Abstract
Background:
Measles is among the most highly infectious human diseases. By virtue of increasingly effective childhood vaccination, together with targeted supplemental immunization activities (SIAs), health authorities in the People’s Republic of China have reduced measles’ reproduction number from about 18 to 2.3. Despite substantial residual susceptibility among young adults, more in some locales than others, sustained routine childhood immunization likely would eliminate measles eventually. To support global eradication efforts, as well as expedite morbidity and mortality reductions in China, we evaluated alternative SIAs via mechanistic mathematical modeling.
Methods:
Our model Chinese population is stratified by immune status (susceptible to measles infection; infected, but not yet infectious; infectious; and recovered or immunized), age (0, 1–4, 5–9, …, 65+ years) and location (31 provinces). Contacts between sub-populations are either empirical or a mixture of preferential and proportionate with respect to age and decline exponentially with distance between locations at age-dependent rates. We estimated initial conditions and most parameters from recent cross-sectional serological surveys, disease surveillance and demographic observations. Then we calculated the reproduction numbers and gradient of the effective number with respect to age- and location-specific immunization rates. We corroborated these analytical results by simulating adolescent and young adult SIAs using a version of our model in which the age-specific contact rates vary seasonally.
Results:
While the gradient indicates that vaccinating young adults generally is the optimal strategy, simulations indicate that a catch-up campaign among susceptible adolescent schoolchildren would accelerate elimination, with timing dependent on uptake.
Conclusion:
These results are largely due to indirect effects (i.e., fewer infections than vaccinated people might otherwise cause), which meta-population models with realistic mixing are uniquely capable of reproducing accurately.
Keywords: measles in China, vaccination strategies, accelerating elimination, meta-population modeling
INTRODUCTION
Measles is among the most highly infectious human diseases.1 Complications include pneumonia, diarrhea and encephalitis.2 Case fatality ratios vary from < 1 to 15% in the most and least developed countries. A global consultation convened by the Western Pacific Regional Office of the World Health Organization in 2010 certified the feasibility of eradication.3
Eradicating pathogens that cause human diseases not only prevents illness and premature death, but also conserves public health resources. Routine vaccination against Variola major and V. minor was no longer necessary once smallpox was eradicated. Poliovirus has since been eliminated from all but a few countries, and measles and rubella viruses were, and rubella virus still has been eliminated from the entire Western hemisphere. Because regions where pathogens remain endemic source reintroductions, eradicating human pathogens requires international cooperation. In several instances, viruses have been reintroduced and become endemic again after elimination. After being eliminated from the United Kingdom, for example, measles virus was reintroduced, and became endemic. While it has since been eliminated again,4 ongoing outbreaks threaten other measles-free European countries.5
Similarly, the Pan American Health Organization had certified elimination of endemic measles and rubella virus transmission in the Region of the Americas,6 but measles transmission was sustained in Venezuela for more than 12 months during 2017–18. Consequently, the entire Region of the Americas is no longer measles-free.7 In the US, outbreaks continue due to unimmunized travelers – frequently children with personal-belief exemptions from vaccination – being infected abroad and, on returning home, exposing members of under-immunized communities.8 In their mid-term review of the Global Measles and Rubella Strategic Plan, 2012–20, experts concluded that progress in other regions had slowed and encouraged countries to continue working toward elimination goals.9
By virtue of an increasingly effective childhood vaccination program, together with targeted supplemental immunization activities (SIAs), health authorities in the People’s Republic of China, the largest and among the most diverse human populations on earth, have substantially reduced endemic measles virus transmission.10,11 Measles elimination would not only reduce morbidity and mortality in China, but also contribute momentum to the global health community’s effort to eradicate measles.
Toward the end of 2016, technical leaders of the National Immunization Program of China, WHO, UNICEF, and US CDC conducted an international consultation to examine measles and rubella surveillance, serological survey, and vaccine uptake data for a mathematical-modeling evaluation of alternative strategies to accelerate elimination of measles and rubella. Results of the work reported here were presented to the National Health Commission and Immunization Advisory Committee for financial and policy support consideration.
BACKGROUND
Routine Vaccination
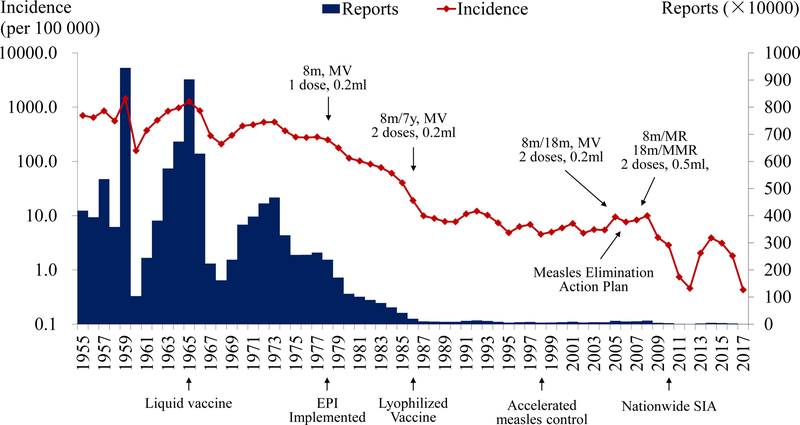
Measles was endemic in China before vaccination began in 1965 (figure 1). In 1978, health authorities established an Expanded Program on Immunization (EPI) whose routine childhood vaccination schedule included single-antigen measles vaccine at eight months of age. Authorities recommended a second routine dose at seven years of age in 1986, but – motivated by the age-distribution of reported infections – in 2005 changed the recommended age to 18 to 23 months. Rubella-containing vaccines – licensed in China in 1993 – were included in the national EPI in 2007, when authorities recommended using combined measles and rubella (MR) and measles-mumps-rubella (MMR) vaccines for the first and second doses, respectively. Due to vaccine shortages, however, this schedule was not fully implemented throughout China until 2011.
FIGURE 1.
Measles in China from 1955 to 2017. Reported cases, incidence and vaccination schedule. MV, MR and MMR denote single-antigen measles, combined-antigen measles and rubella and combined-antigen measles, mumps, and rubella vaccines, respectively.
Disease Surveillance
In the 1960s, the mean annual reported measles incidence in China was 572.0 per 105 persons. Incidence declined to 355.3, 52.9, and 7.6 per 105 persons during successive decades. Since 2000, incidence has remained below 10 per 105 persons, decreasing from 9.9 in 2008 to a nadir of 0.5 per 105 persons in 2012. Since the national case-based system of measles surveillance with laboratory support implemented during 2005 was updated during 2009 – to ensure that all provinces conducted case-based surveillance – performance indicators have steadily improved.12 By 2014, the percentages of suspected measles cases investigated within 48 hours of reporting (98.3%) and providing an adequate serum specimen (96.5%), and of serum specimens with laboratory results within 7 days of collection (95.3%) all exceeded their target values.13 In 2013 and 2014, measles incidence increased to 2.04 and 3.9 per 105 persons, respectively.14,15 In 2015, reported incidence remained as high as 3.1 per 105 persons. Measles-related deaths declined from 103 in 2008 to 9 in 2012, and then increased to 24 deaths in 2013 and 32 in 2015.16
Supplemental Immunization Activities
During the period from 2004 to 2009, 25 provinces supplemented their routine vaccination activities with support from the central government or international organizations. Children aged eight months to 14 years were targeted initially, but seven of the 25 provinces conducted follow-up campaigns targeting children from eight months to six or seven years of age. Doses administered reached 186 million, with more than 97% of eligible children being vaccinated.11 During 2010, the National Health and Family Planning Commission (now National Health Commission) coordinated national SIAs through which 103 million children, 97.5% of those eligible, were vaccinated.11,17 During 2011, in portions of 26 provinces where uptake was low, 19.4 million eligible children were vaccinated.18 During 2012, all 31 provinces conducted SIAs, some selective and others not, vaccinating 22.7 million children.15 During 2014, the China CDC developed a measles risk assessment tool to monitor and guide subsequent elimination efforts. During that year and the next, authorities administered 12.8 and 9.1 million doses of measles-containing vaccine, respectively. In these SIAs, target ages ranged from eight months to four, six or 14 years depending on province (China CDC, unpublished).
METHODS
Immunity
During 1992, 2006 and 2014, health authorities conducted serological surveys throughout China, initially to assess the burden of hepatitis B virus infection and subsequently the impact of escalating mitigation efforts.19 Study populations were composed of persons who had resided for at least six months at locations selected by the Chinese Academy of Preventive Medicine (now Chinese Center for Disease Control and Prevention) by virtue of their representative demographic and socioeconomic characteristics. Authorities used multi-stage cluster sampling to enroll 67 017 and 81 775 persons aged 0–59 years in 1992 and 2006, respectively, and 31 024 persons aged 0–29 years in 2014.
We analyzed residual sera from 47 513 persons aged 0–34 years in 2006 and 30 321 persons aged 0–29 years in 2014 for measles-specific immunoglobulin G antibodies (IgG) via Serion ELISA.20 We considered an IgG titer of 10 IU per ml or higher to be evidence of protective immunity. We performed logistic regressions with single years of age or age and time, respectively, modeled as third- and first-order polynomials.21 Because these main effects are related, our bivariate regressions included all un-aliased interaction terms. From the provincial regressions, we obtained the 2014 proportions immune for our simulations and effective reproduction number calculations, and the vaccination rates for our gradient calculations (supplement V).
Forces of Infection
Using the first of three methods described in supplement I, we calculated hazard rates (often called forces) of measles infection among susceptible people by age from 2005–14. From our 2014 estimates (figure A1b), together with weighted average contact rates from 8 European countries,22 we calculated proportions infectious (often called attack rates) and probabilities of infection upon contact with infectious people aged 0, 1–4, 5–9, …, 65+ years (supplement II).
Then we modeled measles virus transmission in the Chinese population stratified by immune status (susceptible to infection; infected, but not yet infectious; infectious; and recovered or immunized), age (in the 15 groups specified above) and location (31 provinces). This meta-population SEIR model, described in detail elsewhere,23 enabled us to calculate the average numbers of secondary infections per infectious person in wholly or partially susceptible populations and denoted ℜ0 and ℜE (table 1).
Table 1.
Definitions
| Quantity | Symbol | Meaning |
|---|---|---|
| Meta-population | A cross-classified or stratified population (a population composed of sub-populations) | |
| Sub-population | One of n age groups and m spatial locations in our meta-population model of measles in China | |
| Effective reproduction numbers in sub-populations i | ℜEi | Average numbers of secondary infections per primary in sub-populations i = 1, …, n × m |
| Basic reproduction numbers in sub-populations i | ℜ0i | Average numbers in wholly-susceptible sub-populations i (effective contacts while infectious) |
| Meta-population reproduction numbers | ℜE, ℜ0 | Reproduction numbers in a meta-population composed of n × m sub-populations |
| The gradient | ∇ℜE | Multi-variate partial derivative of ℜE with respect to sub-population immunization rates |
Reproduction Numbers
With the exception of the contact rates and mixing matrix (derived from European observations for reasons described in supplement II), we estimated parameters of our meta-population model from recent cross-sectional serological surveys, disease surveillance and demographic observations of the Chinese population. Consequently, for example, the age-specific rates at which contacts decline with distance (figure A2.5) differ from those reported earlier.
Reproduction numbers are properties of next-generation matrices, in turn products of diagonal and mixing matrices, whose respective elements are sub-population reproduction numbers and proportions of the contacts that members of the ith group have with the jth. The sub-population effective reproduction numbers where the pk are proportions of sub-populations k that are immune, slices through the surfaces described in supplement V.
The Gradient
Given an expression for the effective reproduction number, one can calculate the gradient, denoted ∇ℜE and defined as the partial derivative of ℜE with respect to sub-population vaccination rates (table 1). This vector-valued quantity indicates the most expeditious strategy for reducing ℜE,23,25 which must be ≤ 1 to interrupt transmission.
If mixing were proportionate with respect to age, for example, ℜE would be the trace of the next-generation matrix,
where 1/α and 1/γ are the mean latent and recovery periods, 14 and 7 days respectively; the and are immunization and contact rates; the are probabilities of infection on contact with an infectious person; and the are proportions of the contacts that people in location li and age aj have with others in the same location and age group. In this model, u is the rate of turnover (i.e., movement in and out of groups due to unspecified demographic phenomena). We describe our formulation of the and elsewhere23 and our estimation of the and in supplements II and V, respectively.
Simulations
To evaluate the gradient, an analytical result, we simulated a version of our model whose contact rates were seasonally forced. For this purpose, we used a harmonic function whose coefficients we estimated from historical surveillance reports (supplement III). Specifically, we determined if a nationwide catch-up campaign using the MR or MMR vaccine (simultaneously reducing the risk of rubella outbreaks involving young women) among susceptible adolescents entering middle and high school (1/5 of those aged 10–14 and 15–19 years during September of three successive years) could accelerate elimination. We also simulated administering a similar number of doses to susceptible adults aged 20–29 years.
RESULTS
Immunity
Logistic regression equations fitted to the observed proportions immune by single year of age, modeled as a cubic polynomial (figures 2), indicate that immunity was 92% among infants in 2006 and increased to 96% by 2014. Immunity also increased among young adults, from about 80 to 86% during this period. These increases are due to infection (figures A1) as well as immunization, infants via routine services and older children via SIAs. Despite these efforts, however, roughly 15 percent of young adults remain susceptible (figure 2b).
FIGURE 2.
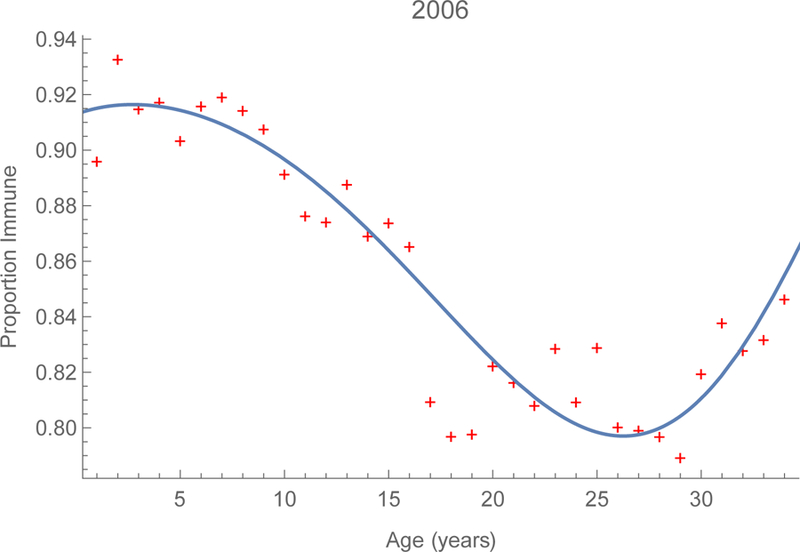
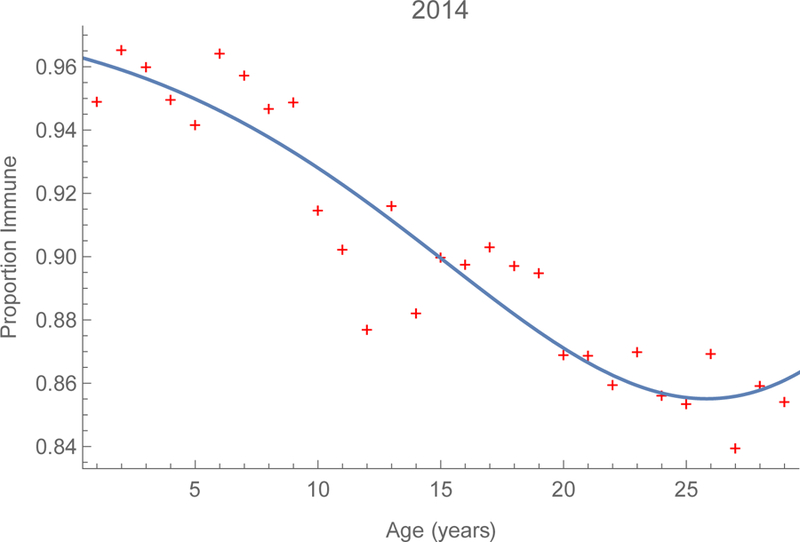
Serological profiles to measles in China. Proportions by age whose sera contained protective titers of measles-specific immunoglobulin G in China during 2006 and 2014 on the left and right, respectively. Supplement V includes 3D surfaces derived from provincial observations.
Forces of Infection
Proportions infectious and probabilities of infection on contact with infectious persons were greatest among infants and young children in 2014, but increased after a nadir among older children and adolescents. They resemble figures A2b and A3b, respectively, which however we calculated from our estimates of the 2006 forces of infection (figure A1a). Consequently, while most infected people were young children, some were reproductive-aged adults.
Reproduction Numbers
We estimate that measles’ ℜ0 is about 18 in China, which is higher than usually reported,24 but our model includes realistic mixing (e.g., between children and reproductive-aged adults), which increases reproduction numbers.25 Sub-population ℜ0i range from 6.9 in Xinjiang to 22.8 in Henan, which differ in population density and proximity to major urban centers (figure 3a, supplement IV). As detailed using the earlier forces of infection in supplement II, we also calculated the contributions to the basic reproduction number of (figure 4a), and equilibrium prevalence in (figure 4b) each of the 15 age groups.26 In 2014, young adults both bore and contributed substantially to the burden of measles in China, especially in the eastern provinces.27 In 2014, we estimate that ℜE was about 2.3 in China, with sub-population ℜEi ranging from 0.78 in Hainan to 3.32 in Guangxi (figure 3a, supplement IV). The ratios ℜEi/ℜ0i, interpretable as proportions of provincial populations that are susceptible, range from 0.04 in Tianjin to 0.25 in Tibet (figure 3b, supplement IV), indicating the effectiveness of vaccination efforts.
FIGURE 3.
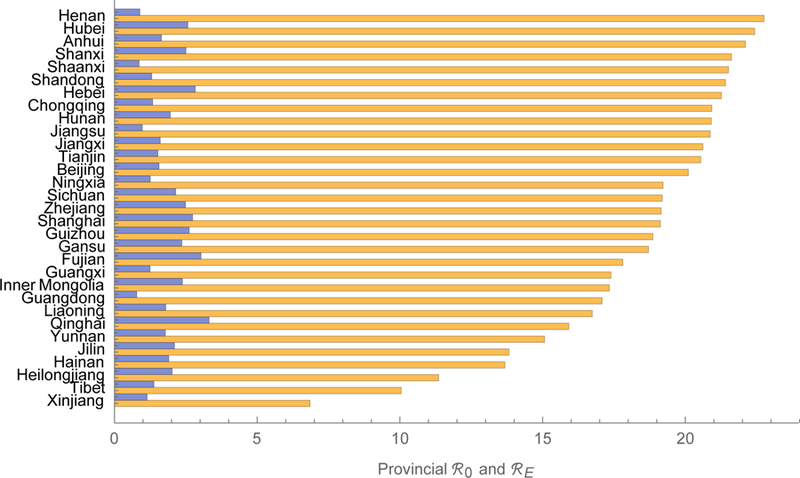
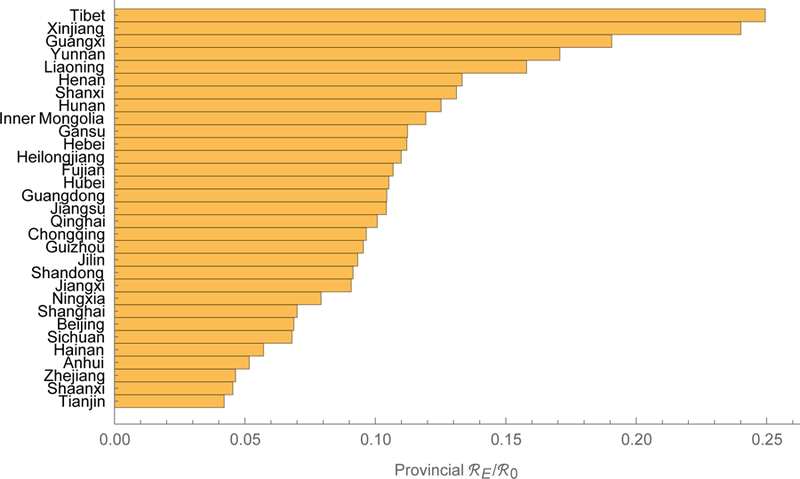
Reproduction numbers for measles in China. The meta-population basic and 2014 effective numbers are 18 and 2.3. Provincial basic and effective reproduction numbers range from 6.9 to 22.8 and from 0.78 to 3.32, the orange and blue bars, respectively. We tabulate these numbers (left) and their ratio (right) in supplement IV.
FIGURE 4.
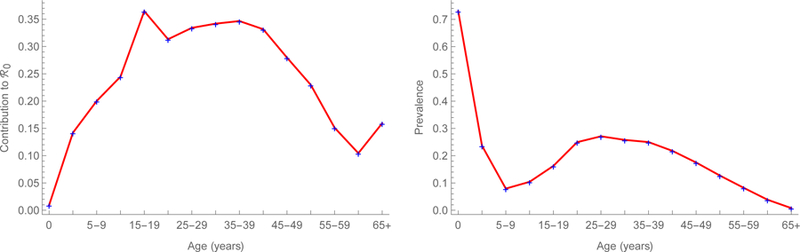
Quantities estimated from an age-structured model of measles transmission in China during 2014. Contributions to the basic reproduction number and equilibrium prevalence on the left and right, respectively. We describe these calculations and provide results for 2006 in supplement II.
The Gradient
Gradient magnitudes identify provinces within regions and directions indicate age groups within provinces where vaccination would reduce ℜE the most (figures 5). Magnitudes are equally modest in large municipalities with excellent vaccination programs, such as Beijing and Shanghai, and isolated rural environs such as Tibet. And they are relatively large in the eastern provinces of Guangdong, Jiangsu, and Shandong and central and western provinces of Henan and Guangxi. Directions are heterogeneous, but resemble on average the age-specific pattern illustrated in figure 4a. In Eastern China, young adults generally are the optimal target for reducing ℜE. Elsewhere, middle-aged adults are more often the optimal target.
FIGURE 5.
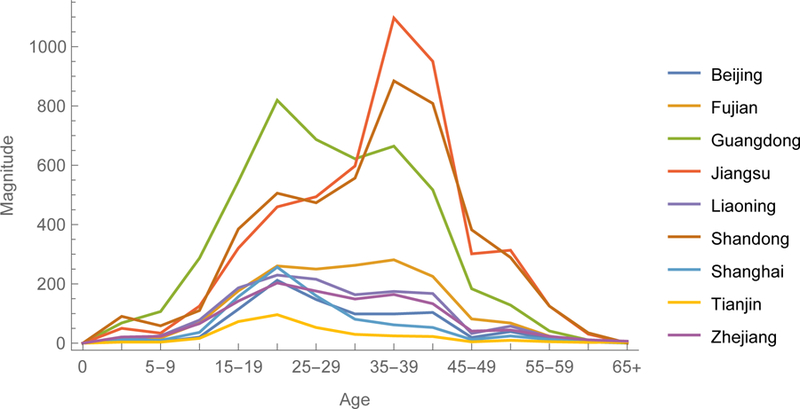
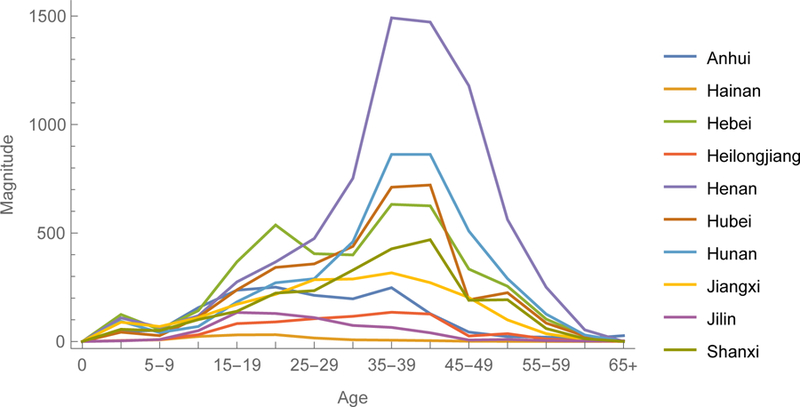
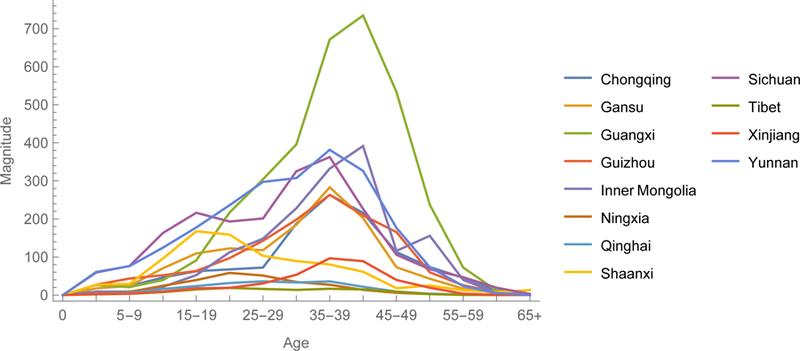
The 2014 gradient among provinces of Eastern, Central and Western China. The y-axes are magnitudes and x-axes directions of this vector-valued function.
Simulations
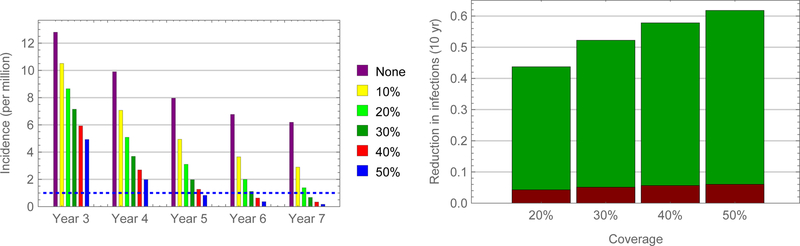
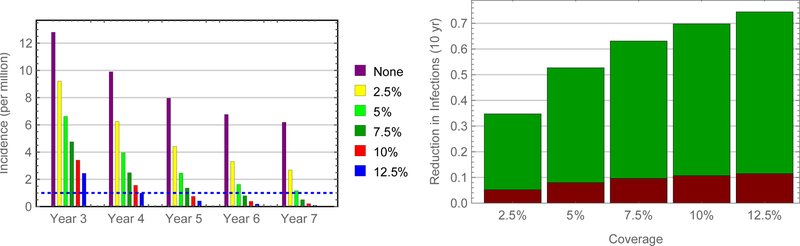
Simulations indicate that authorities could eliminate measles virus from China within a decade – largely by virtue of indirect effects (i.e., infections averted in other age groups) – by vaccinating adolescent schoolchildren (figures 6). Of course, time to elimination (reported incidence less than one per 106 persons) depends on uptake, being about five years if 50% of susceptible adolescent schoolchildren were vaccinated. In contrast, authorities would need to vaccinate only 15% of susceptible young adults to eliminate measles within five years (figures 7 and 8).
FIGURE 6.
Predicted impact of SIAs among adolescents in China. Incidence following vaccination of susceptible adolescent schoolchildren during September of three successive years (left) and proportions of infections averted directly and indirectly, red and green bars, respectively (right).
FIGURE 7.
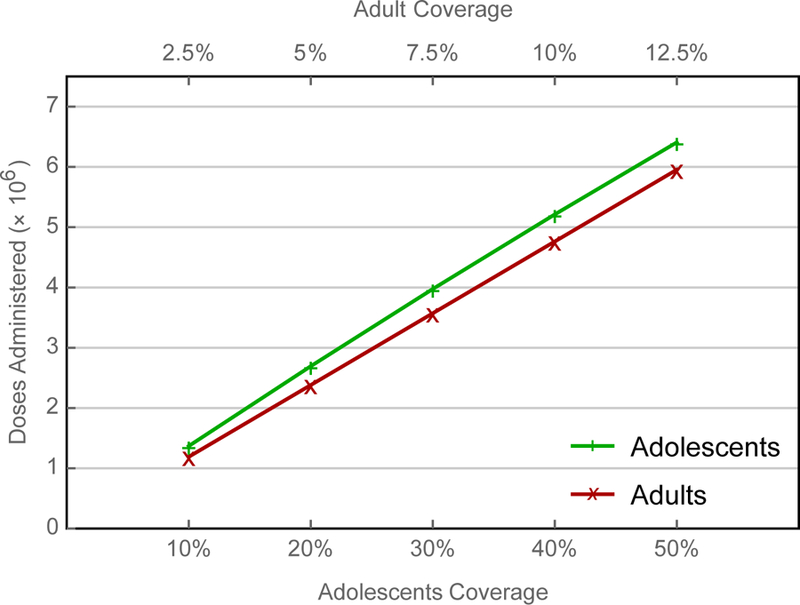
Comparison of alternative SIAs. Because almost four times as many adults aged 20–29 years are susceptible as children aged 10–19 years, the corresponding uptake among susceptible young adults is approximately one quarter that among children.
FIGURE 8.
Predicted impact of SIAs among young adults in China. Incidence following vaccination of susceptible young adults during September of three successive years (left) and proportions of infections averted directly and indirectly, red and green bars, respectively (right).
DISCUSSION
To evaluate alternative vaccination strategies by which health authorities in China might accelerate measles elimination, we modeled transmission of measles virus among members of the host population stratified by immune status, age and location. We estimated initial conditions and most parameters of our meta-population model of the Chinese population from recent cross-sectional serological surveys, disease surveillance and demographic observations. Then we evaluated the effective reproduction number and its multivariate partial derivative with respect to age- and location-specific immunization rates, which indicates the optimal strategy for reducing the effective reproduction number. Finally, we corroborated these analytical results by simulating adolescent and young adult SIAs using a version of our transmission model in which the person-to-person contact rates are seasonally forced.
The indirect effect of vaccination – a reduction in the force of infection that unvaccinated people experience by virtue of the vaccination of others – is evident whenever uptake increases slowly, as it typically does at the beginning of vaccination programs or upon the addition of new vaccines to established programs. In China, measles vaccine began being used in the mid-1960s, but was not added to the routine vaccination program until the mid-1970s, after which uptake increased steadily. Thus, the susceptible young adults illustrated in figures 2 were protected from infection when they were younger by the vaccination of others and not subsequently vaccinated during SIAs. Older adults are immune by virtue of disease. When vaccination is contra-indicated (e.g., live viral vaccines cannot be administered to immunocompromised people), this indirect effect can be beneficial. When infections are more consequential among adults than among children (e.g., with rubella virus), however, it can also be problematic.
In the People’s Republic of China, effective vaccination programs have reduced measles’ reproduction number from 18 to 2.3. Since 2006, measles elimination has been among Five-Year Health Development Plan goals. Sustained routine vaccination likely would eliminate measles eventually, especially if kindergarten and primary school staff reviewed vaccination records and referred children lacking evidence of two doses of measles-containing vaccine to their community health clinics. Optimal SIAs to accelerate this process differ among provinces (figures 5), but – given their immune profiles (supplement V) – invariably involve adults.
As adults are relatively inaccessible, however, we determined if authorities could accelerate elimination via a school-based catch-up campaign among adolescents. Staff from middle and high schools could check the records of three successive entering classes while those from community health clinics vaccinated on site only those children without evidence of two doses of measles-containing vaccine. While school is compulsory only through ninth grade in China, 96% of children completed high or vocational schools in 2017 and 94% of those completing high school attended universities,28 whose staff could help to further increase uptake of measles- and rubella-containing vaccine.
Simulation results indicate that a school-based catch-up campaign could accelerate measles elimination in China (figure 6a), with timing dependent on uptake. This one-off strategy would not only avoid wasting vaccine, but also reinforce the importance of schools in the immunization program and mitigate concern about vaccinating the same children repeatedly. While vaccinating young adults would be more effective (figure 8a), they are relatively inaccessible. Nonetheless, clinic staff could inquire about the vaccination of parents who bring young children for their routine vaccinations and vaccinate them too if indicated.
Vaccinating susceptible adolescents could accelerate measles elimination largely because of its indirect effects (figure 6b). Meta-population models with realistic mixing are uniquely capable of reproducing these effects accurately. Ours has 15 age classes, 31 locations, and a function by which contacts between groups are a mixture of preferential and proportionate with respect to age, but decline exponentially with distance at age-dependent rates.23 We estimated its parameters from observations in Europe that are more detailed than, but otherwise comparable to ones from Southern China (supplement II). We estimated initial conditions and all other parameters from recent observations in China.
Reducing the effective reproduction number may not be the only public health objective. Using the same approach, we compared the optimal and actual H1N1 vaccination programs in the US state by state (and District of Columbia) from October 2009 through June 2010.23 In California, for example, young children and elderly people were over-vaccinated and adolescents and young adults under-vaccinated relative to the optimal for reducing the average number of secondary infections per infectious person. Reducing influenza hospitalizations and deaths among the elderly is a priority for US health authorities. As in China, accessibility also influences US vaccination policy. Young children are more accessible than adolescent ones by virtue of more frequent visits to primary healthcare providers.
Limitations
Serological survey participants were drawn from a representative 1% sample of the population of China. National regulations require that EPI clinics be responsible for the vaccination of children residing in their jurisdictions for more than 3 months. There is considerable internal migration for employment,29 and the degree to which local clinics are unable to find children new to their areas varies. The modelers have proposed to collaborate with demographers knowledgeable about internal migration to evaluate the potential impact of the “floating population” in mainland China. The co-authors have agreed that this phenomenon warrants investigation.
Optimal public health interventions reduce effective reproduction numbers by protecting those most susceptible to infection from those most likely to infect them. Because only heterogeneity affecting sub-population reproduction numbers need be included in meta-population models,25 they are simpler than other approaches and, consequently, relatively tractable analytically. We use the gradient, the multivariate partial derivative of the effective reproduction number with respect to sub-population vaccination rates, to identify optimal vaccination strategies. To ensure that we have not omitted relevant heterogeneity, we corroborate such analytical results by comparing the simulated impact of alternative vaccination strategies using models that are capable of reproducing observed disease dynamics.
Synergisms
Rash illnesses occasionally are misdiagnosed, but measles and rubella are distinct clinical entities for which public health objectives differ. Rubella usually is a mild disease, but measles may be severe. Moreover, because child deaths to which measles contributes often have other proximate causes, measles mortality is under-ascertained. Thus, the public health goal is to prevent measles. Rubella during the first trimester frequently causes spontaneous abortions, stillbirths and congenital rubella syndrome (CRS),30 an array of individually severe afflictions that commonly co-occur. Thus, the public health goal is to prevent infection of young women.
We are writing about rubella in China separately, but would be remiss not to note here that, because of disparate historical efforts, if not public health goals, the best vaccination policy for one disease may not be best for the other. Nonetheless, we can fashion compromise policies for using vaccines that combine antigens to the pathogens causing both diseases. Timely catch-up campaigns among adolescent schoolchildren using the measles-rubella (MR) or measles-mumps-rubella (MMR) vaccines would accelerate measles elimination and avert outbreaks of rubella that, because of the immune profile in China, would greatly increase the incidence of CRS.
Supplementary Material
KEY MESSAGES.
By increasingly effective routine and supplemental immunization activities (SIAs), health authorities in China have reduced measles’ reproduction number from 18 to 2.3
To explore strategies for accelerating elimination, we modeled measles virus transmission in the Chinese population stratified by immune status, age, and location
The gradient of measles’ effective reproduction number with respect to sub-population immunization rates indicates that young adults are the optimal target for SIAs
Simulated adolescent and young adult SIAs using a version of our meta-population model in which the person-to-person contact rates are seasonally forced corroborate this result
Adolescent SIAs would nonetheless accelerate elimination, and insofar as adolescents are more accessible than are adults, this is a more practical strategy
Using a vaccine that also contains rubella antigen might also avert outbreaks that likely would markedly increase the incidence of congenital rubella syndrome
ACKNOWLEDGEMENTS
We are grateful to An Zhijie for helpful discussions, to Aaron Curns and several anonymous reviewers for constructively criticizing earlier drafts of the manuscript, and to Aaron also for drawing choropleth maps of the reproduction numbers.
FUNDING
This work was supported by the World Health Organization [Cooperative Projects 090171 and 090187 to China CDC] and National Science Foundation [DMS-1022758 to ZF].
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or other institutions with which they are affiliated.
Footnotes
SUPPLEMENTARY MATERIAL
In the supplement, we describe methods, perform subsidiary or illustrative calculations, and disaggregate results presented in the main text. In section I (estimating forces of infection in vaccinated populations), we describe three methods, the first of which we use, for estimating forces of infection in vaccinated populations. In section II (probabilities of infection on contact, basic reproduction number and associated eigenvectors), we derive the age-specific probabilities of infection on contact with an infectious person from the 2006 forces of infection, contact rates and mixing matrix (i.e., proportions of the contacts that members of each group have with members of all groups including their own). We also derive ℜ0 for age-structured SEIR population models without demographic dynamics, sub-population contributions and prevalence via the next-generation approach. We perform these calculations using information from contact studies in Southern China and Europe to demonstrate why we use the European observations in our modeling of vaccine-preventable diseases in China.
In section III (evaluating analytical results), we compare the respective impacts of vaccinating the age group contributing most to ℜ0 and older and younger people to illustrate what we mean by evaluating analytical results by simulation. We also describe the harmonic function by which we force the infection rates in our simulation models and compare simulation and surveillance results from 2005 until 2009. For the calculations in subsequent sections and entire main text, we use the 2014 forces of infection and our age- and location-stratified SEIR population model.22 In section IV (sub-population reproduction numbers), we tabulate the provincial reproduction numbers illustrated in the main text (figures 3) and their ratio, an indication of vaccination program effectiveness. In section V (the gradient), we describe the immune profiles required for simulations and to derive the provincial effective reproduction numbers from basic ones and vaccination rates required to evaluate the gradient, presented by province. In the main text, we aggregate constituents of this vector-valued function by region (figures 5).
CONFLICTS OF INTEREST
The authors have no conflicts to disclose.
REFERENCES
- 1.Strebel PM, Papania M, Gastañaduy PA, Goodson JL. Measles vaccines. Pp.579–618 (Chapter 37) in Vaccines, 7th Ed., Plotkin SA, Orenstein WA, Offit PA, Edwards KM, eds. Philadelphia, PA, Elsevier, 2018 [Google Scholar]
- 2.Rota PA, Moss WJ, Takeda M, de Swart RL, Thompson KM, Goodson JL. Measles. Nat Rev Dis Primers 2016; 2:16049. doi: 10.1038/nrdp.2016.49 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization, Western Pacific Regional Office (WPRO). Report of the 19th meeting of the Technical Advisory Group on Immunization and Vaccine-Preventable Diseases in the Western Pacific Region. Manila, Philippines, 24–27 August 2010 (http://www.wpro.who.int/immunization/documents/TAG25_pub/en/). 2011 [Google Scholar]
- 4.World Health Organization, European Regional Office (EURO). Measles no longer endemic in 79% of the WHO European Region (http://www.euro.who.int/en/media-centre/sections/press-releases/2017/measles-no-longer-endemic-in-79-of-the-who-european-region). 2017
- 5.World Health Organization, European Regional Office (EURO). Measles outbreaks across Europe threaten progress towards elimination (http://www.euro.who.int/en/media-centre/sections/press-releases/2017/measles-outbreaks-across-europe-threaten-progress-towards-elimination). 2017
- 6.Pan American Health Organization (PAHO). Region of the Americas declared free of measles (http://www.paho.org/hq/index.php?option=com_content&view=article&id=12528%3Aregion-americas-declared-free-measles). 2016
- 7.Pan American Health Organization (PAHO). PAHO urges rapid increase in vaccination coverage to stop spread of measles in the Americas (https://www.paho.org/hq/index.php?option=com_content&view=article&id=14582:paho-urges-rapid-increase-in-vaccination-coverage-to-stop-spread-of-measles-in-the-americas&Itemid=1926&lang=en). 2018
- 8.Glasser JW, Feng Z, Omer SB, Smith PJ, Rodewald LE. The effect of heterogeneity in uptake of the measles, mumps, and rubella vaccine on the potential for outbreaks of measles: a modelling study. Lancet Infect Dis 2016; 16:599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orenstein WA, Hinman A, Nkowane B, Olive JM, Reingold A. Measles and Rubella Global Strategic Plan 2012–2020 midterm review (http://www.who.int/immunization/diseases/measles/en/). 2016 [DOI] [PubMed]
- 10.Wang L, Zeng G, Lee LA, et al. Progress in accelerated measles control in the People’s Republic of China, 1991–2000. J Infect Dis 2003; 187(Suppl 1):S252–57 [DOI] [PubMed] [Google Scholar]
- 11.Ma C, An Z, Hao L, et al. Progress toward measles elimination in the People’s Republic of China, 2000–2009. J Infect Dis 2011; 204(Suppl 1):S447–54 [DOI] [PubMed] [Google Scholar]
- 12.Ma C, Hao L, An Z, et al. Establishment and performance of measles surveillance system in the People’s Republic of China. Chin J Vaccin Immun 2010; 16:297–306 (in Chinese) [Google Scholar]
- 13.Ma C, Su Q, Wen N, et al. Performance of measles surveillance system in the People’s Republic of China, 2012–2014. Chin J Vaccin Immun 2015; 21:405–9 (in Chinese) [Google Scholar]
- 14.Ma C, Hao L, Zhang Y, et al. Monitoring progress towards the elimination of measles in China: an analysis of measles surveillance data. Bull World Health Organ 2014; 92:340–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma C, Su Q, Hao L, et al. Measles epidemiology characteristics and progress toward measles elimination in China, 2012–2013. Chin J Vaccin Immun 2014; 20:193–9 (in Chinese) [Google Scholar]
- 16.Su Q, Hao L, Ma C, et al. Epidemiology of measles in China, 2015–2016. Chin J Vaccin Immun 2018; 24:146–51 (in Chinese) [Google Scholar]
- 17.Ma C, Hao L, Ma J, et al. Measles epidemiological characteristics and progress of measles elimination in China, 2010. Chin J Vaccin Immun 2011; 17:242–5 (in Chinese) [Google Scholar]
- 18.Ma C, Hao L, Su Q, et al. Measles epidemiology and progress towards measles elimination in China, 2011. Chin J Vaccin Immun 2012; 18:193–9 (in Chinese) [Google Scholar]
- 19.Cui F, Shen L, Li L, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis 2017; 23:765–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao L, et al. A nationally representative serologic survey of measles immunity in China: implications for measles elimination. Manuscript (in preparation)
- 21.McCullagh P, Nelder JA. Generalized Linear Models 2nd Ed. London, UK: Chapman and Hall, 1983 [Google Scholar]
- 22.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 2008; 5:381–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Z, Hill AN, Curns AT, Glasser JW. Evaluating targeted interventions via meta-population models with multi-level mixing. Math Biosci 2017; 287:93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerra FM, Bolotin S, Lim G, et al. The basic reproduction number (ℜ0) of measles: a systematic review. Lancet Infect Dis 2017; 17:e420–28 [DOI] [PubMed] [Google Scholar]
- 25.Feng Z, Hill AN, Smith PJ, Glasser JW. An elaboration of theory about preventing outbreaks in homogeneous populations to include heterogeneity or non-random mixing. J Theor Biol 2015; 386:177–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caswell H Matrix Population Models: Construction, Analysis and Interpretation, 2nd Ed. Sunderland, MA: Sinauer Associates, 2001 [Google Scholar]
- 27.Li S, Ma C, Hao L, Su Q, An Z, Ma F, et al. Demographic transition and the dynamics of measles in six provinces in China: a modeling study. PLoS Med 2017; 14: e1002255. doi: 10.1371/journal.pmed.1002255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.China Statistical Yearbook – 2017. China Statistics Press, Beijing, PRC [Google Scholar]
- 29.Chang LT. Factory Girls: From Village to City in a Changing China New York, NY: Spiegel and Grau, 2008 [Google Scholar]
- 30.Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet 1982; 320:781–84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.