Abstract
Introduction
Natesto®, testosterone nasal gel (TNG), is a testosterone therapy (TTh) indicated for adult male hypogonadism.1 This study allowed titration decisions to be based on physicians’ assessment of patient symptoms.
Methods
Hypogonadal males on active topical testosterone therapy (TThE) or naive to any form of testosterone therapy (TThN) were treated with 22 mg TNG daily (11 mg twice daily) for 90 days. Titration was determined by the physician at day 90 wherein the dose was increased to 33 mg daily if symptoms were not properly managed. Total testosterone (TT) levels were collected at day 90 and 120 and the quantitative Androgen Deficiency in the Aging Male (qADAM) symptom questionnaire was administered on days 0, 30, 60, 90, and 120.
Results
At study endpoint, 77.0% of all patients were in the normal TT range. Mean qADAM scores increased from 30.8 at baseline to 35.5 (6.6) at day 90. Physician assessments resulted in 37% patients being up-titrated for an additional 30 days, however, qADAM scores did not change significantly at the higher dose.
Conclusions
The majority of patients achieved the normal range of testosterone with TNG when physicians based their titration decision on an assessment of symptoms. Sexual function and energy-related symptoms were predictive of improvements resulting from treatment. These symptoms were the most relevant indicators for physicians in making decisions relating to titration.
Introduction
A 2014 U.S. Food and Drug Administration advisory committee reported that only 72% of approximately 240 000 testosterone therapy (TTh) patients had their testosterone levels tested prior to their first prescription, and only 6% of patients returned to the lab after the initial prescription regardless of titration decision, despite the importance of this analysis for safety and efficacy purposes.1 Another study reported that only 9% of men who returned for testing complied with the recommendation that blood samples be taken between 7 am and 12 pm.2 The low compliance to these recommendations is concerning because of the risks of high testosterone levels. For example, one of the more common adverse events with TTh is high hematocrit.3 While excess red blood cell count is readily corrected by phlebotomy, the consequences of inaction can be severe. Due to compliance challenges regarding obtaining serum levels, a drug product that does not produce a supraphysiological testosterone level even at the highest recommended dose for treatment inherently reduces the risks of high testosterone level.
Natesto® testosterone nasal gel (TNG) is indicated for TTh in adult males for conditions associated with deficiency or absence of endogenous testosterone (hypogonadism).4 The starting dose of TNG is 22 mg daily (11 mg twice daily), which can be increased to 33 mg daily (11 mg three times daily). TNG elevates serum total testosterone (TT) levels 40 minutes after application to a peak concentration of 32.5±13.2 nmol/L, and then slowly returns to baseline at six hours post-dose.5–7 The peak concentration is independent of the total daily dose. In a phase 3 study, only 3% of patients were observed to have a single, transient TT excess (<2 hours/day) at any time during pharmacokinetic analysis.8 For patients provided the highest dose in an independent arm of the phase 3 study, values for serum TT, prostate-specific antigen (PSA), and hematocrit remained in safe ranges throughout one year of treatment, demonstrating the safety of the pulsatile, divided daily dose concept of TNG.
MyT was a multisite, single-arm, uncontrolled study that allowed physicians to make titration decisions with TNG based on resolution of hypogonadal symptoms, as the safety risk of testosterone excess is very low. During this study, TT levels were monitored, although the laboratory results remain blinded to the physician. In addition, patient symptoms were independently assessed using a validated, standardized questionnaire — quantitative Androgen Deficiency in the Aging Male (qADAM)9 — and by routine (uncontrolled) physician questions during a medical visit. Only the latter information was used for titration decisions. Patient satisfaction with treatment and patient preference questionnaires were also administered, the results of which are provided in a separate report.
Methods
Study population
Patients were hypogonadal males 18–65 years of age with documented TT levels ≤10.4 nmol/L (300 ng/dL). Approximately 75% of patients selected were receiving treatment with a topical TTh for at least three months prior to enrollment (treatment experienced; TThE); 25% were treatment-naive and had never before taken exogenous testosterone (TThN). Patients were excluded if they had: history of pituitary or hypothalamic tumors or any malignancy within the past five years (excluding basal cell or squamous cell carcinoma of the skin curatively treated by surgery); prostatomegaly or history of abnormal PSA levels (>10.0 ng/mL); history of nasal disorders, nasal or sinus surgery, nasal fracture within the previous six months or nasal fracture that caused a deviated anterior nasal septum surgery, and mucosal inflammatory disorders (specifically Sjogren’s syndrome); use of any intranasal medication delivery other than periodic short-term (less than three days) use of sympathomimetic decongestants; treatment with estrogens, gonadotropin-releasing hormone agonists, or growth hormone within the previous 12 months; or treatment with drugs that interfere with the metabolism of testosterone.
Study design
The My-T Study (NCT02937740) was a multicenter, single-arm study to assess symptom-based titration decisions made by physicians 90 days after initiating treatment in hypogonadal TThE and TThN patients. One hundred and seventeen (117) participants were enrolled at 11 sites in Canada. The first period included a 90-day treatment with 11 mg of TNG twice daily (5.5 mg testosterone per nostril) for a total daily dose of 22 mg. At day 90, the treating physician assessed patient hypogonadism symptoms and decided if a higher dose (11 mg three times daily) was required. At day 120, patients receiving the 33 mg dose returned for a final visit. TT levels of all patients were assessed at day 90, and the TT levels of patients receiving the higher dose were also assessed on day 120. Post-study followup was conducted on day 150 for all patients. Maximum duration of participation was 150 days (~5 months).
The intent-to-treat (ITT) population consisted of all participants who received at least one dose of study drug and had a valid post-dose efficacy measurement. The per-protocol (PP) population consisted of all ITT participants who completed the 90-day treatment period without any major protocol deviations. The safety population included all participants who received at least one dose of the study drug.
This study was conducted under CTA issued by Health Canada and was completed in compliance with the study protocol and in accordance with guidelines set forth by the International Conference on Harmonisation Guidelines for Good Clinical Practice and in accordance with ethical principles that have their origins in the Declaration of Helsinki regarding treatment of human patients in a study.
Monthly visits were performed during treatment to assess patient symptoms (qADAM) and treatment satisfaction (Treatment Satisfaction Questionnaire for Medication [TSQM]; results reported elsewhere). Each qADAM question is scored on a five-point Likert scale (0=terrible/weak, 3=average, and 5=excellent/strong), except for question 8 relating to falling asleep after dinner (0=never to 5=every night) and question 10 relating to height loss (0=2″, 3=1–1.4″, and 5=<0.4″ or none).9 TSQM and patient preference, measured at study initiation and completion, are reported elsewhere.
Analytical methods
Analyses were performed on the complete study population and stratified by dosage or prior TTh experience. Statistical analyses were done using SAS® Version 9.2. Statistical testing was performed at the two-sided 0.05 significance level unless specified. Statistical significance of qADAM results was determined using paired t-tests (two-sided) or Wilcoxon matched pairs signed-rank tests (two-sided). Shapiro-Wilk normality test was used to determine which test was appropriate. Selected secondary efficacy analyses were performed using both total qADAM scores and last observation carried forward (LOCF) total qADAM scores with patient subsets (TThE or TThN).
Safety
Safety assessments included adverse events (AEs), vital signs (blood pressure, heart rate, and temperature), physical examination, total testosterone measurements, hematocrit, lipid profile (total cholesterol, low-density lipoprotein [LDL], high-density lipoprotein [HDL], and triglycerides), hemoglobin, and liver function tests.
Results
Table 1 provides demographics of the 117 patients with hypogonadism who were enrolled from 11 Canadian sites. Of these patients, 24 (20.5%) were TThN and 93 (79.5%) TThE. All were initiated at the starting dose (22 mg/day). A total of 78 patients (67%) completed the study in its entirety. Of the 117 patients enrolled, 100 (85%) were included in the ITT population, including 77 (77.0%) TThE patients. Sixty-two patients were included in the PP population.
Table 1.
Summary of demographic data (ITT analysis)
| ITT | |||
|---|---|---|---|
|
| |||
| Parameter | All patients (n=100) | TThN (n=23) | TThE (n=77) |
| Age, years (mean) (SD) | 52.8 (9.0) | 52.7 (11.6) | 52.9 (8.2) |
| Weight, kg (mean) (SD) | 102.7 (24.6) | 104.0 (20.4) | 102.3 (25.8) |
| Height, cm (mean) (SD) | 176.4 (6.3) | 176.0 (7.7) | 176.6 (5.9) |
| BMI, kg/m2 (mean) (SD) | 32.9 (7.6) | 33.5 (6.0) | 32.8 (8.0) |
| Age of hypogonadism diagnosis, years (mean) (SD) | 49.3 (9.6) | 51.4 (11.1) | 48.7 (9.0) |
| Baseline/historical testosterone, nmol/L (mean) (SD) | 6.9 (2.6) | 6.6 (2.1) | 7.0 (2.8) |
| Race, n (%) | |||
| Caucasian | 87 (87.0) | 20 (87.0) | 67 (87.0) |
| Black | 1 (1.0) | 0 (0.0) | 1 (1.3) |
| Asian | 5 (5.0) | 1 (4.3) | 4 (5.2) |
| Hispanic | 1 (1.0) | 0 (0.0) | 1 (1.3) |
| Middle-Eastern | 5 (5.0) | 1 (4.3) | 4 (5.2) |
| Other | 1 (1.0) | 1 (4.3) | 0 (0.0) |
BMI: body mass index; ITT: intent to treat; SD: standard deviation; TThE: active topical testosterone therapy; TThN: naive to testosterone therapy.
At day 90, as a result of physician decisions based on symptoms, 37% patients were titrated to the higher dose (33 mg/day). At study completion, 70 (70%) patients were on a prescribed dose of 22 mg, of which 60 (85%) were TThE. Thirty (30%) patients were taking 33 mg dose, of which 17 (57%) were TThE. Seventy-seven percent (n=77) of patients were in the normal TT range at the endpoint, with a mean serum TT of 19.4 nmol/L. At the day 150 followup, 42/62 (61%) patients reported that they sought to continue treatment with TNG as a medication of choice after completion of the treatment phase.
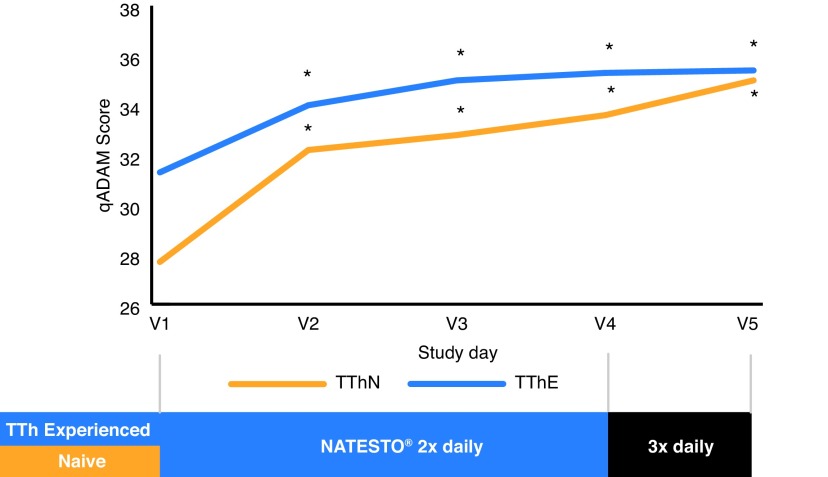
Mean total qADAM scores for each population by visit are provided in Fig. 1, while question-related scores are provided in Table 2. Patients noted significant improvement in qADAM symptom scores from 30.8 (5.9) at baseline to 35.5 (6.6) at day 90 (p<0.0001) and from 30.6 (5.7) to 35.5 (6.1) at study endpoint (all doses; ITT). Patients who were up-titrated and treated for an additional 30 days also showed significant improvement from baseline (30.9 [5.9]) to the end of the treatment period (34.9 [4.8]; p<0.0001); however, there did not appear to be a further significant increase as a result of the up-titration. Overall, 54% patients had total qADAM score ≥30 at study entry and 82% (n=82) patients ≥30 at study completion. A value of 30 indicates that symptoms were generally managed at or above average. The most significant improvements were observed in relation to questions that were lowest at baseline, such as energy level, sports ability, libido, and strength/endurance.
Fig. 1.
Symptoms of androgen deficiency determined by quantitative Androgen Deficiency in the Aging Male (qADAM) questionnaire scores. TThE: active topical testosterone therapy; TThN: naive to testosterone therapy.
Table 2.
qADAM Scores during treatment with TNG
| Question | V1 score | V4 score | Change from V1–4 | p |
|---|---|---|---|---|
| 1: Libido | 2.46 | 3.04 | 0.58 | 0.00000132 |
| 2: Energy level | 2.70 | 3.41 | 0.70 | 0.00000031 |
| 3: Strength/endurance | 2.85 | 3.38 | 0.53 | 0.00001542 |
| 4: Enjoyment of life | 3.26 | 3.57 | 0.31 | 0.00534854 |
| 5: Happiness level | 3.26 | 3.60 | 0.35 | 0.00047582 |
| 6: Strength of erections | 2.60 | 3.05 | 0.44 | 0.00052518 |
| 7: Work performance | 3.10 | 3.58 | 0.48 | 0.00010947 |
| 8: Fall asleep after dinner | 4.12 | 4.28 | 0.16 | 0.14976064 |
| 9: Sports ability | 2.41 | 3.03 | 0.62 | 0.00000033 |
| 10: Height lost | 4.37 | 4.64 | 0.27 | 0.04153504 |
qADAM: quantitative Androgen Deficiency in the Aging Male questionnaire; TNG: testosterone nasal gel.
Post-hoc analyses compared titration decisions made by physicians to theoretical decisions that would have been made based on TT levels. Modeling of the data using the TT lower limit of normal as a criterion for decisions showed that 17 (26%) patients would have been up-titrated. This is slightly less than the 25 (35%) patients who were actually up-titrated in the ITT population. Of note, one site up-titrated 9/11 [89%] of their patients in this study, yet had not up-titrated more than 10% of patients on TNG in clinical practice. When this statistical anomaly was removed from the analysis, the percentage of up-titrations in the study decreased to 26%.
This study further investigated which qADAM questions were the best determinants of successful titration. Questions 1–3, 6, and 9, which were often a concern for patients prior to initiating TNG therapy, had the lowest baseline values and showed the greatest improvement in scores after 90 days (Table 2). Because question 1 (libido) and question 6 (erections) had strong correlation, and because question 9 (sports ability) was not answered by all patients (given that perhaps patients were not actively participating in sports), questions 6 and 9 were removed from consideration in attempting to determine patient benefit from up-titration. A rule was devised and tested based on questions 1–3, wherein, if the sum of values from the three questions was less than 8, up-titration was recommended. Patients who had a cumulative score of ≤8 to these three questions showed the most symptom benefit from three times daily dosing, while patients with cumulative scores >8 did not show consistent improvement at the higher dose. Using this rule, 24% of patients would benefit from up-titration, a value similar to the percentage of actual up-titration decisions made during the study based on TT alone and to decisions made by physicians. Thus, these three questions were selected as most likely symptom queries for physicians to examine when consulting patients treated with TNG.
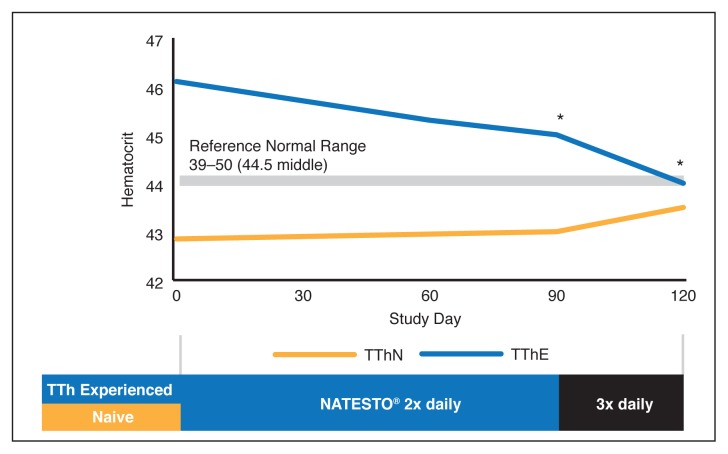
There were no serious treatment-related AEs reported during the conduct of this study. Mean total cholesterol, LDL, HDL, triglycerides, alanine transaminase (ALT), and aspartate aminotransferase (AST) results were all within normal range and mean values observed after dosing were similar to those observed at baseline (visit 1). Table 3 and Fig. 2 show hematocrit levels during the study. There was no significant increase in the hematocrit of TThN patients (mean endpoint hematocrit 42.98%, +0.23% change). TThE (mean endpoint hematocrit 44.92%, −1.27% change) patients experienced a slight but statistically significant (p=0.0052) decrease, however, remained in the normal range.
Table 3.
Mean hematocrit values by visit and dose (safety population)
| Parameter | All patients (n=65) | TThN (n=18) | TThE (n=47) |
|---|---|---|---|
| Baseline (visit 1) (mean) (SD) | 45.36 (5.35) | 45.43 (5.58) | 45.16 (4.72) |
| Day 90 (visit 4 bid dose) (mean) (SD) | 44.43 (3.65) | 44.28 (3.43)* | 44.67 (4.02) |
| Day 120 (visit 5 tid dose) (mean) (SD) | 43.76 (2.44)* | NA | 43.76 (2.44)* |
Statistically significant (p<0.05) from visit 1 to visit 4 and from visit 1 to visit 5.
bid: twice daily; tid: three times daily; SD: standard deviation; TThE: active topical testosterone therapy; TThN: naive to testosterone therapy.
Fig. 2.
Changes in hematocrit during study; 47 males on active topical testosterone therapy (TThE) received twice daily dosing. TThE: active topical testosterone therapy; TThN: naive to testosterone therapy.
Discussion
Most topical testosterone preparations have multiple dose levels and require titration.10 The 2015 Canadian Medical Association Journal guidelines for the diagnosis and treatment of hypogonadism11 state the two key objectives of treatment as: “the improvement in symptoms and the achievement of eugonadal levels of testosterone in the mid-normal range for healthy young men (14–17.5 nmol/L).” These guidelines recognize that higher or lower mid-range serum testosterone concentrations are acceptable when a positive symptom response is observed. When titrating, dose levels are adjusted for safety (dose downward if TT is too high) and efficacy (dose upward if TT is too low). Monitoring TT levels is important because the highest recommended dose of most preparations can produce supraphysiological testosterone levels, which can be unsafe, leading to high levels of hematocrit, which can ultimately require phlebotomy and/or discontinuation.
TNG (4.5% testosterone) is a U.S.- and Canada-approved treatment for hypogonadism that uses a divided dose concept whereby 11 mg are administered either twice or three times daily. The divided dose is efficacious in improving TT levels and symptoms, as discussed here. Furthermore, in an independent arm of the phase 3 program, the three times daily dose was proven safe, with a very low risk of causing supraphysiological TT levels. We have reported additional benefits to divided dosing, including excellent long-term hematology safety and a lessened impact on the hypothalamic-pituitary-gonadal axis (HPG axis), which regulates spermatogenesis and testicular integrity.12,13 While a divided dose can sometimes lead to issues of compliance, we report in an accompanying analysis that patients found that TNG, when given twice or even three times daily, was superior over topical medication, especially with respect to convenience. Thus, TNG is a unique candidate that might allow titrations to be performed within 90 days based on symptoms, with only a confirmatory TT measurement 30 days after finding the most appropriate dose for treatment. Using symptoms as a guide for titration avoids multiple lab analyses for serum TT, reducing the burden on patients and the healthcare system and without increasing the risk to patients.
When using symptoms, absent serum TT values, or a guiding questionnaire, physicians made dose titration decisions during a scheduled routine visit wherein they asked patients routine questions about symptoms, as per training and treatment guidelines. This resulted in 37% of patient being up-titrated. This is similar to the 40% up-titrations made in the phase 3 study. Overall, the majority of patients (>77%) achieved the normal TT range (mean 19.4 nmol/L) when treated with TNG and no excesses of serum TT >1500 ng/dL were observed.
Some treatment-related AEs were reported in this study but these resolved spontaneously. As this is the first instance of a report wherein patients on a daily administered topical medication were switched to TNG, we monitored hematocrit as a measure of safety.14,15 Of particular importance, this study observed that the hematocrit values for TThE patients decreased, while those of TThN patients trended slightly upwards. There appeared to be a convergence of hematocrit values between the two groups toward a single value near the mid-range of normal hematocrit values (Fig. 2).
The qADAM is a good tool for screening patients with hypogonadism,16 as it shows a strong correlation with serum TT levels.17 While it has been reported that total qADAM scores are not good for monitoring changes in hypogonadal symptoms during treatment,18,19 the present study used this questionnaire as a means to try and identify the key symptoms that physicians should be using when titrating patients according to symptoms. Changes in specific qADAM responses were analyzed to determine which questions were most likely to predict a positive symptom improvement that would result from an up-titration and what cumulative response value to those select questions could be used as a threshold for making titration decisions. This analysis identified five questions of particular relevance, 1–3, 6, and 9, which relate to energy level, libido, erections, strength endurance, and sport ability. Because question 6 (libido) asks about related symptoms and its answers strongly correlate to those to question 1 (erections), it was felt to be superfluous. Question 9 (sports) was seen by many patients as irrelevant, as they did not participate in any sports activities. The remaining three questions (1–3) showed the strongest improvement when patients were up-titrated. A value of 8 was found to best correlate to actual titration decisions and benefit derived from those decisions (changes in scores). Inclusion or removal of sports ability and erection questions did not significantly affect the outcome of the analysis, suggesting that a minimal analysis of energy, libido, and endurance was sufficient for titration.
Weaknesses of the study include protocol deviations: one site allowed patients to decide on their preferred dose rather than having the investigator make this decision, resulting in an unusually high titration rate (9/11, 81%) relative to the other 10 sites. Also, nine of 17 discontinuations in the ITT population came from one site where subjects dropped out due to lack of physician followup. These events had slight impact on the numerical results of the ITT analysis; however, when these two sites were removed in PP analyses, the overall study conclusions and observations were not significantly impacted. Analysis of TThE patients showed that the majority had average to above average (≥30) baseline qADAM total scores (32.1 [6.2]), dispelling the notion that these patients were simply dissatisfied with their prior topical medication prior to study entry.
Conclusions
Titration based on symptoms was successful in achieving normal levels of TT in 77% of patients. Statistically significant improvements in symptoms were observed for treatment-naive patients, as well as for patients switching from a topical form of testosterone. Interestingly, 63% of patients remained at the starting 22 mg dose, while 37% were up-titrated. Patients who would most likely benefit from an increased dose can be identified based on assessment of libido, energy, and strength/endurance, which correlated well with testosterone level improvements and symptom reduction. Symptom-based titration facilitates titration decisions without increased risk to patients. Finally, a significant proportion of patients (61%) in the trial who were either initiated on or switched to TNG reportedly sought to continue treatment with TNG as a medication of choice after completion of the treatment phase, suggesting that overall treatment outcomes with TNG, as well as the requirement for repeat dosing, were acceptable to patients.
Acknowledgements
Editorial assistance in the preparation of this manuscript was provided by Christina Sanguinetti.
Footnotes
Competing interests: Dr. Lee has been an advisory board member for Acerus and Paladin; a speakers’ bureau member for Acerus, Astellas, and Paladin; has received honoraria from Acerus, Astellas, Paladin, and Pfizer; and has participated in clinical trials supported by Acerus. Dr. Brock has been an advisory board and speakers’ bureau member for and has received honoraria from Acerus, Lilly, and Pfizer; and holds investments in Boston Scientific and Pfizer. Dr. Barkin has been an advisory board member for Acerus; has received various speaking and study grants; and has participated in the My-T and Natesto Phase 4 trials supported by Acerus. Dr. Bryson, Dr. Gronksi, and Dr. Ormsby are all employees of Acerus.
Funding: This study was supported by Acerus Pharmaceuticals.
References
- 1.Mohamoud M. Comment pre-dates formal release of the final transcript. Hyattsville, Maryland, USA: Sep 17, 2014. Comment made during public meeting of the Bone, Reproductive and Urologic Drugs Advisory Committee (BRUDAC) and The Drug Safety and Risk Management Advisory Committee (Dsarm) on testosterone replacement therapy and the potential for adverse cardiovascular outcomes. [ID no.] [Google Scholar]
- 2.Malik R, Wang CH, Lapin B, et al. Compliance with timing recommendations for testosterone screening and associated treatment choices. J Urol. 2014;191(4S):e336. doi: 10.1016/j.juro.2014.02.945. [DOI] [Google Scholar]
- 3.Ohlander S, Varghese B, Pastuszak AW, et al. Erythrocytosis following testosterone therapy. Sex Med Rev. 2018;6:77–85. doi: 10.1016/j.sxmr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NATESTO® (Testosterone Nasal Gel 4.5%) Product Monograph. Acerus Biopharma Inc; Nov 27, 2017. [Google Scholar]
- 5.Ohdo S. Chronopharmacology focused on biological clock. Drug Metab Pharmacokin. 2007;22:3–14. doi: 10.2133/dmpk.22.3. [DOI] [PubMed] [Google Scholar]
- 6.Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: Timing is everything. Horm Behav. 2006;49:557–74. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lincoln GA, Andersson H, Loudon A. Clock genes in calendar cells as the basis of annual timekeeping in mammals — a unifying hypothesis. J Endocrinol. 2003;179:1–13. doi: 10.1677/joe.0.1790001. [DOI] [PubMed] [Google Scholar]
- 8.Rogol AD, Tkachenko N, Bryson N. Natesto®, a novel testosterone nasal gel, normalizes androgen levels in hypogonadal men. Andrology. 2016;4:46–54. doi: 10.1111/andr.12137. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed O, Freundlich RE, Dakik HK, et al. The quantitative ADAM questionnaire: A new tool in quantifying the severity of hypogonadism. Int J Impot Res. 2010;22:20–4. doi: 10.1038/ijir.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Men’s Health Review Panel. Men’s Health Guidelines for Family Medicine. 1st ed. Toronto: MUMS Guideline Clearinghouse; 2017. [Google Scholar]
- 11.Morales A. Canadian practice recommendations for screening, treatment, and monitoring of aging males with androgen deficiency. Aging Male. 2010;4(Suppl 1):35–7. [Google Scholar]
- 12.Conners W, Morgentaler A, Bryson N, et al. Preservation of normal concentration of pituitary gonadotropins despite achievement of normal serum testosterone levels in hypogonadal men treated with 4.5% nasal testosterone gel (Natesto) J Urol. 2017;197:4S. doi: 10.1016/j.juro.2017.02.2804. [DOI] [Google Scholar]
- 13.Guidry M, Rogol AD, Bryson N. One-year hematologic safety of Natesto® (testosterone) nasal gel in men with hypogonadism. J Sex Med. 2018;15:S76–7. doi: 10.1016/j.jsxm.2017.11.184. [DOI] [Google Scholar]
- 14.Ohlander S, Varghese B, Pastuszak AW, et al. Erythrocytosis following testosterone therapy. Sex Med Rev. 2018;6:77–85. doi: 10.1016/j.sxmr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brækkan SK, Mathiesen EB, Njølstad I, et al. Hematocrit and risk of venous thromboembolism in a general population. The Tromsø study. Haematologica. 2010;95:270–5. doi: 10.3324/haematol.2009.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernie AM, Scovell JM, Ramasamy R. Comparison of questionnaires used for screening and symptom identification in hypogonadal men. Aging Male. 2014;17:195–8. doi: 10.3109/13685538.2014.963041. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed O, Freundlich RE, Dakik HK, et al. The quantitative ADAM questionnaire: A new tool in quantifying the severity of hypogonadism. Int J Impot Res. 2010;22:20–4. doi: 10.1038/ijir.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Jabaloyas JM, Queipo-Zaragoza A, Rodriguez-Navarro R, et al. Relationship between the Saint Louis University ADAM questionnaire and sexual hormonal levels in a male outpatient population over 50 years of age. Eur Urol. 2007;52:1760–7. doi: 10.1016/j.eururo.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Rabah DM, Arafa MA. Validation of an Arabic ADAM questionnaire for androgen deficiency screening in the Arab community. Aging Male. 2009;12:95–9. doi: 10.3109/13685530903265065. [DOI] [PubMed] [Google Scholar]