Abstract
Background:
Crohn’s disease patients are at increased risk of postoperative venous thromboembolism. Historically, extended outpatient prophylaxis has not met conventional measures of societal cost-benefit advantage. However, extended prophylaxis for Crohn’s patients may be more cost-effective due to patients’ high thrombotic risk and long life expectancy.
Objective:
To assess the cost-effectiveness of extended prophylaxis in postoperative Crohn’s patients.
Design:
A decision tree model was used to assess the incremental cost-effectiveness and cost per case averted with extended-duration venous thromboembolism prophylaxis following abdominal surgery.
Setting:
The risk of post-discharge thrombotic event, age at surgery, type of thrombotic event, prophylaxis risk reduction, bleeding complications, and mortality were estimated using existing published sources.
Patients:
Studied were Crohn’s patients versus routine care.
Intervention:
We constructed a decision analysis to compare costs and outcomes in postoperative Crohn’s patients with and without extended prophylaxis over a lifetime horizon.
Main Outcome Measures:
Productivity costs ($) and benefits (quality-adjusted life year) were used to reflect a societal perspective and were time-discounted at 3%. Multivariable probabilistic sensitivity analysis accounted for uncertainty in probabilities, costs, and utility weights.
Results:
Using reference parameters, the individual expected societal total cost of care was $399.83 without and $1,387.95 with prophylaxis. Preventing a single mortality with prophylaxis would cost $43.00 million (number needed to treat: 39,839 individuals). The incremental cost was $1.90 million per quality-adjusted life year. Adjusting across a range of scenarios upheld these conclusions 88% of the time. With further sensitivity testing, subpopulations with post-discharge thrombosis rates greater than 4.9% favors postoperative extended-duration venous thromboembolism prophylaxis.
Limitations:
Further investigation is needed to determine if specific high-risk individuals can be preemptively identified in the Crohn’s surgical population for targeted prophylaxis.
Conclusion:
Extended prophylaxis in postoperative Crohn’s patients is not cost-effective when the cumulative incidence of post-hospital thrombosis remains less than 4.9%. These findings are driven by the low absolute risk of thrombosis in this population and the considerable cost of universal treatment. See Video Abstract at http://links.lww.com/DCR/Axxx.
Keywords: Crohn disease, Cost-benefit analysis, Decision trees, Economic evaluation, Surgery, Venous thrombosis
RESÚMEN
Antecedentes:
los pacientes con enfermedad de Crohn tienen un mayor riesgo de tromboembolismo venoso postoperatorio. Históricamente, la profilaxis ambulatoria prolongada no ha cumplido con las medidas convencionales de ventajas en costo-beneficio para la sociedad. Sin embargo, la profilaxis prolongada en los pacientes con Crohn puede ser más rentable debido al alto riesgo trombótico y a una larga esperanza de vida en estos pacientes.
Objetivo:
evaluar la rentabilidad de la profilaxis prolongada en pacientes postoperados de un Crohn.
Diseño:
se utilizó un modelo de árbol de decisión para evaluar el incremento de rentabilidad y el costo por cada caso evitado con la profilaxis prolongada de tromboembolismo venoso después de cirugía abdominal.
Entorno:
se calcularon utilizando fuentes publicadas el riesgo de evento trombótico posterior al alta, la edad del paciente al momento de la cirugía, el tipo de evento trombótico, la reducción del riesgo de profilaxis, las complicaciones hemorrágicas y la mortalidad.
Pacientes:
se estudiaron los pacientes de atención rutinaria versus aquellos portadores de Crohn.
Intervención:
construimos un arbol de análisis decisional para comparar costos y resultados de pacientes portadores de Crohn, con y sin profilaxis prolongada en el postoperatorio en un horizonte de por vida.
Principales resultados:
los costos de productividad ($) y los beneficios (año de vida ajustado por calidad) se utilizaron para reflejar la perspectiva social y se descontaron en el tiempo de un 3%. El análisis de sensibilidad probabilística multivariable dió cuenta de la incertidumbre en las probabilidades, costos y peso de utilidades.
Resultados:
Usando parámetros de referencia, el costo total social esperado de la atención individual fue de $ 399.83 sin y $ 1,387.95 con profilaxis. La prevención del deceso de un paciente con profilaxis costaría $ 43.00 millones (valor requerido para tratar: 39,839 individuos). El costo incrementado fue de $ 1.90 millones por año de vida ajustado por la calidad. El ajuste a través de una gama de escenarios confirmó estas conclusiones el 88% del tiempo. Con pruebas de sensibilidad adicionales, las subpoblaciones con tasas de trombosis posteriores al alta fueron superiores al 4,9% y favorecían la profilaxis prolongada del tromboembolismo venoso en el postoperatorio.
Limitaciones:
se necesita más investigación para determinar si se puede identificar de manera preventiva los individuos específicos de alto riesgo en la población quirúrgica de Crohn en casos de profilaxis dirigida.
Conclusión:
la profilaxis prolongada en pacientes postoperados de un Crohn no es rentable cuando la incidencia acumulada de trombosis post-hospitalaria sigue siendo inferior al 4,9%. Estos hallazgos son impulsados por el bajo riesgo absoluto de trombosis en esta población y el costo considerable del tratamiento universal.
Vea el Resumen del video en http://links.lww.com/DCR/Axxx.
INTRODUCTION
Venous thromboembolism (VTE) has been a major contributor to perioperative morbidity and mortality with incidence of 6% and a case fatality rate of 3-10%.1,2 Gastrointestinal surgery has been further recognized as a risk factor that with other co-factors may lead to an ongoing risk of postoperative VTE and therefore warrant extended-duration VTE prophylaxis (ePpx) dependent for selected patients.3–5 Typically, a regimen of four weeks of outpatient prophylactic-dose anticoagulation is recommended for high-risk patients to potentiate VTE incidence.6–9 Within the last decade, the rates of clinically-significant VTE following gastrointestinal surgery appear to be lower than historical incidences. These findings along with better risk stratification schemes have led to enhanced patient selection for ePpx, largely limiting its use to elderly patients and those undergoing gastrointestinal cancer operations.3,4,10,11
Recently, studies have identified the potentially overlooked risk of VTE among inflammatory bowel disease (IBD) patients following gastrointestinal surgery.12,13 These mechanistic considerations have been further demonstrated in population-based studies highlighting risks of VTE following gastrointestinal surgery in IBD patients that mirror other high-risk groups such as colorectal cancer patients.14–17 Although the risk has been greater in ulcerative colitis patients than Crohn’s disease (CD) patients, the early age of onset and long life expectancy of the latter group disproportionately weigh on the importance of secondary prevention efforts. These data have driven protocols to increase the use of ePpx in colorectal surgery patients, often including those with IBD.18,19 Avoiding serious, life-threatening pulmonary emboli (PE) in CD patients would permit an otherwise normal life expectancy. Therefore, whether the cost-benefit of ePpx in the CD population leads to meaningful improvement in clinical outcomes has not been determined.
Given the risk of VTE in IBD patients, analyzing the cost(risk)-benefit of ePpx may be useful for societal recommendations on its widespread use. The purpose of this study was to use existing evidence to model the cost-effectiveness of ePpx in postoperative CD patients. Our hypothesis was that the long life expectancy of CD patients and the increased risk of VTE would make this group uniquely suitable for ePpx following colon surgery.
MATERIALS AND METHODS
Study Design
We developed a decision tree model using TreeAge Pro 2018, version 18.2 to assess the incremental cost-effectiveness and cost per case averted with ePpx following abdominal surgery in CD patients versus routine care (VTE prophylaxis through hospital discharge and no ePpx). Key elements included in the model were whether to use ePpx, the impact of generic versus branded anticoagulants, risk of postoperative bleeding, risk of post-discharge VTE, the proportion of higher-morbidity PE versus lower-morbidity lower extremity deep vein thrombi (DVT), and mortality and morbidity of VTE. Figure 1 summarizes the resulting model of risk of post-discharge VTE and associated healthcare events. The reporting methods in this manuscript conform to the recommendations of the Second Panel on Cost Effectiveness in Health and Medicine (see Supplemental Checklist).20,21
FIGURE 1.
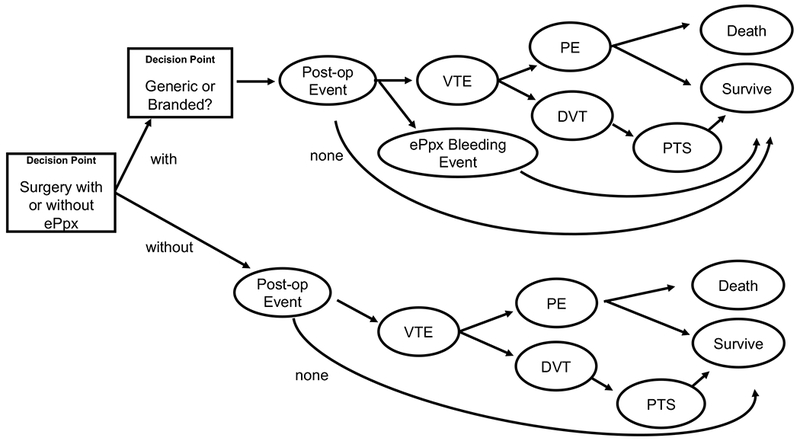
Schematized representation of decision model comparing Crohn’s disease patients undergoing abdominal surgery with and without extended venous thromboembolism prophylaxis. The rectangles represent decision points and the ellipses are chance nodes.
Note: Mirrored paths extending from generic and branded ePpx were omitted for ease of readability.
Our main outcome measure was the incremental cost-effectiveness ratio (ICER): incremental costs of including ePpx divided by the incremental quality-adjusted life years (QALY) gained, relative to no ePpx. We also assessed societal costs per VTE case averted with the equivalent number needed to treat.
We assessed the ICER compared to a willingness-to-pay (cost-effectiveness) threshold of $150,000 per QALY, the current consensus convention for United States-based interventions.22,23 We further assessed the net monetary benefit and cost of ePpx per VTE mortality prevented.
We adjusted all costs to 2017 U.S. dollars by healthcare sector inflation and applied 3% per year discounting for all future costs and QALYs.24,25
Perspective and Time Horizon
We composed our decision model from a United States-based societal perspective incorporating both individual and societal costs and benefits including health sector costs, direct costs, and productivity costs. The time horizon of events incorporated all costs related to VTE events occurring within the 30 days following surgery, costs related to ePpx-related bleeding, and costs through the average life expectancy of a CD patient with and without a VTE event. For model construction, we assumed that all VTE-related events and ePpx-related events occurred during 30 days after surgery and had no long-term sequelae.
Probabilities
We obtained parameter estimates of conditional probabilities from previously published sources and are reported in Table 1. Two authors (I.L. and S.D.) reviewed systematic reviews and primary sources to obtain a broad range of values for sensitivity analysis. Where multiple high-quality sources were identified (e.g., VTE risk reduction estimates for symptomatic VTE versus venographically-screened VTE),26–30 authors I.L., S.D., and B.S. found consensus on a final parameter estimate to be used in the model reported here and broadened sensitivity analyses to account for differences in published effects.
Table 1.
Conditional probabilities, costs, and utilities for cost-effectiveness analysis comparing extended venous thromboembolism prophylaxis following surgery for Crohn’s disease versus routine care
| Parameter | Estimate | SD | Distribution | Reference |
|---|---|---|---|---|
| Probabilities | ||||
| Probability of ePpx compliance | 80.0% | 7.5%a | Beta | 33,34 + Assumption |
| ePpx-related bleeding risks | ||||
| Risk of ePpx-related bleeding | 1.30% | 0.13%b | Beta | 25–29 |
| Death from ePpx-related bleeding | 0.0% | 5.0%a | Beta | 25,26 |
| VTE-related risks | ||||
| Probability of VTE without ePpx | 1.1% | 2.5%a | Gamma | 14–16,25,26,30 |
| Risk reduction of VTE with ePpx | 0.5 | 0.1b | Beta | 3,26–29,53 |
| Probability of DVT versus PE | 77.3% | 7.8%b | Beta | 25 |
| Risk of severe PTS | 3.0% | 0.45%c | Beta | 35 |
| Death from PE | 2.3% | 5.0%a | Beta | 25,54,55 |
| Death from DVT | 0.0% | 5.0% | Beta | Assumption |
| Costs (US 2017$)d | ||||
| Routine ePpx costs | ||||
| Cost of ePpx, branded prescription | $840.94 | $1,080.22 | Gamma | 25,26 |
| Cost of ePpx, generic prescription | $607.34 | $871.93 | Gamma | 25,26 |
| Postoperative complications | ||||
| Hospitalization, ePpx-related bleeding | $52,020 | $76,197 | Gamma | 25,26 |
| Hospitalization, postoperative PE | $40,547 | $51,493 | Gamma | 25,26 |
| Hospitalization, postoperative DVT | $28,754 | $25,098 | Gamma | 25,26 |
| Lifetime costs of PTS | $5,591 | $997 | Gamma | 37 |
| Productivity (human capital lost) | ||||
| Productivity costs of early death | $906,246 | $135,937 | Gamma | 39,40 |
| Utilities(Benefits)d | ||||
| QALYs from decision point | ||||
| Full life | 22.61 | 3.4c | Normal | 44–46 |
| Early death | 0.074 | 0.011c | Normal | 44–46 |
| Disutility adjustments | ||||
| ePpx-related bleeding | 0.0189 | 0.0028c | Normal | 48 |
| VTE with hospitalization | 0.0217 | 0.0033c | Normal | 56 |
| Life-long severe PTS | 1.0015 | 0.1502c | Normal | 49 |
SD: intentionally wider than reported sources by author consensus to capture all clinically realistic possibilities.
SD: +/− 10% of estimate.
SD: +/− 15% of estimate. Projected to long-term with assumed increased variability.
All values discounted to present at 3% per year.
ePpx, extended venous thromboembolism prophylaxis; VTE, venous thromboembolism; PE, pulmonary embolism; DVT, deep vein thrombosis; QALY, quality adjusted life year; PTS, post-thrombotic syndrome
Our preliminary analysis found estimates of postoperative VTE in CD patients to vary widely (Appendix 1). Ultimately, we favored estimates that averaged to 1.1% 30-day outpatient VTE rates in CD patients and similar colorectal surgical patients based on the assumption that all index admission VTEs would have occurred regardless of one’s planned post-discharge ePpx plan.15,16,26,27 Recent estimates of higher rates of VTE in CD patients following surgery than the parameter estimate used in this study were not able to distinguish VTE events that occurred pre- and post-hospital discharge or did not discriminate between CD and ulcerative colitis.14,15,31 Given that recurrent VTE is mediated by intrinsic patient factors rather than history of VTE,32,33 we assumed long-term recurrence of VTE was the same with and without the use of immediate postoperative ePpx. Finally, we used compliance rates previously reported in prior ePpx trials adjusted downward for real-world practice.34,35
We also incorporated a risk of post-thrombotic syndrome (PTS) into the model. We used large-scale observational data of first-time DVT presentation and future development of PTS to predict the incidence of DVT in our study population.36 Since the costs and quality of life limitations of low- and moderate-severity disease are minimal, we only used the risk of severe PTS in our model.37
Costs
We obtained costs of ePpx use and VTE-associated hospitalization from a previously published analysis involving multiple United States-based private payers, generalizable to the country’s privately insured population. The complete methodology of cost capture has been previously described, including both payer and out-of-pocket expenses associated with ePpx use as well as hospitalization costs associated with postoperative VTE in colon surgery and ePpx-related bleeding.26 Costs of inpatient VTE prophylaxis were excluded in both arms of the model due to their being identical. We incorporated post-thrombotic syndrome costs based on prior cost-effectiveness studies conducted in the United States that determined the current present value of future cost of long-term complications from VTE.38
For the purpose of the model, we assumed that all clinically-relevant post-discharge VTE would require an inpatient readmission. We acknowledged that not all VTE in practice are treated with an inpatient stay but such a conservative assumption favored the model preferring the routine ePpx strategy that we were testing.
These private-payer database cost estimates were compared with prior studies using different methodologic approaches and data sources for consistency (Table 1).27 Finally, we estimated societal costs using a human capital approach to lost productivity based on lost lifetime earnings,39,40 currently recommended over friction cost methods.21
Benefits
We assessed the benefits of each decision arm using QALYs. Using a large population-based study,41,42 we estimated the average age of CD onset in the United States population to be 29.5 years old, with an average of 1 year between diagnosis and first surgery. Given that CD in the modern era has no reduction in overall life expectancy,43 we then estimated that the average 31 year-old CD patient would live to an average United States life expectancy of 78 years old.44 Each year of life was quality-adjusted by prior estimates of disutility for a CD diagnosis.45 Life years after the age of 65 years old were further quality-adjusted by convention for aging.46 We describe the complete calculation of available QALYs for the average CD patient in Appendix 2 and summarize in Table 1. We assumed that all deaths from ePpx-related bleeding and postoperative VTE occur at 30 days following surgery (tested variations in the day of death were negligible on outcomes), affording each of these deaths one-twelfth (i.e., one month) of the estimated postoperative QALY. We assumed that patients who had ePpx-related bleeding or postoperative VTE accrued a 30-day disutility for the disability and recovery from the event.47,48 Finally, we assumed that those who developed post-thrombotic syndrome had an annual disutility weight applied to each year of remaining life.49
Statistical Analysis
We performed one-way sensitivity analysis to compare the impact of individual estimates on model conclusions. Given the variable pricing of ePpx, we also performed a one-way sensitivity analysis to determine at what price was the use of ePpx cost-effective.
We incorporated all of the deterministic reference case parameters above into a multivariable probabilistic sensitivity analysis using probability ranges described in (Table 1), including beta distributions for conditional probabilities and gamma distributions for costs. We assumed utilities to be normally distributed based on summated utilities consistently greater 0 and presumed adequate sampling of large population based estimates.50 We assessed the probabilistic distribution of ICERs and net monetary benefits with a 10,000-iteration Monte Carlo simulation.
Finally, we performed a value of perfect information analysis on the most sensitive parameter estimates per current best practices.50 Identifying the value of missing information (e.g., the difference in expected values when perfectly predicting if a specific patient would have a VTE event without prophylaxis versus probability-weighted to a VTE event in the current model) would provide guidance for further efforts to identify the intrinsic value of prediction systems for which CD patient subpopulations may benefit most from ePpx if its use in the entire population were found to not be cost-effective.
RESULTS
Cost-effectiveness of ePpx following surgery in Crohn’s disease
We found that patients undergoing abdominal surgery with CD did not meet conventional measures of cost-effectiveness for ePpx (Table 2). The average expected value of total costs of care without ePpx were $400 per person versus $1,388 per person with ePpx (incremental cost, $988). This incremental cost of ePpx was in comparison to an average expected value QALY benefit of 0.000519 QALYs per person. Thus, the ICER comparing costs to effectiveness was $1,904,328 per QALY compared to a conventional willingness-to-pay threshold of $150,000 per QALY. We also compared the total costs of ePpx per post-discharge VTE mortality prevented which was $43.0 million per death averted, which is equivalent to a number needed to treat of 39,839 individuals to prevent one death (Table 2).
Table 2.
Cost-effectiveness of extended venous thromboembolism prophylaxis following surgery for Crohn’s disease versus routine care
| Strategy | EV cost (US$ 2017) | Incremental EV cost (US$ 2017) | EV effectiveness (QALYs) | Incremental EV effectiveness (QALYs) | ICER ($/QALYs) | Net Monetary Benefit (US$ 2017) | $ per mortality averted |
|---|---|---|---|---|---|---|---|
| With extended prophylaxis | 1,387.95 | 988.63 | 22.60873 | 0.000519 | $1,904,328 | 3,389,921 | 43,003,456 |
| Routine care | 399.832 | 22.60821 | 3,390,832 |
EV, estimated value; QALYs, quality adjusted life years; ICER, incremental cost-effectiveness ratio
Net Monetary Benefit: incremental cost minus willingness-to-pay multiplied by the incremental QALYs
Sensitivity analysis for ePpx following surgery in Crohn’s disease
Figure 2 demonstrates the relative sensitivity of each variable to clinical variation when testing the reliability of our results with variation of individual parameter estimates. With this sensitivity analysis (Figure 2), we found that the baseline incidence of VTE in the model’s population was the only variable that could potentially challenge the conclusions of the mode, with an ICER falling below willingness-to-pay thresholds if the actual incidence of post-discharge VTE in the CD population was greater than 4.9% (Figure 3).
FIGURE 2.
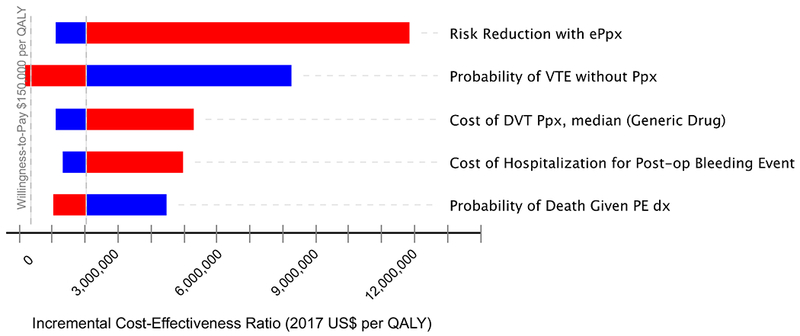
Tornado diagram with incremental cost-effectiveness ratio variation by each model parameter. Red bars represent sensitivity analysis for values greater than parameter estimate. Blue demonstrates sensitivity analysis for values less than parameter estimate.
Variables in Table 1 that are not shown in Figure 2 make up less than 10% of total variation observed in sensitivity analysis and have been omitted for readability.
FIGURE 3.
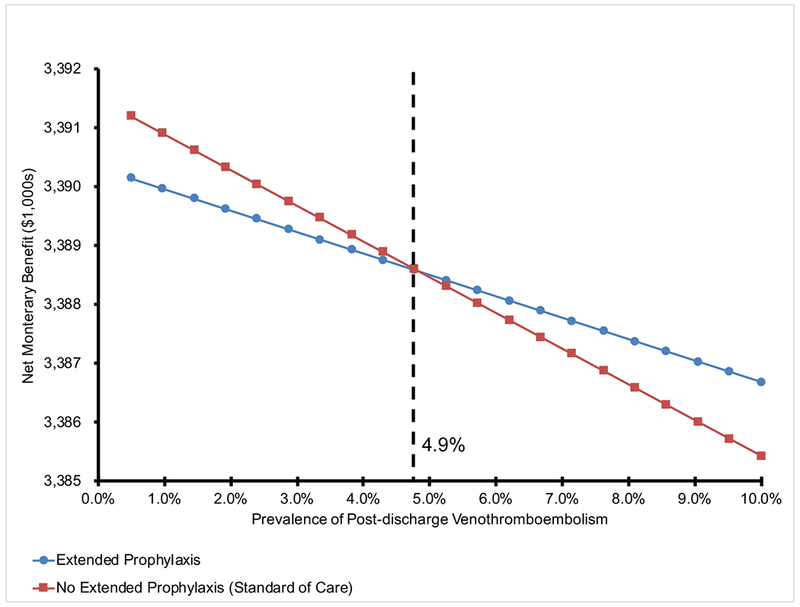
One-way sensitivity analysis of post-discharge venous thromboembolism prevalence comparing net monetary benefit of no extended prophylaxis (red) to extended prophylaxis (blue) following abdominal surgery for Crohn’s disease.
NOTE: For a given venous thromboembolism prevalence, the decision with the greater net monetary benefit is the preferred strategy. The black dashed line denotes at the inflection point for the model where the alternate strategy is now preferred. Below a rate of 4.9% post-discharge venothrombemobolism, no prophylaxis is preferred and vice-versa. Net Monetary Benefit values on the y-axis can be used to compare the relative degree of cost-effectiveness at specific post-discharge venothromboembolism rates.
We also examined a broad range of potential prices for ePpx. Under current incidence rates of postoperative VTE in CD, there was no positive price point for which one-way sensitivity analysis of ePpx cost found ePpx to be cost-effective (results not shown).
Multivariable sensitivity analysis
Multivariable probabilistic sensitivity analysis – varying all model parameters simultaneously in a probability-distributed fashion – demonstrated that the original model’s conclusions were maintained across a wide-range of scenarios. Figure 4 projects how each simulated scenario with varied parameters impacts either the incremental cost or incremental effectiveness, and, thus ultimately the ICER. Across 10,000 of these MonteCarlo simulation scenarios, not offering ePpx was the favored cost-effective strategy 88.9% of the time at a willingness-to-pay threshold of $150,000 per QALY (Appendix 3).
FIGURE 4.
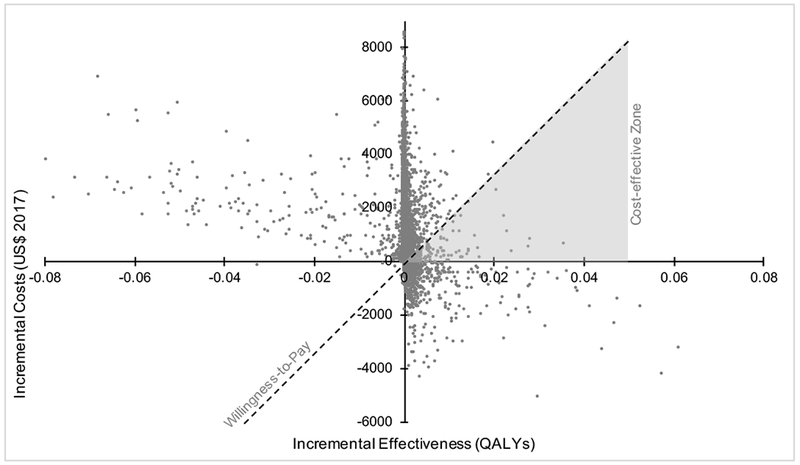
Multivariable probabilistic sensitivity analysis of incremental cost-effectiveness ratio comparing extended venous thromboembolism prophylaxis following surgery for Crohn’s disease versus hospital-only prophylaxis.
NOTE: The x-axis is the additional quality adjusted life years gained with extended prophylaxis versus routine care. The y-axis is the additional costs with extended prophylaxis versus no extended prophylaxis. Each data point represents one of 10,000 iterations of the model with random, probability-distributed variation in model parameters. The dashed line represents a $150,000 per QALY willingness to pay threshold, selected by convention. All data points below the willingness-to-pay threshold represent cases that are cost-effective supporting extended prophylaxis; all points above the line represent cases where no extended prophylaxis is the cost-effective strategy. Furthermore, points in the upper left quadrant are cases where extended prophylaxis reduces total QALYs and is more expensive. Points in the lower right quadrant are cases where extended prophylaxis both increases total QALYs and reduces costs. Thus, the gray shaded region is the only portion of the diagram where cost-effective scenarios for extended prophylaxis will fall. Appendix 3 further highlights that over 88% of all points fall above the willingness-to-pay threshold favoring no extended prophylaxis. For readability, extreme outliers representing less than 5% of the simulated sample have been omitted.
Value of perfect information
Due to the importance of baseline VTE prevalence for the model’s conclusions, we performed value of perfect information analysis on the ability to perfectly predict which specific patients would have go on to suffer a VTE event. The expected value of perfect information for this chance node was $384 suggesting that up to $384 in diagnostic resources would be economically justifiable if such efforts resulted in perfect predictability of who would develop a VTE.
DISCUSSION
In this study, we used a decision analysis model to synthesize the existing literature for the purpose of extrapolating an urgent clinical question: are the additional costs of ePpx and increased risk of bleeding justified by the VTE prevention benefit gained through the use of ePpx in Crohn’s patients undergoing abdominal surgery? We found that when comparing the ratio of costs to benefits for ePpx, the incremental average costs relative to the incremental average quality-adjusted life years gained was over $1.9 million per QALY, 10 times more than the maximum acceptable threshold used in the literature ($150,000 per QALY). When looking at case fatality alone, healthcare payers would need to expend an additional $43 million to prevent one post-discharge VTE mortality in this patient population. These findings were supported in a comprehensive multivariable sensitivity analysis, with more than 88% of scenarios tested supporting the withholding of ePpx in the postoperative CD patient population. The only single variable that strongly challenged these conclusions was the baseline rate of VTE. Only post-discharge VTE rates above 4.9% justified ePpx use. In our review of the literature, institutional rates of post-discharge VTE greater than this have not been published in CD patients undergoing surgery.
These findings should be viewed in the context of the existing published literature. Recent reports have identified unexpectedly high rates of VTE in IBD patients that rival other high-risk groups such as colorectal cancer patients.14–16 VTE events have an established morbidity and mortality associated with them,1,2 and efforts to reduce the risk of VTE in select patient population is critical. However, current aggressive VTE prevention practices such as ePpx incur additional costs and bleeding-related risks.26,27 CD patients undergoing abdominal surgery are a recognized high risk group for VTE. Moreover, in the modern era, CD patients have long life expectancies similar to the general population.43 In CD patients undergoing abdominal surgery, this combination of increased risk for VTE morbidity and potential increased benefit in terms of QALYs gained from avoiding VTE make the population a theoretically attractive target for cost-benefit analysis and economic justification for ePpx.
However, the findings of this study cannot support the use of ePpx in CD patients postoperatively from a societal cost-benefit perspective. The results demonstrate that the cost of an ePpx regimen ($607 for generic – the preferred strategy if choosing ePpx) being accrued by every CD patient undergoing surgery is not justifiable when recognizing the absolute low rate of VTE events even in this high-risk population and increasingly better mortality observed in the developing literature.
Importantly, this method of analysis using probability-weighted decision models is useful when having risk information on a population of patients and making a single decision for the entire group (e.g., whether to universally use ePpx in CD surgical patients). This decision may not necessarily hold for particularly high-risk or, alternatively, particularly inexpensive individuals within the larger population. In other words, the CD patient with multiple other risk factors for VTE or the CD patient with access to unusually cheap ePpx regimens may individually benefit from ePpx use.
Individualized VTE risk-stratification practices supported by current guidelines would be a helpful way of identifying members of the CD patient population best suited for ePpx in a selective fashion.3,4 The value of perfect information analysis performed as part of this study highlights that a significant cost outlay – up to $384 per patient if perfect predictive potential were obtained – would be appropriate to identify those specific patients who would then go on to develop a VTE. This subanalysis provides guidance when considering resource utilization of VTE risk stratification practices on a patient-by-patient basis. For example, the probability-weighted cost of duplex ultrasound detection of DVT may justify its routine use prior to discharge for better-informed risk stratification.51,52 Even more cost-effective, if not currently doing so, clinicians would benefit from using the risk factor scoring systems recommended by existing guidelines.3,4
There are limitations that need to be recognized in this study. First, this study relies on the published literature for nearly all parameter estimates. For example, the low VTE rate in other published studies using administrative data may overlook mild or subclinical VTE. However, we would anticipate that the such ascertainment biases would tend to miss less clinically impactful events and therefore have little effect on cost of hospitalization or an QALYs. Furthermore, the intentional broadening of standard deviations observed in Table 1 should help mitigate this limitation by incorporating possibilities of ascertainment bias into our multivariable sensitivity analysis.
A second limitation of this study is that these findings are specific to CD patients, not IBD patients more generally. Importantly, UC patients undergoing abdominal surgery have been found to have some of the highest rates of VTE of any studied subgroup.4,14 These two groups have markedly different VTE risk profiles as well as postoperative life expectancies due to average age at time of surgery and the indications for operations.
Finally, this study used enoxaparin as the intended intervention agent given its guideline-recommended role in colorectal cancer surgery. Oral anticoagulants have not been investigated in this patient population, and this cost-effectiveness analysis’ direct conclusions are specific to enoxaparin-based regimens.
CONCLUSIONS
CD patients represent a group with both increased VTE risk and long life expectancy following abdominal surgery. Even with these characteristics, we have demonstrated in a decision analysis model that ePpx is not justifiable on a cost-effectiveness basis when the cumulative incidence of post-hospital VTE is less than 4.9%. CD patients should not universally receive ePpx following abdominal surgery and would likely benefit from VTE risk-stratification practices for selective use of ePpx only.
Supplementary Material
Acknowledgments
Funding/Support: I.L.L. received salary support for the preparation of this manuscript from a National Cancer Institute T32 Institutional Training Grant (5T32CA126607) and a Research Foundation of the American Society of Colon and Rectal Surgeons Resident Research Initiation Grant (GSRRIG-031). E.R.H. received salary support through grants and contracts from the Patient Centered Outcomes Research Institute (CE-12-11-4489, DI-1603-34596, PCS-1511-32745), the Agency for Healthcare Research and Quality (1R01HS024547), and the National Heart, Lung, and Blood Institute (R21HL129028).
Footnotes
Financial Disclaimer: None reported.
This study is scheduled to be presented as podium presentation W28 at the American Society of Colon and Rectal Surgeons Annual Scientific Meeting, June 1-5, 2019 in Cleveland, Ohio.
REFERENCES
- 1.Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest. 2001;119(suppl):132S–175S. [DOI] [PubMed] [Google Scholar]
- 2.Wu C, Alotaibi GS, Alsaleh K, Linkins LA, McMurtry MS. Case-fatality of recurrent venous thromboembolism and major bleeding associated with aspirin, warfarin, and direct oral anticoagulants for secondary prevention. Thromb Res 2015;135:243–248. [DOI] [PubMed] [Google Scholar]
- 3.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. CHEST J. 2012;141:e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming F, Gaertner W, Ternent CA, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14–20. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee D, Lidor AO, Chu KM, Gearhart SL, Haut ER, Chang DC. Postoperative venous thromboembolism rates vary significantly after different types of major abdominal operations. J Gastrointest Surg 2008;12:2015–2022. [DOI] [PubMed] [Google Scholar]
- 6.Holwell A, McKenzie J-L, Holmes M, et al. Venous thromboembolism prevention in patients undergoing colorectal surgery for cancer. ANZ J Surg 2014;84:284–288. [DOI] [PubMed] [Google Scholar]
- 7.Vedovati MC, Becattini C, Rondelli F, et al. A randomized study on 1-week versus 4-week prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer. Ann Surg 2014;259:665–669. [DOI] [PubMed] [Google Scholar]
- 8.Kakkar VV, Balibrea JL, Martínez-González J, Prandoni P; CANBESURE Study Group. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost 2010;8:1223–1229. [DOI] [PubMed] [Google Scholar]
- 9.Akl EA, Kahale LA, Hakoum MB, et al. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst Rev 2017;9:CD006652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA Jr, Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg 2010;251:344–350. [DOI] [PubMed] [Google Scholar]
- 11.Rogers SO Jr, Kilaru RK, Hosokawa P, Henderson WG, Zinner MJ, Khuri SF. Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 2007;204:1211–1221. [DOI] [PubMed] [Google Scholar]
- 12.Bargen JA. Extensive arterial and venous thrombosis complicating chronic ulcerative colitis. Arch Intern Med (Chic). 1936;58:17. [Google Scholar]
- 13.Giannotta M, Tapete G, Emmi G, Silvestri E, Milla M. Thrombosis in inflammatory bowel diseases: what’s the link? Thromb J. 2015;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali F, Al-Kindi SG, Blank JJ, Peterson CY, Ludwig KA, Ridolfi TJ. Elevated venous thromboembolism risk following colectomy for IBD is equal to those for colorectal cancer for ninety days after surgery. Dis Colon Rectum. 2018;61:375–381. [DOI] [PubMed] [Google Scholar]
- 15.Benlice C, Holubar SD, Gorgun E, et al. Extended venous thromboembolism prophylaxis after elective surgery for IBD patients: nomogram-based risk assessment and prediction from nationwide cohort. Dis Colon Rectum. 2018;61:1170–1179. [DOI] [PubMed] [Google Scholar]
- 16.Wallaert JB, De Martino RR, Marsicovetere PS, et al. Venous thromboembolism after surgery for inflammatory bowel disease: are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum. 2012;55:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol 2011;106:713–718. [DOI] [PubMed] [Google Scholar]
- 18.Najjar PA, Madenci AL, Zogg CK, et al. Implementation of a Comprehensive post-discharge venous thromboembolism prophylaxis program for abdominal and pelvic surgery patients. J Am Coll Surg 2016;223:804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrow NE, Aboagye JK, Lau BD, et al. The role of extended/outpatient venous thromboembolism prophylaxis after abdominal surgery for cancer or inflammatory bowel disease. J Patient Saf Risk Manag 2018;23:19–26. [Google Scholar]
- 20.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 21.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine. Second Edi New York, NY: Oxford University Press; 2017. [Google Scholar]
- 22.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med 2003;163:1637–1641. [DOI] [PubMed] [Google Scholar]
- 23.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bureau of Labor Statistics. Employment, Hours, and Earnings from the Current Employment Statistics survey (National). https://data.bls.gov/timeseries/CES0500000008 Published 2015. Accessed April 6, 2018.
- 25.Data US. Bureau of Labor Statistics FRED: Federal Reserve Economic. Consumer Price Index for All Urban Consumers: Medical Care. Federal Reserver Bank of St. Louis; https://fred.stlouisfed.org/series/CPIMEDNS Published 2018. Accessed April 10, 2018. [Google Scholar]
- 26.Leeds IL, Canner JK, DiBrito SR, Safar B. Justifying total costs of extended venothromboembolism prophylaxis after colorectal cancer surgery. J Gastrointest Surg 2019. doi: 10.1007/s11605-019-04206-z [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iannuzzi JC, Rickles AS, Kelly KN, et al. Defining high risk: cost-effectiveness of extended-duration thromboprophylaxis following major oncologic abdominal surgery. J Gastrointest Surg 2014;18:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergqvist D, Agnelli G, Cohen AT, et al. ; ENOXACAN II Investigators. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975–980. [DOI] [PubMed] [Google Scholar]
- 29.Lausen I, Jensen R, Jorgensen LN, et al. Incidence and prevention of deep venous thrombosis occurring late after general surgery: randomised controlled study of prolonged thromboprophylaxis. Eur J Surg 1998;164:657–663. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen MS, Jorgensen LN, Wille-Jørgensen P, et al. ; FAME Investigators. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost 2006;4:2384–2390. [DOI] [PubMed] [Google Scholar]
- 31.Merrill A, Millham F. Increased risk of postoperative deep vein thrombosis and pulmonary embolism in patients with inflammatory bowel disease: a study of National Surgical Quality Improvement Program patients. Arch Surg 2012;147:120–124. [DOI] [PubMed] [Google Scholar]
- 32.Fahrni J, Husmann M, Gretener SB, Keo HH. Assessing the risk of recurrent venous thromboembolism--a practical approach. Vasc Health Risk Manag 2015;11:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Aken BE, den Heijer M, Bos GM, van Deventer SJ, Reitsma PH. Recurrent venous thrombosis and markers of inflammation. Thromb Haemost. 2000;83:536–539. http://www.ncbi.nlm.nih.gov/pubmed/10780312 [PubMed] [Google Scholar]
- 34.Haac BE, Van Besien R, O’Hara NN, et al. Post-discharge adherence with venous thromboembolism prophylaxis after orthopedic trauma: results from a randomized controlled trial of aspirin versus low molecular weight heparin. J Trauma Acute Care Surg 2018;84:564–574. [DOI] [PubMed] [Google Scholar]
- 35.Bergqvist D, Arcelus JI, Felicissimo P; ETHOS investigators. Post-discharge compliance to venous thromboembolism prophylaxis in high-risk orthopaedic surgery: results from the ETHOS registry. Thromb Haemost. 2012;107:280–287. [DOI] [PubMed] [Google Scholar]
- 36.Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008;149:698–707. http://www.ncbi.nlm.nih.gov/pubmed/19017588 [DOI] [PubMed] [Google Scholar]
- 37.Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost. 2009;7:884–888. [DOI] [PubMed] [Google Scholar]
- 38.Caprini JA, Botteman MF, Stephens JM, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health. 2003;6:59–74. [DOI] [PubMed] [Google Scholar]
- 39.Liu G Measuring the stock of human capital for international and intertemporal comparisons In: Jorgenson DW, Landefeld S, Schreyer P, eds. Measuring Economic Sustainability and Progress. Cambridge, MA: University of Chicago; 2014:493–544. http://www.nber.org/chapters/c12832. [Google Scholar]
- 40.Leeds IL, Namasivayam V, Bamogo A, Sankhla P, Thayer WM. Cost effectiveness of meningococcal serogroup B vaccination in college-aged young adults. Am J Prev Med 2019;56:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV Jr. Incidence and prevalence of crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol 2017;15:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn’s disease. Br J Surg 2000;87:1697–1701. [DOI] [PubMed] [Google Scholar]
- 43.Hovde Ø, Kempski-Monstad I, Småstuen MC, et al. Mortality and causes of death in Crohn’s disease: results from 20 years of follow-up in the IBSEN study. Gut. 2014;63:771–775. [DOI] [PubMed] [Google Scholar]
- 44.Social Security Administration. Actuarial Life Table. https://www.ssa.gov/oact/STATS/table4c6.html Published 2014. Accessed April 6, 2018.
- 45.Saito S, Shimizu U, Nan Z, et al. Economic impact of combination therapy with infliximab plus azathioprine for drug-refractory Crohn’s disease: a cost-effectiveness analysis. J Crohn’s Colitis. 2013;7:167–174. [DOI] [PubMed] [Google Scholar]
- 46.Brogan AJ, Talbird SE, Davis AE, Thommes EW, Meier G. Cost-effectiveness of seasonal quadrivalent versus trivalent influenza vaccination in the United States: A dynamic transmission modeling approach. Hum Vaccin Immunother 2017;13:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heisen M, Treur MJ, Heemstra HE, Giesen EBW, Postma MJ. Cost-effectiveness analysis of rivaroxaban for treatment and secondary prevention of venous thromboembolism in the Netherlands. J Med Econ 2017;20:813–824. [DOI] [PubMed] [Google Scholar]
- 48.Monahan M, Ensor J, Moore D, Fitzmaurice D, Jowett S. Economic evaluation of strategies for restarting anticoagulation therapy after a first event of unprovoked venous thromboembolism. J Thromb Haemost. 2017;15:1591–1600. [DOI] [PubMed] [Google Scholar]
- 49.de Jong LA, Dvortsin E, Janssen KJ, Postma MJ. Cost-effectiveness analysis for apixaban in the acute treatment and prevention of venous thromboembolism in the Netherlands. Clin Ther 2017;39:288–302.e4. [DOI] [PubMed] [Google Scholar]
- 50.Briggs A, Sculpher M, Claxton K. Decision Modeling for Health Economic Evaluation. Oxford, England: Oxford University Press; 2006. [Google Scholar]
- 51.Wilson RD, Murray PK. Cost-effectiveness of screening for deep vein thrombosis by ultrasound at admission to stroke rehabilitation. Arch Phys Med Rehabil 2005;86:1941–1948. [DOI] [PubMed] [Google Scholar]
- 52.Brasel KJ, Borgstrom DC, Weigelt JA. Cost-effective prevention of pulmonary embolus in high-risk trauma patients. J Trauma. 1997;42:456–460. http://www.ncbi.nlm.nih.gov/pubmed/9095113 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


