases cartilage tensile stiffness initially at the surface, but eventually progresses to the deeper layers with a reduction of cartilage mechanical integrity6–8, thinning of the cartilage tissue, and formation of cartilage lesions. The higher frictional loading9 and severe wearing10 eventually erode the cartilage surfaces, and movement can become painful as bone-on-bone contact occurs. Therefore, early OA treatments that reduce excessive cartilage wear would be beneficial.
Current OA treatments range in complexity and invasiveness depending on the severity of the disease. Typical treatments for early-stage OA include weight loss, exercise, activity modification, assistive devices (e.g., canes), and non-steroidal anti-inflammatory drugs. When these methods are unsuccessful or the disease progresses, prescription anti-inflammatory drugs (e.g., intraarticular corticosteroids) or surgical techniques (including partial or total joint arthroplasty) are required. Joint arthroplasty is typically considered a last resort for patients and is the most invasive. Microfracture, osteochondral transfer, and autologous chondrocyte implantation are less invasive and are moderately effective at treating cartilage focal defects of up to 1 cm2 in size.11–13 Despite their moderate success at addressing small-to-medium cartilage defects, these invasive treatments are only typically considered once significant cartilage surface erosion has occurred. Therefore, minimally invasive treatments that reduce or prevent cartilage erosion are of interest as early treatments.
One such approach is the use of injectable SF supplements. In an effort to delay or obviate surgical intervention, HA solutions are injected intra-articularly (i.e., viscosupplementation) to lubricate and cushion cartilage14, 15. Yet, there is significant controversy surrounding the use of viscosupplements (e.g., Synvisc, Orthovisc, Hyalgan, and Supartz) due to their limited clinical efficacy, susceptibility to hyaluronidase enzymatic degradation, and short residence time (t1/2 = 1–3 days) in the synovial joint16, 17. Moreover, such viscosupplements have demonstrated chondroprotection only in ex vivo and limited small animal models.18, 19 Hence, there is a need for new treatments that reduce cartilage friction and wear to provide chondroprotection.
We hypothesize that supplementing SF with a polymer that reduces the coefficient of friction (μ) will minimize cartilage wear, and ultimately delay or mitigate the need for more invasive OA treatments. Towards this goal, we are investigating high molecular weight polyelectrolytes as viscous biolubricants for cartilage. Several classes of cartilage protectants are under preclinical investigation20 and include lubricin21, 22 and lubricin mimics,23–25 liposomes,26 phospholipid coated silk microspheres,27 and high molecular weight lubricious polymers,28, 29 the latter of which can form viscous aqueous solutions due to polymer chain entanglement and/or crosslinking. From a tribological design perspective, a viscous lubricant creates a thin film between the cartilage surfaces to ensure that a pressurized fluid film more easily forms. This thin film reduces the prevalence of boundary lubrication, thus minimizing the harshest frictional loads. We recently reported the synthesis of poly(7-oxanorbornene-2-carboxylate)30 as an effective biolubricant in cartilage-on-cartilage torsional friction tests ex vivo31 and in a rat model of osteoarthritis.32 However, this polymer is highly anionic. To better match the osmolality of healthy SF (400 mOsm/kg,33), to reduce the overall negative charge of the polymer when dissolved in saline, and to examine the effect of altering the polymer architecture/composition, we synthesized a linear poly(7-oxanorbornene-2-carboxylate) of molecular weight and covalently conjugated pendent triethyleneglycol (TEG) chains to prepare a bottle-brush polymer lubricant of 2.8 MDa referred to as “2.8M TEG” after its molecular weight and TEG attachments (Figure 1). Herein, we report the synthesis of 2.8M TEG, its performance in three ex vivo long-duration friction tests compared to saline and bovine SF using intact and previously worn bovine cartilage, and initial biocompatibility studies after intra-articular injection in murine and canine models.
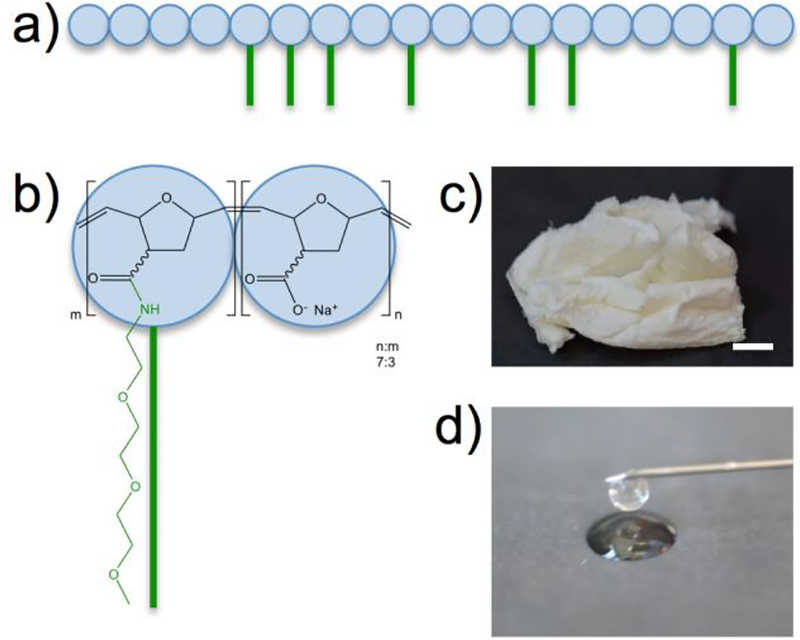
Figure 1.
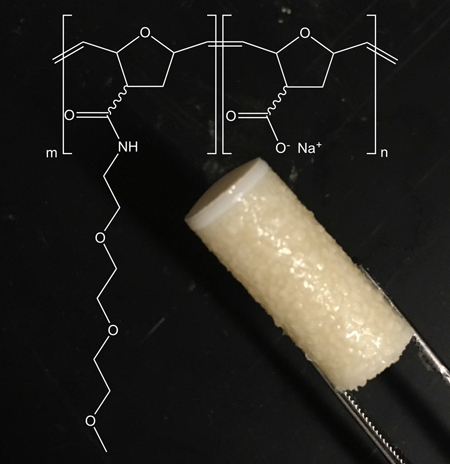
a) Graphical representation of the bottle brush 2.8M TEG lubricant. b) Chemical structure of the 2.8M TEG polymer. c) Photograph of the isolated 2.8M TEG polymer after synthesis. Scale bare = 1 cm d) Photograph of an aqueous solution of the 2w/v% 2.8M TEG ejecting from a 26G needle.
EXPERIMENTAL
Specimen Preparation and Study Design
Twenty-six mated, osteochondral plug pairs were cored from the femoral groove (12mm diameter) and patella (7mm) of seven freshly slaughtered, skeletally mature cows. All plugs were then frozen at −20 °C in 400 mOsm/kg saline containing GIBCO Anti-Anti stock solution (5x; Invitrogen, Grand Island, NY), ethylenediaminetetraacetic acid (5 mM; Sigma, St. Louis, MO), and benzamidine HCl (5 mM; Sigma B6506, St. Louis, MO). These same additives were included in all solutions that were exposed to the samples to prevent nonspecific cartilage degradation during the experiments as a result of the samples being left at room temperature or at 4 °C for extended periods. Prior to testing, all samples were thawed overnight at 4 °C. Twelve plug pairs were used to evaluate the frictional properties and wear prevention of saline, bovine synovial fluid (BSF), and 2.8M TEG during a long-duration torsional friction test (Study 1). In Study 2, three plug pairs were used to directly compare 2.8M TEG to BSF by testing the pairs in 2.8M TEG following testing in BSF. Finally, eleven plug pairs were used to evaluate the ability of BSF, Synvisc, and 2.8M TEG to improve the frictional properties of previously worn cartilage (Study 3). Group bias was controlled by using neighboring plug pairs from the same knees for each lubricant group.
Contrast Agent (CA4+) and 2.8M TEG Preparation
The computed tomography (CT) contrast agent CA4+ was synthesized as previously reported34. The contrast agent solution was prepared by dissolving the dry compound in deionized water, balancing the pH to 7.4 using NaOH, and adjusting the osmolality to 400 mOsm/kg using sodium chloride to match the in situ osmolality of articular cartilage (350–450 mOsm/kg33).
The linear 2 MDa poly(7-oxanorbornene-2-carboxylate) polymer was synthesized as previously reported30, 31 (see Supporting Information, SI, for details), and triethyleneglycol (TEG) chains were added to a third of the available carboxylic acids using carbodiimide coupling in phosphate buffer at pH=6 to afford the 2.8 MDa polymer referred to as 2.8M TEG (Figure SI–1). The 2.8M TEG lubricant solution was then prepared by dissolving the polymer in deionized water at 2 w/v%, balancing the pH to 7.4 using NaOH, and adjusting the osmolality to 400 mOsm/kg using sodium chloride.
Baseline Surface Photographing and microCT Imaging
For all studies, the congruent cartilage surfaces of the osteochondral plugs were photographed (1x and 2.25x magnification) using a microscope camera (PL-B681CU, PixeLINK, Ottawa, ON) to ensure all plugs had comparably smooth cartilage surfaces prior to friction testing. For Study 1, the plugs were then immersed in the CA4+ contrast agent at 12 mgI/mL (patella: 1 mL, groove: 3 mL) for 24 h at room temperature. The samples were then imaged on a microCT scanner (μCT40, Scanco Medical AG, Brüttisellen, Switzerland), and the microCT data were post-processed in Analyze™ (Analyze Direct, Overland Park, KS) using our previously developed protocols35 (see SI). The thicknesses of the segmented cartilage object maps were then measured at 5 points across the width of the tissue for all coronal slices. Following contrast-enhanced CT (CECT) imaging, each sample was washed in excess saline for 24 hr to desorb the contrast agent. Since sample surface roughness was not examined in Studies 2 & 3, the cartilage thicknesses for these studies were measured using the same procedure as for Study 1, except the thicknesses were obtained from non-contrast-enhanced microCT scans.
Baseline Coefficient of Friction (μ) Testing
The baseline frictional properties of all plug pairs were evaluated at room temperature using saline as the test solution and the same mechanical testing procedure36 (see SI). Briefly, each plug pair was compressed 18% (Enduratec 3230, BOSE, Eden Prairie, MN), relaxed for 70 min (stress equilibrium), and then the femoral groove plug was rotated against the patellar plug for 720° at 5°/sec36. Static and dynamic coefficients of friction (μstatic, μstatic_eq, and μkinetic) were calculated (see SI), and all samples were then allowed to recover in saline overnight.
Long-Duration Friction Testing
The plug pairs in Study 1 were immersed in either the 2.8M TEG biolubricant (2 w/v%), BSF, or saline (n=4 plug pairs each; all plugs from the same animal) for 4 h at room temperature prior to long-duration testing as described previously (see SI for details).32 Briefly, plugs from the femoral groove were rotated against the plugs from the patella at 360°/sec (22 mm/sec) for 10,080 rotations, while under 0.78 MPa compressive stress at room temperature (see SI). Every 160 rotations, there was a “lift-off” to allow lubricant re-introduction between the surfaces. The average μ (μmean) was calculated for each of the sixty-three 160-rotation periods between lift-offs. For Study 1 only, the rotations were paused every hour for extraction of a 100-μL lubricant aliquot for later analysis. After each test, the samples were allowed to recover for >16 h in saline.
For Study 2, three plug pairs were subjected to the same long-duration friction test on four sequential days. Using BSF as the lubricant, the first test was for preconditioning the samples. The lubricants for the subsequent tests (Tests 1–3) were BSF, 2.8M TEG (2 w/v%), and BSF, respectively.
For Study 3, all the plug pairs were subjected to the long-duration testing once per day on three sequential days: the first two days (“wear tests,” lubricated by saline) were intended to progressively “wear” the cartilage, and the third day (“lubricant test”) was to evaluate the efficacy of each lubricant (either BSF (n=4), Synvisc (n=3), or 2.8M TEG (2 w/v%) (n=4)) on “worn” cartilage.
Analysis of Wear
For all studies, the cartilage surface roughness of each plug was evaluated by photography as well as CECT as described previously32 (see SI). Briefly, after the long-duration testing, circular grooves (rings) developed on some of the samples, indicating surface wear.32 Two observers, blinded to the experimental groups, counted the number of rings in the surface photographs for the samples from Study 1 to evaluate the ability of the different lubricants to prevent cartilage surface wear. The average number of rings and standard deviation for each lubricant group was then computed.
Additionally, five sequential coronal and five sequential sagittal CECT images pre- and post-long-duration testing were evaluated to measure the change in each plug’s cartilage surface roughness (see SI)32, 37.
The mass of GAG in the lubricant solutions from Study 1 was measured using the 1,9-dimethylmethylene blue (DMMB) colorimetric assay38 (see SI).
In Vivo Studies
Both preliminary in vivo studies were performed by Bolder BioPATH (Boulder, CO), approved by the IACUC at Bolder BioPATH, and were conducted in accordance with the recommendations in the Guide for Care and Use of Laboratory Animals of the National Institutes of Health. For the first in vivo study (Study 4), male Lewis rats were injected in the right knee with 2 w/v% 2.8M TEG (N=4) or saline (N=4) and observed for any changes (left knee served as un-injected control). On day 3, animals were evaluated semiqualitatively for effect on gait and gait deficiency (no loads measured; see SI and Figure SI–2) and then euthanized on day 5. In Study 5, ten female beagle dogs were injected with 2 w/v% 2.8M TEG (N=5) or saline (N=5) into one knee joint. On day 4, animals were euthanized, and the synovium was sectioned and stained with H&E (See Table SI–1). All sections were examined by a board-certified veterinary pathologist for signs of inflammation (Dr. Alison Bendele).
Statistics
One-way ANOVA with Tukey post-hoc tests was used to evaluate differences between: 1) baseline μ for all lubricant groups within each study; 2) μmean for each lubricant group in Study 1; 3) number of “wear rings” on apposing cartilage surfaces for each lubricant group in Study 1; 4) percent increase in surface roughness after long-duration testing for each lubricant in Study 1; 5) mass of GAG in lubricant solutions from Study 1; 6) differences in μmean for Test 2 and Test 3 each normalized to Test 1 in Study 2; and, 7) the percent change in μmean for the “lubricant test” on day 3 relative to the “wear test” on day 2 for all time points and lubricants in Study 3. Significance level was set as two-tailed p<0.05.
RESULTS
Synthesis
The poly(7-oxanorbornene-2-carboxylate) polymer was synthesized with a Mn of 2 MDa g/mol (PDI=1.2), and the TEG chains were successfully added to 30% of the polymer’s carboxylate groups to give the 2.8 MDa polymer (2.8M TEG). Reaction yields for the two steps ranged between 80–95% (n=3), and the final 2.8M TEG (Figures 1 and SI–1) polymer was isolated as a white solid, similar in texture to HA (Figure 1c). The 2.8M TEG was completely soluble in water at 2 w/v%, and administered through a small 26G needle (Figure 1d). The viscosity of the 2.8M TEG 2 w/v% solution is ≈1.5 Pa·s at a shear rate of 1 Hz. For comparison, the viscosity of Synvisc is ≈1000 Pa·s, and does not readily pass through a 26G needle.
Long-Duration Friction Properties (Study 1)
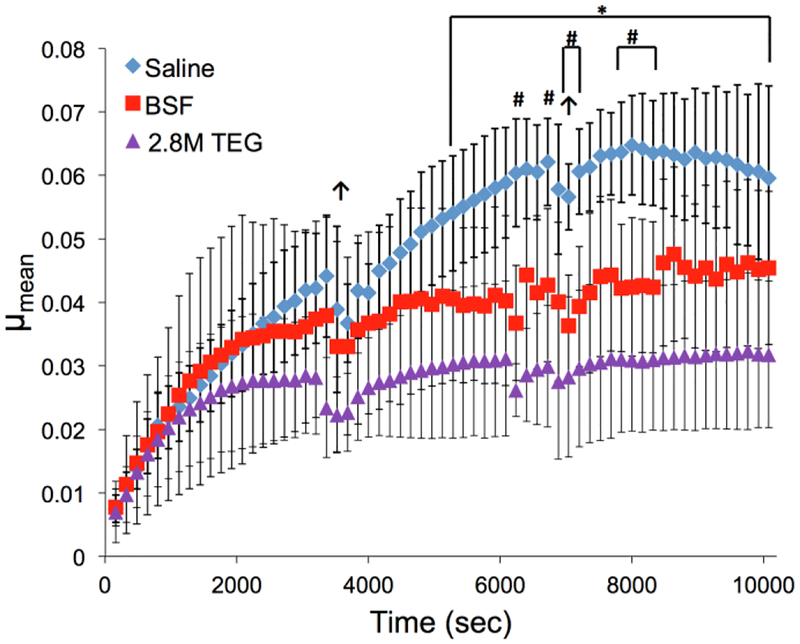
There were no significant differences in the initial baseline coefficient of friction among the plug pairs assigned to saline, BSF or 2.8M TEG (2 w/v%) test groups for Study 1. The saline and BSF data reported herein were also used for comparison with the non-TEGylated predecessor to this lubricant32 using plug pairs from the same bovine knees as this study to ensure cartilage plug consistency. Long-duration friction testing of plug pairs lubricated with 2.8M TEG exhibited the lowest μmean values, being statistically significantly lower for all time points after 5280 sec compared to plugs lubricated with saline, which had the greatest μmean results (Figure 2). The non-TEGylated polymer (2.4 MDa)32 exhibited a lower μmean value of 0.016 lower compared to 0.027 for 2M TEG. The samples lubricated with BSF exhibited μmean values greater than those of 2.8M TEG and less than those of saline, being significantly lower than saline for 8 time points after 6240 sec of testing.
Figure 2.
Coefficients of friction (μmean) for bovine cartilage plug pairs (patella against femoral groove) tested in three lubricants (n=4 each) during the long-duration torsional friction regimen (>10,000 rotations) of Study 1. Error bars indicate standard deviation. *2.8M TEG vs Saline (p<0.05), #BSF vs Saline (p<0.05), ↑ indicates pause for lubricant extraction (plugs held apart for ~30 sec). Saline and BSF data reproduced with permission from ref 32. Copyright 2018 Elsevier.
Wear Properties (Study 1)
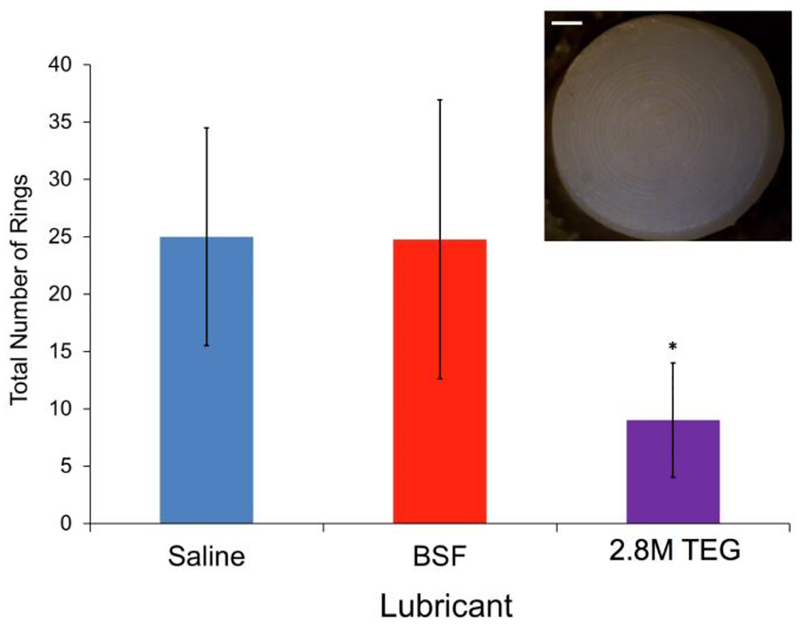
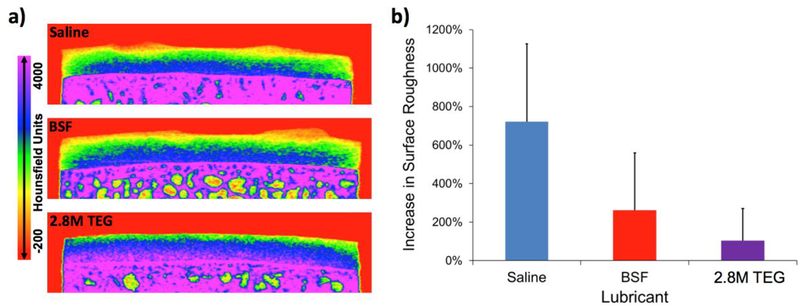
Cartilage plug pairs lubricated with 2.8M TEG (2 w/v%) had fewer circular wear grooves on their surface compared to samples lubricated in saline but not BSF (Figure 3 and insert). Additionally, the samples lubricated with 2.8M TEG had noticeably less rough surfaces following long-duration friction testing than those tested in saline and BSF (Figure 4a). Comparing each femoral groove plug’s post-testing surface roughness to its baseline value, the percent change in roughness after friction testing was significantly lower for samples tested in 2.8M TEG compared to samples lubricated with saline (Figure 4b). The average mass of GAG (a known wear debris product39, 40) in the 2.8M TEG test solutions was undetectable (<3 μg), while the mass of GAG found in the saline wear solutions was substantial at 121.1±33.1 μg (mean ± SD).
Figure 3.
Total number of rings on cartilage plug surfaces from each pair tested (example photo shown in inset) following long-duration torsional friction testing in three lubricants (n=4 each) in Study 1. Error bars indicate standard deviation. *2.8M TEG vs saline (p<0.05). Scale bar is 1 mm.
Figure 4.
a) Representative CECT color maps of femoral groove plugs subjected to long-duration torsional friction testing in Study 1 using saline, BSF, and 2.8M TEG. b) Percent increase in cartilage surface roughness following long-duration torsional friction testing in three lubricants (n=4 each) during Study 1. 2.8M TEG vs Saline (p<0.05). Error bars indicate standard deviation.
Directly Comparing 2.8M TEG to BSF (Study 2)
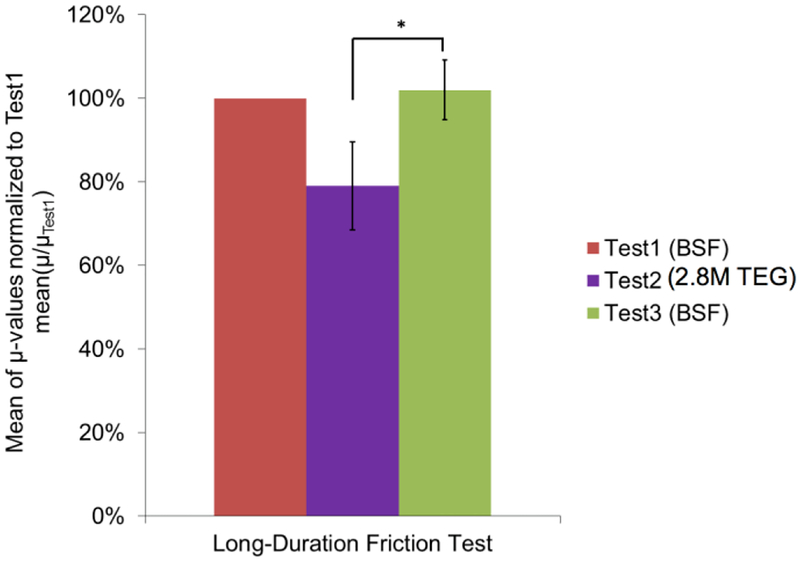
For Study 2, the overall mean coefficient of friction of Test2 (2.8M TEG; 2 w/v%) reduced by 20.9 ± 10.5% (mean ± SD) relative to that of Test1 (BSF), while the mean coefficient of friction of Test3 (BSF) returned to approximately the same value as that in Test1, being 2.0 ± 7.1% greater relative to Test1 (Figure 5), thereby demonstrating the reversibility of 2.8M TEG’s lubricating effect. Further, the normalized Test2 mean μ value was significantly less than the normalized Test3 μ value (p<0.05).
Figure 5.
Mean of each long-duration friction test’s μ-values normalized to the μ-values of Test1 from Study 2 (n=3). The mean coefficient of friction of Test2 (2.8M TEG) reduced by 20.9 ± 10.5% (mean ± SD) relative to that of Test1 (BSF), while the mean coefficient of friction of Test3 (BSF) increased by 2.0 ± 7.1% relative to Test1. Error bars indicate standard deviation. *The normalized Test2 mean μ-value was significantly less than the normalized Test3 μ-value (p<0.05).
Effectiveness of 2.8M TEG on Previously Worn Cartilage Surfaces (Study 3)
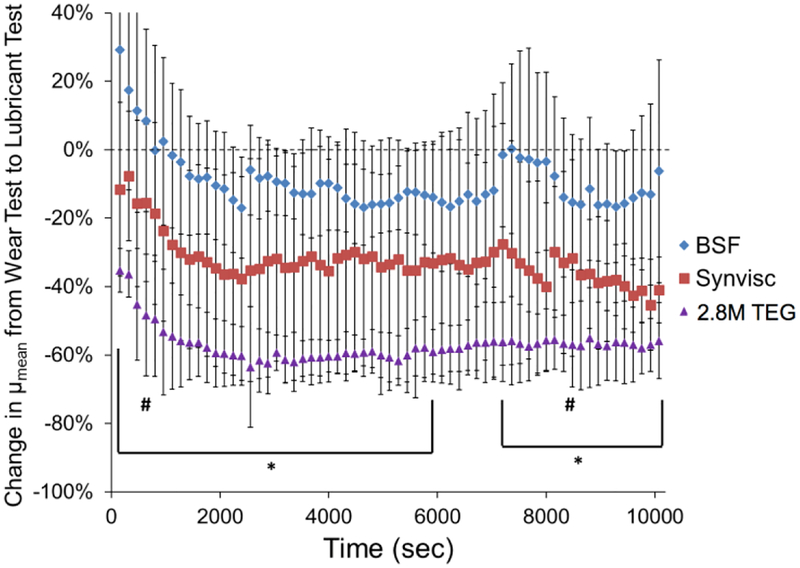
For Study 3, there were no significant differences in baseline μ among the three paired-plug groups assigned to BSF, Synvisc or 2.8M TEG (2 w/v%). The percent reduction in μmean for the day 3 “lubricant test” relative to the day 2 “wear test” was the greatest for 2.8M TEG, followed by Synvisc, then BSF (Figure 6), being significantly greater for 2.8M TEG than for BSF for all time points except 800, 6080, 6240, 6400, 6560, 6720, 6880, 7040 and 8480 sec (p<0.05).
Figure 6.
Percent change in μmean from the day 2 “wear test” to the day 3 “lubricant test” in BSF (n=4), Synvisc (n=3) and 2.8M TEG (n=4) during Study 3. Error bars indicate standard deviation. The percent change in μmean values for 2.8M TEG were significantly lower than those for BSF for all time points except 800, 6080, 6240, 6400, 6560, 6720, 6880, 7040 and 8480 sec. *2.8M TEG vs BSF (p<0.05), except for the two time points indicated with # (800 and 8480 sec).
Rat and Dog In Vivo Studies
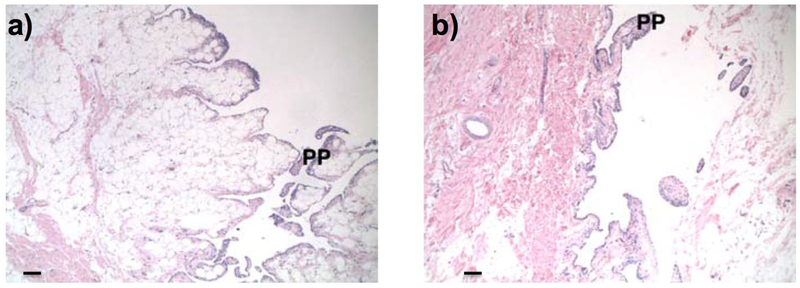
Rats injected intra-articularly with 2 w/v% 2.8M TEG gained approximately 7–8 grams of body weight over the course of the study and had no observed swelling, inflammation, or clinical abnormalities. The results were similar to animals injected with saline (N=4 per group). The rats treated with 2.8M TEG or saline had gait scores of 0 on day 3 after injection (see SI), with mean gait deficiency percentages of 3% for the 2.8M TEG treated animals compared to the saline treated animals. Dog knees injected with saline exhibited normal synovia, with one animal having minimal papillary proliferation (Figure 7a) (N=5 per group). Knees injected with 2 w/v% 2.8M TEG were comparable to saline-injected knees with two animals having minimal papillary proliferation following an established grading system (See SI; Table SI–1; Figure 7b).
Figure 7.
Representative H&E histological sections (50x) of synovium from beagle knees injected with a) saline or b) 2 w/v% 2.8M TEG. Scale bar = 100 μm. Both knees experienced minimal papillary proliferation (PP).
DISCUSSION
To improve upon the shortcomings of current hyaluronic acid viscosupplements, we report a novel, synthetic bottle-brush polyelectrolyte based on poly(7-oxanorbornene-2-carboxylate) possessing TEG sidechains (2.8M TEG) and its efficacy as a cartilage lubricant (Figure 1). From a biomaterials and chemistry perspective, a polyelectrolyte that: 1) possesses an overall fixed negative charge will remain at the cartilage surface due to electrostatic repulsion from the internal cartilage negative fixed charge; 2) incorporates ethylene glycol units within the polymeric structure will enable a highly hydrated state; 3) adopts a bottle-brush polymer architecture reminiscent of lubricin to lubricate the surface; and, 4) possesses a molecular weight greater than 1 MDa to increase its joint space residence time by retarding its diffusion through the synovial membrane. Assuming a 2.8M molecular weight (16,495 repeat units) and TEGylation of 30% of the polymer repeat units, the polymer is highly charged with more than 10,000 formal negative charges. A 2 w/v% aqueous solution of the polymer is lubricious to the touch. Figure 2 shows the significant reduction in coefficient of friction (μmean) for plug pairs lubricated with 2.8M TEG (2 w/v%) compared to samples tested in saline and BSF. The observed plateau formation in the μmean curves indicates the cartilage interstitial fluid pressure has subsided (corroborated by a plateau formation at the same time in each group’s corresponding creep curves). Such a plateau indicates a transition from predominantly hydrodynamic/elastohydrodynamic lubrication during the beginning portion of the test to predominantly either viscous lubrication (for a high-viscosity lubricant, e.g., 2.8M TEG) or boundary lubrication (for a low-viscosity lubricant) during the remainder of the test41. The equilibrium values of μmean (defined as the mean of μmean in the fully plateaued region from 8000 to 10080 sec) for saline, BSF, and 2.8M TEG are 0.0626 ± 0.0015 (mean ± SD), 0.0448 ± 0.0016, and 0.0316 ± 0.0005, respectively. Several reports describe long-term coefficients of friction experiments using a cartilage-on-cartilage setup,39, 42–44 and the most common configuration is linearly reciprocating sliding. However, our predecessor lubricant was evaluated using a torsional configuration, so the same setup was used to evaluate this lubricant for ease of comparison. Although it is difficult to compare μ values directly between torsional and linearly oscillating configurations, our results with saline are within an order of magnitude of these prior reports. For example, the lubricin mimic23, 25, 45 lubricants afford μkinetic values ranging 0.1–0.3 on cartilage, while the liposomes26 exhibit μkinetic values ranging 0.02–0.1 and phospholipid coated silk microspheres range 0.02–0.06.27 A high molecular weight lubricious polymer with poly(2‐methyl‐2‐oxazoline) sidechains gives μkinetic values spanning 0.02–0.1.28 The 2.8M TEG biolubricant provides superior lubrication even after the interstitial fluid pressure has subsided, indicating the viscous lubricant is superior to healthy BSF and saline under these loading conditions.
Fitting an exponential of the form μmean=a*e−b*time+c (MATLAB 2011a, MATLAB, Natick, MA) to the curves, the tau values (1/b), representing the time at which 63.2% of the plateau μmean value is reached46, 47, are 4527 ± 2321 sec (mean ± SD), 3253 ± 4112, and 1070 ± 281 for saline, BSF, and 2.8M TEG, respectively. Although the samples were harvested from neighboring locations, the differences in μ curves and tau values for each lubricant are rationalized based on Stribeck principles. Since 2.8M TEG is substantially more viscous than saline, and the mechanical testing parameters are the same for both solutions, samples tested in the 2.8M TEG maintain mixed-mode lubrication as their interstitial-fluid pressure subsides, while samples tested in saline transition to boundary lubrication. During mixed-mode lubrication, the thin film between the cartilage surfaces reduces friction below that of boundary lubrication, hence the μ values for 2.8M TEG plateau sooner and lower than those of saline.
The presence of the polyelectrolyte biolubricant 2.8M TEG also affords significantly less cartilage surface wear than saline. The circular grooves that formed on the cartilage surfaces during testing (Figure 3) likely developed as thicker regions of the cartilage tissues shear against each other during the rotations. We hypothesize that when testing in saline, after the interstitial fluid pressure has subsided, the macroscopically smooth cartilage wears at these high-stress regions as any remaining hyaluronic acid (HA) or lubricin molecules on the cartilage surfaces are forced out of the interface. However, when testing in BSF, HA and lubricin macromolecules can re-deposit between the cartilage surfaces during liftoffs, thus preventing as much direct matrix-matrix contact, which results in less surface wear. When the 2.8M TEG is used, these large polymers reside between the cartilage surfaces, affording fewer wear rings (Figure 3). This hypothesis of a lubricating polymer’s resistance to being squeezed away from an articulating surface is supported by other investigations of polymer morphology under tribological load, with polymer chains flattening yet not dissipating from the interface.48, 49 To further analyze the cartilage surface roughness, we extended a technique originally developed for analyzing Safranin-O stained histology slices37 to our cartilage CECT data. The samples tested in saline experience a ~7.22x increase in surface roughness compared to baseline, while samples tested in BSF and 2.8M TEG undergo a 2.62x and 1.05x increase, respectively (Figure 4). These results agree with the mass of GAG released into the lubricant solutions during testing. More GAG is lost from the cartilage samples tested in saline (121.1 ± 33.1 μg (mean ± SD; consistent with literature reports39, 40) than from those tested in 2.8M TEG (<3 μg). Hence, use of the 2.8M TEG biolubricant minimizes cartilage surface wear.
To directly compare the performance of BSF and 2.8M TEG (2 w/v%), the same cartilage samples are used with both lubricants (BSF, wash, 2.8M TEG, wash, BSF) to determine if: 1) 2.8M TEG reduces μ below that of BSF; and, 2) 2.8M TEG is washed from the cartilage surfaces after testing such that when the samples are tested in BSF again their μ values return to the original BSF μ values. Pilot studies using BSF confirm that after the first pre-conditioning long-duration test, the next three tests afford comparable μmean values (data not shown). Treatment of the cartilage surfaces with 2.8M TEG reduces μ by 20.9 ± 10.5% (mean ± SD) from that of BSF (Figure 5). The ability of the 2M TEG to reduce the μ value is not hindered by a plug coated or previously treated with BSF. Further, the percent change between the two BSF tests is 2.0 ± 7.1%, indicating that 2.8M TEG is likely washed from the cartilage surfaces and BSF is the active lubricant in the final test as the μ value returned to its original level. The 2.8M TEG is rinsed away, because it does not bind to or alter the cartilage surface. Its negative charge electrostatically repels it from the fixed negative charge in cartilage. Hence, the polyelectrolyte acts as a viscous lubricant and forms a layer interposed between the two cartilage surfaces during testing. The 2.8M TEG biolubricant will likely be slow to diffuse from an intact joint, as its molecular weight is greater than the largest pores of the joint capsule (~750 kDa molecular weight cut-off).50
Finally, the performance of BSF, Synvisc, and 2.8M TEG (2 w/v%) are compared using previously worn cartilage. Since samples tested in saline during the long-duration test become substantially rougher than at baseline, the samples in this study are intentionally worn via two long-duration tests using saline to impart surface roughness prior to a third test with one of the lubricant groups. Despite the increased roughness, 2.8M TEG still reduces the coefficient of friction by ~60%, compared to ~35% for Synvisc and ~15% for BSF, being significantly different than BSF for most of the time points (Figure 6). The 2.8M TEG is the first reported lubricant, to our knowledge, to reduce friction of roughened ex vivo cartilage surfaces, while HA-based viscosupplements are relatively less effective at lubricating degraded cartilage. For example, the coefficient of friction of HA treated samples increases by approximately 25% after cartilage is worn,18 where as the coefficient of friction of 2.8M TEG treated samples decreases by approximately 20% after the cartilage was worn.
The 2.8M TEG (2 w/v%) polymer also exhibits positive preliminary safety in vivo with respect to gait and inflammation. There is no change in gait score (compared to untreated animals) or signs of abnormalities after intra-articular administration in rats. Likewise, intra-articular injection of 2.8M TEG affords effects similar to the saline control in dog knees (Figure 7). Although extended-duration timepoints were not investigated, the preliminary data from this pilot study indicate minimal acute safety concerns, thus providing motivation for further in vivo safety and efficacy evaluation.
CONCLUSIONS
In osteoarthritis, both cartilage and synovial fluid progressively deteriorate, which reduces joint lubrication. In this study, the 2.8M TEG polyelectrolyte reduces cartilage wear by lowering the coefficient of friction during long-duration testing and is superior to BSF and Synvisc for lubrication of ex vivo worn cartilage. Future studies using healthy and osteoarthritic human cartilage are planned to validate our findings and further challenge this biolubricant as a potential treatment for early-stage OA. Nevertheless, the present studies demonstrate 2.8M TEG’s enhanced lubrication for both healthy and worn cartilage in more abrasive loading conditions than those encountered in vivo, as articulating joint surfaces move at slower speeds than those tested herein and are more continuously re-coated with lubricant than the present samples were. Treatment with the 2.8M TEG affords minimal acute in vivo synovitis similar to treatment with saline warranting further in vivo safety and efficacy studies. In summary, a long-lasting, intra-articularly injected biolubricant, such as 2.8M TEG, potentially fulfills the unmet need for a minimally invasive treatment option for patients with early-stage cartilage wear who wish to decelerate their OA and delay a joint replacement.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the Coulter Foundation, the Harvard Catalyst Program, the NIH (R01AR066621), the Boston University Undergraduate Research Opportunities Program (L.Z. and D.J.G.), an NSF Graduate Research Fellowship (DGE-1247312) (B.G.C.), and the NIH (T32 GM008541) Pharmacology Training Grant (J.D.F.).
FUNDING SOURCES
This work was supported in part by the Coulter Foundation, the Harvard Catalyst Program, the NIH (R01GM098361), the Boston University Undergraduate Research Opportunities Program (L.Z. and D.J.G.), an NSF Graduate Research Fellowship (DGE-1247312) (B.G.C.), and the NIH (T32 GM008541) Pharmacology Training Grant (J.D.F.).
Footnotes
SUPPORTING INFORMATION AVAILABLE
The Supporting Information (SI) document is available free of charge and includes greater experimental details on: the synthesis of the polymer, the contrast-enhanced μCT imaging, the baseline coefficient of friction (μ) testing, the analysis of GAG content in the wear solutions, the safety and gait analysis of the male Lewis rats, and acute safety study in beagles.
COMPETING INTERESTS
M.W.G., M.W., and B.D.S. had a conflict of interest, as they owned shares in FlexBiomedical, Inc, a company that specializes in the design and development of biolubricants for OA treatment, as well as have grant funding from the NIH and Coulter Foundation.
REFERENCES
- 1.Felson DT; Lawrence RC; Dieppe PA; Hirsch R; Helmick CG; Jordan JM; Kington RS; Lane NE; Nevitt MC; Zhang Y; Sowers M; McAlindon T; Spector TD; Poole AR; Yanovski SZ; Ateshian G; Sharma L; Buckwalter JA; Brandt KD; Fries JF, Osteoarthritis: new insights. Part 1: the disease and its risk factors. Annals of Internal Medicine 2000, 133 (8), 635–46. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA; Mankin HJ, Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instructional Course Lectures 1998, 47, 487–504. [PubMed] [Google Scholar]
- 3.Silvast TS; Kokkonen HT; Jurvelin JS; Quinn TM; Nieminen MT; Toyras J, Diffusion and near-equilibrium distribution of MRI and CT contrast agents in articular cartilage. Physics in Medicine and Biology 2009, 54, 6823–36. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig TE; McAllister JR; Lun V; Wiley JP; Schmidt TA, Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: Restoration through proteoglycan 4 supplementation. Arthritis and Rheumatism 2012, 64 (12), 3963–71. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt TA; Gastelum NS; Nguyen QT; Schumacher BL; Sah RL, Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis and Rheumatism 2007, 56 (3), 882–891. [DOI] [PubMed] [Google Scholar]
- 6.Kempson G; Muir H; Swanson S; Freeman M, Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochimica et Biophysica Acta (BBA)-General Subjects 1970, 215 (1), 70–77. [DOI] [PubMed] [Google Scholar]
- 7.Treppo S; Koepp H; Quan EC; Cole AA; Kuettner KE; Grodzinsky AJ, Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. Journal of Orthopaedic Research 2000, 18 (5), 739–748. [DOI] [PubMed] [Google Scholar]
- 8.Bansal PN; Joshi NS; Entezari V; Grinstaff MW; Snyder BD, Contrast enhanced computed tomography can predict the glycosaminoglycan content and biomechanical properties of articular cartilage. Osteoarthritis and Cartilage 2010, 18, 184–91. [DOI] [PubMed] [Google Scholar]
- 9.Hunziker E, Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis and cartilage 2002, 10 (6), 432–463. [DOI] [PubMed] [Google Scholar]
- 10.Meachim G, Light microscopy of Indian ink preparations of fibrillated cartilage. Ann Rheum Dis 1972, 31 (6), 457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalson NS; Gikas PD; Briggs TWR, Current strategies for knee cartilage repair. International Journal of Clinical Practice 2010, 64 (10), 1444–1452. [DOI] [PubMed] [Google Scholar]
- 12.Gillogly SD; Voight M; Blackburn T, Treatment of articular cartilage defects of the knee with autologous chondrocyte implantation. Journal of Orthopaedic & Sports Physical Therapy 1998, 28 (4), 241–251. [DOI] [PubMed] [Google Scholar]
- 13.Alford JW; Cole BJ, Cartilage restoration, part 1 - Basic science, historical perspective, patient evaluation, and treatment options. American Journal of Sports Medicine 2005, 33 (2), 295–306. [DOI] [PubMed] [Google Scholar]
- 14.Agerup B; Berg P; Akermark C, Non-animal stabilized hyaluronic acid: a new formulation for the treatment of osteoarthritis. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy 2005, 19, 23–30. [DOI] [PubMed] [Google Scholar]
- 15.Genzyme, US2006/0148755 A1.
- 16.Rutjes AWS; Jüni P; da Costa BR; Trelle S; Nüesch E; Reichenbach S, Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Annals of internal medicine 2012, 157, 180–191. [DOI] [PubMed] [Google Scholar]
- 17.Lo GH; LaValley M; McAlindon T; Felson DT, Intra-articular hyaluronic acid in treatment of knee osteoarthritis - A meta-analysis. Journal of the American Medical Association 2003, 290 (23), 3115–3121. DOI: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- 18.Bonnevie ED; Galesso D; Secchieri C; Bonassar LJ, Degradation alters the lubrication of articular cartilage by high viscosity, hyaluronic acid-based lubricants. Journal of Orthopaedic Research 2018, 36 (5), 1456–1464. [DOI] [PubMed] [Google Scholar]
- 19.Ando A; Hagiwara Y; Chimoto E; Hatori K; Onoda Y; Itoi E, Intra-articular injection of hyaluronan diminishes loss of chondrocytes in a rat immobilized-knee model. Tohoku Journal of Experimental Medicine 2008, 215 (4), 321–331. [DOI] [PubMed] [Google Scholar]
- 20.Cooper BG; Bordeianu C; Nazarian A; Snyder BD; Grinstaff MW, Active agents, biomaterials, and technologies to improve biolubrication and strengthen soft tissues. Biomaterials 2018, 181, 210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waller KA; Chin KE; Jay GD; Zhang LX; Teeple E; McAllister S; Badger GJ; Schmidt TA; Fleming BC, Intra-articular Recombinant Human Proteoglycan 4 Mitigates Cartilage Damage After Destabilization of the Medial Meniscus in the Yucatan Minipig. American Journal of Sports Medicine 2017, 45 (7), 1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flannery CR; Zollner R; Corcoran C; Jones AR; Root A; Rivera-Bermudez MA; Blanchet T; Gleghorn JP; Bonassar LJ; Bendele AM; Morris EA; Glasson SS, Prevention of Cartilage Degeneration in a Rat Model of Osteoarthritis by Intraarticular Treatment With Recombinant Lubricin. Arthritis and Rheumatism 2009, 60 (3), 840–847.. [DOI] [PubMed] [Google Scholar]
- 23.Bayer IS, Advances in Tribology of Lubricin and Lubricin-Like Synthetic Polymer Nanostructures. Lubricants 2018, 6 (2). [Google Scholar]
- 24.Samaroo KJ; Tan M; Putnam D; Bonassar LJ, Binding and lubrication of biomimetic boundary lubricants on articular cartilage. Journal of Orthopaedic Research 2017, 35 (3), 548–557. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence A; Xu X; Bible MD; Calve S; Neu CP; Panitch A, Synthesis and characterization of a lubricin mimic (mLub) to reduce friction and adhesion on the articular cartilage surface. Biomaterials 2015, 73, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivan S; Schroeder A; Verberne G; Merkher Y; Diminsky D; Priev A; Maroudas A; Halperin G; Nitzan D; Etsion I; Barenholz Y, Liposomes Act as Effective Biolubricants for Friction Reduction in Human Synovial Joints. Langmuir 2010, 26 (2), 1107–1116. [DOI] [PubMed] [Google Scholar]
- 27.Zheng R; Zhan J; Wang X; Kaplan D; Pesika N; John VT, Lubrication Properties of Phospholipid Liposome Coated Silk Microspheres. Particle & Particle Systems Characterization 2013, 30 (2), 133–137.. [Google Scholar]
- 28.Morgese G; Cavalli E; Rosenboom JG; Zenobi-Wong M; Benetti EM, Cyclic Polymer Grafts That Lubricate and Protect Damaged Cartilage. Angewandte Chemie-International Edition 2018, 57 (6), 1621–1626. [DOI] [PubMed] [Google Scholar]
- 29.Tnibar A; Schougaard H; Camitz L; Rasmussen J; Koene M; Jahn W; Markussen B, An international multi-centre prospective study on the efficacy of an intraarticular polyacrylamide hydrogel in horses with osteoarthritis: a 24 months follow-up. Acta Veterinaria Scandinavica 2015, 57, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wathier M; Stoddart SS; Sheehy MJ; Grinstaff MW, Acidic polysaccharide mimics via ring-opening metathesis polymerization. Journal of the American Chemical Society 2010, 132 (45), 15887–9. [DOI] [PubMed] [Google Scholar]
- 31.Wathier M; Lakin BA; Bansal PN; Stoddart SS; Snyder BD; Grinstaff MW, A large-molecular-weight polyanion, synthesized via ring-opening metathesis polymerization, as a lubricant for human articular cartilage. Journal of the American Chemical Society 2013, 135, 4930–4933. [DOI] [PubMed] [Google Scholar]
- 32.Wathier M; Lakin BA; Cooper BG; Bansal PN; Bendele AM; Entezari V. a.; Suzuki H; Snyder BD; Grinstaff MW, A synthetic polymeric biolubricant imparts chondroprotection in a rat meniscal tear model. Biomaterials 2018, 182, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumgarten M; Bloebaum RD; Ross SD; Campbell P; Sarmiento A, Normal human synovial fluid: osmolality and exercise-induced changes. The Journal of bone and joint surgery. American volume 1985, 67, 1336–1339. [PubMed] [Google Scholar]
- 34.Joshi NS; Bansal PN; Stewart RC; Snyder BD; Grinstaff MW, Effect of contrast agent charge on visualization of articular cartilage using computed tomography: exploiting electrostatic interactions for improved sensitivity. Journal of the American Chemical Society 2009, 131 (37), 13234–13235. [DOI] [PubMed] [Google Scholar]
- 35.Lakin BA; Grasso DJ; Stewart RC; Freedman JD; Snyder BD; Grinstaff MW, Cationic agent contrast-enhanced computed tomography imaging of cartilage correlates with the compressive modulus and coefficient of friction. Osteoarthritis and Cartilage, 2013, 21, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt TA; Sah RL, Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis and Cartilage 2007, 15 (1), 35–47. DOI: S1063-4584(06)00178-6. [DOI] [PubMed] [Google Scholar]
- 37.Tofte J; Elsaid K; Zhang L; Waller K; Fleming BC; Jay GD, Exercise in ACL-Transected Rats Increases Cartilage Roughness Measured by a Novel Digital Method In Orthopedic Research Society Annual Meeting, San Francisco, CA, 2012. [Google Scholar]
- 38.Farndale RW; Buttle DJ; Barrett AJ, Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta 1986, 883 (2), 173–7. [DOI] [PubMed] [Google Scholar]
- 39.Katta J; Jin Z; Ingham E; Fisher J, Effect of nominal stress on the long term friction, deformation and wear of native and glycosaminoglycan deficient articular cartilage. Osteoarthritis and Cartilage 2009, 17 (5), 662–668. [DOI] [PubMed] [Google Scholar]
- 40.Verberne G; Merkher Y; Halperin G; Maroudas A; Etsion I, Techniques for assessment of wear between human cartilage surfaces. Wear 2009, 266 (11–12), 1216–1223. [Google Scholar]
- 41.Gleghorn JP; Bonassar LJ, Lubrication mode analysis of articular cartilage using Stribeck surfaces. Journal of Biomechanics 2008, 41 (9), 1910–8. [DOI] [PubMed] [Google Scholar]
- 42.Lee DW; Banquy X; Israelachvili JN, Stick-slip friction and wear of articular joints. Proceedings of the National Academy of Sciences of the United States of America 2013, 110 (7), E567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basalo IM; Chen FH; Hung CT; Ateshian GA, Frictional response of bovine articular cartilage under creep loading following proteoglycan digestion with chondroitinase ABC. Journal of Biomechanical Engineering-Transactions of the Asme 2006, 128 (1), 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishnan R; Kopacz M; Ateshian GA, Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. Journal of Orthopaedic Research 2004, 22 (3), 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samaroo KJ; Tan M; Putnam D; Bonassar LJ, Binding and lubrication of biomimetic boundary lubricants on articular cartilage. Journal of Orthopaedic Research 2016, 35 (3), 548–557. doi: 10.1002/jor.23370 [DOI] [PubMed] [Google Scholar]
- 46.Bansal PN; Joshi NS; Entezari V; Malone BC; Stewart RC; Snyder BD; Grinstaff MW, Cationic contrast agents improve quantification of glycosaminoglycan (GAG) content by contrast enhanced CT imaging of cartilage. Journal of Orthopaedic Research 2011, 29 (5), 704–709. [DOI] [PubMed] [Google Scholar]
- 47.Palmer AW; Guldberg RE; Levenston ME, Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proceedings of the National Academy of Sciences of the United States of America 2006, 103 (51), 19255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller M; Lee S; Spikes HA; Spencer ND, The influence of molecular architecture on the macroscopic lubrication properties of the brush-like co-polyelectrolyte poly(L-lysine)-g-poly(ethylene glycol) (PLL-g-PEG) adsorbed on oxide surfaces. Tribology Letters 2003, 15 (4). [Google Scholar]
- 49.Kenausis GL; Voros J; Elbert DL; Huang NP; Hofer R; Ruiz-Taylor L; Textor M; Hubbell JA; Spencer ND, Poly(L-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: Attachment mechanism and effects of polymer architecture on resistance to protein adsorption. Journal of Physical Chemistry B 2000, 104 (14). [Google Scholar]
- 50.Simkin PA; Bassett JE, Pathways of microvascular permeability in the synovium of normal and diseased human knees. Journal of Rheumatology 2011, 38 (12), 2635–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.