SUMMARY
Calcium is an abundant intracellular ion and calcium homeostasis plays crucial roles in several cellular processes. The calcineurin signaling cascade is one of the major pathways governed by intracellular calcium. Calcineurin, a conserved protein from yeast to humans, is a calcium–calmodulin-dependent serine/threonine-specific phosphatase that orchestrates cellular stress responses.. In eukaryotic microbial pathogens, calcineurin controls essential virulence pathways, such as the ability to grow at host temperature, morphogenesis to enable invasive hyphal growth, drug tolerance and resistance, cell wall integrity, and sexual development. Therefore, the calcineurin cascade is an attractive target in drug development against eukaryotic pathogens. In the present review, we summarize and discuss the current knowledge on the roles of calcineurin in eukaryotic microbial pathogens, focusing on fungi and parasitic protists.
INTRODUCTION
Eukaryotic microbial pathogens, including protists and fungi, are a major concern for human health because of their prevalence and higher resistance rates against currently available drugs (Fairlamb et al., 2016). Eukaryotic pathogens are more closely related to their hosts than bacterial or viral pathogens, and this higher level of shared similarity poses significant challenges to the development of novel drugs specific to eukaryotic pathogens with no or minimal adverse effects on their hosts. It is necessary to understand both the eukaryotic pathogen and host systems and their respective signal transduction mechanisms in order to identify specific druggable targets and develop novel antimicrobial compounds.
The calcium (Ca2+)-calcineurin signaling cascade has been extensively studied in eukaryotic systems (Clapham, 2007) (Figure 1). Calcium is a key factor in myriad biological and cellular processes that require the initiation of rapid responses and adaptation to diverse environmental cues (Carafoli, 2002; Clapham, 2007). In response to internal or external signals, cellular cytosolic calcium concentrations increase by influx through pumps in the cell membrane or release from intracellular calcium reservoirs (i.e., vacuoles and the endoplasmic reticulum) via channels (Carafoli, 2002). Cytosolic calcium ions bind to and activate proteins containing the calcium-binding motif EF hands (Nowycky and Thomas, 2002). Calmodulin (calcium-modulated protein) is a representative sensor protein for cytosolic calcium ions that transduces calcium signals into appropriate outputs through calmodulin-binding proteins (including calcineurin), calmodulin-dependent protein kinases, and histone deacetylases (Stull, 2001).
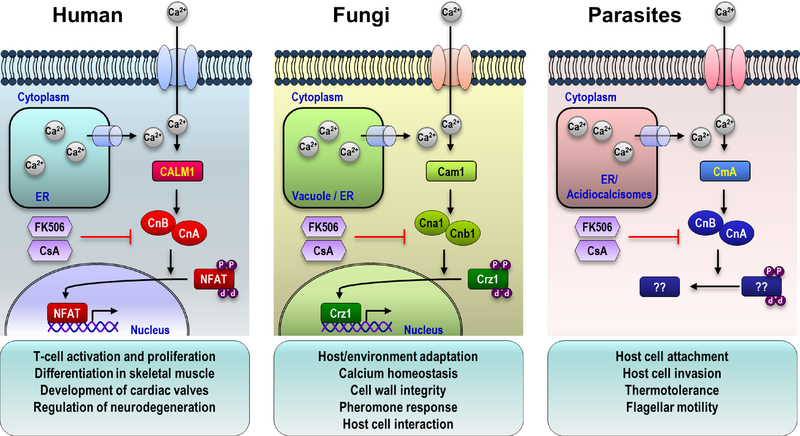
Figure 1. The calcineurin signaling pathway in mammals, fungi, and protists.
In response to external or internal signals, intracellular calcium concentrations increase via uptake from the extracellular milieu or by release from intracellular stores. Calcium ions bind to the calcium binding protein calmodulin, which in turn binds to and stimulates calcineurin protein phosphatase activity. Activated calcineurin dephosphorylates target proteins, including NFAT and Crz1, enabling appropriate cellular responses.
Calcineurin is a serine/threonine phosphatase that functions by dephosphorylating target proteins in eukaryotes (Cyert, 2003; Rusnak and Mertz, 2000). In mammals, calcineurin controls the activity of the key transcription factor NFAT (nuclear factor of activated T cells), which is required for T-cell activation and interleukin (IL)-2 expression at the onset of immune responses (Hogan et al., 2003). In addition, calcineurin is involved in many other cellular processes, including differentiation of skeletal muscle, development of cardiac valves, and regulation of neurodegeneration (Mukherjee and Soto, 2011; Schulz and Yutzey, 2004; Swoap et al., 2000). Importantly, calcineurin is the target of two immunosuppressants, cyclosporine A (CsA, a cyclic peptide) and FK506 (tacrolimus, a macrolide), which are used for organ transplant recipients. CsA and FK506 bind to the immunophilins cyclophilin A and FKBP12, respectively, and each complex blocks access of the target proteins to the active site of calcineurin (Ho et al., 1996).
The calcium-calcineurin signaling pathway is also conserved in eukaryotic microbial organisms (Juvvadi et al., 2017). In fungi and protists, the calcium-calcineurin signaling pathway is involved in biological processes, such as calcium homeostasis, stress responses, maintenance of cell wall integrity, and flagellar motility. In pathogenic fungi and parasitic protists, the calcium-calcineurin cascade is also involved in their virulence through a variety of mechanisms, including adaptation to host and/or different environments, formation of infectious propagules, and interaction with hosts (Table 1). In addition, calcineurin is associated with antifungal drug resistance, suggesting that calcineurin is an attractive target for the treatment of fungal infections (Juvvadi et al., 2017). Thus, a fuller understanding of the components, functions, and molecular mechanisms of the calcium-calcineurin signaling cascade will provide insights into host-pathogen interactions and facilitate development of novel therapeutic approaches. Here, we describe and discuss the components of the calcium-calcineurin pathway, highlighting the roles of this pathway in the virulence mechanisms of eukaryotic microbial pathogens, pathogenic fungi and parasitic protists. We also discuss strategies for the development of new drugs targeting the calcium-calcineurin pathway.
Table 1.
Pathogenicity mechanisms of eukaryotic microbial pathogens controlled by calcineurin signaling.
| Pathogenicity mechanisms | Animal/human pathogenic fungi | Plant pathogenic fungi | Insect pathogenic fungi | Protists |
|---|---|---|---|---|
| Host/environment adaptation | ||||
| Thermotolerance | Cryptococcus neoformans* | Metarhizium acridum | Leishmania major | |
| Cryptococcus gattii* | Beauveria bassiana | |||
| Cryptococcus deneoformans* | ||||
| Candida glabrata | ||||
| Arthroderma vanbreuseghemii | ||||
| Growth in serum | Candida albicans | |||
| Candida dubliniensis | ||||
| Candida lusitaniae | ||||
| Candida tropicalis | ||||
| Candida glabrata | ||||
| Cell wall integrity | Cryptococcus spp. | Sclerotinia sclerotiorum | Metarhizium acridum | |
| Candida spp. | Beauveria bassiana | |||
| Mucor circinelloides | ||||
| Infectious structure formation | ||||
| Dimorphic transitions | Mucor circinelloides | |||
| Spore formation | Aspergillus fumigatus | Verticillium dahlia | Metarhizium acridum | |
| Valsa pyri | Beauveria bassiana | |||
| Ustilago maydis | ||||
| Sclerotinia sclerotiorum | ||||
| Appressorium formation | Magnaporthe oryzae | Metarhizium acridum | ||
| Magnaporthe grisea | ||||
| Cochliobolus miyabeanus | ||||
| Host cell interaction | ||||
| Host cell attachment | Plasmodium berghei | |||
| Plasmodium falciparum | ||||
| Toxoplasma gondii | ||||
| Host cell invasion | Botrytis cinerea | Plasmodium berghei | ||
| Magnaporthe oryzae | Plasmodium falciparum | |||
| Magnaporthe grisea | Trypanosoma cruzi | |||
| Other | ||||
| Hyphal extension | Aspergillus fumigatus | |||
Components of the calcium-calcineurin signaling pathway
In most eukaryotic systems including major eukaryotic pathogens, the calcium-calcineurin signaling pathway is composed of calcium channels, transporters, a calcium sensory protein (calmodulin), a calcium-calmodulin dependent phosphatase (calcineurin), and calcineurin interacting proteins (i.e. cyclophilins).
Calcium pumps or transporters in the cell membrane or calcium storage organelles are crucial for maintaining Ca2+ homeostasis (Clapham, 2007), calcium-dependent signaling pathways, and host-microbe interactions in pathogenic fungi and protists (Moreno et al., 2011). In fungal cell membranes, the high-affinity Ca2+ influx system (HACS) is the major calcium entry route. HACS consists of two proteins, Cch1 (calcium channel) and Mid1 (mating-induced death), which interact to form calcium channels in the plasma membrane and regulate calcium homeostasis, thereby impacting fungal growth, conidiation, and sexual development. HACS is also required for virulence in the human pathogenic fungi Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus (de Castro et al., 2014; Vu et al., 2015). Along with the plasma membrane calcium influx system, calcium storage organelles, such as vacuoles, the endoplasmic reticulum (ER), and the Golgi apparatus, are involved in regulating calcium homeostasis in the cytosol (Gao et al., 2015). Vacuoles serve as a primary Ca2+ storage depot, and in yeast, the vacuolar membrane contains several Ca2+ transporters including Yvc1 (yeast vacuolar conductance), Vcx1 (a H+/Ca2+ exchanger), and Pmc1 (a Ca2+-ATPase). These transporters are involved in the control of intracellular Ca2+ concentrations, stress responses, and virulence of pathogenic fungi such as C. albicans and A. fumigatus (Gao et al., 2015). In the Golgi and ER, the Ca2+ pumps Pmr1, Cod1, and Eca1 also function to restore basal levels of cytosolic Ca2+ and thus contribute to virulence (Gao et al., 2015).
Increased cytosolic Ca2+ concentrations are sensed by calmodulin and other proteins containing calcium-binding motifs (Stull, 2001). The Ca2+-calmodulin complex interacts with targets that regulate cellular fuctions such as mitosis, growth, actin-based processes, and stress responses in fungi and protists. Importantly, the Ca2+ -calmodulin complex binds to calmodulin-binding proteins, including calcineurin, calmodulin-dependent protein kinases, and histone deacetylases. Calcineurin is a Ca2+/calmodulin-activated serine/threonine-specific protein phosphatase that is a core member of the calcium-calcineurin signaling cascade (Rusnak and Mertz, 2000). Calcineurin is a heterodimeric complex consisting of a catalytic A (Cna1) subunit and a regulatory B subunit (Cnb1) (Steinbach et al., 2007). The Cna1 subunit contains an N-terminal catalytic domain, a C-terminal regulatory domain for Cnb1 and calmodulin binding, and an autoinhibitory domain, which together with the calmodulin-binding domain, controls calcineurin catalytic activity (Rusnak and Mertz, 2000). At low calcium concentrations, the Cnb1 binding helix interacts with the calmodulin-binding domain, and the autoinhibitory domain blocks the catalytic site of Cna1. When Ca2+ concentrations are high, calcium binds to calmodulin and Cnb1, resulting in a conformational change of Cnb1. Calmodulin then binds to Cna1, releasing the autoinhibitory domain from the substrate-binding site, thereby activating calcineurin to dephosphorylate targets.
Several components of the calcineurin signaling cascade are similar between parasitic protists and fungi. For example, most fungi and protists, with the exception of piroplasmids, contain calcineurin and calmodulin orthologs with similar domains and functions. The plasma membrane Ca2+-ATPase (PMCA) is a major channel for Ca2+ influx from the extracellular milieu that plays a similar role in parasitic protists as the HACS channel in fungi. However, unlike fungi, apicomplexan parasitic protists possess acidic calcium storage organelles, acidocalcisomes and vacuoles related to those of plants. In these acidic organelles, Ca2+-ATPases and Ca2+/H+ exchangers play a central role in maintaining Ca2+ homeostasis (Moreno et al., 2011).
Downstream regulatory networks of the calcium-calcineurin signaling pathway
Although the core components of the calcium-calcineurin signaling pathway, such as calmodulin and calcineurin, are conserved in eukaryotes, the functions of this pathway differ between organisms. Indeed, downstream targets of this pathway, including calcineurin-dependent transcription factors, are diverse in pathogenic fungi and may be missing altogether in some parasitic protists (Table 2). The Crz1 transactivator, which contains one or more C-terminal DNA binding C2H2 zinc finger domains, is a conserved calcineurin target in fungi and the calcineurin-Crz1 pathway is a major signaling module for calcium-induced cellular responses in many pathogenic fungi (Thewes, 2014). Dephosphorylated Crz1 translocates into the nucleus and activates the expression of Crz1-dependent genes, including those involved in chitin synthesis (CHS7) and membrane transport (PMC1) (Chow et al., 2017).
Table 2.
Components of the calcium-calcineurin signaling pathway in C. neoformans, A. fumigatus, C. albicans, and P. falciparum.
| Components | Cryptococcus neoformans | Candida albicans | Aspergillus fumigatus | Plasmodium falciparum |
|---|---|---|---|---|
| Ca++ pumps or channels | ||||
| Cell membrane | Mid1/Cch1 | Mid1/Cch1/Ec m7, Rch1, | CchA/MidA, YvcA | PMCA |
| Vacuolar | Vcx1 | Yvc1 | YvcA | V-type H(+)- |
| Pmc1 | Vcx1 | VcxA | ATPase | |
| Pmc1 | PmcA | |||
| Tfp1 | ||||
| Golgi | Pmr1 | PmrA | ND | |
| Gdt1 | ||||
| ER | Eca1 | Spf1 | ATP6 | |
| Calmodulin | Cam1 | Cmd1 | CamA | CmA |
| Calcineurin | ||||
| Catalytic subunit | Cna1 | Cna1/Cmp1 | CnaA/CalA | CnA |
| Regulatory subunit | Cnb1 | Cnb1 | CnaB | CnB |
| Calcineurin binding proteins | Cbp1 | Rcn1 | CbpA | Cyp |
| Calcineurin targets | ||||
| Calcineurin-regulated TFs | Crz1/SP1 | Crz1 | CrzA | ND |
| Other calcineurin targets | Pbp1, Puf4, Lhp1 | Actin# | ||
| Crz1 target genes | CHS7 | CHS7 | chsA | ND |
| PMC1 | PMC1 | pmcA | ||
Potential target
ND, Not determined.
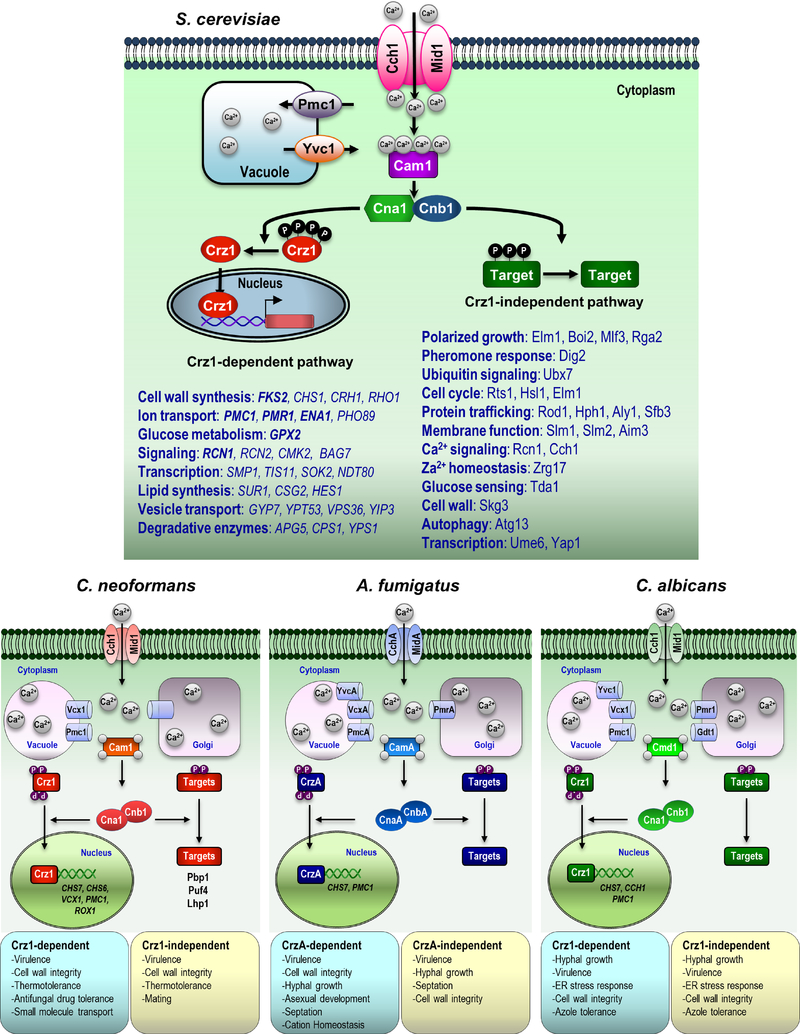
In the model budding yeast Saccharomyces cerevisiae, dephosphorylated Crz1 enters the nucleus and binds to the calcineurin-dependent, Crz1-dependent response elements (CDREs) in target promoters, regulating mRNA expression of genes such as FKS2, PMC1, PMR1, ENA1, GPX2, and RCN1 (Yoshimoto et al., 2002). These Crz1-dependent genes have related functions, such as cell wall integrity, ion transport, and glucose metabolism. Although Crz1-dependent genes have similar roles in different fungal species, a small subset of calcineurin-Crz1-dependent genes is conserved among fungi. Some fungi, including S. cerevisiae, A. fumigatus, and C. albicans, share similar CDRE motifs, but Crz1 orthologs in other fungi recognize different CDRE sequence(s) (Chow et al., 2017). The function of Crz1 and its orthologs is apparently conserved, but Crz1 regulatory networks are plastic and species-specific and respond differently to various stresses.
Although Crz1 and its orthologs are key calcineurin targets in many fungi, crz1 mutant strains have less severe phenotypes in terms of virulence or the pheromone response compared to those of calcineurin mutant strains, indicating that calcineurin also coordinates cellular functions in a Crz1-independent manner (Chow et al., 2017; Thewes, 2014). Putative calcineurin targets that are involved in the calcineurin-Crz1-independent pathway have been identified in two model organisms, S. cerevisiae and C. neoformans (Goldman et al., 2014; Li et al., 2011; Park et al., 2016). In S. cerevisiae, calcineurin dephosphorylates its substrates, including Crz1, Dig2, Rcn1, and Atg13, thereby controlling many cellular functions (Figure 2). Dig2, which is involved in pheromone responses during mating is one such substrate in the calcineurin-Crz1-independent pathway. (Goldman et al., 2014).
Figure 2. Functions of the calcineurin signaling pathway in S. cerevisiae, C. neoformans, A. fumigatus, and C. albicans.
The phenotypes and functions attributed to calcineurin signaling in fungal species occur via Crz1-dependent and Crz1-independent pathways. In each species, some calcineurin functions are mediated via Crz1, while others are independent of Crz1 and mediated by other calcineurin targets.
In C. neoformans, 44 putative calcineurin targets, including Crz1 and several RNA-binding proteins (Pbp1, Puf4, Pab1 and Lhp1), were identified by a phosphoproteomic analysis during thermal stress (Park et al., 2016). Importantly, several targets, including Pbp1 and Puf4, colocalize with Cna1 in P-bodies and stress granules in response to thermal stress, suggesting that calcineurin governs post-transcriptional processes that regulate stress survival. Epistasis analysis has revealed that Crz1 and the RNA-binding proteins (Pbp1, Puf4, and Lhp1) function in a branched pathway that orchestrates thermotolerance and virulence (Park et al., 2016), and an RNA-seq analysis found 393 genes that are regulated by calcineurin during thermal stress in a Crz1-independent manner (Chow et al., 2017). Overall, these results indicate that calcineurin coordinates a branched signaling pathway to control its targets at both the transcriptional and post-transcriptional levels (Chow et al., 2017; Park et al., 2016). The calcineurin-Crz1-independent pathway also plays an essential role in various cellular processes, but calcineurin substrates and regulatory networks have not been studied in other eukaryotic pathogenic organisms.
Parasitic protists on the other hand, do not seem to have orthologs of yeast Crz1 and mammalian NFAT in their known genomes. Interestingly, many protists appear to lack broad classes of transcription factors (Balaji et al., 2005), suggesting that the calcineurin cascade may operate in ways other than transcription factor mediated regulatory mechanisms controlling virulence of parasitic protists. This could be due to the substantial evolutionary distance between fungi and protists, leading to highly divergent rewiring of the calcineurin pathway. Although direct targets of calcineurin remain to be identified in parasitic protists, several potential targets have been proposed in the malaria parasite Plasmodium falciparum including HSP90, actin-1, and phosphoglycerate kinase (Singh et al., 2014).
Additional studies are therefore needed to further illuminate the roles of calcineurin in pathogenic fungi and to gain a thorough understanding of how this pathway operates to control the virulence of parasitic protists. A combination of phospho-proteomic analysis and genome wide CRISPR/Cas9 approaches will help identify additional calcineurin-Crz1 activated genes and Crz1-independent Calcineurin targets, and provide a more comprehensive view of this signaling cascade (Goldman et al., 2014; Li et al., 2011; Park et al., 2016).
Functions of the calcium-calcineurin signaling pathway in eukaryotic pathogens
Calcineurin plays a crucial role in the ability of eukaryotic pathogens to adapt to and survive in the host and/or under certain environmental conditions. In most fungi, calcineurin regulates mRNA expression of genes associated with cell wall integrity. Second, calcineurin is involved in the formation of infectious structures or propagules (spores or appressoria), and during dimorphic transitions that play critical roles in many pathogenic fungi. For example, calcineurin-defective fungal strains exhibit reduced formation of asexual spores and sexual structures, and cannot complete the morphogenic transition (ie. yeast to hypha and vice versa) (Cao et al., 2014; Choi et al., 2009; Huang et al., 2015; Juvvadi and Steinbach, 2015; Lee et al., 2013). Calcineurin signaling pathway also controls host-pathogen interactions in both fungi and protists, such as thehydrophobicity of spores, host cell receptor engagement, actin depolymerization, and cell invasion (Alshahni et al., 2016; Paul et al., 2015; Philip and Waters, 2015). In summary, calcineurin is involved in virulence via multiple mechanisms, which we discuss in more detail below.
Human/animal pathogenic fungi
C. neoformans is a global human fungal pathogen and its ability to adapt to host environments is a prerequisite for its survival and virulence (Cooney and Klein, 2008). In C. neoformans, calcineurin is essential for growth at host body temperature (37°C), and is also required for survival in respon se to host environmental or non-host environmental cues, such as osmotic and oxidative stresses (Cruz et al., 2001; Odom et al., 1997). Calcineurin is also involved in hyphal elongation and survival during mating and thus affects the production of infectious basidiospores that can readily disseminate and can be inhaled to cause infection (Cruz et al., 2001; Kozubowski et al., 2011). The calcineurin-Crz1 dependent pathway contributes to thermal stress survival, cell wall integrity, small molecule transport, and virulence via transcriptional regulation (Chow et al., 2017). In addition, Crz1-independent targets such as Puf4 and Lhp1 are involved in controlling high-temperature growth and virulence (Park et al., 2016). Identification of Pbp1 as a calcineurin target also provides insight into how calcineurin regulates sexual reproduction (Park et al., 2016). (Figure 2). The function of calcineurin has been characterized in other basidiomycetous Cryptococcus species such as C. deneoformans (Fu et al., 2018), C. gattii (Chen et al., 2013), and C. humicola (Zhang et al., 2017). Similar to its role in C. neoformans, calcineurin is crucial for thermotolerance and virulence in these species (Chen et al., 2013; Fu et al., 2018). Fu et al. examined the role of calcineurin in unisexual reproduction and concluded that this process is affected at different stages of sexual development via both Crz1- dependent and Crz1-independent pathways (Fu et al., 2018).
Calcineurin plays a crucial role in cell wall integrity, stress response, asexual spore production, and phosphate transfer, all of which are associated with the virulence of A. fumigatus, a leading cause of invasive fungal infections (Steinbach et al., 2007). Several studies have shown that deletion of the individual calcineurin A or B subunit or both subunits in A. fumigatus results in defects in hyphal growth, conidial germination, stress adaptation, septation, and cell wall formation (Juvvadi and Steinbach, 2015). In addition, when compared to a wildtype strain, the calcineurin catalytic subunit ΔcnaA mutant is either avirulent or exhibits attenuated virulence in animal models, where it shows decreased fungal burden in lung tissues (Steinbach et al., 2007). Mutations in calcineurin cause inhibition of hyphal extension during tissue invasions and as a result, decreased host mortality.
Candida species are components of the human microbiota that colonize the mucosal surfaces of humans, and also cause superficial and invasive systemic fungal diseases (Pfaller and Diekema, 2007). Calcineurin holds conserved roles in several Candida species including C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei, in which calcineurin is required for morphogenesis (hyphal or pseudohyphal growth), cell wall integrity, and responses to ER stress (Chen et al., 2011; Chen et al., 2012; Chen et al., 2014; Zhang, J. et al., 2012). In addition, deletion of the calcineurin catalytic subunit causes attenuated virulence of Candida spp. in a murine systemic infection model, and other model systems, suggesting that calcineurin is essential for fungal virulence (Chen et al., 2011; Chen et al., 2013; Chen et al., 2014; Yu et al., 2015). Importantly, calcineurin mutants cannot survive in sera, implying that calcineurin is required to survive in the host bloodstream(Steinbach et al., 2007). Unlike other Candida species, C. glabrata calcineurin mutants only exhibit growth defects at human body temperature, which is similar to calcineurin mutants in C. neoformans and C. gattii (Chen et al., 2012). The mechanisms underlying the species specificity to temperature stress remain to be fully elucidated.
In Mucor circinelloides, one of the key Mucorales responsible for mucormycosis, calcineurin coordinates the yeast-hyphal dimorphic transition and fungal spore size, which are crucial processes that contribute to virulence (Lee et al., 2013) and alter host-pathogen interactions (phagosome maturation and host cell death) (Lee et al., 2015). In Trichophyton mentagrophytes (Arthroderma vanbreuseghemii), a dermatophyte species that causes skin and nail infections in animals and humans, calcineurin is also involved in hyphal morphogenesis, thermal stress resistance, and spore hydrophobicity (Alshahni et al., 2016). Importantly, calcineurin inhibitors in combination with other antifungal agents against T. mentagrophytes and Mucorales showed synergistic effects, suggesting that calcineurin is an attractive anti-fungal target (Shirazi and Kontoyiannis, 2013). These pathways and calcineurin targets are summarized in Figure 2.
Plant pathogenic fungi
Similar to human pathogenic fungi, calcineurin plays important roles in the pathogenicity of fungi that infect plants, which cause substantial economic losses in agriculture and contribute to food shortages (Fisher et al., 2012). Calcineurin controls fungal growth and spore formation, resulting in decreased production of asexual or sexual spores, which are major infectious propagules. For example, deletion of the calcineurin catalytic A subunit gene leads to the production of abnormal hyphae and the absence of mature conidia in the citrus fungal pathogen Alternaria alternate (Tsai and Chung, 2014). In the soil borne pathogen Verticillium dahliae, Crz1 is crucial for the production of microsclerotia to survive in the environment (Xiong et al., 2015). In several fungi, including Valsa pyri, Ustilago maydis, and Sclerotinia sclerotiorum, calcineurin mutants produce abnormal or reduced sexual structures (Egan et al., 2009; Harel et al., 2006; He et al., 2016). Calcineurin is also essential for fungi to penetrate plant hosts. The appressorium is a specialized infectious structure in many plant pathogenic fungi and Δcrz1 mutants of Magnaporthe oryzae, Magnaporthe grisea, and Botrytis cinerea exhibit abnormal appressorium maturation and defects in host penetration, resulting in reduced pathogenicity (Choi et al., 2009; Schumacher et al., 2008; Zhang et al., 2009). Further calcineurin inhibition blocks appressorium formation in Cochliobolus miyabeanus (Ahn and Suh, 2007). In Puccinia striiformis f. sp. tritici (Pst), calcineurin regulates the expression of pathogenicity genes involved in the wheat-Pst interaction (Zhang, H. et al., 2012). Similar to human pathogenic fungi, calcineurin is required for cell wall integrity and stress responses in several plant pathogenic fungi. In S. sclerotiorum, inhibition of the calcineurin pathway causes reduced β−1,3-glucan in the cell wall and thus increased sensitivity to glucan synthase inhibitors and cell wall-degrading enzymes (Harel et al., 2006).
Insect pathogenic fungi
The roles of calcineurin have been studied in two entomopathogenic fungi: Beauveria bassiana and Metarhizium acridum (Cao et al., 2014; Li et al., 2015). Deletion of the calcineurin catalytic subunit leads to a decrease in the number of conidia produced and an increased sensitivity to various stressful conditions in both species. In addition, mutations in calcineurin lead to altered cell wall composition and a defect in conidial surface hydrophobicity, resulting in a decreased ability to bind to hydrophobic surfaces in B. bassiana or M. acridum. Calcineurin mutants produced weakened cell walls and thus had increased susceptibility to host defenses. In addition, calcineurin mutants were more sensitive to heat and other stresses. Calcineurin also regulates the germination and appressorium formation required for cell invasion in insect hemocoels by M. acridum. These results provide insights into the mechanistic roles of calcineurin in the virulence of two insect pathogenic fungi.
Parasitic protists
As in fungi, the calcium-dependent calcineurin signaling cascade plays diverse roles in the life and virulence cycles of protists (Billker et al., 2009). Interestingly, the role of calcineurin in the pathogenicity of parasitic protists is similar to that in plant-pathogenic fungal calcineurin, in that calcineurin is involved in host cell attachment and invasion (Table 1). In the malaria-causing protist P. falciparum, Singh et al found that calcineurin controls actin depolymerization prior to actin-dependent entry into host cells, and also modulates microneme exocytosis during erythrocyte invasion (Singh et al., 2014). Furthermore, calcineurin regulates the ability of P. falciparum to recognize and engage surface receptors in diverse host cells (Paul et al., 2015). The function of calcineurin is highly conserved in other apicomplexans, including Toxoplasma gondii, in which depletion of calcineurin results in a decrease in protist attachment to host cells and defects in cellular entry (Paul et al., 2015). This was also observed in Plasmodium berghei, a protist that causes malaria in certain rodents, where deletion of calcineurin causes reduced colonization of diverse host cell types and impaired erythrocyte invasion (Philip and Waters, 2015). In Trypanosoma cruzi, the causative agent of Chagas disease, inhibition of calcineurin leads to reduced host cell entry, suggesting that calcineurin is required for cell invasion (Araya et al., 2008). These results demonstrate that calcineurin plays essential roles in host cell attachment and cell invasion in the pathogenesis of diverse protists.
Calcineurin has additional roles in the adaptation to environmental or host stresses in some protists. Similar to the role of calcineurin in C. neoformans, calcineurin is required for thermotolerance to host temperature and proper responses to environmental stresses in Leishmania major (Naderer et al., 2011). In Leishmania donovani, calcineurin is involved in pathogenesis by controlling flagellar motility and wave polarity (Mukhopadhyay and Dey, 2017). Overall, these studies illustrate that calcineurin plays a global role in virulence but via distinct virulence mechanisms in different eukaryotic microbial pathogens.
Calcineurin as a potential drug target against pathogenic fungi and parasitic protists
Because the calcineurin pathway is important for fungal virulence, its components are promising antifungal drug targets (Fox and Heitman, 2002). FK506 and CsA have potent antifungal and immunosuppressive properties (Ho et al., 1996). These natural compounds interact with the immunophilins FKBP12 and cyclophilin A (CypA) and form immunophilin-immunosuppressant complexes (FK506-FKBP12 and CsA-CypA), which bind to and inhibit calcineurin (Cardenas et al., 1995; Heitman et al., 1994). While these two drugs are currently available as immunosuppressants, neither is effective for treating systemic fungal infections because both show host cross-reactivity (Azzi et al., 2013). Various strategies have been proposed to reduce the side effects and/or increase the antifungal activity of these calcineurin inhibitors. L-685,818 is an FK506 analog with reduced immunosuppressive activity that exhibits antifungal activity against C. neoformans and C. albicans (Del Poeta et al., 2000; Lee et al., 2018). Nambu and colleagues developed a novel strategy using FK506 analogs that act as an antagonist by binding to FKBP12 (Nambu et al., 2017). These analogs could enter mammalian, but not fungal, cells and did not inhibit calcineurin activity in mammalian cells. The combination of excess antagonist with FK506 is not immunosuppressive and has potent antifungal activity against A. fumigatus (Nambu et al., 2017).
Although calcineurin is essential for virulence in eukaryotic pathogens, its effects on the pathogenicity of fungi and protists are somewhat varied. Mutants lacking calcineurin are avirulent in mouse models in some fungal species such as C. neoformans (Odom et al., 1997) and C. gattii (Chen et al., 2013), whereas absence of calcineurin results in significant attenuation of virulence in other fungal species including A. fumigatus (Steinbach et al., 2006), C. albicans (Blankenship et al., 2003), and M. circinelloides (Lee et al., 2013). These results suggest that calcineurin inhibitors can be useful as combination antifungal therapy with other antifungal agents (Johnson et al., 2004). A variety of studies demonstrated that calcineurin inhibitors show synergistic effects with other anti-fungal agents (Azzi et al., 2013). For example, FK506 is synergistic with fluconazole against C. neoformans (Del Poeta et al., 2000), and calcineurin inhibitors have potent fungicidal activity against azole-resistant A. fumigatus in combination with Hsp90 inhibitors (Cowen et al., 2009). Further, FK506 and posaconazole exhibit synergistic efficacy in heterologous animal mucormycosis model systems (Lewis et al., 2013). FK506 also exhibits synergistic growth inhibition in combination with micafungin against M. circinelloides (Lee et al., 2015), and a lower incidence of mucormycosis was observed among solid organ transplant recipients receiving immunosuppressant FK506 treatment(Singh et al., 2009). In C. albicans, calcineurin inhibitors can perturb biosynthesis of the membrane lipid ergosterol, and combination of calcineurin inhibitors with membrane fluconazole is highly synergistic for fungicidal activity (Cruz et al., 2002). These results suggest that combination treatment with calcineurin inhibitors and other anti-fungal agents is a promising strategy.
Beyond the calcineurin complex, other components of the calcium signaling pathway, such as calcium channels, pumps, and downstream calcineurin targets, are promising targets for new antifungal agents (Gao et al., 2015). Notably, compounds that block calcium channels or inhibit calmodulin activity show antifungal activity alone or in combination with other antifungal agents (Butts et al., 2014; Spitzer et al., 2011). However, several components of the calcium signaling pathway are conserved in the host and thus, it is necessary to identify components unique to eukaryotic pathogens. Overall, the findings summarized here highlight the importance of the calcineurin pathway as a promising target for novel antifungal agents and for the treatment of invasive fungal infections.
Conclusions
Because the calcium-calcineurin signaling pathway plays crucial roles in fungal biology and pathogenicity, it has been the subject of extensive studies over the past two decades. In eukaryotic pathogens, calcineurin governs processes including morphogenesis, stress responses, drug tolerance, cell wall integrity, and mating. Importantly, the calcium-calcineurin signaling cascade is associated with virulence via various mechanisms in fungi and protists (Table 1). Therefore, the components of the calcineurin signaling pathway are considered attractive targets for novel antifungal agents. Further elucidation of the molecular mechanisms of the calcineurin signaling pathway is required to develop novel antifungal or antiparasitic agents. In both mammals and fungi, the core components of the calcineurin pathway are highly conserved, implying that inhibitors for these components may have host cross-reactivity. However, downstream components of the pathway such as calcineurin targets or calcineurin-Crz1-controlled genes are diverse between eukaryotic pathogens and mammals. Further molecular and functional characterization of these calcineurin fungal-specific targets could aid in the development of antifungal drugs with reduced host toxicity.
In this Review, Park et al. provide an overview of the calcium-calmodulin-calcineurin signaling pathways in eukaryotic microbial pathogens, with a focus on fungal pathogens and parasitic protists. They highlight both the conserved and unique aspects of this important pathway, and explore their potential role as therapeutic target against such pathogens.
Acknowledgments
We thank Ci Fu and Michael Hoy for critical comments on the manuscript and Cecelia Wall for editorial assistance. HSP is supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2019R1F1A1048574). The work at Duke University was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) R01 AI50438-10 to J.H. and M.E.C., NIH/National Cancer Institute (NCI) R01 CA154499 to M.E.C., and R01 AI112595-04, P01 AI104533-05, and R21 AI141080-01 to J.H. This work was also supported in part by NIH R03 AI119617 and a Korean Food Research Institution (KFRI) grant to S.C.L. S.C.L. holds a Voelcker Fund Young Investigator Pilot Award from the MAX AND MINNIE TOMERLIN VOELCKER FUND.to S.C.L. J.H. is co-director and fellow of the Canadian Institute for Advanced Research (CIFAR) program Fungal Kingdom: Threats & Opportunities. We apologize to authors of papers related to this topic that were not cited due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn IP, and Suh SC (2007). Calcium restores prepenetration morphogenesis abolished by methylglyoxal-bis-guanyl hydrazone in Cochliobolus miyabeanus infecting rice. Phytopathology. 97(3), 331–337. Published online 2008/l0/24 DOI: 10.1094/PHYTO-97-3-0331. [DOI] [PubMed] [Google Scholar]
- Alshahni MM, Shimizu K, Yoshimoto M, Yamada T, Nishiyama Y, Arai T, and Makimura K (2016). Genetic and phenotypic analyses of calcineurin A subunit in Arthroderma vanbreuseghemii. Med Mycol. 54(2), 207–218. DOI: 10.1093/mmy/myv088. [DOI] [PubMed] [Google Scholar]
- Araya JE, Cornejo A, Orrego PR, Cordero EM, Cortez M, Olivares H, Neira I, Sagua H, da Silveira JF, Yoshida N, et al. (2008). Calcineurin B of the human protozoan parasite Trypanosoma cruzi is involved in cell invasion. Microbes Infect. 10(8), 892–900. Published online 2008/07/29 DOI: 10.1016/j.micinf.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Azzi JR, Sayegh MH, and Mallat SG (2013). Calcineurin inhibitors: 40 years later, can’t live without. J Immunol. 191(12), 5785–5791. Published online 2013/12/10 DOI: 10.4049/jimmunol.1390055. [DOI] [PubMed] [Google Scholar]
- Balaji S, Babu MM, Iyer LM, and Aravind L (2005). Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res. 33(13), 3994–4006. Published online 2005/07/26 DOI: 10.1093/nar/gki709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker O, Lourido S, and Sibley LD (2009). Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 5(6), 612–622. Published online 2009/06/17 DOI: 10.1016/j.chom.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JR, Wormley FL, Boyce MK, Schell WA, Filler SG, Perfect JR, and Heitman J (2003). Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot Cell. 2(3), 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts A, Koselny K, Chabrier-Rosello Y, Semighini CP, Brown JC, Wang X, Annadurai S, DiDone L, Tabroff J, Childers WE Jr., et al. (2014). Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio. 5(1), e00765–00713. Published online 2014/02/13 DOI: 10.1128/mBio.00765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Du M, Luo S, and Xia Y (2014). Calcineurin modulates growth, stress tolerance, and virulence in Metarhizium acridum and its regulatory network. Appl Microbiol Biotechnol. 98(19), 8253–8265. DOI: 10.1007/s00253-014-5876-3. [DOI] [PubMed] [Google Scholar]
- Carafoli E (2002). Calcium signaling: a tale for all seasons. Proc Natl Acad Sci U S A. 99(3), 1115–1122. DOI: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas ME, Muir RS, Breuder T, and Heitman J (1995). Targets of immunophilin-immunosuppressant complexes are distinct highly conserved regions of calcineurin A. EMBO J. 14(12), 2772–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Brand A, Morrison EL, Silao FG, Bigol UG, Malbas FF Jr., Nett JE, Andes DR, Solis NV, Filler SG, et al. (2011). Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryot Cell. 10(6), 803–819. DOI: 10.1128/EC.00310-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Konieczka JH, Springer DJ, Bowen SE, Zhang J, Silao FG, Bungay AA, Bigol UG, Nicolas MG, Abraham SN, et al. (2012). Convergent evolution of calcineurin pathway roles in thermotolerance and virulence in Candida glabrata. G3 :Genes, Genomes, Genetics. 2(6), 675–691. DOI: 10.1534/g3.112.002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Lehman VN, Lewit Y, Averette AF, and Heitman J (2013). Calcineurin governs thermotolerance and virulence of Cryptococcus gattii. G3 :Genes, Genomes, Genetics. 3(3), 527–539. DOI: 10.1534/g3.112.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Yu SJ, Huang HY, Chang YL, Lehman VN, Silao FG, Bigol UG, Bungay AA, Averette A, and Heitman J (2014). Calcineurin controls hyphal growth, virulence, and drug tolerance of Candida tropicalis. Eukaryot Cell. 13(7), 844–854. DOI: 10.1128/EC.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Kim Y, Kim S, Park J, and Lee YH (2009). MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal Genet Biol. 46(3), 243–254. DOI: 10.1016/j.fgb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Chow EW, Clancey SA, Billmyre RB, Averette AF, Granek JA, Mieczkowski P, Cardenas ME, and Heitman J (2017). Elucidation of the calcineurin-Crz1 stress response transcriptional network in the human fungal pathogen Cryptococcus neoformans. PLoS Genet. 13(4), e1006667 DOI: 10.1371/journal.pgen.1006667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE (2007). Calcium signaling. Cell. 131(6), 1047–1058. DOI: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Cooney NM, and Klein BS (2008). Fungal adaptation to the mammalian host: it is a new world, after all. Curr Opin Microbiol. 11(6), 511–516. DOI: 10.1016/j.mib.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen LE, Singh SD, Kohler JR, Collins C, Zaas AK, Schell WA, Aziz H, Mylonakis E, Perfect JR, Whitesell L, et al. (2009). Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A. 106(8), 2818–2823. DOI: 10.1073/pnas.0813394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MC, Fox DS, and Heitman J (2001). Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 20(5), 1020–1032. DOI: 10.1093/emboj/20.5.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, Cardenas ME, Perfect JR, McCusker JH, and Heitman J (2002). Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 21(4), 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert MS (2003). Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem Biophys Res Commun. 311(4), 1143–1150. Published online 2003/11/19. [DOI] [PubMed] [Google Scholar]
- de Castro PA, Chiaratto J, Winkelstroter LK, Bom VL, Ramalho LN, Goldman MH, Brown NA, and Goldman GH (2014). The involvement of the Mid1/Cch1/Yvc1 calcium channels in Aspergillus fumigatus virulence. PLoS One. 9(8), e103957 DOI: 10.1371/journal.pone.0103957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Poeta M, Cruz MC, Cardenas ME, Perfect JR, and Heitman J (2000). Synergistic antifungal activities of bafilomycin A(1), fluconazole, and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob Agents Chemother. 44(3), 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JD, Garcia-Pedrajas MD, Andrews DL, and Gold SE (2009). Calcineurin is an antagonist to PKA protein phosphorylation required for postmating filamentation and virulence, while PP2A is required for viability in Ustilago maydis. Mol Plant Microbe Interact. 22(10), 1293–1301. DOI: 10.1094/MPMI-22-10-1293. [DOI] [PubMed] [Google Scholar]
- Fairlamb AH, Gow NA, Matthews KR, and Waters AP (2016). Drug resistance in eukaryotic microorganisms. Nat Microbiol. 1(7), 16092 Published online 2016/08/31 DOI: 10.1038/nmicrobiol.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, and Gurr SJ (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature. 484(7393), 186–194. Published online 2012/04/14 DOI: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DS, and Heitman J (2002). Good fungi gone bad: the corruption of calcineurin. Bioessays. 24(10), 894–903. DOI: 10.1002/bies.10157. [DOI] [PubMed] [Google Scholar]
- Fu C, Donadio N, Cardenas ME, and Heitman J (2018). Dissecting the roles of the calcineurin pathway in unisexual reproduction, stress responses, and virulence in Cryptococcus deneoformans. Genetics. 208(2), 639–653. DOI: 10.1534/genetics.117.300422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Li W, Liu X, Gao F, and Zhao X (2015). Reversing effect and mechanism of soluble resistance-related calcium-binding protein on multidrug resistance in human lung cancer A549/DDP cells. Mol Med Rep. 11(3), 2118–2124. DOI: 10.3892/mmr.2014.2936. [DOI] [PubMed] [Google Scholar]
- Goldman A, Roy J, Bodenmiller B, Wanka S, Landry CR, Aebersold R, and Cyert MS (2014). The calcineurin signaling network evolves via conserved kinase-phosphatase modules that transcend substrate identity. Mol Cell. 55(3), 422–435. DOI: 10.1016/j.molcel.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel A, Bercovich S, and Yarden O (2006). Calcineurin is required for sclerotial development and pathogenicity of Sclerotinia sclerotiorum in an oxalic acid-independent manner. Mol Plant Microbe Interact. 19(6), 682–693. DOI: 10.1094/MPMI-19-0682. [DOI] [PubMed] [Google Scholar]
- He F, Zhang X, Mafurah JJ, Zhang M, Qian G, Wang R, Safdar A, Yang X, Liu F, and Dou D (2016). The transcription factor VpCRZ1 is required for fruiting body formation and pathogenicity in Valsa pyri. Microb Pathog. 95, 101–110. DOI: 10.1016/j.micpath.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Heitman J, Cardenas ME, Breuder T, Hemenway C, Muir RS, Lim E, Goetz L, Zhu D, Lorenz M, and Dolinski K (1994). Antifungal effects of cyclosporine and FK506 are mediated via immunophilin-dependent calcineurin inhibition. Transplant Proc. 26(5), 2833–2834. [PubMed] [Google Scholar]
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, and Crabtree GR (1996). The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 80(3 Pt 2), S40–45. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, and Rao A (2003). Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17(18), 2205–2232. DOI: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Huang S, He Z, Zhang S, Keyhani NO, Song Y, Yang Z, Jiang Y, Zhang W, Pei Y, and Zhang Y (2015). Interplay between calcineurin and the Slt2 MAP-kinase in mediating cell wall integrity, conidiation and virulence in the insect fungal pathogen Beauveria bassiana. Fungal Genet Biol. 83, 78–91. DOI: 10.1016/j.fgb.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, and Rex JH (2004). Combination antifungal therapy. Antimicrob Agents Chemother. 48(3), 693–715. Published online 2004/02/26 DOI: 10.1128/aac.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi PR, Lee SC, Heitman J, and Steinbach WJ (2017). Calcineurin in fungal virulence and drug resistance: Prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence. 8(2), 186–197. DOI: 10.1080/21505594.2016.1201250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi PR, and Steinbach WJ (2015). Calcineurin orchestrates hyphal growth, septation, drug resistance and pathogenesis of Aspergillus fumigatus: where do we go from here? Pathogens. 4(4), 883–893. DOI: 10.3390/pathogens4040883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L, Aboobakar EF, Cardenas ME, and Heitman J (2011). Calcineurin colocalizes with P-bodies and stress granules during thermal stress in Cryptococcus neoformans. Eukaryot Cell. 10(11), 1396–1402. DOI: 10.1128/EC.05087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Li A, Calo S, and Heitman J (2013). Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 9(9), e1003625 DOI: 10.1371/journal.ppat.1003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Li A, Calo S, Inoue M, Tonthat NK, Bain JM, Louw J, Shinohara ML, Erwig LP, Schumacher MA, et al. (2015). Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol Microbiol. 97(5), 844–865. DOI: 10.1111/mmi.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee KT, Lee SJ, Beom JY, Hwangbo A, Jung JA, Song MC, Yoo YJ, Kang SH, Averette AF, et al. (2018). In Vitro and In Vivo Assessment of FK506 Analogs as Novel Antifungal Drug Candidates. Antimicrob Agents Chemother. 62(11). Published online 2018/09/06 DOI: 10.1128/AAC.01627-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RE, Ben-Ami R, Best L, Albert N, Walsh TJ, and Kontoyiannis DP (2013). Tacrolimus enhances the potency of posaconazole against Rhizopus oryzae in vitro and in an experimental model of mucormycosis. J Infect Dis. 207(5), 834–841. Published online 2012/12/18 DOI: 10.1093/infdis/jis767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang ZL, Zhang LB, Ying SH, and Feng MG (2015). The role of three calcineurin subunits and a related transcription factor (Crz1) in conidiation, multistress tolerance and virulence in Beauveria bassiana. Appl Microbiol Biotechnol. 99(2), 827–840. DOI: 10.1007/s00253-014-6124-6. [DOI] [PubMed] [Google Scholar]
- Li H, Rao A, and Hogan PG (2011). Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 21(2), 91–103. DOI: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno SN, Ayong L, and Pace DA (2011). Calcium storage and function in apicomplexan parasites. Essays Biochem. 51, 97–110. DOI: 10.1042/bse0510097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, and Soto C (2011). Role of calcineurin in neurodegeneration produced by misfolded proteins and endoplasmic reticulum stress. Curr Opin Cell Biol. 23(2), 223–230. DOI: 10.1016/j.ceb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay AG, and Dey CS (2017). Role of calmodulin and calcineurin in regulating flagellar motility and wave polarity in Leishmania. Parasitol Res. 116(11), 3221–3228. Published online 2017/09/09 DOI: 10.1007/s00436-017-5608-6. [DOI] [PubMed] [Google Scholar]
- Naderer T, Dandash O, and McConville MJ (2011). Calcineurin is required for Leishmania major stress response pathways and for virulence in the mammalian host. Mol Microbiol. 80(2), 471–480. Published online 2011/02/16 DOI: 10.1111/j.1365-2958.2011.07584.x. [DOI] [PubMed] [Google Scholar]
- Nambu M, Covel JA, Kapoor M, Li X, Moloney MK, Numa MM, Soltow QA, Trzoss M, Webb P, Webb RR, 2nd, et al. (2017). A calcineurin antifungal strategy with analogs of FK506. Bioorg Med Chem Lett. 27(11), 2465–2471. DOI: 10.1016/j.bmcl.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, and Thomas AP (2002). Intracellular calcium signaling. J Cell Sci. 115(Pt 19), 3715–3716. [DOI] [PubMed] [Google Scholar]
- Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, and Heitman J (1997). Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16(10), 2576–2589. DOI: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Chow EW, Fu C, Soderblom EJ, Moseley MA, Heitman J, and Cardenas ME (2016). Calcineurin targets involved in stress survival and fungal virulence. PLoS Pathog. 12(9), e1005873 DOI: 10.1371/journal.ppat.1005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AS, Saha S, Engelberg K, Jiang RH, Coleman BI, Kosber AL, Chen CT, Ganter M, Espy N, Gilberger TW, et al. (2015). Parasite calcineurin regulates host cell recognition and attachment by apicomplexans. Cell Host Microbe. 18(1), 49–60. Published online 2015/06/30 DOI: 10.1016/j.chom.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, and Diekema DJ (2007). Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 20(1), 133–163. DOI: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N, and Waters AP (2015). Conditional degradation of Plasmodium calcineurin reveals functions in parasite colonization of both host and vector. Cell Host Microbe. 18(1), 122–131. Published online 2015/06/30 DOI: 10.1016/j.chom.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F, and Mertz P (2000). Calcineurin: form and function. Physiol Rev. 80(4), 1483–1521. Published online 2000/10/04 DOI: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Schulz RA, and Yutzey KE (2004). Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol. 266(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Schumacher J, de Larrinoa IF, and Tudzynski B (2008). Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot Cell. 7(4), 584–601. DOI: 10.1128/EC.00426-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi F, and Kontoyiannis DP (2013). The calcineurin pathway inhibitor tacrolimus enhances the in vitro activity of azoles against Mucorales via apoptosis. Eukaryot Cell. 12(9), 1225–1234. Published online 2013/07/16 DOI: 10.1128/EC.00138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Aguado JM, Bonatti H, Forrest G, Gupta KL, Safdar N, John GT, Pursell KJ, Munoz P, Patel R, et al. (2009). Zygomycosis in solid organ transplant recipients: a prospective, matched case-control study to assess risks for disease and outcome. J Infect Dis. 200(6), 1002–1011. Published online 2009/08/08 DOI: 10.1086/605445. [DOI] [PubMed] [Google Scholar]
- Singh S, More KR, and Chitnis CE (2014). Role of calcineurin and actin dynamics in regulated secretion of microneme proteins in Plasmodium falciparum merozoites during erythrocyte invasion. Cell Microbiol. 16(1), 50–63. Published online 2013/08/06 DOI: 10.1111/cmi.12177. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, Rossi L, De Pascale G, Curak J, Brown E, Tyers M, et al. (2011). Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol. 7, 499 DOI: 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, Cramer RA Jr., Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, Kirkpatrick WR, Patterson TF, Benjamin DK Jr., Heitman J, et al. (2006). Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 5(7), 1091–1103. DOI: 10.1128/EC.00139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, Reedy JL, Cramer RA Jr., Perfect JR, and Heitman J (2007). Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol. 5(6), 418–430. DOI: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]
- Stull JT (2001). Ca2+-dependent cell signaling through calmodulin-activated protein phosphatase and protein kinases minireview series. J Biol Chem. 276(4), 2311–2312. DOI: 10.1074/jbc.R000030200. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Hunter RB, Stevenson EJ, Felton HM, Kansagra NV, Lang JM, Esser KA, and Kandarian SC (2000). The calcineurin-NFAT pathway and muscle fiber-type gene expression. Am J Physiol Cell Physiol. 279(4), C915–924. DOI: 10.1152/ajpcell.2000.279.4.C915. [DOI] [PubMed] [Google Scholar]
- Thewes S (2014). Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot Cell. 13(6), 694–705. Published online 2014/04/01 DOI: 10.1128/EC.00038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, and Chung KR (2014). Calcineurin phosphatase and phospholipase C are required for developmental and pathological functions in the citrus fungal pathogen Alternaria alternata. Microbiology. 160(Pt 7), 1453–1465. Published online 2014/04/26 DOI: 10.1099/mic.0.077818-0. [DOI] [PubMed] [Google Scholar]
- Vu K, Bautos JM, and Gelli A (2015). The Cch1-Mid1 high-affinity calcium channel contributes to the virulence of Cryptococcus neoformans by mitigating oxidative stress. Eukaryot Cell. 14(11), 1135–1143. DOI: 10.1128/EC.00100-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D, Wang Y, Tang C, Fang Y, Zou J, and Tian C (2015). VdCrz1 is involved in microsclerotia formation and required for full virulence in Verticillium dahliae. Fungal Genet Biol. 82, 201–212. Published online 2015/08/04 DOI: 10.1016/j.fgb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Yoshimoto H, Saltsman K, Gasch AP, Li HX, Ogawa N, Botstein D, Brown PO, and Cyert MS (2002). Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J Biol Chem. 277(34), 31079–31088. DOI: 10.1074/jbc.M202718200. [DOI] [PubMed] [Google Scholar]
- Yu SJ, Chang YL, and Chen YL (2015). Calcineurin signaling: lessons from Candida species. FEMS Yeast Res. 15(4), fov016 DOI: 10.1093/femsyr/fov016. [DOI] [PubMed] [Google Scholar]
- Zhang H, Guo J, Voegele RT, Zhang J, Duan Y, Luo H, and Kang Z (2012). Functional characterization of calcineurin homologs PsCNA1/PsCNB1 in Puccinia striiformis f. sp. tritici using a host-induced RNAi system. PLoS One. 7(11), e49262 Published online 2012/11/10 DOI: 10.1371/journal.pone.0049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao Q, Liu K, Zhang Z, Wang Y, and Zheng X (2009). MgCRZ1, a transcription factor of Magnaporthe grisea, controls growth, development and is involved in full virulence. FEMS Microbiol Lett. 293(2), 160–169. Published online 2009/03/06 DOI: 10.1111/j.1574-6968.2009.01524.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Heitman J, and Chen YL (2012). Comparative analysis of calcineurin signaling between Candida dubliniensis and Candida albicans. Commun Integr Biol. 5(2), 122–126. DOI: 10.4161/cib.18833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang JJ, Liu S, Nian HJ, and Chen LM (2017). Characterization of calcineurin from Cryptococcus humicola and the application of calcineurin in aluminum tolerance. BmC Biotechnol. 17(1), 35 DOI: 10.1186/s12896-017-0350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]