Summary
Ductal carcinoma in situ is a non-invasive form of breast cancer. Its incidence is increasing due to widespread use of mammographic screening. It presents several diagnostic and management challenges in part due to its relatively indolent behaviour. Most series analysing biomarkers in these lesions are small (<100 patients) and large clinical trials have not been frequent. Herein, we review the recent progress made in understanding the biology of this entity and the tools available for prognostication.
Keywords: Breast cancer, DCIS, ductal carcinoma in situ, pathology, molecular features
INTRODUCTION
The biological basis of micro-calcifications within the breast is poorly defined. In spite of this, it is common to observe micro-calcifications in benign ducts as well as in pre-invasive lesions of the breast. It has long been recognised that breast cancer can be associated with micro-calcifications. Although mammography also detects architectural distortion and masses, the science of mammography is based on the detection of presence and pattern of calcifications. As in situ lesions tend to exhibit micro-calcifications more frequently than invasive carcinoma, it is natural that the incidence of detecting pre-invasive lesions has dramatically increased (up to 20%) following the routine use of mammographic screening.1
In the pre-mammographic era, ductal carcinoma in situ (DCIS) was a palpable lesion accounting for approximately 1–2% of cases.2 It is believed that palpable DCIS may be biologically different from screen detected DCIS. Although peri-ductal fibrosis is frequently observed in DCIS, its aetiology is far from clear. One possible explanation is that it represents a reaction to ‘early’ invasion either in the form of cells or cellular products which might play a critical role in this process. Pathologists should regard this lesion with caution and generous sampling of the tissues to identify small foci of invasion is prudent. On the clinical side, particularly in the United States, many surgeons prefer to perform a priori sentinel node biopsies for these lesions. From the biological aspect, these lesions are of great interest for the study of tumour microenvironment and the changes associated with the process of invasion.
There has been significant progress in the last few years in our understanding of DCIS and its management. The focus of this review is to highlight some of these aspects while underlining the large number of unknowns and the need to devise better methods for studying this entity. We presume that the readers of this review are conversant with the basics of DCIS and these issues will not be reviewed in detail.
DIAGNOSTIC CRITERIA
The morphological changes in DCIS lie within in the spectrum of hyperplasia and invasive cancer. The criteria for differentiation of DCIS from hyperplastic lesions are well described. Stains for high molecular weight keratins (HMWK) can be used to assist the distinction of DCIS from usual hyperplasia. The distinction of DCIS from atypical ductal hyperplasia (ADH) is based on examination of architectural, cytological and nuclear features. Size of the lesion is also an important consideration, with diagnosis of ADH being favoured for smaller lesions. Immunohistochemistry has little role to play in the distinction of DCIS from ADH. However, the strong and uniform expression of oestrogen receptor (ER), indicative of clonal proliferation, can been used to support the diagnosis of DCIS in difficult cases. Stains for basal and myoepithelial markers aid in the identification of invasion. However, some forms of non-invasive lesions such solid papillary carcinoma might not retain myoepithelial staining.
TERMINOLOGY FOR DCIS
Long-term follow-up studies have documented high overall survival rates (>95% at 10 years) for DCIS; this includes death due to any cause such as road traffic accidents and cardiovascular events. This has raised the important issue of whether DCIS be considered ‘cancer’? The term ‘cancer’ is associated with considerable emotional baggage including the fear of chemotherapy, and death. The logic of applying such a charged word to something that is indolent and very unlikely to cause significant impediment to life has been questioned. This is particularly true for lobular carcinoma in situ (LCIS), which undergoes spontaneous regression in the vast majority of cases. Although it has been suggested that the term carcinoma should be avoided,3 there is poor consensus on the replacement terminology. The term ‘indolent lesions of epithelial origin (IDLE)’4 has been suggested but is not yet widely adopted. There are concerns that the term ‘indolent’ might not convey the marked increase in risk for the development of invasive carcinoma and might result in undertreatment of patients.
ASSESSING THE BIOLOGY OF DCIS
The wide variations in series size and the treatments offered has added to the confusion regarding the prognostic factors in DCIS. Most series of DCIS are small (100–200 patients), limited by follow-up data and have variable endpoints for outcome assessment, i.e., recurrence of DCIS or development of invasive carcinoma or both. Despite this a few generalisations can be made and these are discussed below.
Clinical and histopathological features
Younger age is associated with increased risk of recurrence of DCIS. This has been observed in almost all the series and clinical trials. The size of DCIS is also a poor prognostic factor with larger size being associated with greater risk of recurrence. The measurement of size of DCIS is not always easy and multiple methods for measurement have been described (for review see Lester et al.5). Accurate assessment of size is greatly aided by a good mammographic correlation.
Margin status is another important consideration. A number of different definitions have been used to define ‘clear’ margins. The NSABP trials have used absence of ‘ink-on-tumour’ for margin negativity, whereas other groups have used 1 mm, 3 mm, 5 mm and even 1 cm margins. The precise definition was quite arbitrary until the recent Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology (SSO-ASTRO-ASCO) DCIS consensus on margin status.6 These guidelines recommend adoption of greater than 2 mm as definition of clear margins, and use of clinical judgement for re-excision of lesions with 0–2 mm margin status; these have been successfully implemented within the surgical community.7
Architectural features are used to classify DCIS into comedo, solid, cribriform, micropapillary and other subtypes.8 Comedo DCIS has been associated with earlier recurrences and micropapillary pattern with extensive/large disease volume. A number of systems have been used to determine histological grade of DCIS.8 Some of these evaluate cellular features as well as cell polarity, while others are based entirely on nuclear grade. The role of grade in determination of likelihood of recurrence has been controversial. In the subset of patients (n=327) from the E5194 clinical trial that was used to develop/validate the DCIS Score (Genomic Health, USA), grade [College of American Pathologists (CAP) grading system] and histological features were not associated with recurrence.9 In contrast, in the parent trial (n=665), high grade was predictive of outcome at 10 and 12 years.10 The importance of grade was also confirmed in analysis of 57,222 DCIS cases from the SEER database by Sagara et al.11 The weighted 10-year breast cancer-specific survival hazard ratios of low grade DCIS cases were significantly different for intermediate and high grade DCIS [low grade hazard ratio (HR) 0.85, 95% confidence interval (CI) 0.21–3.52; intermediate grade HR 0.23, 95% CI 0.14–0.42; and high grade HR 0.15, 95% CI 0.11–0.23].11
Stromal features have also been used for prognostication. The presence of periductal fibrosis has been associated with increased likelihood of recurrence.12 The mechanisms associated with the development of this feature is uncertain. Some studies have implicated cancer associated fibroblasts (CAFs) as the basis for the altered stroma and for making the tumour microenvironment more conducive for the development of invasive cancer.13,14 Periductal angiogenesis is also often seen in DCIS; the significance of this feature is uncertain. The failure of anti-angiogenic therapies in the treatment of invasive breast cancer has led to a diminishing interest in understanding the molecular basis of this feature.15
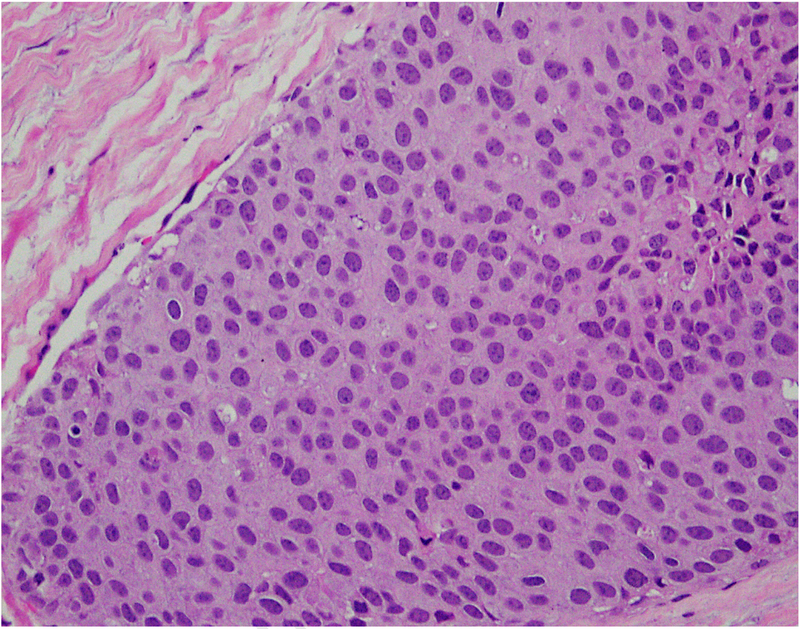
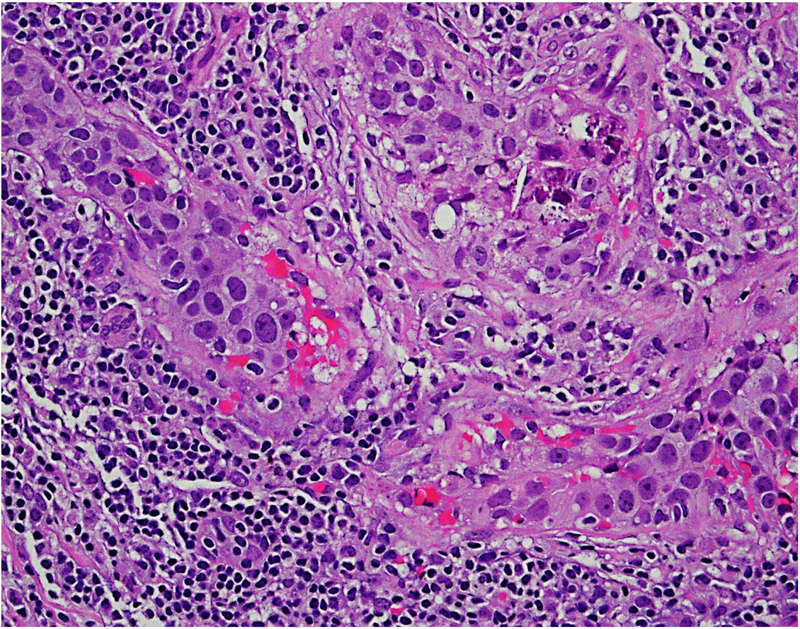
The recognition importance of stromal tumour infiltrating lymphocytes (TILs) in breast cancer, particularly triple negative breast cancer,16–18 has led to a number of studies investigating immune cells in DCIS. DCIS cases harbour stromal TILs, where they are associated with younger age, symptomatic presentation, larger size, higher nuclear grade, comedo necrosis and ER negativity as well as shorter recurrence-free interval.19 Similarly, high grade DCIS cases are also likely to show expression of FOXP3, CD68, CD4, CD20, HLA-DR20 and PD-L1.21 Further parsing of the data is necessary to identify the exact role of TILs in DCIS. The association of immune cells with poor prognosis DCIS could be indicative of a breach of the integrity of the basement membrane, at least at a molecular level. Figure 1 shows infiltration by immune cells in ductal carcinoma in situ (DCIS).
Fig. 1.
Focal infiltration by immune cells in ductal carcinoma in situ (DCIS) (20× objective using a Nikon DP27 camera using CellSens software).
Biological parameters
Protein analysis
Immunohistochemistry has been extensively used to identify prognostic factors in DCIS; however, most studies are limited by small sample size and limited follow-up. In spite of this, there are several important parameters that can be used for prognostication. A number of markers related to proliferation, cell cycle regulation, apoptosis, angiogenesis, extracellular matrix proteins (CD10, SPARC) and inflammation including COX2 have been analysed in DCIS. A detailed meta-analysis of the biological markers and risk of recurrence was performed by Lari and Kuerer22 in 2011; much of the data stands to date and will not be reproduced herein but salient features will be highlighted.
Expression of ER is observed in greater than 80% of cases and in some series 90–95% of cases (E5194 and Toronto cohorts23–26) while progesterone receptor (PR) expression is observed in approximately 60% of cases.22 ER negative DCIS has been associated with increased likelihood of recurrence. The analysis of expression of ER and PR has become commonplace in the management of DCIS. This in part due to the data from the NSABP B-24 study, which assessed the benefit of tamoxifen in 732 patients treated with surgery and radiation.27 The patients with ER+ DCIS (76%) had significant benefit from tamoxifen (HR 0.64) at 10 years of follow-up. However, the 22 patients with ER negative DCIS did not obtain any benefit. Although this data has not been further validated, it was deemed practice changing with resultant implementation in clinical practice.
The expression of HER2 has been analysed in a number of cohorts reviewed by Sanati.26 Expression has been associated with increased likelihood of development of recurrence. Of interest, expression of HER2 is observed in around 40% of cases of DCIS which is a significantly higher rate than in invasive cancer. Theories to explain this finding include loss of HER2 during progression, invasion arising from a distinct clone with HER2 positive cells acting as ‘enablers’ during the process.
The expression of Ki-67 has been analysed in a number of series. The expression in DCIS, in contrast to invasive cancer, tends to be low. In most series, this results in poor correlation with outcomes. In a large series of patients, Kerlikowske et al. demonstrated that the combination of Ki-67 with COX2 and p16 expression could predict development of recurrence.28 More recently, this set of markers has been expanded to include HER2, PR, Ki-67, COX2, p16/INK4A, FOXA1 and SIAH2 and available as a commercial assay DCISionRT from PreludeDx (discussed later).29,30
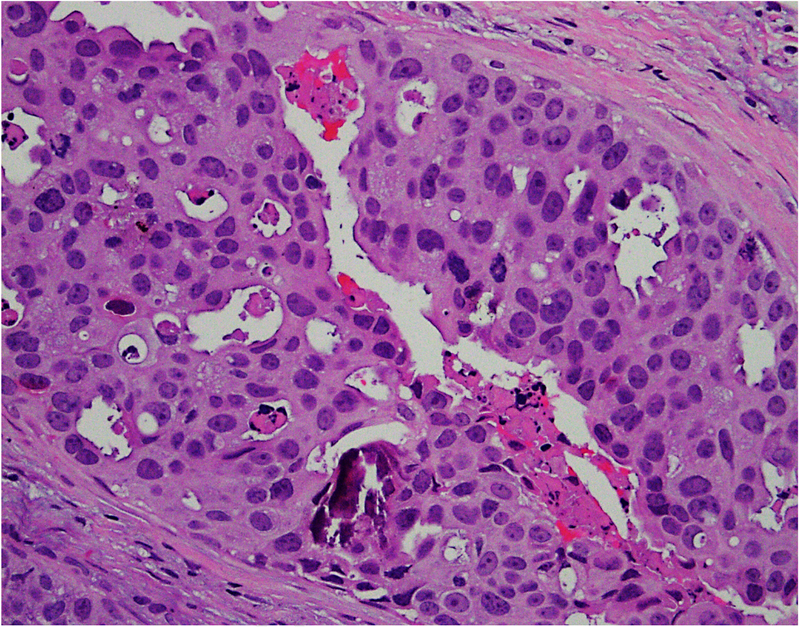
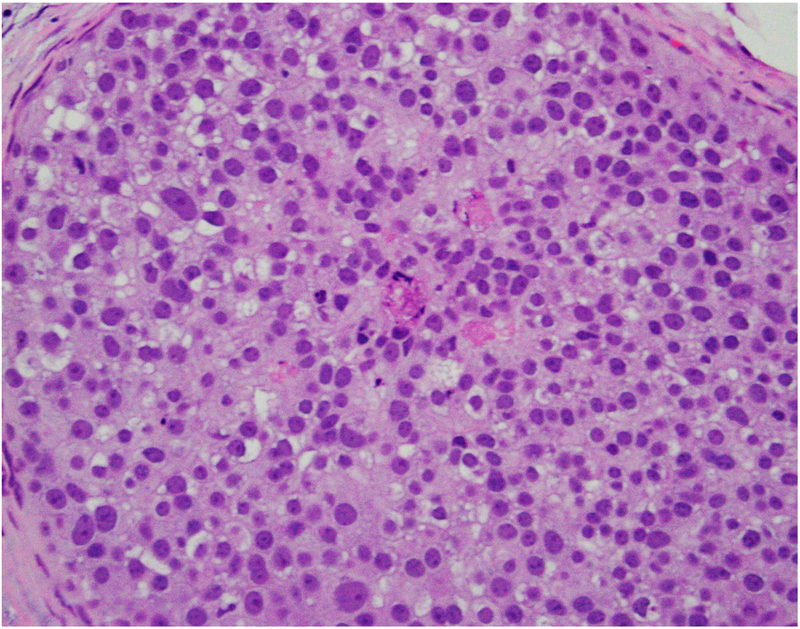
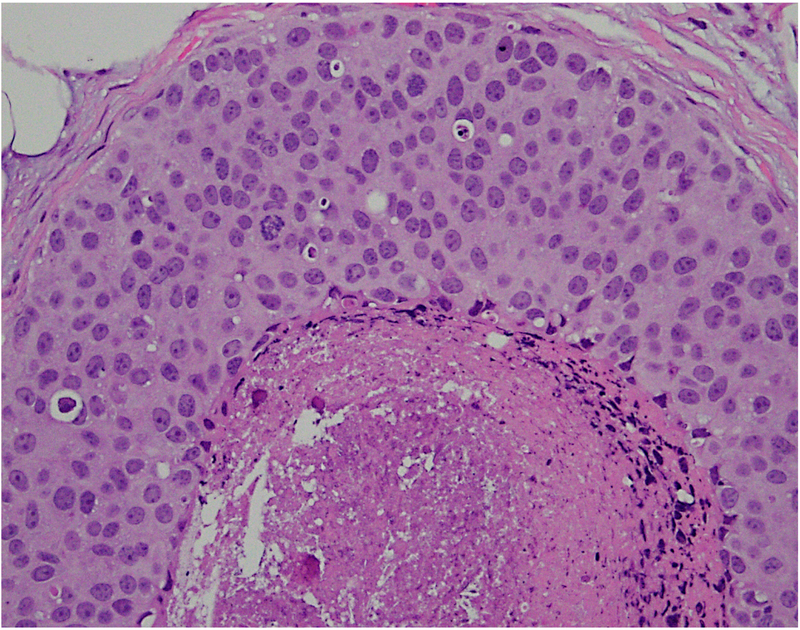
We have analysed the co-expression of multiple proteins in DCIS using multiplex immunofluorescence on a single 5 μm section. The study revealed marked heterogeneity in expression of oncologically relevant proteins in cells located within the same duct.31 In addition, there was variability in degree of heterogeneity with some ducts containing predominantly a single subtype of cells while others showing the presence of multiple subpopulations. Further analyses seeking to understand the biological meaning of these findings are ongoing. Figure 2 shows heterogeneity in the morphological patterns of DCIS.
Fig. 2.
(A–D) Heterogeneity in the morphological patterns of ductal carcinoma in situ (DCIS). Images are taken from a needle biopsy of a single patient with DCIS (same patient as Fig. 1; 20× objective using a Nikon DP27 camera using CellSens software). Note the range of architectural patterns ranging from solid to cribriform as well as the variable amounts of necrosis in the ducts.
Genomic analysis
Copy number analysis has been used to study the biology of DCIS (reviewed by Rane et al.32). Array comparative genomic hybridisation (aCGH) analysis has confirmed the presence of low and high grade pathways in DCIS. The low grade pathway is associated with loss of 16q and variable gain of 1q. Deletions of 16q are rare in the high grade pathway, while alterations of 8p, 11q, 13q, 17q and 20q are more frequent. Multiple studies have used fluorescent in situ hybridisation (FISH) to study DCIS and have found alteration in copy numbers of HER2, C-MYC, CCND1, COX2, CDH1, and TP53.33–36
A number of studies have sought to analyse the mutational patterns in DCIS and compare them with those of invasive carcinomas. Some of these studies have compared concurrent DCIS with invasive lesions from the same patient, while others have compared ‘pure’ DCIS lesions with DCIS associated with invasive carcinoma from different patients or a combination of the two.37 The pattern of mutations in DCIS appears to be identical to that in invasive carcinomas with high frequency mutations in PI3K and p53.38–40 These studies have also noted a significant intratumoural heterogeneity in DCIS. An important study in this area was undertaken by Navin and colleagues wherein single cells from concurrent DCIS and IDC were analysed for their mutation status.41 They developed and applied topographical single cell sequencing (TSCS) to analyse the genomic copy number profiles of 1,293 cells from 10 patients. The study documented nearly identical mutational patterns in these lesions, suggesting the development of invasion does not need additional mutations. It also suggested a multi-clonal invasion model in which one or more clones assist each other in order to escape the ducts and establish the invasive carcinomas. Epigenetic events in tumour cells might be critical drivers for the progression. A number of studies have analysed DNA methylation, non-coding RNA, histone modification and chromatin remodelling patterns in DCIS and associated these with progression (reviewed by DeVaux and Herschkowitz42). Alterations in the character and function of the tumour microenvironment could play a major role.43–45 There are no data currently available to justify routine genomic analysis of DCIS.
RNA expression analysis
RNA expression analysis compares DCIS with concurrent invasive lesions or pure DCIS with DCIS associated with invasive cancers.46–50 The patterns of expression have been found to be fairly similar. Hannemann et al. analysed a series of 40 in situ lesions and compared them with 40 invasive carcinomas using an 18K expression array.51 They identified 300 differentially expressed genes and developed a 43-gene classifier that could distinguish low grade DCIS from high grade and a 35-gene classifier to distinguish DCIS from invasive carcinoma. Interestingly, many of the genes identified in the classifier belong to the signal transduction and cell growth and maintenance pathways. Muggerud et al. studied a set of genes that identified a group that was most similar to invasive cancers.52 This group was enriched in genes related to reorganisation of the microenvironment. Kristensen et al., applied an integrated approach by modeling mRNA, copy number alterations, microRNAs, and methylation in a pathway context utilising the pathway recognition algorithm using data integration on genomic models (PARADIGM).53 They found a great degree of similarity in the expression pattern of the five subtypes (PDGM1–5) in DCIS and invasive carcinoma with the exception of PDGM2, which was not identified in any of the 36 cases of (pure) DCIS. They did not find an association between PDGMs and grade, Ki-67 or HER2 staining. Lesurf et al.,39 integrated mRNA, miRNA and copy number analyses to identify features that are unique to each of the intrinsic subtypes of DCIS. Their analysis suggests that there are subtype specific features that determine likelihood of recurrence.
Multi-parametric tools including commercial assays
Nomograms
The need for predictors of likelihood of presence of invasive carcinoma or development of recurrence has resulted in the development of multiple nomograms. The Van Nuys prognostic index (VNPI) is based on patient’s age, lesion size and grade, and margin width.54,55 It stratifies patients into three risk groups and provides treatment guidelines to achieve less than 20% recurrence rate at 12 years. The limitations of this nomogram include lack of robust independent validation and failure to include molecular characteristics. A more recent modification of this nomogram incorporates Genomic Grade Index (GGI) but needs further validation.56 The Memorial Sloan Kettering (MSKCC) nomogram57 is based on age, family history, clinical features at initial presentation, nuclear grade, presence of necrosis, margins, number of excisions, type of surgery, radiation and endocrine therapy. It is widely used and validated in multiple independent studies. However, there are some concerns regarding under-estimation of heterogeneity in DCIS.58 More recently, the Mayo group has described a nomogram to predict upstaging of DCIS to invasive carcinoma.59
Oncotype Dx DCIS score
The Eastern Co-operative Oncology Group (ECOG) had undertaken a registration (non-randomised) clinical trial of DCIS wherein patients were offered complete surgical excision (with centrally confirmed clean margins) but no radiation therapy.60 Patients were enrolled onto one of two study cohorts (not randomly assigned): cohort 1, low or intermediate grade DCIS, tumour size ≤2.5 cm (n=561); or cohort 2, high-grade DCIS, tumour size ≤1 cm (n=104). Blocks were obtained from a subset of patients (n=327) for Oncotype DX recurrence score and DCIS Score. The 21-gene recurrence score was not associated with likelihood of ipsilateral breast event (IBE). In contrast, DCIS score was prognostic for identifying both the likelihood of recurrent DCIS and/or development of invasive disease.24 These findings have been independently confirmed by Rakovitch’s group in a large study from Toronto.23 One of the limitations of these studies is that the vast majority (>95%) of the DCIS cases were ER positive; the relevance of the signature in ER negative DCIS has not been established.
DCISionRT (PreludeDx)
DCISionRT from PreludeDx analyses the expression of a set of markers (HER2, PR, Ki-67, COX2, p16/INK4A, FOXA1 and SIAH2) to identify benefit of radiotherapy. It has been validated in multiple independent cohorts from the USA (Kaiser Permanente and UMass), Sweden (Uppsala) and Australia (Melbourne).30 The test has been recently used in cohort of 526 patients with DCIS treated with breast conserving surgery with or without radiotherapy (RT). In this cohort, it was prognostic for risk and predicted RT benefit.29
MANAGEMENT ISSUES AND NEWER CLINICAL TRIALS
The management of DCIS has evolved considerably within last few decades. Until the mid-1980s, DCIS was routinely treated with mastectomy.61 The National Surgical Adjuvant Breast Project (NSABP) B06 clinical trial compared the role of lumpectomy with mastectomy in the treatment of breast cancer.62 Retrospective analysis of these cases showed that a small percentage of the 2072 patients (n=76) had (mostly palpable) DCIS. Twenty-one of these patients had been treated with lumpectomy; of these nine patients (43%) developed recurrence. The recurrence rate for patients with lumpectomy plus radiation (XRT) was 7%, while that for patients undergoing mastectomy was 0%. The value of addition of radiation to lumpectomy was further confirmed in the NSABP B17 clinical trial in which 81% of the patients had non-palpable DCIS.61,63 A dramatic decrease in rates of recurrent DCIS (13.4% to 8.2%) and development of invasive disease (13.4% to 3.2%) was observed in patients receiving radiation. The compilation of results from multiple clinical trials by the early breast cancer trialists groups has documented a 50% reduction in IBEs after addition of radiation to lumpectomy.64 Further reduction in recurrence rates also has been observed following the addition of tamoxifen to surgery and radiation (L+ RT+ Tam), making this the standard of care for treatment of DCIS. In a relatively recent series, Palazzo’s group studied the outcomes of 141 patients treated with surgery only. Despite negative margins, 60 recurrences (42.5%) were observed after a median follow-up of 122 months.65 In contrast, Tadros et al. reported that for patients with close margins (<2 mm), the addition of radiation was associated with dramatic reduction in recurrence rates at 10 years (30.9% vs 4.8%).66 There was marginal benefit in adding radiation therapy to patients with greater than 2 mm margins (5.4% and 3.3%, respectively) in this study.
There is a major concern that the standard of care therapy for DCIS (L+ RT+ Tam) might be unnecessarily aggressive for patients with small screen detected DCIS. This has resulted in a number of clinical trials that are addressing the question of reduction of therapy for these patients. The Comparison of Operative to Monitoring and Endocrine Therapy (COMET) trial enrols patients with non-comedo DCIS for medical (non-surgical) management.67 Similarly, LORIS (Low RIsk DCIS) and LORD (Low Risk DCIS) trials are being conducted around the world to address the issues.68,69 Trial enrolment is based on the subjective assessment of grade and absence of comedo necrosis. A recent study by Schnitt’s group documented the poor correlation amongst pathologists in determining ‘comedo necrosis’.70 In this vein, it is important to remember that a significant number of DCIS lesions, approximately 25%, are upstaged to invasive carcinoma on excision;71–75 these might be missed in these trials. Based on archival data, it has been estimated that approximately 20% of patients enrolled in the LORIS trial will have invasive cancer.69 However, a recent analysis found the upstaging rates to invasive disease were only 6% (5/81), 7% (5/74), and 10% (1/10) for the COMET, LORIS, and LORD trials, respectively.67 It is critical that all conservatively managed patients should be closely monitored.
CONCLUSION AND FUTURE CHALLENGES
There has been steady, albeit slow, progress in understanding the biology of DCIS. There are multiple lines of evidence to document that DCIS is not a single entity but a collection of biologically heterogeneous lesions. Biologically, the cells in these lesions seem to have acquired all the necessary mutations and are poised to invade. However, the critical event necessary for invasion is not yet identified but could be an epigenetic event involving tumour-stromal interactions.
Although histology, tumour markers such as ER and HER2, and stromal markers such as TILs, provide some clues regarding the likelihood of recurrence, our knowledge regarding the mechanisms associated with progression of DCIS to invasive cancer are still in their infancy. There is a critical need to understand intra-lesional and inter-lesional heterogeneity and their impact on likelihood of recurrence/progression. There is a clear and pressing need for development of well-annotated cohorts with treatment information and follow-up data to understand the molecular basis of recurrence and progression. This will require a collaborative multi-centre, multi-disciplinary approach, such as the Cancer Research UK Grand Challenge program. It will also be critical to perform integrative analysis (histology, mutations, copy number, mRNA, and protein levels) to predict likelihood of progression and impact of endocrine/radiotherapy. Ultimately, it might become necessary to have large multi-centre trials based on biomarkers of recurrence to convince the breast community regarding the utility of these predictive tools.
Conflicts of interest and sources of funding:
SB is supported by Susan G Komen for the Cure and NIH R01 CA194600. The authors state that there are no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017; 67: 439–48. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin 2011; 61: 409–18. [DOI] [PubMed] [Google Scholar]
- 3.Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and Management of Ductal Carcinoma In Situ September 22–24, 2009. J Natl Cancer Inst 2010; 102: 161–9. [DOI] [PubMed] [Google Scholar]
- 4.Esserman LJ, Thompson IM, Reid B, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol 2014; 15: e234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lester SC, Connolly JL, Amin MB. College of American Pathologists protocol for the reporting of ductal carcinoma in situ. Arch Pathol Lab Med 2009; 133: 13–4. [DOI] [PubMed] [Google Scholar]
- 6.Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Ductal Carcinoma In Situ. J Clin Oncol 2016; 34: 4040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeSnyder SM, Hunt KK, Dong W, et al. American Society of Breast Surgeons’ practice patterns after publication of the SSO-ASTRO-ASCO DCIS Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation. Ann Surg Oncol 2018; 25: 2965–74. [DOI] [PubMed] [Google Scholar]
- 8.Badve S, A’Hern RP, Ward AM, et al. Prediction of local recurrence of ductal carcinoma in situ of the breast using five histological classifications: a comparative study with long follow-up. Hum Pathol 1998; 29: 915–23. [DOI] [PubMed] [Google Scholar]
- 9.Badve SS, Gray R, Baehner FL, et al. Correlation between the DCIS score and traditional clinical and pathologic features in the prospectively-designed E5194 clinical validation study. J Clin Oncol 2012; 30 (Suppl 1). [Google Scholar]
- 10.Solin LJ, Gray R, Hughes LL, et al. Surgical excision without radiation for ductal carcinoma in situ of the breast: 12-year results from the ECOG-ACRIN E5194 study. J Clin Oncol 2015; 33: 3938–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagara Y, Mallory MA, Wong S, et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA Surg 2015; 150: 739–45. [DOI] [PubMed] [Google Scholar]
- 12.Sneige N, McNeese MD, Atkinson EN, et al. Ductal carcinoma in situ treated with lumpectomy and irradiation: histopathological analysis of 49 specimens with emphasis on risk factors and long term results. Hum Pathol 1995; 26: 642–9. [DOI] [PubMed] [Google Scholar]
- 13.Van Bockstal M, Lambein K, Van Gele M, et al. Differential regulation of extracellular matrix protein expression in carcinoma-associated fibroblasts by TGF-beta1 regulates cancer cell spreading but not adhesion. Oncoscience 2014; 1: 634–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osuala KO, Sameni M, Shah S, et al. Il-6 signaling between ductal carcinoma in situ cells and carcinoma-associated fibroblasts mediates tumor cell growth and migration. BMC Cancer 2015; 15: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aalders KC, Tryfonidis K, Senkus E, Cardoso F. Anti-angiogenic treatment in breast cancer: Facts, successes, failures and future perspectives. Cancer Treat Rev 2017; 53: 98–110. [DOI] [PubMed] [Google Scholar]
- 16.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015; 26: 259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol 2019; 37: 559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014; 32: 2959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toss MS, Miligy I, Al-Kawaz A, et al. Prognostic significance of tumor-infiltrating lymphocytes in ductal carcinoma in situ of the breast. Mod Pathol 2018; 31: 1226–36. [DOI] [PubMed] [Google Scholar]
- 20.Campbell MJ, Baehner F, O’Meara T, et al. Characterizing the immune microenvironment in high-risk ductal carcinoma in situ of the breast. Breast Cancer Res Treat 2017; 161: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson E, Taube JM, Elwood H, et al. The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol 2016; 29: 249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lari SA, Kuerer HM. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer 2011; 2: 232–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rakovitch E, Nofech-Mozes S, Hanna W, et al. Multigene expression assay and benefit of radiotherapy after breast conservation in ductal carcinoma in situ. J Natl Cancer Inst 2017; 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst 2013; 105: 701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villanueva H, Grimm S, Dhamne S, et al. The emerging roles of steroid hormone receptors in ductal carcinoma in situ (DCIS) of the breast. J Mammary Gland Biol Neoplasia 2018; 23: 237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanati S. Morphologic and molecular features of breast ductal carcinoma in situ. Am J Pathol 2019; 189: 946–55. [DOI] [PubMed] [Google Scholar]
- 27.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol 2012; 30: 1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerlikowske K, Molinaro AM, Gauthier ML, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst 2010; 102: 627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bremer T, Whitworth PW, Patel R, et al. A biological signature for breast ductal carcinoma in situ to predict radiotherapy benefit and assess recurrence risk. Clin Cancer Res 2018; 24: 5895–901. [DOI] [PubMed] [Google Scholar]
- 30.Zhou W, Jirstrom K, Johansson C, et al. Long-term survival of women with basal-like ductal carcinoma in situ of the breast: a population-based cohort study. BMC Cancer 2010; 10: 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerdes MJ, Gokmen-Polar Y, Sui Y, et al. Single-cell heterogeneity in ductal carcinoma in situ of breast. Mod Pathol 2018; 31: 406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rane SU, Mirza H, Grigoriadis A, Pinder SE. Selection and evolution in the genomic landscape of copy number alterations in ductal carcinoma in situ (DCIS) and its progression to invasive carcinoma of ductal/no special type: a meta-analysis. Breast Cancer Res Treat 2015; 153: 101–21. [DOI] [PubMed] [Google Scholar]
- 33.Vincent-Salomon A, Lucchesi C, Gruel N, et al. Integrated genomic and transcriptomic analysis of ductal carcinoma in situ of the breast. Clin Cancer Res 2008; 14: 1956–65. [DOI] [PubMed] [Google Scholar]
- 34.Heselmeyer-Haddad K, Berroa Garcia LY, Bradley A, et al. Single-cell genetic analysis of ductal carcinoma in situ and invasive breast cancer reveals enormous tumor heterogeneity yet conserved genomic imbalances and gain of MYC during progression. Am J Pathol 2012; 181: 1807–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorringe KL, Hunter SM, Pang JM, et al. Copy number analysis of ductal carcinoma in situ with and without recurrence. Mod Pathol 2015; 28: 1174–84. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen MA, Doebar SC, van Deurzen CHM, Martens JWM, van Diest PJ, Moelans CB. Copy number profiling of oncogenes in ductal carcinoma in situ of the male breast. Endocr Relat Cancer 2018; 25: 173–84. [DOI] [PubMed] [Google Scholar]
- 37.Afghahi A, Forgo E, Mitani A, et al. Chromosomal copy number alterations for associations of ductal carcinoma in situ with invasive breast cancer. Breast Cancer Res 2015; 17: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakr RA, Weigelt B, Chandarlapaty S, et al. PI3K pathway activation in high-grade ductal carcinoma in situ––implications for progression to invasive breast carcinoma. Clin Cancer Res 2014; 20: 2326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesurf R, Aure MR, Mork HH, et al. Molecular features of subtype-specific progression from ductal carcinoma in situ to invasive breast cancer. Cell Rep 2016; 16: 1166–79. [DOI] [PubMed] [Google Scholar]
- 40.Pang JB, Savas P, Fellowes AP, et al. Breast ductal carcinoma in situ carry mutational driver events representative of invasive breast cancer. Mod Pathol 2017; 30: 952–63. [DOI] [PubMed] [Google Scholar]
- 41.Casasent AK, Schalck A, Gao R, et al. Multiclonal invasion in breast tumors identified by topographic single cell sequencing. Cell 2018; 172: 205–17e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeVaux RS, Herschkowitz JI. Beyond DNA: the role of epigenetics in the premalignant progression of breast cancer. J Mammary Gland Biol Neoplasia 2018; 23: 223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendry S, Pang JB, Byrne DJ, et al. Relationship of the breast ductal carcinoma in situ immune microenvironment with clinicopathological and genetic features. Clin Cancer Res 2017; 23: 5210–7. [DOI] [PubMed] [Google Scholar]
- 44.Nelson AC, Machado HL, Schwertfeger KL. Breaking through to the other side: microenvironment contributions to DCIS initiation and progression. J Mammary Gland Biol Neoplasia 2018; 23: 207–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha VC, Piwnica-Worms H. Intratumoral heterogeneity in ductal carcinoma in situ: chaos and consequence. J Mammary Gland Biol Neoplasia 2018; 23: 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seth A, Kitching R, Landberg G, Xu J, Zubovits J, Burger AM. Gene expression profiling of ductal carcinomas in situ and invasive breast tumors. Anticancer Res 2003; 23: 2043–51. [PubMed] [Google Scholar]
- 47.Elias EV, de Castro NP, Pineda PH, et al. Epithelial cells captured from ductal carcinoma in situ reveal a gene expression signature associated with progression to invasive breast cancer. Oncotarget 2016; 7: 75672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdalla M, Tran-Thanh D, Moreno J, et al. Mapping genomic and transcriptomic alterations spatially in epithelial cells adjacent to human breast carcinoma. Nat Commun 2017; 8: 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santpere G, Alcaraz-Sanabria A, Corrales-Sanchez V, Pandiella A, Gyorffy B, Ocana A. Transcriptome evolution from breast epithelial cells to basal-like tumors. Oncotarget 2018; 9: 453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz S, Bartsch H, Sotlar K, et al. Progression-specific genes identified in microdissected formalin-fixed and paraffin-embedded tissue containing matched ductal carcinoma in situ and invasive ductal breast cancers. BMC Med Genomics 2018; 11: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hannemann J, Velds A, Halfwerk JB, Kreike B, Peterse JL, van de Vijver MJ. Classification of ductal carcinoma in situ by gene expression profiling. Breast Cancer Res 2006; 8: R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muggerud AA, Hallett M, Johnsen H, et al. Molecular diversity in ductal carcinoma in situ (DCIS) and early invasive breast cancer. Mol Oncol 2010; 4: 357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kristensen VN, Vaske CJ, Ursini-Siegel J, et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci USA 2012; 109: 2802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silverstein MJ, Poller DN, Waisman JR, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet 1995; 345: 1154–7. [DOI] [PubMed] [Google Scholar]
- 55.Silverstein MJ, Barth A, Poller DN, et al. Ten-year results comparing mastectomy to excision and radiation therapy for ductal carcinoma in situ of the breast. Eur J Cancer 1995; 31A: 1425–7. [DOI] [PubMed] [Google Scholar]
- 56.Altintas S, Toussaint J, Durbecq V, et al. Fine tuning of the Van Nuys prognostic index (VNPI) 2003 by integrating the genomic grade index (GGI): new tools for ductal carcinoma in situ (DCIS). Breast J 2011; 17: 343–51. [DOI] [PubMed] [Google Scholar]
- 57.Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol 2010; 28: 3762–9. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Perez C, Turnbull AK, Ekatah GE, et al. Current treatment trends and the need for better predictive tools in the management of ductal carcinoma in situ of the breast. Cancer Treat Rev 2017; 55: 163–72. [DOI] [PubMed] [Google Scholar]
- 59.Jakub JW, Murphy BL, Gonzalez AB, et al. A validated nomogram to predict upstaging of ductal carcinoma in situ to invasive disease. Ann Surg Oncol 2017; 24: 2915–24. [DOI] [PubMed] [Google Scholar]
- 60.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009; 27: 5319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer 1999; 86: 429–38. [DOI] [PubMed] [Google Scholar]
- 62.Fisher ER, Anderson S, Redmond C, Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06. 10-year pathologic and clinical prognostic discriminants. Cancer 1993; 71: 2507–14. [DOI] [PubMed] [Google Scholar]
- 63.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst 2011; 103: 478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ebctcg, McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014; 383: 2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holmes P, Lloyd J, Chervoneva I, et al. Prognostic markers and long-term outcomes in ductal carcinoma in situ of the breast treated with excision alone. Cancer 2011; 117: 3650–7. [DOI] [PubMed] [Google Scholar]
- 66.Tadros AB, Smith BD, Shen Y, et al. Ductal carcinoma in situ and margins <2 mm: contemporary outcomes with breast conservation. Ann Surg 2019; 269: 150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grimm LJ, Ryser MD, Partridge AH, T, et al. Surgical upstaging rates for vacuum assisted biopsy proven DCIS: implications for active surveillance trials. Ann Surg Oncol 2017; 24: 3534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elshof LE, Tryfonidis K, Slaets L, et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ - The LORD study. Eur J Cancer 2015; 51: 1497–510. [DOI] [PubMed] [Google Scholar]
- 69.Pilewskie M, Stempel M, Rosenfeld H, Eaton A, Van Zee KJ, Morrow M. Do LORIS trial eligibility criteria identify a ductal carcinoma in situ patient population at low risk of upgrade to invasive carcinoma? Ann Surg Oncol 2016; 23: 3487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison BT, Hwang ES, Partridge AH, Thompson AM, Schnitt SJ. Variability in diagnostic threshold for comedo necrosis among breast pathologists: implications for patient eligibility for active surveillance trials of ductal carcinoma in situ. Mod Pathol 2019; Apr 12: (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 71.Dillon MF, McDermott EW, Quinn CM, O’Doherty A, O’Higgins N, Hill AD. Predictors of invasive disease in breast cancer when core biopsy demonstrates DCIS only. J Surg Oncol 2006; 93: 559–63. [DOI] [PubMed] [Google Scholar]
- 72.Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology 2011; 260: 119–28. [DOI] [PubMed] [Google Scholar]
- 73.Park AY, Gweon HM, Son EJ, Yoo M, Kim JA, Youk JH. Ductal carcinoma in situ diagnosed at US-guided 14-gauge core-needle biopsy for breast mass: preoperative predictors of invasive breast cancer. Eur J Radiol 2014; 83: 654–9. [DOI] [PubMed] [Google Scholar]
- 74.Sim YT, Litherland J, Lindsay E, et al. Upgrade of ductal carcinoma in situ on core biopsies to invasive disease at final surgery: a retrospective review across the Scottish Breast Screening Programme. Clin Radiol 2015; 70: 502–6. [DOI] [PubMed] [Google Scholar]
- 75.Shi B, Grimm LJ, Mazurowski MA, et al. Prediction of occult invasive disease in ductal carcinoma in situ using deep learning features. J Am Coll Radiol 2018; 15: 527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]