Abstract
Objective:
The benefits of antibiotic treatment during pregnancy are immediate, but there may be long-term risks to the developing child. Prior studies show an association between early life antibiotics and obesity, but few have examined this risk during pregnancy.
Subjects:
To evaluate the association of maternal antibiotic exposure during pregnancy on childhood BMI-z at five years, we conducted a retrospective cohort analysis. Using electronic health record data from seven health systems in PCORnet, a national distributed clinical research network, we included children with same-day height and weight measures who could be linked to mothers with vital measurements during pregnancy. The primary independent variable was maternal outpatient antibiotic prescriptions during pregnancy (any versus none). We examined dose response (number of antibiotic episodes), spectrum and class of antibiotics, and antibiotic episodes by trimester. The primary outcome was child age- and sex-specific BMI-z at age five years.
Results:
The final sample was 53 320 mother-child pairs. During pregnancy, 29.9% of mothers received antibiotics. In adjusted models, maternal outpatient antibiotic prescriptions during pregnancy were not associated with child BMI-z at age five years (β=0.00, 95% CI −0.03, 0.02). When evaluating timing during pregnancy, dose-response, spectrum and class of antibiotics, there were no associations of maternal antibiotics with child BMI-z at age five years.
Conclusion:
In this large observational cohort, provision of antibiotics during pregnancy was not associated with childhood BMI-z at five years.
Background
Identifying early determinants of obesity, which now affects 36.5% of adults and 17.0% of children in the United States, is necessary for developing prevention strategies that can ameliorate this public health crisis.(1, 2) One area of emerging research focuses on the potential role of in utero and early childhood antibiotic exposure on childhood metabolism and growth.(3, 4) Mothers receive antibiotics frequently during pregnancy with clear and immediate benefit, most commonly for indications like urinary tract infections.(5) With advances in understanding potential mechanistic pathways such as modification of the intestinal microbiome or epigenetic programming, recent investigations have focused on potential longer-term consequences of in utero exposure to antibiotics for childhood growth.
Several prospective cohort studies have found that in utero and early life exposures are associated with an increased risk of childhood obesity. For example, maternal pre-pregnancy BMI, gestational weight gain, smoking, and cesarean delivery all are independently associated with an increased risk of childhood obesity.(6–9) Four studies have explored maternal antibiotic exposure during pregnancy and childhood obesity risk.(10–13) Two of the largest studies had divergent findings. In a retrospective cohort study by Poulsen et al., the risk of childhood obesity at age three years was higher if mothers received antibiotics during pregnancy, but only because antibiotics in pregnancy were associated with children receiving antibiotics early in life.(13) Effect modification by maternal weight status was present; associations were largest for children born to mothers who were overweight/obese before pregnancy. In a separate cohort study of Danish school students, Mor et al., reported a higher adjusted prevalence for childhood obesity at age 7–16 years among children exposed to prenatal antibiotics.(11) With the variability of these findings, the objective in this study was to assess these relationships in a sample large enough to explore dose response, trimester-specific associations, and associations across important sub-groups.
We conducted a large retrospective cohort analysis among maternal-child pairs from seven health systems to estimate the associations between maternal outpatient antibiotic exposure during pregnancy on childhood body weight and overweight and obesity risk at age five years, controlling for multiple pregnancy risk factors, including maternal age at delivery, pre-pregnancy BMI, total gestational weight gain, pregnancy smoking status, glucose status, and mode of delivery.
Methods
The National Patient Centered Clinical Research Network (PCORnet) is a large, distributed research network (DRN) enabling multi-institutional research.(4, 14–16) Most of the data in PCORnet comes from electronic health records (EHRs) with some additional data from other healthcare data sources, such as insurance claims. The network utilizes a Common Data Model (CDM) in which each institution standardizes their data in the same format, allowing for cross-institutional querying and analysis.
This study utilizes data from seven healthcare institutions embedded within three PCORnet Clinical Data Research Networks (Supplemental Table 1). The Institutional Review Boards from each of the contributing institutions approved the study. These seven institutions transferred de-identified patient-level data to Harvard Pilgrim Health Care Institute, where the statistical analyses were conducted. The Harvard Pilgrim Institutional Review Board also approved the study.
Cohort Formation
The inclusion criteria for children in the study were a valid birth date and patient identifier, male or female sex, and a valid same-day height and weight measurement at 0 to <12 months, 12 to <30 months, and 48 to <72 months of age. Full details on the creation of the cohort are available elsewhere.(17) These requirements ensured that children were longitudinally connected to the healthcare system where data was available, allowing us to more readily capture their exposures throughout early childhood. To capture data on mothers, we required children to be linked electronically to their mothers and for mothers to have any encounter with vital measures during their pregnancy, similarly ensuring that we have at least some opportunity to capture pregnancy exposures.
Linking Mothers to Children
Each institution included in the study used separate processes to link mothers to children. Five of the institutions are fully integrated or partially integrated delivery systems that serve as both the provider and insurer for patients. These institutions have family subscriber insurance identification numbers that were used to link mothers and their children. One institution has a care delivery policy requiring mothers to be linked to children at the time of birth. Therefore, linkages were available for any children who were born in the system. The final institution adapted a multi-tiered linkage algorithm that utilized information available outside of the CDM, including child IDs listed on maternal records, geocoded residential location or home phone numbers in structured fields, and free text emergency contact information.(18)
Development of Study Specifications and Variables
Maternal Exposures
The PCORnet CDM utilized the US National Library of Medicine’s (NLM) RxNorm terminology as the standard classification system for medications. We used NLM resources and other systematic look-ups tools to create a list of systemic antibiotics. In PCORnet, many institutions do not have information on medications administered while inpatient. As a result, we included only oral and commonly-used intramuscular formulations (e.g., penicillin, ceftriaxone).
We created antibiotic episodes to define exposure during pregnancy. We utilized episodes, encompassing all prescriptions ordered within 10 days of a prior prescription, because prescribing records often do not have days’ supply or end date, precluding a determination of the exact dose or number of days for each individual prescription. If multiple antibiotics were given during an episode, the episode was defined as broad or narrow based on the broadest spectrum antibiotic prescribed.
For capturing outpatient maternal exposures during pregnancy, we defined pregnancy as nine months (273 days) prior to the index child’s date of birth. We also created trimester-specific exposures (1st trimester as days 1–90, 2nd trimester as days 91–181, 3rd trimester as days 182–273). We used this definition for pregnancy because child gestational age was not included in the PCORnet CDM and was not available on all children.
Our main independent variable was outpatient maternal antibiotic prescriptions during pregnancy, defined as any versus no antibiotic prescription. To further assess for a dose response to antibiotics, we created a categorical count of antibiotic episodes (0 to 4+). We also separately examined narrow (amoxicillin, penicillin, dicloxacillin) and broad-spectrum exposures, including the most-commonly prescribed classes of broad spectrum antibiotics – penicillin combinations (i.e., amoxicillin/clavulanic acid), 1st/2nd generation cephalosporins, 3rd generation cephalosporins, sulfa drugs, nitrofurans, and macrolides.
Child Outcomes
The primary outcome was child age- and sex-specific BMI-z from 48 to <72 months (“age five years”); if multiple measures were available, we chose the BMI-z value that was closest to 60 months of age as the outcome measure. We used standard CDC statistical macros to calculate BMI-z and percentiles using same-day heights and weights; we excluded outlier BMI-z values that were >+8 or <−4, as is recommended by the CDC.(19) The secondary outcome was overweight or obesity from 48 to <72 months, defined as an age- and sex-specific BMI ≥85th percentile; we again chose the value closest to 60 months of age.
Child Covariates and Effect Modifiers
We chose all covariates included in models a priori. Because children with complex chronic conditions are likely to have substantially different patterns of growth and interactions with the health care system than counterparts, we conducted a secondary analysis where we stratified all models by whether children had complex chronic conditions. We used the list of complex chronic conditions reported by Feudtner, et al; to this we added some growth-related conditions, including hypothyroidism and pituitary disorders, that could be related to antibiotics and weight.(20)
Covariates in models included child sex, race, and ethnicity ascertained from the patient EHR record. We collapsed race into Asian, Black or African American, White, Other, or Unknown and Hispanic ethnicity as yes or no/unknown.
Maternal Covariates
We extracted maternal age at delivery, pre-pregnancy BMI, diagnoses of diabetes or gestational diabetes (GDM), maternal smoking, method of delivery (cesarean vs. vaginal delivery), and gestational weight gain.
To calculate maternal pre-pregnancy BMI (kg/m2), we used any height that was available after age 18 and the weight closest to the beginning of the defined pregnancy period; we made use of any weight from 21 to eight months prior to delivery. We calculated total gestational weight gain as the last weight during pregnancy (within 31 days before delivery) minus pre-pregnancy weight.
We classified mothers as having diabetes if they met two or more of the following pre-pregnancy criteria (during the period 21 to nine months before the child’s date of birth): glycosylated hemoglobin (HbA1c) ≥6.5%, ICD-9 code for diabetes, or receipt of any diabetes prescription. We classified the mother as having GDM if she did not have Type 1 or Type 2 DM and she had an ICD-9 code for GDM during pregnancy.
Smoking status was available in the CDM and was defined according to the status recorded during the pregnancy period, including never/former vs. active smoker. We classified mothers as active smokers if they had any such categorization during pregnancy. We captured delivery method (cesarean vs. vaginal) using diagnostic codes and Healthcare Common Procedure Coding System (HCPCS) and Current Procedural Terminology (CPT) procedure codes.
Statistical analyses
We fit linear mixed effects regression models to examine associations of any antibiotics during pregnancy with BMI-z values at five years and logistic mixed effect regression models for the outcome of overweight or obesity (BMI ≥85th v. <85th percentile). We accounted for clustering by institution and adjusted models for child sex, race, ethnicity, age in months at outcome, maternal age at delivery, pre-pregnancy BMI, total gestational weight gain, pregnancy smoking status, glucose status and mode of delivery. To examine dose-response associations, we examined associations of 0 to 4+ antibiotic episodes during pregnancy with these outcomes.
The final set of models included separate assessments of narrow and broad-spectrum antibiotics during pregnancy. For narrow spectrum exposures, we limited the analysis to participants with no maternal broad-spectrum exposures during the same exposure time window or before. For the secondary analyses assessing trimester-specific exposures, we adjusted for antibiotic exposures during prior trimesters.
In sensitivity analysis, we re-ran models limited to mother-child pairs with no missing covariate values. To determine if pre-pregnancy BMI categories (<25, 25-<30, ≥30 kg/m2), mode of delivery, and complex chronic condition status were effect modifiers of the relationship, we ran stratified models. To assess associations of the most commonly prescribed classes of broad-spectrum antibiotics during pregnancy, we ran models treating each class of broad-spectrum antibiotics as an independent variable (any of this class of antibiotic vs. none of this class, as the reference). To address the potential for selection bias, we compared characteristics of the included vs. excluded sample.
We performed all analyses using software SAS version 9.4 (SAS Institute Inc, Cary, NC), using proc MIXED for linear and GLIMMIX for logistic regression models.
Results
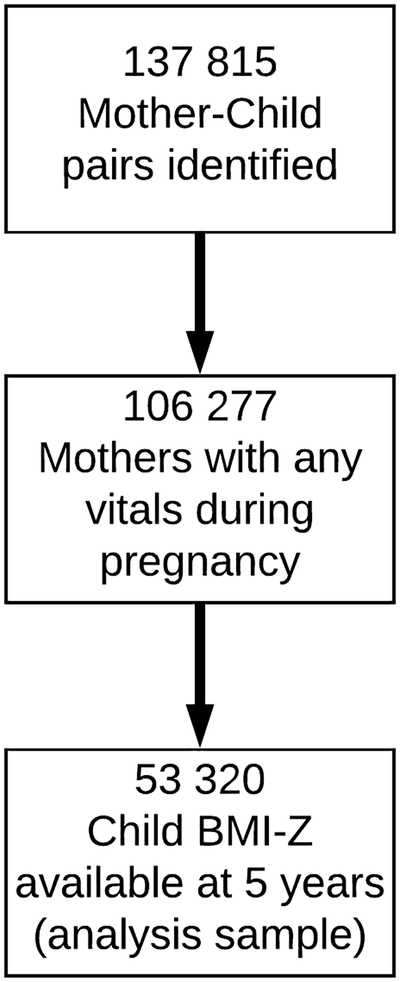
Of the 137 815 maternal-child pairs identified in the seven health systems, 53 320 mother-child pairs met inclusion criteria for the study (Figure 1). There were no differences in sociodemographic characteristics or antibiotic prescribing rates between the included and excluded participants (Supplemental Table 2). For the study sample, the average age of mothers at the time of delivery was 29.6 (SD 5.9) years. The average pre-pregnancy BMI was 27.0 (SD 6.4) kg/m2. According to their pre-pregnancy BMI, 28.3% of mothers were overweight, and 25.9% had obesity. The average total maternal gestational weight gain was 13.4 (SD 6.7) kg. Among children included in the cohort, 48.4% were female, 65.5% were White, 15.6% were Black, 8.9% were Asian, 5.1% were multiracial/other, and 24.5% were Hispanic. The average birth weight was 3336 (554 SD) g, and 12.2% of the children were preterm (Table 1).
Figure 1. Flow-Diagram of All Cohort Participants.
Of the 137 815 maternal-child pairs identified in the seven health systems, 53 320 mother-child pairs met inclusion criteria for the study.
Table 1.
Demographic and clinical characteristics of the study 504 population (N=53,320)
| Total study population N=53,320 | |
|---|---|
| N (%) or mean (SD) | |
| Mother | |
| Pregnancy smoking status, n (%) | |
| No | 50123 (94.0) |
| Yes | 3197 (6.0) |
| Pre-pregnancy BMI category, kg/m2, n (%) | |
| <25 | 11901 (45.8) |
| 25–<30 | 7346 (28.3) |
| ≥30 | 6729 (25.9) |
| Pre-pregnancy BMI, kg/m2 | 27.0 (6.4) |
| Total gestational weight gain, kg | 13.4 (6.7) |
| Age at delivery, years Glucose status, n (%) | 29.6 (5.9) |
| Normal | 46297 (86.8) |
| Gestational diabetes | 6277 (11.8) |
| History of type 1 or type 2 diabetes | 746 (1.4) |
| Antibiotic prescribing episodes during pregnancy*, n (%) | |
| 0 | 37797 (70.9) |
| 1 | 10812 (20.3) |
| 2 | 3174(6.0) |
| 3 | 1018 (1.9) |
| 4+ | 519 (1.0) |
| Broad spectrum antibiotic prescribing episodes during pregnancy*, n (%) | |
| 0 | 40587 (76.1) |
| 1 | 9245 (17.3) |
| 2 | 2380 (4.5) |
| 3 | 742 (1.4) |
| 4+ | 366 (0.7) |
| Narrow spectrum antibiotic prescribing episodes during pregnancy*, n (%) | |
| 0 | 48833 (91.6) |
| 1 | 3970 (7.4) |
| 2 | 462 (0.9) |
| 3 | 45 (0.1) |
| 4+ | 10 (0.0) |
| Trimester-specific antibiotics | |
| Any antibiotics 1 st trimester, n (%) | 6606 (12.4) |
| Any antibiotics 2nd trimester, n (%) | 6472 (12.1) |
| Any antibiotics 3rd trimester, n (%) | 6543 (12.3) |
| Cesarean delivery, n (%) | 10691 (20.1) |
| Child | |
| Preterm, n (%) | 6503 (12.2) |
| Gestational age at delivery, weeks | 39.0 (1.9) |
| Child birth weight, grams | 3336 (554) |
| Female, n (%) | 25823 (48.4) |
| Race, n (%) | |
| Asian | 4746 (8.9) |
| Black or African American | 8316 (15.6) |
| White | 34950 (65.5) |
| Other | 2694 (5.1) |
| Unknown | 2614 (4.9) |
| Hispanic ethnicity, n (%) | 13054 (24.5) |
| Child age 5 years | |
| BMI category at 5 years of age, n (%) | |
| Underweight (<5th percentile) | 2464 (4.6) |
| Normal weight (5th to <85th percentile) | 36919 (69.2) |
| Overweight (85th to <95th percentile) | 7415 (13.9) |
| Obese (≥95th percentile) | 6522 (12.2) |
| Age, months | 57.0 (5.4) |
| BMI-z score (SD) | 0.35 (1.17) |
Abbreviations: BMI, body mass index
Sample size for maternal pre-pregnancy BMI is 25976, gestational weight gain is 24290, gestational age is 31328, and birthweight is 27468
Multiple antibiotics given on the same day or within 10 days of each other were considered a single prescribing episode.
During pregnancy, 29.1% of mothers received at least one course of antibiotics; 20.3% received 1 course, and 1.0% received 4+ courses. Antibiotic prescribing was relatively constant across trimesters with approximately 12% of mothers receiving an antibiotic prescription in each trimester. Antibiotics were most often broad spectrum; 23.9% of mothers received 1+ broad spectrum antibiotics versus 8.4% for narrow spectrum antibiotics.
The average child age at final follow-up (“age five years”) was 57.0 (SD 5.4) months. Over 2/3 of children were normal weight (69.2%), while 13.9% had overweight, and 12.2% had obesity. The average BMI-z for children at 5-year follow-up was 0.35 (SD 1.17).
In unadjusted models (i.e., models including in utero antibiotic exposure and a fixed effect term for site only) there was an association between maternal antibiotic exposure during pregnancy and child BMI-z at age five years (β=0.06, 95% CI 0.04, 0.09). This association was completely attenuated with the addition of the covariates to the model (β=0.00, 95% CI −0.03, 0.02) (Table 2). When covariates were added sequentially to the model, child race/ethnicity and maternal pre-pregnancy BMI were primarily responsible for the attenuation of the unadjusted association (i.e., biased by confounding of these factors). In fully-adjusted logistic regression models, there was no association between maternal antibiotics during pregnancy and childhood overweight/obesity at age five years for children without complex medical conditions (AOR 0.96, 95% CI 0.90, 1.03) or children with these conditions (AOR 1.02, 95% CI 0.82, 1.27). When we stratified analyses by pre-pregnancy BMI and mode of delivery (Supplemental Table 3), we found no associations. When evaluating variation in associations according to timing during pregnancy, dose-response, or broad vs. narrow spectrum antibiotics, there were no associations of maternal antibiotics with childhood BMI-z at age five years (Table 3). There were no differences when further stratifying by complex chronic conditions (Supplemental Table 4). Associations between classes of broad-spectrum antibiotics with BMI-z at age five demonstrated variability in effect sizes. Confidence intervals for all included 0, except for penicillin combinations, which had a marginally significant association but with a small effect size large confidence interval (Supplemental Table 5). Results were the same in sensitivity analyses including only maternal-child pairs with complete data.
Table 2.
Multivariable linear regression results for the associations of antibiotics during pregnancy with BMI-z at ages 48 to <72 months (N=24,290)
| Adjusted β (95% CI) | |
|---|---|
| Mother | |
| Any antibiotics during pregnancy | 0.00 (−0.03, 0.02) |
| Pre-pregnancy BMI, kg/m2 | 0.05 (0.05, 0.05) |
| Total gestational weight gain, kg | 0.02(0.01,0.02) |
| Smoked during pregnancy (yes v. no) | 0.15(0.09,0.21) |
| Glucose status | |
| Gestational diabetes | 0.00 (−0.05, 0.04) |
| History of type 1 or type 2 diabetes | 0.10(0.01,0.19) |
| Normal | 0.0 (ref) |
| Age at delivery, years | 0.00 (−0.01, 0.00) |
| Cesarean delivery (yes v. no) | 0.01 (−0.03,0.04) |
| Child | |
| Race | |
| Asian | −0.05 (−0.10,0.00) |
| Black or African American | 0.02 (−0.02, 0.06) |
| Other | 0.01 (−0.05,0.07) |
| Unknown | 0.14(0.06,0.21) |
| White | 0.0 (ref) |
| Hispanic ethnicity (yes v. no) | 0.24(0.19,0.28) |
| Female v. male | 0.03 (0.00, 0.06) |
| Age at outcome, months | 0.00(0.00,0.01) |
Table 3.
Dose, timing, and spectrum of maternal antibiotics during pregnancy and child BMI-z at 48 to <72 months
| Adjusted* β (95% CI) | |
|---|---|
| Pregnancy exposures | |
| Trimester-specific antibiotics | |
| Any antibiotics 1 st trimester | 0.00 (−0.03, 0.04) |
| Any antibiotics 2nd trimester | −0.01 (−0.04, 0.03) |
| Any antibiotics 3rd trimester | 0.01 (−0.03,0.05) |
| Any broad-spectrum antibiotics | 0.00 (−0.03, 0.03) |
| Any narrow spectrum antibiotics | −0.01 (−0.07, 0.04) |
| Antibiotic prescribing episodes | |
| 0 | 0.0 (ref) |
| 1 | 0.00 (−0.03, 0.04) |
| 2 | −0.02 (−0.07, 0.03) |
| 3 | −0.08 (−0.16, 0.01) |
| 4+ | 0.05 (−0.06,0.16) |
Results shown for 6 separate models, each with the outcome of child BMI-Z at 48 to <72 months and the reference group of no antibiotics during pregnancy.
Model 1: Exposure is any antibiotics in the 1st trimester
Model 2: Exposure is any antibiotics in the 2nd trimester (any antibiotics in the 1st trimester as a covariate).
Model 3: Exposure is any antibiotics in the 3rd trimester (any antibiotics in the 1st or second trimester as covariates)
Model 4: Exposure is any broad-spectrum antibiotics during pregnancy.
Model 5: Exposure is any narrow spectrum antibiotics during pregnancy (limited to participants with no broad-spectrum antibiotics during pregnancy)
Model 6: Exposure is the number of antibiotic prescribing episodes during pregnancy (categorized as 0 [ref], 1, 2, 3, 4+)
All models were corrected for clustering by site plus adjusted for maternal pre-pregnancy BMI, total gestational weight gain, smoking during pregnancy, glucose status, age at delivery, and mode of delivery and child race, ethnicity, sex, and age at outcome.
Discussion:
In this large multi-center retrospective cohort study, there was no association between maternal outpatient antibiotic use during pregnancy and BMI-z or childhood obesity at age five years. This was true even among mothers who received four or more antibiotics during pregnancy. The large number of mother-child pairs included in this study facilitated investigation into important sub-populations, including children with complex health conditions and mothers who had pre-pregnancy overweight or obesity. Results in these subgroups did not differ from the overall results. In addition, the precision of the effect estimates indicates that even if the association between maternal antibiotics during pregnancy and child BMI-Z at age 5 were at the upper limit of the 95% confidence interval, it would not represent a clinically meaningful association. Taken together, these results do not support the hypothesis that maternal outpatient antibiotic exposure during pregnancy is associated with later childhood obesity.
This study is one of the first to utilize the National Patient Centered Clinical Research Network (PCORnet) to conduct large epidemiological studies. The demographic characteristics of participants who met inclusion criteria for this study was diverse, mirroring the national population, allowing for robust population inferences. In addition, the rate of outpatient prescribing practices among pregnant women was similar to previously published rates.(5) To conduct this study, we applied several different methods of linking mothers to children, and we pulled in data outside of the PCORnet Common Data Model (e.g., maternal-child linkage). This was the first study across the network to employ these approaches and shows the feasibility of expanding on PCORnet’s CDM for multi-institutional research.
Previous studies have found conflicting results regarding a potential association between maternal antibiotics during pregnancy and later child obesity. One investigation of 436 maternal-child pairs by Mueller et al., identified an 84% increased risk of offspring obesity at age seven years for 2nd and 3rd trimester antibiotic exposure.(10) Among 9 886 Danish school children ages 7–16 years, Mor et al., reported that maternal antibiotic use during pregnancy was associated a with higher adjusted prevalence ratio of 1.29 (95% CI 1.03, 1.62) for child obesity. (11) A recent study of 303 maternal-child pairs also demonstrated a small increase in mean BMI-z (0.2, 95% CI 0.1, 0.3) over 2 years of follow-up, if exposed to prenatal antibiotics.(12) However, Poulsen et al., found no overall association among 8,793 maternal-child pairs from the Geisinger Clinic.(13) One reason for the differing findings among these studies could be the various methodological approaches, including both the timing of follow-up and the method for evaluating maternal antibiotic exposure. One study used maternal self-report to define antibiotic exposure,(10) but others (including the current study) have used electronic health record data.(11–13) Similarly, the length of follow-up has varied from 2 −16 years. The current results advance the field by demonstrating no association between maternal outpatient antibiotic use during pregnancy and later child overweight and obesity at age five among a large, diverse sample, using electronic health record data.
This study had several limitations. In studies using electronic health records, missing or incomplete data could lead to misclassification of either the exposure or the outcome. Specifically, we were only able to identify antibiotic prescribing and not dispensing data. If we missed prescriptions given to mothers outside of the healthcare institutions from which we had data (e.g., in urgent care clinics), antibiotic prescribing rates would be underrepresented; in contrast, having prescribing data alone precludes a determination of prescription filling, which may lead to overrepresentations of exposure. We also used weight and height data collected in the course of routine clinical care, measures that may not be as precise as data collected specifically for research purposes. Inaccurate weight and weight status would be non-differential misclassification, biasing results toward the null. In addition, we were only able to capture outpatient antibiotic prescribing, which may have overlooked important potential contributions of more broad-spectrum antibiotics that would typically be prescribed in inpatient settings, especially peripartum antibiotic use. Because we could only use data from maternal-child linkages in which mothers had vitals during pregnancy, we selected only for mothers receiving prenatal care; mothers not receiving this care are likely different for our study population. The pre-term delivery rate in the included sample was 12%, which is higher than the national average, and may limit generalizability of the findings. This difference is attributable to higher pre-term delivery rates in some of the hospital systems within PCORnet that provide high-risk obstetric care. Finally, as in any observational study, confounding by unmeasured variables such as socio-economic status, insurance status, and dietary intake is of concern.
Strengths of this study include use of multiple health systems with a broad geographic distribution and diverse patient population, ability to incorporate maternal and child data, and a large sample size allowing for assessments of subgroups.
Conclusions:
In this large retrospective cohort there was no association between maternal outpatient antibiotic exposure during pregnancy and childhood obesity at age five years. Antibiotics should always be prescribed cautiously and appropriately during pregnancy. However, the long-term risk of childhood obesity from maternal antibiotic use during pregnancy appears to be negligible.
Supplementary Material
Acknowledgements
This work was supported through the Patient-Centered Outcomes Research Institute (PCORI) Program Award (OBS-1505-30699). All statements in this manuscript are solely those of the authors and do not necessarily represent the views of PCORI, its Board of Governor, or Methodology Committee. The PCORnet Childhood Antibiotic Study Team includes a diverse group of investigators, research staff, clinicians, community members, and parent caregivers. All members of the team including the study’s Executive Antibiotic Stakeholder Advisory Group (EASAG) contributed to the study design, data acquisition, and interpretation of results. The Study Team would like to thank the leaders of the participating PCORnet Clinical Data Research Networks (CDRNs), Patient Powered Research Networks (PPRNs), and PCORnet Coordinating Center as well as members of the PCORI team for their support and commitment to this project.
Abbreviations:
- PCORnet
National Patient-Centered Clinical Research Network
- BMI
Body mass index
- CDRNs
Clinical data research networks
- CDM
Common Data Model
- HER
Electronic health record
- NLM
National Library of Medicine
- HCPCS
Healthcare Common Procedure Coding System
- CPT
Current Procedural Terminology
Appendix 1
Members of the PCORnet Antibiotics and Childhood Growth Study Group:
David Arterburn MD, Kaiser Permanente Washington Health Research Institute, Seattle, Washington; Lauren P. Cleveland, MS, MPH, Division of Chronic Disease Research Across the Lifespan (CoRAL), Department of Population Medicine, Harvard Pilgrim Health Care Institute, Harvard Medical School, Boston, Massachusetts; Jonathan Finkelstein, MD, MPH, Boston Children’s Hospital, Department of Population Medicine, Harvard Pilgrim Health Care Institute, Harvard Medical School, Boston, Massachusetts; Stephanie L. Fitzpatrick, PhD, Kaiser Permanente Center for Health Research, Portland, Oregon; Andrea Goodman, MSW, MPH, Genetic Alliance, Washington DC; Michael Horberg, MD, MAS, Kaiser Permanente Mid-Atlantic Permanente Research Institute, Rockville, Maryland; Jenny Ingber, PhD, no affiliation, New York, New York; Kathleen Murphy, MPH, Genetic Alliance, Washington DC; Holly Landrum Peay, MS, PhD, RTI International, Research Triangle Park, North Carolina; Pedro Rivera, MS, OCHIN Inc, Portland, Oregon; Juliane S. Reynolds, MPH, Therapeutics Research and Infectious Disease Epidemiology Group, Department of Population Medicine, Harvard Pilgrim Health Care Institute, Harvard Medical School, Boston, Massachusetts; Jessica L. Sturtevant, MS, Therapeutics Research and Infectious Disease Epidemiology Group, Department of Population Medicine, Harvard Pilgrim Health Care Institute, Harvard Medical School, Boston, Massachusetts; Ivette Torres, BA, Valley Baptist Medical Center - Harlingen, Harlingen, Texas
Footnotes
List of members of the PCORnet Antibiotics and Childhood Growth Study Group is listed as Appendix 1
Competing interests
The authors have no conflicts of interest to report.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity among adults and young: United States, 2011–2014. NCHS Data Brief, no 219. Hyattsville, MD: National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- 2.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am J Prev Med. 2016;50(6):761–79. [DOI] [PubMed] [Google Scholar]
- 3.Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nature reviews Endocrinology. 2015;11(3):182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block JP BL, Gillman MW, Lunsford D, Daley MF, Eneli I, Finkelstein J, Heerman WJ, Horgan CE, Hsia DS, Jay M, Rao G, Reynolds JS, Rifas-Shiman SL,,Sturtevant JL, Sengwee T, Trasande L, Young JG, Forrest CB, on behalf of PCORnet Antibiotics and Childhood Growth Study Group. Early Antibiotic Exposure and Weight Outcomes in Young Children. Pediatrics. 2018; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ailes EC, Summers AD, Tran EL, Gilboa SM, Arnold KE, Meaney-Delman D, et al. Antibiotics Dispensed to Privately Insured Pregnant Women with Urinary Tract Infections - United States, 2014. MMWR Morb Mortal Wkly Rep. 2018;67(1):18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond). 2013;37(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135(4):617–26. [DOI] [PubMed] [Google Scholar]
- 8.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA pediatrics. 2014;168(11):1063–9. [DOI] [PubMed] [Google Scholar]
- 9.Scott FI, Horton DB, Mamtani R, Haynes K, Goldberg DS, Lee DY, et al. Administration of antibiotics to children before age 2 years increases risk for childhood obesity. Gastroenterology. 2016;151(1):120–9.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. International journal of obesity. 2015;39(4):665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mor A, Antonsen S, Kahlert J, Holsteen V, Jorgensen S, Holm-Pedersen J, et al. Prenatal exposure to systemic antibacterials and overweight and obesity in Danish schoolchildren: a prevalence study. International journal of obesity. 2015;39(10):1450–5. [DOI] [PubMed] [Google Scholar]
- 12.Cassidy-Bushrow AE, Burmeister C, Havstad S, Levin AM, Lynch SV, Ownby DR, et al. Prenatal antimicrobial use and early-childhood body mass index. International journal of obesity. 2018;42(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulsen MN, Pollak J, Bailey-Davis L, Hirsch AG, Glass TA, Schwartz BS. Associations of prenatal and childhood antibiotic use with child body mass index at age 3 years. Obesity (Silver Spring, Md). 2017;25(2):438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient-centered clinical research network. J Am Med Inform Assoc. 2014;21(4):578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis LH, Brown J, Platt R. Four health data networks illustrate the potential for a shared national multipurpose big-data network. Health affairs. 2014;33(7):1178–86. [DOI] [PubMed] [Google Scholar]
- 16.Brown JS, Holmes JH, Shah K, Hall K, Lazarus R, Platt R. Distributed health data networks: a practical and preferred approach to multi-institutional evaluations of comparative effectiveness, safety, and quality of care. Med Care. 2010;48(6 Suppl):S45–51. [DOI] [PubMed] [Google Scholar]
- 17.Block JP, Bailey LC, Gillman MW, Lunsford D, Boone-Heinonen J, Cleveland LP, et al. The PCORnet Antibiotics and Childhood Growth Study: Process for Cohort Creation and Cohort Description. Acad Pediatr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angier H, Gold R, Crawford C, J POM, C JT, Marino M, et al. Linkage methods for connecting children with parents in electronic health record and state public health insurance data. Maternal and child health journal. 2014;18(9):2025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years) [cited 2018. Available from: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 20.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.