Abstract
Cortisol has many roles not only in mediating the response to stress but also in the circadian rhythm, and it does so by both genomic and nongenomic cellular and molecular mechanisms. Yet, it is common to associate cortisol only with stress and, in particular, with the negative aspects of stress even though we would not survive without it. This commentary provides a brief overview not only of the diverse roles of cortisol but also of how to measure it to get meaningful information in the context of other mediators of stress and adaptation and the concepts of allostasis and allostatic load and overload. In particular, the adaptive plasticity of the brain mediated by glucocorticoids and excitatory amino acids is discussed in relation to misconceptions about what constitutes brain damage. Thus the confusion with cortisol is that it does too many important things both positive and negative!
Keywords: circadian, adaptive plasticity, protection, damage, development
When we talk about stress and think about measuring stress, we usually focus on cortisol, not fully understanding what it does. Cortisol is one of many mediators of allostasis, the active process of adaptation to our environment and experiences, whether or not we consider them “stressful.”1 The sympathetic and parasympathetic nervous system, the immune system, metabolic hormones, and many mediators in the brain and throughout the body form a nonlinear network that responds to experiences and changes in the environment to promote adaptation and maintain homeostasis. When these responses are used frequently, or when the network is unbalanced (e.g., too much or too little cortisol or too much or too little inflammatory mediators), then there is a gradual cumulative change in systems throughout the body, including the brain, that can result in disease and is referred to as allostatic load or overload.2,3
Yet, we often hear that measuring our cortisol level will tell us if we are stressed. This reflects a lack of information at multiple levels. First, a single measure of cortisol will tell us nothing since cortisol levels go up and down within minutes. In fact, that ultradian pulsatility is key to many of its adaptive effects in the brain and body, including aspects of gene expression and turnover of synaptic connections in the cerebral cortex.4,5 Moreover, there is the day–night (diurnal) rhythm of pulsating cortisol that goes up in the early morning and wakes us up, then declines, except for a rise at lunch time, and which is normally low in the evening before we go to bed. This is important not only to coordinate daily activity and metabolism6 but also for a normal stress response (turning it on when needed and turning it off when stress is over)7 and for synaptic turnover that promotes motor learning.8 A flat circadian cortisol variation occurring after sleep deprivation, after shift work, and in depression undermines all these adaptive actions.6,9
There are several ways around the measurement problem—we can collect urine overnight or over the day or we can measure cortisol in hair from the forehead10 which gives us an even longer, over multiple days, index of our cortisol production. Or we can measure cortisol in our saliva at multiple times during the day or before, during and after a stressful challenge, such as talking about something personal before a group of strangers.11 The stressful challenge gives us a picture of the efficiency of our allostasis, namely, turning on our cortisol when we are challenged and need it and then turning off our cortisol response when the stressor is over.7 Failure to turn it on when needed is deleterious—other systems that balance it, like inflammation, may over-react; failure to turn it off when stress over produces negative effects on metabolism and contributes to other processes, like obesity, diabetes, anxiety, depression and other psychopathologies and eventual heart disease, that contribute to allostatic load/overload.1,2
A second thing we need to know is that the cortisol response is not the “bad guy,” as it is often implied by its elevation in the context of a bad experience; rather, cortisol has a normal physiological role not only helping us adapt to stressors but also coordinating our metabolism with our daily activity and sleep patterns.12 We would not live very long or well without our cortisol! So let us not blame it for our problems unless it stays elevated when it should not be or is not turned on when we need it! For example, we have noted that diurnal fluctuations of cortisol promote the formation and elimination of synapses in the brain, and this helps us learn and adapt.5,8 Moreover, the diurnal early morning rise of cortisol as well as a stress response activates adaptive immune function. That is, an acute stress-induced rise of cortisol and adrenaline actually helps the immune system to respond to a pathogen or repair a wound.13 Likewise, the morning awakening rise of cortisol enhances the body’s response to an immunization.14 Beside cortisol and adrenaline involvement in this important adaptive function, key biochemicals of the immune system are also necessary and, together, they help immune cells “go to their battle stations” where they are needed15! The body’s response is like an orchestra involving many players that need to work in harmony! But, indeed, too much cortisol also causes problems as it does with a flat diurnal rhythm (Jacobson et al.7) and in Cushing’s Disease when excess cortisol is produced by a pituitary gland tumor and produces long-lasting changes even if some recovery is possible16,17!
Third, it is important to know that glucocorticoids do not play the same role across the life course but rather serve different functions, starting with their role in “wakening” of the amygdala in neonatal life18 to their role in promoting ponderal growth in adolescence and their influence on the vulnerability of the adolescent brain to stressful experiences19 as well as the age-accelerating role of excess cortisol in aging.20,21 Moreover, they work synergistically with other mediators, for example, oxytocin in the case of elevated glucocorticoids stimulating, rather than inhibiting, dentate gyrus neurogenesis during sexual activity.22
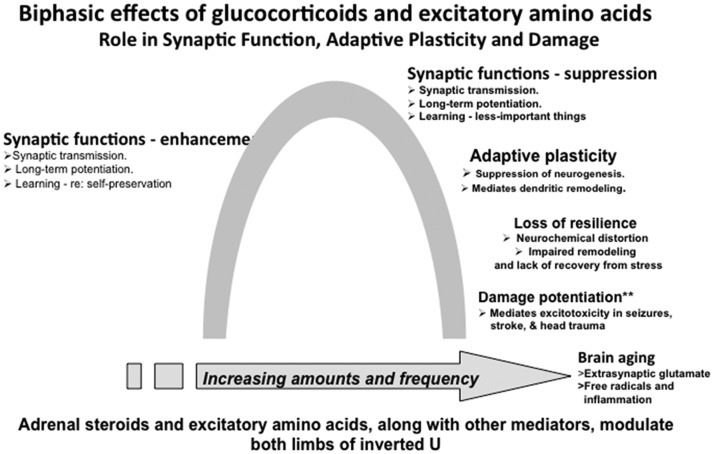
Finally, there is the concept of adaptive plasticity in which both glucocorticoids and excitatory amino acids participate in remodeling of neural circuits as a result of stressful and other life experiences (see Figure 1). These changes are not “brain damage” but rather adaptations to those experiences3,23 even though unregulated glucocorticoid secretion and massive overactivity of glutamatergic systems in stroke, seizures, and head trauma do produce irreversible brain damage.24 The key question is when conditions change, and the stressor is gone, can the brain further adapt to the new situation? When it gets “stuck” and does not show resilience, then an external intervention is needed to promote recovery. All of this should be considered in the context of the multiple genomic and nongenomic mechanisms by which cortisol mediates many processes for better or sometimes, for worse.3,23–25
Figure 1.
Inverted U representing multiple, biphasic effects in which glucocorticoids are involve along with excitatory amino acids and other mediators.
Indeed, the confusion with cortisol is that it does too many important beneficial things but can also cause problems!
Declaration of Conflicting Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received support from the Hope for Depression Research Foundation, New York.
References
- 1.McEwen BS. Protective and damaging effects of stress mediators. New England J Med 1998; 338: 171–179. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav 2003; 43: 2–15. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks, Calif.) 2017; 1 doi:10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stavreva DA, Wiench M, John S, et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 2009; 11: 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A 2011; 108: 16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen BS, Karatsoreos IN. Sleep deprivation and circadian disruption: stress, allostasis, and allostatic load. Sleep Med Clin 2015; 10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson L, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology 1988; 122: 1343–1348. [DOI] [PubMed] [Google Scholar]
- 8.Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci 2013; 16: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrin 2004; 25: 69–76. [DOI] [PubMed] [Google Scholar]
- 10.Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology 2013; 38: 1220–1235. [DOI] [PubMed] [Google Scholar]
- 11.Kirschbaum C, Prüssner JC, Stone AA, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med 1995; 57: 468–474. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS, Sakai RR, Spencer RL. Hormonally-Induced Changes in Mind and Brain (ed J. Schulkin). Cambridge, MA: Academic Press; 1993:157–189.
- 13.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun 1997; 11: 286–306. [DOI] [PubMed] [Google Scholar]
- 14.Dhabhar F, Miller AH, Stein M, McEwen BS, Spencer R. Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun 1994; 8: 66–79. [DOI] [PubMed] [Google Scholar]
- 15.Dhabhar FS, Satoskar AR, Bluethmann H, David JR, McEwen BS. Stress-induced enhancement of skin immune function: a role for g interferon. Proc Natl Acad Sci 2000; 97: 2846–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in partiens with Cushing’s syndrome. Biol Psychiatry 1992; 32: 756–765. [DOI] [PubMed] [Google Scholar]
- 17.van der Werff SJ, Pannekoek JN, Andela CD, et al. Resting-state functional connectivity in patients with long-term remission of Cushing’s disease. Neuropsychopharmacology 2015; 40: 1888–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriceau S, Sullivan RM. Corticosterone influences on mammalian neonatal sensitive-period learning. Behav Neurosci 2004; 118: 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romeo RD. The impact of stress on the structure of the adolescent brain: implications for adolescent mental health. Brain Res 2017; 1654: 185–191. [DOI] [PubMed] [Google Scholar]
- 20.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev 1986; 7: 284–301. [DOI] [PubMed] [Google Scholar]
- 21.Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998; 1: 69–73. [DOI] [PubMed] [Google Scholar]
- 22.Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus 2012; 22: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci 2015; 18: 1364–1375. [DOI] [PubMed] [Google Scholar]
- 24.Sapolsky R. Stress, the aging brain, and the mechanisms of neuron death. Yale J Biol Med 1993; 66(2): 109–110. [Google Scholar]
- 25.McEwen BS, Bowles NP, Gray JD, et al. Mechanisms of stress in the brain. Nat Neurosci 2015; 18: 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]