Abstract
The acquisition of ligand-independent ESR1 mutations during aromatase inhibitor (AI) therapy in metastatic estrogen receptor (ER)-positive breast cancer is a common mechanism of hormonal therapy resistance. Preclinical and clinical studies have demonstrated that ESR1 mutations can pre-exist in primary tumors and can be enriched during metastasis. Furthermore, ESR1 mutations express a unique transcriptional profile that favors tumor progression, suggesting that selected ESR1 mutations may influence metastasis. Several groups have used sensitive detection methods using patient liquid biopsies to track ESR1 or truncal somatic mutations to predict treatment outcome and tumor progression, and some of these techniques may eventually be used to guide sequential treatment options in patients. Further development and standardization of mutation tracking in circulating tumor DNA (ctDNA) is ongoing. Clinically, patients with ESR1 mutations derive clinical benefit when treated with fulvestrant and CDK4/6 targeted therapies, but the development of more potent selective estrogen receptor degraders (SERDs), and/or new targeted biotherapies are needed to overcome the endocrine-resistant phenotype of ESR1 mutant-bearing tumors. In this review, we discuss mechanisms of resistance and dissemination of ESR1 mutations, as well as detection methods for ESR1 mutation tracking, newly discovered potential therapeutic targets, and the clinical implications and treatment options for treating patients with ESR1 mutant-bearing tumors.
Keywords: estrogen receptor, mutation, breast cancer, metastasis
Precis: A concise review of mechanisms of resistance and dissemination of mutations in the ESR1 gene in breast cancer. Topics include detection methods, preclinical modeling, new therapeutic targets and the clinical implication for patients with ESR1 mutations.
Introduction
About 70% of breast cancers are ER-positive, and many of these patients are effectively cured of their disease1. However, despite effective hormonal and targeted therapies, half of these patients will relapse or progress to incurable metastatic disease. Several mechanisms of de novo and acquired ET resistance have been described, including loss of ER expression, ER crosstalk with growth factor receptors, subclonal genomic alterations of tumor suppressors or drivers, and acquisition of ESR1 fusions or activating ESR1 missense mutations2–4.
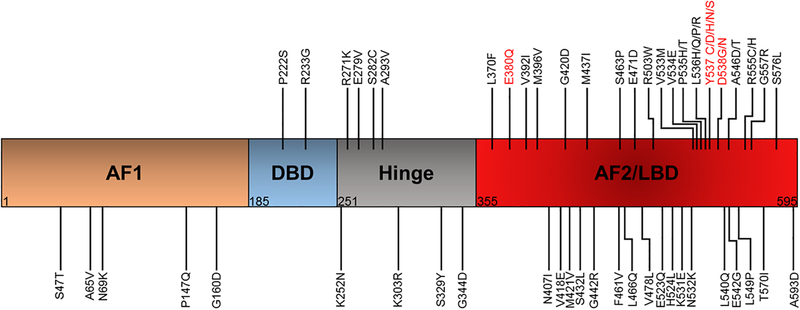
Current clinical strategies to effectively treat and prevent recurrence of ER-positive breast cancer is with endocrine therapies (ET) which target the ER through hormone deprivation or antagonistic binding of the receptor. ET cures about half of patients in the non-metastatic setting, and clinical benefit is seen in about 30% of metastatic patients. However, most metastatic breast cancer (MBC) patients will eventually progress on ET and succumb to their disease. Other targeted agents such as the CDK4/6 inhibitors palbociclib, ribociclib, or abemaciclib, and the mTOR inhibitor everolimus are combined with ET in the metastatic setting, as these have shown improved progression-free survival (PFS) in patients compared to ET alone5–8. However, patients with ESR1 mutant-positive metastases are resistant to standard-of-care ET, and exhibit a worse overall survival9, 10. Figure 1 shows the location within the ER where these mutations occur in clinical samples. The most common and well-characterized mutations occur at the Y537 and D538 residues. However, missense mutations have been identified in at least 51 other residues, with most of these within the ER ligand binding domain (LBD). The most common mutations have been functionally characterized as hormone-independent activating mutations (Y537, D538), while others result in estrogen hypersensitivity (K303R, E380Q)11, 12 or neutral, retaining hormone-dependent activation function (S432L, V534E)12. Furthermore, many of these mutations are rare and have not yet been functionally annotated. There is a current clinical need to identify additional effective hormonal and targeted therapies, as well as novel therapeutic sequencing strategies to best treat MBC. This review will focus on the biology, detection, and treatment strategies of ESR1 LBD mutations as one of the most common mechanisms of acquired endocrine resistance.
Figure 1.
Location of ESR1 missense mutations found in clinical samples. 47/62 identified mutations occur in the LBD, and several are associated with ligand-independent activation of ER. AF2, activation function 1; DBD, DNA binding domain; AF2, activation function 2; LBD, ligand binding domain. COSMIC database and cBioPortal accessed 03/11/2019
Biology of ESR1 mutations in preclinical studies
Several studies have shown that ESR1 LBD mutations are constitutively active and are less sensitive to ER antagonists tamoxifen and fulvestrant13–16. In vitro studies have shown that the Y537S and D538G mutations required higher concentrations of antagonist to decrease ER signaling compared to the wild-type (WT) receptor. Molecular modeling of the Y537S and D538G ESR1 LBD mutations showed that these mutations are in an apo-receptor conformation, and are constitutively active even upon antagonist binding15, 17. These mutations induced changes in protein structure, which resulted in reduced ligand affinity, and this may be one potential mechanism for their ligand-independent activity and ET resistance. In the absence of ligand, the mutant receptors exhibited increased hydrogen bonding between Y537S and N348, which was similar to estrogen-bound WT receptor. Mutant receptors also had enhanced protein stability compared to WT when bound to fulvestrant. Several studies have shown that the most common ESR1 LBD mutations also recruited coactivators, such as SRC-1 and SRC-3, in the absence of ligand that further potentiated ER transcription18, 19. Therefore, it was concluded that the altered structure of ESR1 mutations conferred ET resistance and potentiated distinct mechanisms of resistance through enhanced coactivator recruitment.
The transcriptomes of WT and mutant ERs have been described by several groups, showing there are shared, classical ER signaling signatures, as well as mutant-specific transcriptional regulation20–22. Using in vitro derived ESR1 mutant cell line models generated using CRISPR/Cas9 technology, or through natural selection of cells in hormone-deprived conditions, Martin et al. showed there was a high overlap between ER chromatin binding sites of estrogen-stimulated WT receptor and hormone-deprived mutant receptors. They further showed that estrogen treatment of both the WT and Y537S models exhibited a 74% concordance in ER binding sites23. These results suggest that there are hormone-dependent, but also independent mechanisms of mutant gene regulation, and that the unique constitutive mutant-specific ER binding sites and transcriptional regulation should be further studied to better understand ESR1 mutant’s role in tumor growth and progression.
Recent studies by Jeselsohn et al. demonstrated that the models expressing the Y537S mutation was relatively more resistant to growth inhibition when treated with tamoxifen or fulvestrant compared to D538G and WT, which is consistent with many published studies20. The Y537S and D538G mutations exhibited different cistromes and transcriptomes compared to WT ESR1. Specifically, gene expression analyses comparing estrogen-bound WT receptor with hormone-deprived Y537S and D538G mutant receptors showed little overlap of shared gene expression (18% and 33%, respectively). Furthermore, there was minimal upregulation of gene expression when Y537S mutant cells were treated with estrogen (increase in expression of only 12 genes), but there was a significant increase in gene expression when D538G mutant-expressing cells were treated with estrogen (an increase in 416 genes was observed), and many of these genes were unique to each mutation. Transcriptome analysis of MBC patient tumors harboring ESR1 mutations showed a high correlation with profiles obtained from these cell line models as analyzed by gene set enrichment analysis, validating the significance of the in vitro-derived models. Collectively, these data demonstrate that ESR1 mutations mediate unique and allele-specific transcriptional programs that do not just mimic estrogen-regulated WT ER expression. A better mechanistic understanding of how mutant receptors drive unique gene expression in metastatic disease could provide insight into not only the subclonal evolution of ESR1 mutants, but may also identify novel strategies to target tumor progression.
Other studies have extended clinical findings of acquired ESR1 mutations in hormone-deprived MBC patients by confirming these observations in two-dimensional culture systems. For instance, Martin et al.23 were the first to model in vitro the natural acquisition of ESR1 mutations in ER-positive breast cancer cells. Culturing breast cancer cells with WT ESR1 for long-term in hormone-depleted media resulted in gradual acquisition of the Y537C mutation in MCF-7 cells, and the Y537S mutation in SUM44 cells. Interestingly, analysis of parental cells from each line demonstrated that a subpopulation of Y537S mutant cells pre-existed in SUM44 cells at a very low frequency, and that this population was then enriched with long-term estrogen deprived (LTED) in vitro conditions. The Y537C mutation was not identified in the parental MCF-7 cells, suggesting that these mutations can either pre-exist, or be acquired depending on the cell line background. Furthermore, tamoxifen or fulvestrant-resistant long term treated cell lines did not acquire ESR1 mutations, further supporting clinical evidence that most tumors acquiring ESR1 mutations did so during estrogen withdrawal with AIs. Integrated transcriptomic and cistromic analyses demonstrated that the cell line models with naturally-occurring ESR1 mutations exhibited enriched chromatin binding, which correlated with enhanced estrogen-independent transcriptional activity. These results in LTED models are also confirmatory of studies that used CRISPR/Cas9 knock-in or lentiviral engineered ESR1 mutation models20, 23.
Non-genomic functions of ESR1 mutations have also been described. Gelsomino et al. have shown that insulin growth factor 1 receptor (IGF-1R) signaling was upregulated in ESR1 mutant overexpression models, and was involved in ET resistance to tamoxifen14. Interestingly, this mechanism of resistance was cell-type specific and was dependent on the expression of the PI3K regulators, PI3K3R1 and PI3K3R3, since siRNA knockdown of these regulators restored tamoxifen sensitivity. Treatment with specific inhibitors of the IGF-1R pathway also sensitized ESR1 mutant cells to tamoxifen. Furthermore, ER immunoprecipitation and proximity ligation assays demonstrated enhanced co-localization and crosstalk between mutant ER and IGF-1R14. More recently, Li et al. confirmed a role for the IGF-1R pathway using similar mutant models24. RNA-Seq analyses showed an enhanced IGF gene signature in the mutant models compared to WT receptor expressing models. These cells exhibited an enhanced growth response to IGF1, which was common between the mutant models, but also tamoxifen-resistant, and long-term estrogen deprived models. Targeting the IGF-1R pathway through siRNA knockdown or targeted inhibitors sensitized ESR1 mutant cells to ET, as demonstrated in the earlier study14. Unfortunately, IGF-1R inhibitors have not yet proven clinically useful in MBC, and thus are not a viable targeted clinical approach at this time for patients with ESR1 mutations. Other growth factor receptors, including the HER1–3 family members, need to be evaluated as potential mechanisms of ET resistance in ESR1 mutation models, since expression of different growth factor receptor family members have been shown to be enhanced in these models14, 24.
Martin et al. performed rapid immunoprecipitation with tandem mass spectrometry of endogenous proteins (RIME) to delineate ESR1 WT and mutant interactomes23. These analyses demonstrated that many of the proteins bound by mutant ESR1 were also bound by WT ESR1, but that there were increased interactions between mutant receptors and selected transcriptional regulators, such as GREB1 and FOXA1. ChIP-Seq analyses also demonstrated a ligand-independent enrichment of FOXA1 motifs in ESR1 mutant cells. Targeted knockdown of FOXA1 in WT and mutant cells resulted in a greater growth inhibition in ESR1 mutant cells compared to WT, suggesting a role for FOXA1 in mutant-specific biology. In contrast, Jeselsohn et al. found that the FOXA1 motif was not enriched in hormone-deprived Y537S cells compared to estrogen treated WT cells, and that knockdown of FOXA1 did affect growth of mutant ESR1 cells compared to WT cells20. These discordant findings could be explained by different treatment conditions between the groups, different modeling of ESR1 mutations through CRISPR/Cas9 or overexpression systems, or divergent cellular backgrounds. Studies of the interactome of mutant ESR1 from clinical samples needs to be more thoroughly studied, as these may help resolve these discrepancies and potentially identify new approaches for targeting direct, mutant-specific transcriptional regulators in ESR1 mutant samples.
Implications of ESR1 mutations in metastasis and tumor progression
There is considerable preclinical and clinical evidence demonstrating metastatic tumor cells with ESR1 mutations are most frequently acquired under the selective pressure of AI therapy20, 25. These mutations are rare, or are not present in primary tumors. ESR1 mutant cells may display an enhanced “aggressive phenotype” which could provide an enrichment of t subclonal cell populations in circulating tumor cells and metastatic sites10, 26.
Gu et al.25 were the first to report that the Y537S ESR1 mutation drives spontaneous ligand-independent distant metastasis in vivo using CRISPR-Cas9 engineered ESR1 mutant xenograft models. Jeselsohn et al. have recently shown using doxycycline-inducible models of the Y537S and D538G ESR1 mutations that these cells express a transcriptional network that promotes metastasis20. ESR1 Y537S and D538G mutant cells both metastasized, and withdrawal of doxycycline in the Y537S models resulted in regression of metastatic tumors, demonstrating that metastases resulted from mutant ER populations. Transcriptional profiling from this and other studies have shown that ESR1 mutations promote upregulation of Hallmark cancer pathways including estrogen response, the p53 pathway, and MTORC1 signaling, suggesting a role for mutant ERs in promoting an ET-resistant and metastatic phenotype20, 23.
Sensitive detection methods for ESR1 mutations in clinical biopsies
It has been reasoned that the detection of ESR1 mutations in clinical samples could provide important prognostic information over the treatment course of ER-positive metastatic disease. ER-positive breast cancer patients can recur many years after completion of adjuvant therapy. Zhang et al. were the first to identify an ESR1 mutation, Y537N, in a metastatic ER-positive tumor biopsy in 1997 using ER-specific exon primer PCR and Sanger sequencing13. Fifteen years after this seminal discovery, many laboratories have now confirmed the presence of ESR1 mutations in MBC biopsy samples using deep sequencing, and collectively these studies have identified a hotspot for ESR1 mutations within the LBD region using various DNA sequencing methods9, 10, 12, 15, 16, 26–28. Early studies using next generation sequencing (NGS) as the detection method for ESR1 mutations found that mutations were relatively rare in clinical samples. However, the development of droplet digital PCR (ddPCR) technology has allowed for more sensitive and reliable detection of these mutations. With current data, it is thought that the acquisition of ESR1 mutations are the most common mechanism of ET resistance in MBC. One of the challenges in breast cancer is the development of prognostic and predictive biomarkers for monitoring MBC patients. Next we will discuss the different sequencing approaches that have been recently developed using retrospective analysis of clinical trial samples to evaluate the emergence of ESR1 mutations during tumor progression.
NGS Detection of ESR1 mutations in patient biopsies
Some of the early studies that analyzed ESR1 mutations from clinical samples utilized NGS platforms. NGS using Illumini Hiseq 2000 technology identified lower mutation frequencies of 25% (9/36) and 11% (5/44) in two independent cohorts of MBC patient tumors15. Only 3% (6/183) of primary tumors contained ESR1 mutations, providing some of the first data that these mutations may emerge during metastasis. NGS of sequential tumor biopsies over time would allow for the identification of genomic alterations as potential mechanisms of tumor progression, but the burden of multiple biopsies on patients, and the cost and complexity of the assay over other techniques, such as targeted ddPCR, limits its routine use as a standardized assay for patient management.
The Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) panel was developed as an alternative to NGS to detect selected common cancer gene mutations with high sensitivity4. Using this mutational profiling technology, ESR1 mutation status was analyzed in archival samples from a cohort of 929 breast cancer treated at MSK; mutations were found in 3.5% (11/313) primary breast tumors and 13.6% of metastatic tumors. Furthermore, ESR1 mutations were found in ER-positive samples, but not triple negative cancers12. ESR1 mutations were also identified in 7.9% (10/126) of ER-positive/HER2-positive patients, suggesting that ESR1 mutations might also play a role in HER2 patients undergoing HER2-targeted therapy, thus further investigation will be needed to evaluate ESR1 mutant-specific biology in a HER2-positive background to determine if patients with mutations have a different treatment outcome.
Additional studies utilizing the MSK-IMPACT platform compared genomic alterations between early and MBC29. These analyses demonstrated that ER-positive, but not HER2-positive or triple negative breast cancers, had a significant enrichment of additional driver mutations in metastatic tumors compared to early breast cancer. Some of these mutated genes are downstream of, or are involved in the regulation of ER itself, including the transcriptional regulators KMT2C, NCOR1, and AKT1. These findings suggest that ESR1 mutant-positive breast cancers evolve, acquire distinct, and perhaps targetable mechanisms driving tumor evolution that directly affect or are a consequence of ER signaling.
ddPCR detection of ESR1 mutations in patient biopsies
ddPCR technologies are increasingly being used to detect ESR1 mutations in both solid tumor and liquid biopsies due to its enhanced sensitivity and lower cost compared to NGS technology. Thus the increased sensitivity of ddPCR is allowing for more accurate positive detection of ESR1 mutations from patient biopsies. Takeshita et al. were one of the first groups to evaluate ESR1 mutation status (Y537S, D538G, Y537N, or Y537C) from formalin-fixed, paraffin-embedded (FFPE) samples (270 primary and 55 MBC tumors), and reported ESR1 mutation frequencies of 2.5% and 20%, respectively30. Of the 11 MBC patients with ESR1 mutations, 5 had been treated with an AI, 4 were treated with tamoxifen, and 2 had not had received prior ET before biopsy. Furthermore, Gelsomino et al. analyzed ESR1 mutation status in a larger cohort of 203 primary breast cancers treated with tamoxifen monotherapy, and found that the frequency of ESR1 mutations was higher (12% Y537N, 5% Y537S, and 2% for the D538G mutations) compared to other reports in the literature of unselected primary tumors14. In addition, the presence of ESR1 mutations was associated with a better progression free survival in these tamoxifen-treated patients, suggesting that the use of tamoxifen might not select for or may be effective at preventing the emergence of mutation. This possibility needs to be explored.
In the CARMINA02 clinical trial, which evaluated the efficacy of neoadjuvant anastrozole versus fulvestrant, ESR1 mutations were found in 3.4% (3/89) of treatment-naïve tumors31. Technologies for assessing liquid biopsies have also recently been developed, and have there are now commercial assays to monitor ESR1 mutation status in metastatic patients. Schiavon et al. used ddPCR of liquid biopsy samples from advanced breast cancer (ABC) patients, but did not detect ESR1 mutations in a 22 patient cohort that were previously treated with tamoxifen in the adjuvant setting26. However, they detected ESR1 mutation frequencies in 0% (0/32) to 5.8% (3/52) in two cohorts of patients who were treated with an AI in the adjuvant setting. Mutation frequencies were enriched to 36.4% (16/44) in patients who were treated with an AI in the metastatic setting. These data demonstrate that ESR1 mutations can pre-exist at low frequencies in primary tumors, but are enriched in the metastatic setting, especially in AI treated patients.
Whether the analysis of circulating tumor cells (CTCs) or circulating tumor DNA (ctDNA) will become a superior technical approach to detect ESR1 mutations, and monitor treatment response or progression is still being evaluated. Shaw el al. compared paired CTCs and ctDNA samples from the same patient cohort32. More mutations were discovered in ctDNA than the paired CTC sample, and not all CTCs had the same mutations that were found in the ctDNA. This suggests ctDNA analyses may be more sensitive at tracking gene mutations rather than CTCs. However, there is a need to evaluate mutations in liquid biopsies to those found in distant metastatic tumors biopsies to draw definitive conclusions about discordances. Thus, digital PCR using liquid biopsies has been applied to evaluate ESR1 mutation frequencies in several large Phase II/III clinical trials such as BOLERO 2, SoFEA, PALOMA3 and FERGI9, 10, 28. With increasing use, the potential advantages of ctDNA vs CTC sampling should become apparent.
In the BOLERO-2 clinical trial, MBC patients who had prior exposure to a non-steroidal AI were randomized to the steroidal AI exemestane, or in combination with the mTOR inhibitor everolimus33. Y537S and D538G ESR1 mutations were found in 29% (156/541) of patients10. Thirty patients exhibited polyclonal Y537S and D538G mutations, and other studies have also confirmed that ESR1 mutations are frequently polyclonal9, 28, 34–37. Survival analyses showed that patients with Y537S or D538G mutations exhibited a worse overall survival compared to patients with only WT ER (32.1 vs 20.7 months). Patients with polyclonal ESR1 mutations also exhibited a worse overall survival (15.2 months). Treatment with everolimus was associated with an improved PFS for patients with the D538G mutation that was similar to WT (5.78 months for D538G, 8.48 months for WT), but the analysis of PFS in patients with the Y537S mutation was underpowered to determine if clinical benefit was achieved in this treatment arm. A potential limitation of this study was that only two ESR1 mutations were assessed using ddPCR, and the acquisition of other polyclonal ESR1 mutations may have impacted the prognostic results obtained in this trial.
Fribbens et al.9 developed a multiplex ddPCR assay to simultaneously study seven ESR1 mutations in ctDNA from the SoFEA and PALOMA3 clinical trials. In the SoFEA clinical trial, patients who exhibited prior sensitivity to non-steroidal AIs were randomized to receive fulvestrant plus anastrozole, fulvestrant plus placebo, or the steroidal AI exemestane38. In this cohort of patients, ESR1 mutations were found overall in 39.1% (63/161) of patient’s ctDNA, and 49.1% (27/55) of these patients exhibited polyclonal mutations. Patients who had an ESR1 mutation and were treated with exemestane had a worse PFS compared to patients who did not have detectable ESR1 mutations (2.6 vs 8.0 months). Patients who were treated with fulvestrant derived significant clinical benefit whether they had detectable ESR1 mutations or not (PFS 5.7 months for ESR1 mutations, 5.4 months for WT ER). Therefore, this study suggests that patients with ESR1 mutations were relatively sensitive to a fulvestrant-containing regiment, and should be treated with fulvestrant if there were detectable ESR1 mutations.
In the PALOMA-3 clinical trial, patients who progressed during ET were randomized to receive fulvestrant or fulvestrant plus palbociclib5. ESR1 mutations were detected overall in 25.3% (91/360) of patients in this cohort. Patients with ESR1 mutations had an improved PFS when treated with fulvestrant plus palbociclib versus fulvestrant monotherapy (9.4 vs. 3.6 months), as well as patients with WT ESR1 (9.5 vs 5.4 months). These results suggest that patients who have progressed on a non-steroidal AI derive clinical benefit from fulvestrant and palbociclib, regardless of whether the patient ctDNA exhibited ESR1 mutations. However, a more recent analysis of the PALOMA-3 clinical trial demonstrated that although there was no overall enrichment of ESR1 mutations during treatment, the Y537S mutation was selectively enriched in fulvestrant-based treatments in the metastatic setting, suggesting relative resistance to fulvestrant and palbociclib39.
In the FERGI clinical trial, AI-resistant, locally advanced or MBC patients were treated with fulvestrant alone or in combination with the pan-PI3K inhibitor pictilisib.40 BEAMing Digital PCR assays were developed to detect 12 ESR1 mutations using ctDNA. Liquid biopsies were evaluated at progression on an AI, and ESR1 mutations were found in 37% (57/153) of patient’s ctDNA.28 The authors also compared ctDNA data with that found using tumor tissue from a subset of the patients. No ESR1 mutations were detected in primary tumor tissue from biopsies collected at diagnosis (0/81). However 9.7% (3/31) of metastatic samples obtained before AI therapy contained ESR1 mutations and the frequency increased 63% (12/19) in tumor samples collected after AI progression. In patients with both ctDNA and tumor tissues, the ctDNA often showed increased ESR1 mutant content than match tumor tissue. This suggests that ctDNA might better reflect the total ESR1 mutation burden across multiple metastatic sites.
There was no significant difference in PFS between patients according to ESR1 mutation status in both the fulvestrant only or the combination fulvestrant plus pictilisib arms of FERGI. These findings support that patients derive benefit from fulvestrant treatment regardless of ESR1 mutation status when mutations are analyzed in aggregate. This is in contrast to the retrospective analysis of PALOMA-35 where end of treatment ctDNA was used to demonstrate that the Y537S was significantly increased and associated with resistance to fulvestrant or fulvestrant plus palbociclib.
Collectively, these results demonstrate a need for the routine use of targeted sequencing of liquid biopsies, and if possible tumor samples. These collective results showed that ESR1 mutations represent a small subclonal population in primary breast tumors that are most probably enriched during the course of treatment in the metastatic setting. Table 1 summarizes the frequencies of ESR1 mutations found in clinical tumor biopsies. These frequencies show that overall, the use of ctDNA may be a more reliable and representative detection of total ESR1 mutation status compared to genomic DNA from primary or metastatic tumors.
Table 1.
ESR1 mutation frequency in primary or metastatic tumors, or ctDNA.
| Reference | Sequencing Method | ESR1 Mutation Prevalence | Mutations Detected |
|---|---|---|---|
| Primary tumor | |||
| Toy et al.15 | NGS | 3.3% (6/183) | E380Q, V392I, L536R, Y537C, Y537N, D538G |
| Toy et al.12 | Targeted sequencing | 3.5% (11/313) | S329Y, G344D, E380Q, V418E, S463P, S432L, F461D, L466Q, V478L, N532K, V534E, L536H, L536P, L536R, Y537S, Y537N, Y537C, Y537D, D538G, E542G, A576D |
| Liang et al.31 | ddPCR | 3.4% (3/89) | Mutations not specified |
| Metastatic tumor | |||
| Toy et al.12 | Targeted sequencing | 14% (84/616) | S329Y, G344D, E380Q, V418E, S463P, S432L, F461D, L466Q, V478L, N532K, V534E, L536H, L536P, L536R, Y537S, Y537N, Y537C, Y537D, D538G, E542G, A576D |
| Toy et al.15 | NGS | 25% (9/36) and 11% (5/44) |
S463P, V534E, L536R, Y537N, Y537S, D538G |
| Robinson et al.16 | NGS | 55% (6/11) | L536Q, Y537S, D538G |
| Takeshita et al.36 | ddPCR | 17% (6/35) | Y537C, Y537N, Y537S, D538G |
| ctDNA | |||
| Schiavon et al.26 | ddPCR | 14% (24/171) 4.1% (2/49) 22% (4/18) |
L536R, Y537C, Y537N, Y537S, D538G |
| Clatot et al.27 | ddPCR | 31% (44/144) | Y537C, Y537N, Y537S, D538G |
| Spoerke et al.28 | ddPCR | 37% (57/153) | E380Q, S463P, P535H, L536Q, L536R, L536H, L536P, Y537C, Y537N, Y537S, D538G |
| Chandarlapaty et al.10 | ddPCR | 29% (156/541) | Y537S, D538G |
| Fribbens et al.9 | ddPCR | 39% (63/161) and 25% (91/360) |
E380Q, S463P, L536R, Y537C, Y537N, Y537S, D538G |
| Chung et al.35 | NGS | 34% (67/197) | T347A, R352M, H356D, M357V, L379I, E380Q, M388I, M388L, T431S, G442R, S463P, H476N, H524L, H524Y, P535T, L536F, L536H, L536P, L536R, L536V, Y537S, Y537N, Y537C, Y537D, D538G, L539P, L541M, E542D, E542K |
| Takeshita et al.36 | ddPCR | 14% (5/35) | Y537C, Y537N, Y537S, D538G |
| Cristofanilli et al.84 | BEAMing ddPCR | 27% (106/396) | Mutations not specified |
| O’Leary et al.43 | ddPCR | 31% (61/195) | E380Q, S463P, L536R, Y537C, Y537N, Y537S, D538G |
| Lok et al.85 | ddPCR | 30% (10/33) | E380Q, S463P, I514V, Y537C, Y537S, D538G |
| Allouchery et al.86 | ddPCR | 33% (7/21) | Y537C, Y537N, Y537S, D538G |
| Fribbens et al.34 | ddPCR | 56% (22/39) | E380Q, S463P, L536R, Y537C, Y537N, Y537S, D538G |
Clinical implications and therapeutic strategies to treat MBC with ESR1 Mutations
There are effective ETs to treat patients with ER-positive metastatic disease. ET and biotherapy combinations are now commonly used to treat ET-resistant MBC, as opposed to sequencing of single endocrine agents. There are two biological targets which have been approved for ER-positive breast cancer — mammalian target of rapamycin (mTOR) and cyclin dependent kinases (CDK) 4/6. Treatment guidelines recommend that patients who develop MBC 12 months after adjuvant ET, or are de novo metastatic should be treated in the first line setting with an AI in combination with a CDK4/6 inhibitor41. This recommendation is based on the results of the PALOMA-2 trial that showed the combination of letrozole and palbociclib extended PFS by 13.1 months compared to letrozole alone (27.6 vs. 14.5 months)42. However, other approaches, such as the SERD fulvestrant alone or in combination with a CDK4/6 inhibitor, are also often used in the first line metastatic setting. Subsequent therapy of an AI and/or CDK4/6 inhibitor-treated patient includes fulvestrant monotherapy (or in combination with a CDK4/6 inhibitor in treatment-naïve patients), a steroidal AI with or without an mTOR inhibitor, tamoxifen, or chemotherapy. Preclinical development and proof of clinical utility of additional targeted biotherapies are needed to delay the eventual progression to unresponsive metastatic disease. A better understanding of when to use targeted therapies and the sequence of combination therapies is critical to effectively manage ER-positive disease. Importantly, there has not been a direct comparison of the efficacy of fulvestrant monotherapy versus an AI with a CDK4/6 inhibitor. Furthermore, there has not been a direct comparison between the CDK4/6 inhibitors palbociclib, ribociclib, and abemaciclib in clinical trials. These comparisons will help us understand effective sequencing of therapies in order to delay acquired resistance to targeted therapies and manage metastatic disease.
Current treatment guidelines for ER-positive MBC do not stratify patients based on ESR1 mutation status. Although many preclinical studies have demonstrated that ESR1 mutant cells respond to fulvestrant, but with less sensitivity, recent retrospective analyses of the PALOMA-2 clinical trial published by O’Leary et al. trial showed that patients treated with fulvestrant monotherapy alone, or in combination with palbocicilb continued to acquire the Y537S ESR1 mutation during treatment39. Furthermore, a more recent retrospective correlative analysis of the PALMOA-3 trial evaluated whether early changes in ESR1 or PIK3CA mutations measured using ddPCR of ctDNA were predictive of response to therapy. Although total ESR1 mutant abundance was shown to decrease in both treatment arms, these changes were not predictive of response to fulvestrant43. In contrast, PIK3CA mutation frequency was lower in the fulvestrant and palbociclib-treated group, and was significantly predictive of PFS. This study suggests that truncal mutations, such as PIK3CA, may be more useful to predict treatment responses. Differences in the predictive value of these two genetic biomarkers may be due to the clinical resistance of selected ESR1 mutant cells to fulvestrant, and the truncal nature of PIK3CA mutations which are shared by all subclones in the metastatic tumor. O’Leary et al. also showed that other driver mutations in RB1, growth factor receptors, TP53, and PIK3CA were acquired over the course of treatment39. The acquisition of these mutations was associated with a longer time of treatment, and acquired mutations at the end of treatment correlated with a longer PFS. These data support the conclusion that driver mutations may be acquired later in therapy as a consequence of therapeutic pressures, but perhaps not always in the early treatment setting. These studies also suggest there may be limited clinical utility to stratify patients to treatment based on ESR1 mutation status alone and that concurrent acquisition of other driver mutations may play a significant role in therapeutic resistance.
There are several considerations for the development of new targeted agents that may prove effective in suppressing ESR1 mutant-bearing tumors. Firstly, preclinical studies rely on the generation of ESR1 mutation models through genetic manipulation using CRISPR/Cas9 or through overexpression systems, and thus any results must be validated in patient-derived material. Patient derived xenografts (PDX) can preserve clonal representation and are becoming a useful tool to study ESR1 mutations and test new therapeutic strategies44. Indeed, several studies have shown treatment responses in PDX models in in vivo transplant experiments or ex vivo organoid culture strongly correlated to treatment responses seen in patients45–47. Unfortunately, there is a paucity of ER-positive PDX models from MBC patients, especially patients with ESR1 mutations. There is an urgent need to establish more mutation-positive PDX models from metastatic patients.
It is hoped that the development of novel oral and more potent SERDs will provide a more effective ET backbone to target WT and mutant ERs. Preclinical studies have shown the effectiveness of the newer SERDs AZD9496, GDC-0810, and elacestrant (RAD1901) in reducing tumor growth of ESR1 WT and mutant tumors48–51. AZD9496 exhibited higher activity against ESR1 mutant cells than fulvestrant as demonstrated through transcriptional and growth assays, and in xenograft growth models. However, the short half-life of AZD9496 and reduced efficacy of GDC0810 are significant limitations for clinical development of these agents. Elacestrant is an orally-available SERD currently in phase III clinical trial to compare its efficacy and safety to standard of care ET (EMERALD trial; ). Patients in this study who have WT or mutant ESR1 and have progressed on up to two lines of ET with a CDK4/6 inhibitor were randomized to either elacestrant or standard of care ET (fulvestrant, anastrozole, letrozole, or exemestane). Preclinical studies demonstrated elacestrant inhibited both WT and mutant ESR1 signaling and reduced the growth of mutant ESR1-bearing PDX models. If clinical studies demonstrate significant efficacy in the elacestrant arm, it would be one of the first clinically approved oral SERDs that may also be useful for patients with ESR1 mutations.
Proteolysis targeting chimeras (PROTACs) are being developed to degrade the ER and may prove useful in patients with ER-positive breast cancer52. PROTACs molecules contain an ER ligand covalently linked to an E3 ligase, which promotes proteasomal degradation of ER. Preclinical studies showed that the ER PROTAC ARV-471 promoted potent degradation of ER in multiple ER-positive cell line models53. Furthermore, ARV-471 showed robust growth inhibition of WT and mutant ESR1 xenograft models. Clinical development of ARV-471 is ongoing and if successful, will represent a novel class of ER protein degraders that can also be applied to targeting other proteins in breast and other cancers.
The development of additional targeted therapies for vulnerable pathways in breast cancer is needed to prolong progression and survival of patients with metastatic disease. Clinical trials using pan-PI3K inhibitors and isoform-specific inhibitors have been recently developed. A phase I trial in ER-positive MBC patients using the pan-PI3K inhibitor buparlisib in combination with fulvestrant showed that two patients with ESR1 mutations derived clinic benefit54. Larger studies using fulvestrant with buparlisib, such as BELLE-2 and BELLE-3, are currently being evaluated. The BELLE-2 clinical trial was a phase III trial where patients who had ER-positive breast cancer and progressed on AI therapy received either fulvestrant or fulvestrant plus buparlisib55. The BELLE-3 trial evaluated the efficacy of these therapies in patients who progressed after being treated with an mTOR inhibitor56. Although combinations exhibited a marginally better PFS (BELLE-2: 6.9 vs 5.0 months and BELLE-3: 3.9 vs 1.8 months) the toxicity profile of buparlisib plus fulvestrant does not support its further development in this setting. However, the use of isoform-specific PI3K inhibitors are showing promising clinical benefit. In the Phase III SOALR-1 clinical trial, patients with ER-positive MBC who progressed on AI therapy were randomized to fulvestrant with or without the mutant PI3K-alpha specific inhibitor alpelisib57. Patients whose tumors contained PIK3CA mutations exhibited an improved PFS when treated with the combination of fulvestrant and alpelisib compared to fulvestrant monotherapy (11.1 vs 3.7 months). There was no significant difference in PFS for patients who had WT PIK3CA. In the SANDPIPER Phase III clinical trial where patients were randomized to receive either fulvestrant monotherapy or fulvestrant plus the mutant PI3K-alpha, beta-sparing inhibitor taselisib, the addition of taselisib extended PFS by only 2 months compared to fulvestrant alone (7.4 vs 5.4 months) and was associated with more adverse events58. Importantly, there has not yet been a retrospective analysis of ESR1 mutations in these patient populations. Understanding whether these therapies can effectively target tumors with ESR1 mutations, or prevent the acquisition or subclonal evolution of these mutations, could provide alternative treatments for patients with ESR1 mutations.
AKT inhibitors may be another approach for targeting patients with ESR1 mutations since this pathway can be activated. A single arm, phase II neoadjuvant clinical trial was conducted using the AKT inhibitor MK-2206 in combination with anastrozole in advanced breast cancer patients with a PIK3CA mutation59. There were no pathological complete responses (pCR) in the ER-positive population, and there was no additional suppression of tumor cell proliferation with the addition of MK-220659. Furthermore, a patient in this study acquired an ESR1 mutation detected at the time of surgery. Although not statistically powered for analysis, this observation could suggest clinical resistance of ESR1 mutations to AKT inhibitors. This is an important clinical question to be resolved. Another phase II/III clinical trial, IPATunity130 (), is investigating the use of the AKT inhibitor ipatasertib in combination with paclitaxel to determine if patients with advanced or ER-positive MBC or TNBC who have a genomic alteration in PIK3CA, AKT1, or PTEN will benefit from ATK inhibition. The LOTUS phase II clinical trial found that patients with TNBC treated with ipatasertib and paclitaxel had an improved PFS compared to paclitaxel alone (6.2 vs 4.9 months)60 providing the rationale for IPATunity130. It will be valuable to determine if ER-positive breast cancer patients in this trial will also derive benefit from this combination. However, mTOR inhibitors currently remain the best option for treating ER-positive patients with activation of the PI3K/AKT/mTOR pathway.
The combination of fulvestrant and everolimus has been tested in the PrE0102 () clinical trial, where patients with ER-positive, AI-resistant MBC were treated with fulvestrant, with or without everolimus61. Patients treated with the combination showed a significant extension of PFS compared to fulvestrant alone (10.3 vs 5.1 months). However, there are no projected differences in the overall survival. These results demonstrate that targeting the PI3K pathway through PI3K or mTOR selective inhibitors may be clinically useful, however further analysis of whether patients with ESR1 mutations derive benefit from these combinations will have to be investigated.
Immunotherapy using checkpoint inhibitors is approved for many cancers including melanoma, lung and bladder cancer. In recent years antibodies to PD-L1, PD-1, or CTLA4 have been developed to target immune suppressive pathways, allowing for cytotoxic T-cells to infiltrate and kill tumor cells62, 63. In breast cancer, immunotherapy clinical trials have been mainly focused on metastatic TNBC, a genomically unstable subtype of breast cancer that is believed to be the most immunogenic64, 65. The multicenter I-SPY 2, phase II clinical trial is evaluating novel neoadjuvant therapies and comparing immune therapy in combination with chemotherapy. In TNBC there were higher pCR rates in patients treated with the combination of the anti-PD-1 therapy pembrolizumab with paclitaxel compared to paclitaxel treatment alone (est. 60% vs. 20%)66. In ER-positive/HER2-negative breast cancers, there was also an increase in pCR in patients treated with the pembrolizumab-containing regimen (est. 34% vs. 13%). Furthermore, in the phase I KEYNOTE-028 clinical trial in which ER-positive breast cancer patients were treated with pembrolizumab monotherapy after progressing on several lines of therapies (median of 9 therapies before pembrolizumab treatment), 20% (5/25) attained a partial response and 52% (13/25) had stable disease. Pembrolizumab is currently being tested in combination with fulvestrant in MBC patients () and may give insight into efficacy of immunotherapies in the first- or second-line metastatic disease.
Since many patients with ER-positive MBC will be treated with a CDK4/6 inhibitor, it is important to understand how systemic inhibition of CDK4/6 alters immunotherapy responses. A recent study reported that CDK4/6 inhibitors enhanced PD-L1 expression on tumor cells and augmented responsiveness to PD-L1 immune therapy67. In a separate study, it was demonstrated that systemic CDK4/6 inhibition resulted in suppression of regulatory T cells, but had a lesser effect on CD8+ T cells, suggesting there may be an enhanced T cell-mediated anti-tumor response68. These preclinical data suggest that systemic CDK4/6 inhibition may upregulate PD-L1 expression on tumor cells and inhibit regulatory T cells, which may augment responses to immunotherapy in patients after or during treatment with CDK4/6 inhibitors. The palbociclib after CDK inhibitor and ET (PACE, ) phase II clinical trial is testing the efficacy of combining ET, CDK4/6 inhibition, and a PD-1 inhibitor, in MBC patients who have progressed on ET. Further, since ESR1 mutant cells have been shown to upregulate pathways involved in inflammatory response20, it is possible that tumors expressing ESR1 mutations may be sensitive to immunotherapies. Analysis of ESR1 mutations in patients being treated in these clinical trials should be performed to determine whether these therapies prevent the acquisition of ESR1 mutations during therapy.
Preclinical strategies to identify actionable targets in ESR1 mutant breast cancer
Genome-wide CRISPR knockout screens and transcriptomic analyses identified several genes that are essential for growth of ESR1 mutant tumors20. Potential candidates identified in these preclinical studies are classified as ER coregulators, kinases and receptors involved in growth factor signaling and downstream ER phosphorylation, and epigenetic modifying proteins (Table 2)14, 18, 20, 21, 24, 54, 69–72. Importantly, many of these proteins are targetable, and combinations of specific inhibitors and ET have either an additive or synergistic growth reduction in preclinical models.
Table 2.
Potential therapeutic targets discovered in preclinical studies for treating ESR1 mutant-bearing tumors.
| Target Name | Targeted Therapy | Reference |
|---|---|---|
| CDK7 | THZ1 | Harrod et al, 201721; Jeselsohn et al, 201820 |
| BET family | JQ1 | Ladd et al, 201669 |
| Class I and II HDAC | Vorinostat | Ladd et al, 201669 |
| SRC-3 | SI-2 | Gates et al, 201818 |
| CDK2 | Dinaciclib | Scott et al, 201770 |
| IGF-1R | OSI-906 | Gelsomino et al, 201614; Li et al, 201824 |
| UPR | BHPI | Mao et al, 201671 |
| Notch | RO4929097 | Gelsomino et al, 201872 |
The identification of mutant-selective co-regulators is one strategy to target mutant ER transcriptional activity. Gates et al. performed mass spectrometry-based proteomic profiling of WT-, Y537S-, and D538G-protein complexes to identify coactivators for mutant ER18. Pharmacological inhibition of the coactivator SRC-3 in combination with ET synergistically reduced ER transcriptional activity and growth of mutant ER-expressing models. Furthermore, the SRC inhibitor SI-2 in combination with AZD9496 significantly inhibited growth of a Y537S ESR1 mutant PDX model in vivo, suggesting that the SRC family of co-activators may be useful therapeutic targets for blocking mutant ER growth.
In addition to targeting ER co-regulators, several studies have shown that targeting kinases that phosphorylate mutant ER can augment growth inhibition by ET. CDK7 phosphorylates ER at S118 and is also required for cell cycle progression73. In a study reported by Harrod et al., the selective CDK7 inhibitor THZ1 significantly reduced the growth of MCF-7 cells expressing the Y537S ESR1 mutation21. The combination of THZ1 with fulvestrant resulted in further decreased growth, reduction of S118 phosphorylation, and reduction of ER-mediated gene expression. Jeselsohn et al. confirmed these findings, and also reported that the growth of MCF-7 ESR1 Y537S xenograft tumors were significantly reduced with fulvestrant in combination with THZ120. A study reported by Scott et al. reported that CDK2 can phosphorylate ER serine 294, which led to ligand-independent ER-mediated gene transcription70. ER S294 was also found to be hyper-phosphorylated in MCF-7 ESR1 Y537S and D538G cells compared to WT ESR1 cells. Treatment with the CDK2 inhibitor dinaciclib resulted in a reduction of S294 phosphorylation, and in combination with tamoxifen resulted in tumor regression in a MCF-7 ESR1 Y537S xenograft model. These data demonstrate that inhibition of selective CDKs, such as CDK7 and CDK2, could potentially benefit patients with ESR1 mutations and supports their clinical development of these alternative treatment approaches.
ESR1 mutant models have been utilized to demonstrate other potential targetable pathways, such as the unfolded protein response (UPR) and stemness pathways. Mao et al. showed that Y537S and D538G mutant cells have constitutive hyperactivation of the UPR pathway, and that this may contribute to the ET-resistant phenotype of ESR1 Y537S and D538G mutant cells71. The ER biomodulator BHPI activates UPR, which causes inhibition of protein synthesis and cell death. Treatment of T47D ESR1 WT and mutant cells with BHPI decreased estrogen-stimulated growth, and further enhanced growth reduction with tamoxifen or fulvestrant. Furthermore, activation of progesterone receptor with progestin was associated with increased resistance to anti-estrogen therapies, and further activation of the UPR pathway in ESR1 mutant cells as measured by downstream markers, such as spliced XBP1. Treatment with BHPI reduced progestin-stimulated growth. Collectively, this study demonstrated that UPR activation contributes to the ET-resistant phenotype associated with ESR1 mutation expression. Gelsomino et al. demonstrated that the Y537S ESR1 mutation increases a stem cell-like phenotype72. Y537S ESR1 mutant cells exhibited a higher percentage of CD44+/CD24- cells compared to WT ESR1 cells, and had enhanced Notch signaling indicating that these cells represent a more stem-like progenitor. Therapeutic screening of stem cell pathway inhibitors enhanced Notch signaling in ESR1 mutant cells which was mediated through phosphorylation of residue S118 in Y537S ER. Furthermore, inhibition of Notch signaling using the RO4929097 selective inhibitor reduced mammosphere formation efficiency, but inhibitors of other stem cell pathways such as the Wnt/B-catenein and Sonic Hedgehog were not effective. This collective results show that ESR1 mutant cells have enriched stem cell properties through Notch signaling, and that this pathway could be potentially targeted in patients with ESR1 mutant-expressing tumors.
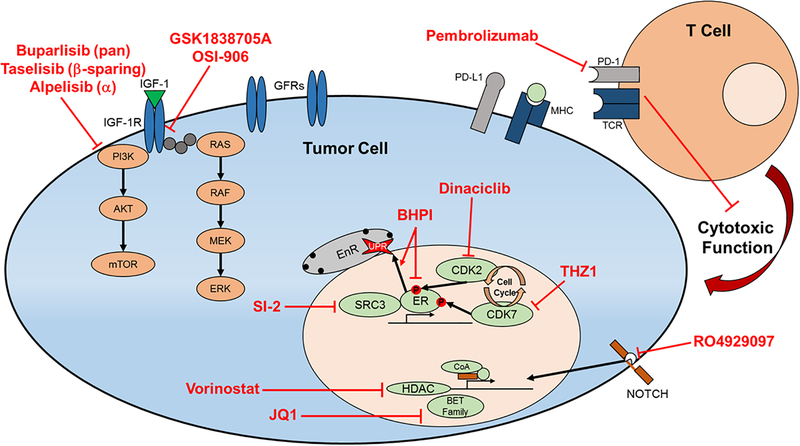
An additional novel strategy to overcome ET resistance is to therapeutically target epigenetic modifying proteins to either inhibit ER transcriptional activity, or to resensitize tumor cells to ET by modifying ER expression and chromatin binding. The JQ1 inhibitor targets the BET family of bromodomain proteins, and the HDAC inhibitor vorinostat have been tested in an ESR1 D538G model, and demonstrated effective reduction of tumor growth and ER transcriptional activity when treated in combination with fulvestrant69. Unfortunately the BET inhibitors have shown widespread toxicity in clinical trials. The efficacy of the HDAC inhibitor entinostat has been tested in clinical trials in combination with ET. In the phase II ENCORE301 trial, patients with ER-positive ABC were treated with either exemestane or in combination with entinostat74, 75. There was an improvement in PFS (4.3 vs 2.3 months) and OS (28.1 vs 19.8 months) with the combination, leading to the initiation of the larger phase III E2112 trial ()76. Since preclinical studies suggest that ESR1 mutant cells are sensitive to HDAC inhibition in combination with ET, it would be valuable to see whether clinical trials with tamoxifen or fulvestrant can be further developed, and whether patients with ESR1 mutations derive benefit from these combinations. Figure 2 shows potential targetable pathways in ESR1m tumors and highlights several inhibitors used in preclinical and clinical studies to target these pathways. However, the use of PI3K inhibitors alpelisib and taselisib will have to be further evaluated to see if patients with ESR1 mutant-bearing tumors derive clinical benefit. These studies demonstrate there are several new targets that could potentially be actionable in the clinic, however there is an urgent need to continue developing clinical trials of inhibitors of these novel targets.
Figure 2.
Targetable pathways in ESR1 mutant cells identified in preclinical studies. GFR signaling through IGF-1R activates the PI3K pathway, which is inhibited with GFR inhibitors or PI3K inhibitors. CDK2 and CDK7 phosphorylate and activate ER. NOTCH, HDACs, BET family proteins, ER coactivators, or ER itself are altered and implicated in resistance of ER mutant cancers. Immunotherapies targeting PD-1 are currently in breast cancer clinical trials. GFRs, growth factor receptors; EnR, endoplasmic reticulum; CoA, coactivator; HDAC, histone deacetylase; MHC, major histocompatibility complex; TCR, T cell receptor
An additional consideration for the development of effective therapies to target ESR1 mutant tumors will be to target common acquired mechanisms of resistance between mutant and WT ER. The majority of patients with advanced or ER-positive MBC will be treated with a CDK4/6 or mTOR inhibitor in combination with ET during the course of their disease. Indeed, several studies now demonstrate differential mechanisms of acquired resistance to either mTOR or CDK4/6 inhibitors in breast and other cancers, including upregulation of MAPK signaling in everolimus- and palbociclib-resistant models, as well as mutations in RB1, and upregulation of CDK2, CCNE1, or PDK1 in palbociclib-resistant cells77–83. These aberrations associated with targeted therapy resistance could be utilized to develop an effective treatment sequence to delay disease progression. Certainly, several recent and ongoing clinical trials testing investigational agents are recruiting patients who have been previously treated with mTOR or CDK4/6 inhibitors, and comparison of response between these patient populations is necessary to provide insight into whether certain patient populations benefit from sequencing of targeted therapies or are inherently resistant to therapy.
Conclusions
Collectively, it has been demonstrated that ESR1 mutations are frequently acquired during AI therapy in the metastatic setting, and may play a role in metastatic progression. Advances in DNA sequencing technology has led to more sensitive detection of ESR1 mutations in clinical samples, and there are now several studies applying sequencing and ddPCR methods to track ESR1 and other mutations during treatment and progression. Targeted DNA sequencing and ddPCR technologies have shown that ESR1 mutations can pre-exist in about 5% of primary tumors and are significantly enriched by 30–40% in the metastatic setting. Analysis of ctDNA allows for an easy, noninvasive and relatively inexpensive method to monitor driver mutations that may arise during treatment, which may eventually be used to guide treatment decisions. Importantly, monitoring ESR1 mutations alone has not shown to be clinically predictive of treatment, however monitoring the acquisition of truncal or other mutations may predict response and/or progression of treated cancers. Currently, patients who have tumors expressing ESR1 mutations are best treated with the combination of fulvestrant and palbocicilb, since this combination has significantly improved PFS in patients with the majority of identified ESR1 mutations. Ongoing clinical trials using fulvestrant with PI3K-alpha specific inhibitors are showing promising clinical results, but the analysis of whether patients with specific ESR1 mutations will benefit from this treatment have yet to be published. Furthermore, immunotherapies are becoming increasingly more effective in solid tumors, and it is hoped that ongoing clinical trials may show clinical benefit in selected ER-positive MBC patients. Preclinical studies have identified novel targets that may be clinically important for targeting in these patients. Further discovery and development of targeted inhibitors is needed, and ongoing and future clinical trials are necessary to discover new treatment options for patients with MBC.
Acknowledgements:
This work was supported by NIH/NCI R01-CA72038 and R01-CA207270, CPRIT MIRA RP120732, BCRF 18–055, and 5P30 CA125123.
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Society AC. Breast Cancer Facts & Figures 2017–2018, 2017. [Google Scholar]
- 2.Barone I, Iacopetta D, Covington KR, et al. Phosphorylation of the mutant K303R estrogen receptor alpha at serine 305 affects aromatase inhibitor sensitivity. Oncogene. 2010;29: 2404–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuqua SA, Gu G, Rechoum Y. Estrogen receptor (ER) alpha mutations in breast cancer: hidden in plain sight. Breast Cancer Res Treat. 2014;144: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razavi P, Chang MT, Xu G, et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell. 2018;34: 427–438 e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner NC, Ro J, Andre F, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373: 209–219. [DOI] [PubMed] [Google Scholar]
- 6.Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016;375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 8.Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017;35: 3638–3646. [DOI] [PubMed] [Google Scholar]
- 9.Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J Clin Oncol. 2016;34: 2961–2968. [DOI] [PubMed] [Google Scholar]
- 10.Chandarlapaty S, Chen D, He W, et al. Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A Secondary Analysis of the BOLERO-2 Clinical Trial. JAMA Oncol. 2016;2: 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuqua SA, Wiltschke C, Zhang QX, et al. A hypersensitive estrogen receptor-alpha mutation in premalignant breast lesions. Cancer Res. 2000;60: 4026–4029. [PubMed] [Google Scholar]
- 12.Toy W, Weir H, Razavi P, et al. Activating ESR1 Mutations Differentially Affect the Efficacy of ER Antagonists. Cancer Discov. 2017;7: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang QX, Borg A, Wolf DM, Oesterreich S, Fuqua SA. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 1997;57: 1244–1249. [PubMed] [Google Scholar]
- 14.Gelsomino L, Gu G, Rechoum Y, et al. ESR1 mutations affect anti-proliferative responses to tamoxifen through enhanced cross-talk with IGF signaling. Breast Cancer Res Treat. 2016;157: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45: 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45: 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlin M, Spinello A, Pennati M, et al. A Computational Assay of Estrogen Receptor alpha Antagonists Reveals the Key Common Structural Traits of Drugs Effectively Fighting Refractory Breast Cancers. Sci Rep. 2018;8: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gates LA, Gu G, Chen Y, et al. Proteomic profiling identifies key coactivators utilized by mutant ERalpha proteins as potential new therapeutic targets. Oncogene. 2018;37: 4581–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merenbakh-Lamin K, Ben-Baruch N, Yeheskel A, et al. D538G mutation in estrogen receptor-alpha: A novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res. 2013;73: 6856–6864. [DOI] [PubMed] [Google Scholar]
- 20.Jeselsohn R, Bergholz JS, Pun M, et al. Allele-Specific Chromatin Recruitment and Therapeutic Vulnerabilities of ESR1 Activating Mutations. Cancer Cell. 2018;33: 173–186 e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrod A, Fulton J, Nguyen VTM, et al. Genomic modelling of the ESR1 Y537S mutation for evaluating function and new therapeutic approaches for metastatic breast cancer. Oncogene. 2017;36: 2286–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahreini A, Li Z, Wang P, et al. Mutation site and context dependent effects of ESR1 mutation in genome-edited breast cancer cell models. Breast Cancer Res. 2017;19: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin LA, Ribas R, Simigdala N, et al. Discovery of naturally occurring ESR1 mutations in breast cancer cell lines modelling endocrine resistance. Nat Commun. 2017;8: 1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Levine KM, Bahreini A, et al. Upregulation of IRS1 Enhances IGF1 Response in Y537S and D538G ESR1 Mutant Breast Cancer Cells. Endocrinology. 2018;159: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu G, Rechoum Y, Gelsomino L, et al. The Y537S ESR1 mutation is a dominant driver of distant ER-positive breast cancer metastasis. SABCS 2016 Abstract. 2016. [Google Scholar]
- 26.Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7: 313ra182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clatot F, Perdrix A, Augusto L, et al. Kinetics, prognostic and predictive values of ESR1 circulating mutations in metastatic breast cancer patients progressing on aromatase inhibitor. Oncotarget. 2016;7: 74448–74459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spoerke JM, Gendreau S, Walter K, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun. 2016;7: 11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andre F, Filleron T, Ng C, et al. Genomic Characterization of Metastatic Breast Cancers. SABCS 2018. 2018. [Google Scholar]
- 30.Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, et al. Droplet digital polymerase chain reaction assay for screening of ESR1 mutations in 325 breast cancer specimens. Transl Res. 2015;166: 540–553 e542. [DOI] [PubMed] [Google Scholar]
- 31.Liang X, Briaux A, Becette V, et al. Molecular profiling of hormone receptor-positive, HER2-negative breast cancers from patients treated with neoadjuvant endocrine therapy in the CARMINA 02 trial (UCBG-0609). J Hematol Oncol. 2018;11: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw JA, Guttery DS, Hills A, et al. Mutation Analysis of Cell-Free DNA and Single Circulating Tumor Cells in Metastatic Breast Cancer Patients with High Circulating Tumor Cell Counts. Clin Cancer Res. 2017;23: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fribbens C, Garcia Murillas I, Beaney M, et al. Tracking evolution of aromatase inhibitor resistance with circulating tumour DNA analysis in metastatic breast cancer. Ann Oncol. 2018;29: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung JH, Pavlick D, Hartmaier R, et al. Hybrid capture-based genomic profiling of circulating tumor DNA from patients with estrogen receptor-positive metastatic breast cancer. Ann Oncol. 2017;28: 2866–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, et al. Comparison of ESR1 Mutations in Tumor Tissue and Matched Plasma Samples from Metastatic Breast Cancer Patients. Transl Oncol. 2017;10: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P, Bahreini A, Gyanchandani R, et al. Sensitive Detection of Mono- and Polyclonal ESR1 Mutations in Primary Tumors, Metastatic Lesions, and Cell-Free DNA of Breast Cancer Patients. Clin Cancer Res. 2016;22: 1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14: 989–998. [DOI] [PubMed] [Google Scholar]
- 39.O’Leary B, Cutts RJ, Liu Y, et al. The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer Discov. 2018;8: 1390–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krop IE, Mayer IA, Ganju V, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma C Treatment approach to metastatic hormone receptor-positive, HER2-negative breast cancer: Endocrine therapy and targeted agents. Available from URL: https://www.uptodate.com/contents/treatment-approach-to-metastatic-hormone-receptor-positive-her2-negative-breast-cancer-endocrine-therapy-and-targeted-agents?topicRef=767&source=see_link [accessed December 18, 2018, 2018].
- 42.Finn RS, Dieras V, Rugo HS, et al. Palbociclib (PAL) + letrozole (L) as first-line (1L) therapy (tx) in estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC): Efficacy and safety across patient (pt) subgroups. Journal of Clinical Oncology. 2017;35: 1039–1039. [Google Scholar]
- 43.O’Leary B, Hrebien S, Morden JP, et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018;9: 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Shen D, Shao J, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 2013;4: 1116–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hidalgo M, Bruckheimer E, Rajeshkumar NV, et al. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol Cancer Ther. 2011;10: 1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao H, Korn JM, Ferretti S, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21: 1318–1325. [DOI] [PubMed] [Google Scholar]
- 47.Aboulkheyr Es H, Montazeri L, Aref AR, Vosough M, Baharvand H. Personalized Cancer Medicine: An Organoid Approach. Trends Biotechnol. 2018;36: 358–371. [DOI] [PubMed] [Google Scholar]
- 48.Weir HM, Bradbury RH, Lawson M, et al. AZD9496: An Oral Estrogen Receptor Inhibitor That Blocks the Growth of ER-Positive and ESR1-Mutant Breast Tumors in Preclinical Models. Cancer Res. 2016;76: 3307–3318. [DOI] [PubMed] [Google Scholar]
- 49.Joseph JD, Darimont B, Zhou W, et al. The selective estrogen receptor downregulator GDC-0810 is efficacious in diverse models of ER+ breast cancer. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bihani T, Patel HK, Arlt H, et al. Elacestrant (RAD1901), a Selective Estrogen Receptor Degrader (SERD), Has Antitumor Activity in Multiple ER(+) Breast Cancer Patient-derived Xenograft Models. Clin Cancer Res. 2017;23: 4793–4804. [DOI] [PubMed] [Google Scholar]
- 51.Garner F, Shomali M, Paquin D, Lyttle CR, Hattersley G. RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs. 2015;26: 948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Guillen VS, Sharma N, et al. New Class of Selective Estrogen Receptor Degraders (SERDs): Expanding the Toolbox of PROTAC Degrons. ACS Med Chem Lett. 2018;9: 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flanagan J, Qian Y, Gough S, et al. ARV-471, an oral estrogen receptor PROTAC™ protein degrader for breast cancer. SABCS 2018. 2018. [Google Scholar]
- 54.Ma CX, Luo J, Naughton M, et al. A Phase I Trial of BKM120 (Buparlisib) in Combination with Fulvestrant in Postmenopausal Women with Estrogen Receptor-Positive Metastatic Breast Cancer. Clin Cancer Res. 2016;22: 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baselga J, Im SA, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18: 904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Leo A, Johnston S, Lee KS, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19: 87–100. [DOI] [PubMed] [Google Scholar]
- 57.Alpelisib Andre F. (ALP) + fulvestrant (FUL) for advanced breast cancer (ABC): results of the Phase 3 SOLAR-1 trial. ESMO 2018 Congress. 2018;LBA3_PR. [Google Scholar]
- 58.Baselga J, Faye Dent S, Cortes J, et al. Phase III study of taselisib (GDC-0032) plus fulvestrant versus fulvestrant in patients with estrogen receptor-positive, PIK3CA-mutant, locally advanced or metastatic breast cancer: Primary analysis from SANDPIPER. J Clin Oncol. 2018;36: abstr LBA1006. [Google Scholar]
- 59.Ma CX, Suman V, Goetz MP, et al. A Phase II Trial of Neoadjuvant MK-2206, an AKT Inhibitor, with Anastrozole in Clinical Stage II or III PIK3CA-Mutant ER-Positive and HER2-Negative Breast Cancer. Clin Cancer Res. 2017;23: 6823–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SB, Dent R, Im SA, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18: 1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kornblum N, Zhao F, Manola J, et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J Clin Oncol. 2018;36: 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19: 1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front Oncol. 2018;8: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14: R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31: 860–867. [DOI] [PubMed] [Google Scholar]
- 66.Nanda R, Liu M, Yao C, Asare S, Hylton N, Van’t Veer L. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J. Clin Oncol. 2017;35: Abstract 506. [Google Scholar]
- 67.Zhang J, Bu X, Wang H, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng J, Wang ES, Jenkins RW, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018;8: 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ladd B, Mazzola AM, Bihani T, et al. Effective combination therapies in preclinical endocrine resistant breast cancer models harboring ER mutations. Oncotarget. 2016;7: 54120–54136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scott GK, Chu D, Kaur R, et al. ERpS294 is a biomarker of ligand or mutational ERalpha activation and a breast cancer target for CDK2 inhibition. Oncotarget. 2017;8: 83432–83445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mao C, Livezey M, Kim JE, Shapiro DJ. Antiestrogen Resistant Cell Lines Expressing Estrogen Receptor alpha Mutations Upregulate the Unfolded Protein Response and are Killed by BHPI. Sci Rep. 2016;6: 34753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gelsomino L, Panza S, Giordano C, et al. Mutations in the estrogen receptor alpha hormone binding domain promote stem cell phenotype through notch activation in breast cancer cell lines. Cancer Lett. 2018;428: 12–20. [DOI] [PubMed] [Google Scholar]
- 73.Chen D, Riedl T, Washbrook E, et al. Activation of estrogen receptor alpha by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Mol Cell. 2000;6: 127–137. [PubMed] [Google Scholar]
- 74.Yardley D, Ismail-Khan R, Klein P. Results of ENCORE 301, a randomized, phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive (ER+) breast cancer progressing on a nonsteroidal aromatase inhibitor (AI). Journal of Clinical Oncology. 2011;29: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol. 2013;31: 2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yeruva SLH, Zhao F, Miller KD, et al. E2112: randomized phase iii trial of endocrine therapy plus entinostat/placebo in patients with hormone receptor-positive advanced breast cancer. NPJ Breast Cancer. 2018;4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kimura M, Hanamura T, Tsuboi K, et al. Acquired resistance to everolimus in aromatase inhibitor-resistant breast cancer. Oncotarget. 2018;9: 21468–21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herrera-Abreu MT, Palafox M, Asghar U, et al. Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2016;76: 2301–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Condorelli R, Spring L, O’Shaughnessy J, et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2018;29: 640–645. [DOI] [PubMed] [Google Scholar]
- 80.de Leeuw R, McNair C, Schiewer MJ, et al. MAPK Reliance via Acquired CDK4/6 Inhibitor Resistance in Cancer. Clin Cancer Res. 2018;24: 4201–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jansen VM, Bhola NE, Bauer JA, et al. Kinome-Wide RNA Interference Screen Reveals a Role for PDK1 in Acquired Resistance to CDK4/6 Inhibition in ER-Positive Breast Cancer. Cancer Res. 2017;77: 2488–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malorni L, Piazza S, Ciani Y, et al. A gene expression signature of retinoblastoma loss-of-function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancer. Oncotarget. 2016;7: 68012–68022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Min A, Kim JE, Kim YJ, et al. Cyclin E overexpression confers resistance to the CDK4/6 specific inhibitor palbociclib in gastric cancer cells. Cancer Lett. 2018;430: 123–132. [DOI] [PubMed] [Google Scholar]
- 84.Cristofanilli M, DeMichele A, Giorgetti C, et al. Predictors of prolonged benefit from palbociclib plus fulvestrant in women with endocrine-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer in PALOMA-3. Eur J Cancer. 2018;104: 21–31. [DOI] [PubMed] [Google Scholar]
- 85.Lok SW, Whittle JR, Vaillant F, et al. A Phase Ib Dose-Escalation and Expansion Study of the BCL2 Inhibitor Venetoclax Combined with Tamoxifen in ER and BCL2-Positive Metastatic Breast Cancer. Cancer Discov. 2019;9: 354–369. [DOI] [PubMed] [Google Scholar]
- 86.Allouchery V, Beaussire L, Perdrix A, et al. Circulating ESR1 mutations at the end of aromatase inhibitor adjuvant treatment and after relapse in breast cancer patients. Breast Cancer Res. 2018;20: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]