Abstract
Russia has a widespread injection drug use epidemic with high prevalence of HIV and HCV among people who inject drugs (PWID). We conducted a mixed methods study of young (age 18–26) hard drug users in St. Petersburg. Thirty-nine structured and 10 semi-structured interviews were conducted. No HIV cases and two HCV cases were detected among the PWID subsample (n=29). Amphetamine and other stimulants were common (70%), opioid use was rare and episodic. Consistent condom use was 10%. No PWID reported syringe-sharing, 51% reported other drug paraphernalia sharing. Most (89%) never or rarely communicated with older (30+) opiate users. A new cohort of drug users in St. Petersburg may have emerged, which is much safer in its injection practices compared to previous cohorts. However, risky sexual practices among this new cohort may expose them to the possibility of sexual transmission of HIV and widespread drug paraphernalia sharing to the HCV epidemic.
Keywords: People who inject drugs (PWID), HIV, Russia, mixed-methods study, young drug users
Resumen
Rusia tiene una epidemia generalizada de consumo de drogas inyectables con una alta prevalencia de VIH y Hepatitis C (VHC) entre las personas que se inyectan drogas (PQID). Realizamos un estudio de métodos mixtos de jóvenes (de 18 a 26 años) usuarios de drogas duras en San Petersburgo. Se realizaron 39 entrevistas estructuradas y 10 semiestructuradas. Entre la submuestra de PQID (n = 29) se detectaron dos casos de VHC y ninguno de VIH. La anfetamina y otros estimulantes eran comunes (70%), el uso de opioides era raro y episódico. El uso consistente del condón fue del 10%. Ninguna PQID reportó uso compartido de jeringas, el 51% reportó uso compartido de otra parafernalia para inyección de drogas. El 89% nunca o rara vez se comunicó con usuarios de opiáceos mayores de 30 años. Es posible que haya surgido una nueva cohorte de usuarios de drogas en San Petersburgo que, en comparación con cohortes anteriores, es mucho más segura en sus prácticas de inyección. Sin embargo, las prácticas sexuales de riesgo de esta nueva cohorte pueden exponerlas a la posibilidad de transmisión sexual de VIH y compartir la parafernalia de drogas puede exponerla a la epidemia del VHC.
Keywords: Personas que se inyectan drogas (PQID), VIH, Rusia, estudio de métodos mixtos, jóvenes usuarios de drogas
INTRODUCTION
Russia is one of the countries with the highest incidence rates of HIV infection (1). While for some time the HIV epidemic in Russia has been less concentrated among people who inject drugs (PWID) than previously (2), PWID are still a major driver of Russia’s HIV epidemic (3,4), particularly in places with large numbers of PWID like St. Petersburg with its estimated 80 000 PWID (5).
The HIV and HCV epidemics among PWID in Russia in general, and St. Petersburg in particular, are well documented (6–12). Figure 1 presents the distribution of participants’ age and HIV prevalence among studies conducted on PWID in St. Petersburg from 2000 to 2012 (7,11–17). It clearly demonstrates a rise of both participants’ age and HIV prevalence until 2009 and relative stabilization of the latter on a high level thereafter. Additionally, the HCV prevalence rate also increased from 78.2% in 2000 (14) to 90% in 2009 (7,13,17).
Figure 1.
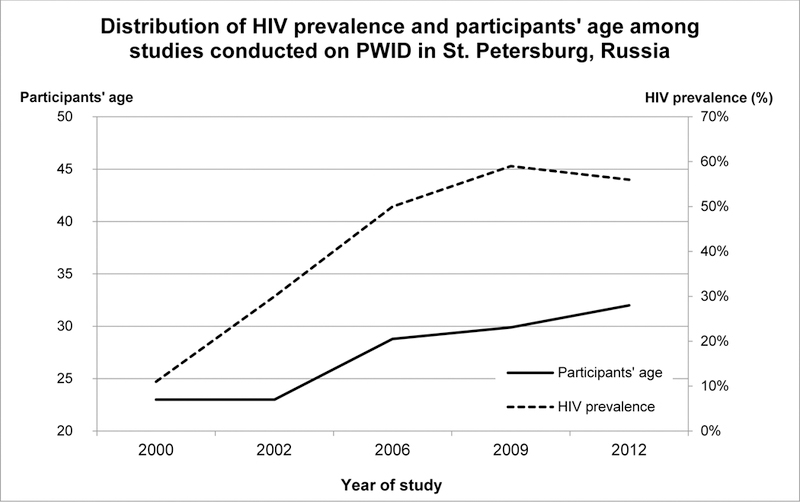
Distribution of HIV prevalence and participants’ age among studies conducted on PWID in St. Petersburg, Russia1
Despite a massive police crackdown on the heroin trade in 2010 – 2015, 96% of respondents in a St Petersburg study of PWID between November 2012 and June 2013 named opiates as their drug of choice (69% heroin and 27% methadone) (12). This is similar to previous studies that found the overwhelming majority of respondents named heroin as their drug of choice (15,16). It is noteworthy that in this same study only 3% of the respondents named amphetamine as their main drug - thus, this population of PWID consisted primarily of opiate users (12).
Summarizing the previous research on PWID in St. Petersburg, we can see that it examined roughly the same generational-grouping of drug users – those who felt the full brunt of the effects of the transition that occurred in Russia in the 1990s, including the opioid and HIV epidemics, that were in their early twenties at the turn of the century, turning 30s in 2010, and are now in their mid-thirties. In terms of a useful conceptualization proposed by Golub, Johnson and Dunlap (18) we can characterize these cohorts as a “heroin generation” (or “opiates generation” if we take into account the latest shift in opiate market from heroin to methadone). However, there are few studies (either in published academic or grey literature) that focused on the younger PWID or non-injecting drug users (NIDU) cohorts in St. Petersburg, those whose age is currently in the range of 18–29 years.
Many studies have found that young drug users, and especially young PWID, are particularly at risk for HIV-infection (19–21). For instance, studies conducted among young (under 25 years) new injectors in Vietnam (22) and heroin injectors in United Republic of Tanzania aged 17–25 years (23) provide evidence of high HIV prevalence - varying from 25.6% to 28% among these populations. Moreover, in St. Petersburg, Russia, HIV prevalence among street youth (age 15–19) who injected drugs was 68 % in 2007 (24). In a replication study conducted in 2012 (25), HIV prevalence among young street PWID aged 15–19 was essentially unchanged at 67%.
A number of studies in different countries have found that young PWID are less likely to know or apply safer injection practices (26) and are more inclined to share syringes and other drug paraphernalia than older PWID (23,27–29). Similarly, a number of studies have found that many PWID become HCV-infected early in their injection careers; 51% of young drug injectors aged 18–30 years residing in a neighborhood of New York City, USA were found to have HCV (30), in Sarajevo, Bosnia and Herzegovina (31,32), among PWID aged 18–24 years HIV prevalence was 36.0% and HCV prevalence was 47.6%, respectively.
Given the lack of studies of young drug users in St. Petersburg in recent years and the fact that this group is particularly vulnerable to HIV infection and other risks, we undertook a mixed-methods pilot study of young (age 18 – 26) hard drug users in St. Petersburg as a precursor to attempting to organize a major respondent driven sampling (RDS) study informed by the results of the pilot project. Although there is no agreed upon definition of what constitutes “hard drugs” or “hard drug use” (33), for the purposes of this study we define “hard drugs” as any illegal psychoactive drugs save cannabinoids. In this pilot we sought to explore what substances are used by young drug users, their drug use patterns and trajectories, drug use and sexual network characteristics, risk injection and sexual practices, overdose risks, HIV and HCV prevalence, and other issues related to drug use.
METHODS
We employed a mixed-methods research design to capitalize on the relative strengths of both quantitative and qualitative research perspectives. Two types of mixed-methods research were combined – Exploratory Design – a two-phase sequential procedure where qualitative data are used to develop a quantitative questionnaire (34) and Complementarity Design, whose purpose is ‘to use the results from one method to elaborate, enhance or illustrate the results from the other’ (35). Thus, after collecting and analyzing all the data we treated quantitative data as the “primary” data in our study, while qualitative data served a complementary role.
Participants and Data Collection
Qualitative
We started the pilot by collecting and conducting preliminary analysis of qualitative data (phase 1). To be eligible for the qualitative study, informants had to be: 18–26 years old, report any drug use (apart from cannabinoids) in the past 30 days, reside in St. Petersburg, speak Russian, and able to provide written informed consent. We used maximum variation sampling strategy (36) and tried to cover the diversity of informants’ experiences. We thus made special efforts to recruit HIV and HCV-positives and participants who used opioids regularly.
We first tried to recruit informants from a local harm reduction service and the local HIV-treatment clinic (“AIDS-Center”), with whom we have good working relationships and which had served as our recruitment sites for numerous previous projects. However, we were unable to recruit young (age 18 – 26) hard drug users through these venues. We then tried to recruit young users though referral by older ones who attended these venues, offering 500 rubles (~$7 USD) incentive per subject. To our surprise, the older PWID told us that they “don’t hang out with the young folk.” (The implications of this recruitment failure are discussed in the Discussion section). We extended our search for informants by incorporating the City Drug Treatment Center and exploiting our good relations with the local Narcotics Anonymous (NA) community, again offering 500 rubles as an incentive for recruitment of a study subject eligible for the study. We also asked our research interns to bring to the study their friends and acquaintances, if they were eligible for the study and ready to take part in it. These strategies proved fruitful and we managed to recruit 10 participants. Two were recruited at the City Drug Treatment Center, both at the higher end of our age range (26 years old); eight were recruited through our NA connections. Four respondents were women and six were men, with an age range from 20 to 26 (mean = 22.4). Two participants were HCV infected, one of them was also co-infected with HIV. Serological status was self-reported. Amphetamine was the drug of choice for three of the subjects, one regularly used methadone, and the rest reported polydrug use. Nine out of ten respondents reported injecting drug use (methadone, amphetamine, mephedrone, or their combination).
Data collection, consisting of semi-structured interviews, was conducted in February 2016. Interview guide topics included participants’ background, drug use initiation and trajectories, characteristics of drug and injection networks, injection practices, attitudes towards opiates and older opiate users, sexual practices, overdose history, practices of embedding safety within their injection and sexual networks, and HIV and HCV-testing (and for HIV and/or HCV-positive participants – behaviors related to serological status after having been diagnosed infected by either virus). Informants were interviewed in cafes or a private room at the City Drug Treatment Center. Interviews were conducted in Russian language, recorded, and lasted on average around 90 minutes. All participants provided written informed consent and were reimbursed for their time and effort with the sum in rubles equal to 15 USD. Interviews were conducted by two members of the research team (PM and AD). The names of the informants have been changed to pseudonyms. The qualitative part of the study was approved by the Ethical Committee of the St. Petersburg Association of Sociologists.
Quantitative
Participants were recruited from February 2017 through April 2017 using RDS, a method designed to overcome some of the biases inherent in the non-random nature of sampling hard-to-reach populations without a clear sampling frame (37,38). Eligibility criteria were the same as in the qualitative part, with one addition: participants had to be willing both to self-test for HIV and HCV in the presence of the interviewers immediately after completing structured interviews and also to disclose their test results to them. Participants who looked 25 or older were asked to produce their photo ID for age verification. Drug use was self-reported; those who reported recent injection use were visually assessed for injection marks.
Three eligible participants were recruited as RDS “seeds” from the sample of informants, who had participated in the qualitative part, and five seeds were recruited via our NA connections and research interns’ ties. (It is noteworthy that we tried to use as seeds any of the three participants who reported regular opiate use in the qualitative part but we failed since all of them told us that they associated only with older (30+) opiate users and thus could not recruit young users). Seeds were provided with three coupons with which to recruit other eligible participants. This process was repeated with recruited participants until we had engaged 39 subjects, after which we stopped recruiting subjects due to the budget considerations. We used a dual-incentives system: each participant received the ruble equivalent of 8 USD for taking part in the study and the ruble equivalent of 8 USD for each study participant successfully recruited.
Structured interviews lasted between 90 to 120 minutes and included the same thematic blocks that were used in the qualitative phase. In writing the questionnaire items, we drew on language used by the participants in semi-structured interviews, where necessary, to help word questions appropriately. Additionally, based on results of preliminary analysis of semi-structured interviews, we introduced questions related to social distance between different categories of drug users with respect to types of drugs used, routes of their use, injection practices and serological status. Following Karakayali (39), we view social distance as a multidimensional phenomenon and adopt the concepts of affective and interactive distances as separate aspects of social distance; thus affective distance is measured in terms of attitudes and emotions toward the group, whereas interactive distance reflects the frequency of interaction between the groups. After interviewing, OraQuick rapid HIV and HCV saliva tests were provided to the participants for self-testing to determine serostatus.
The quantitative part of the study was approved by the Institutional Review Board of National Development and Research Institutes, Inc. and the Ethical Committee of the St. Petersburg Association of Sociologists.
Analysis
Qualitative
Participant interviews were transcribed verbatim. Thematic analysis (40) was conducted with the aid of a free software program called Open Code 3.6 for qualitative data analysis. All interviews were initially coded using a priori codes developed on the basis of the interview guide, and then data were coded again inductively based on emergent findings from the data. The coding was performed independently by two Russian-speaking researchers (PM and AM). To exclude possible discrepancies in interpretations, the coders met after every third transcript was coded and disputes in interpretation were resolved on a consensus basis.
Quantitative
Standard descriptive analyses were performed using IBM SPSS Statistics, version 22.0 software (41). Variables were summarized using medians and interquartile ranges (IQR) or relative frequencies and percentages, as appropriate. Due to the small sample size, the Mann-Whitney U test was conducted for continuous variables and Fisher’s exact test was used for categorical variables to compare sociodemographic characteristics of PWID and NIDU respondents as well as to compare sexual practices among male and female respondents.
RESULTS
In this section we first present the results of the quantitative part of the study and then elaborate them or illustrate them with qualitative data in accordance with Complementarity Design. We report percentages for the quantitative data only. Sociodemographic characteristics, HIV testing behaviors and drug use behaviors of the quantitative sample are described for two categories of respondents – PWID (74%) and NIDU (26%) (See Table 1.) The sample was 56% male. Median age was 22 years (IQR=20–25). Around half (56%) were permanent residents of St. Petersburg and 91% identified themselves as Russian. Participants were relatively highly educated – 75% either had some college experience or had graduated from a university, predominantly unmarried (70%), and 95% lived with parents, partner or a roommate. Compared to PWID, NIDU were more likely to be university students than to have secondary (p=0.015) or higher education (p=0.038). Economically, 72% of respondents had full or part-time jobs, 59% earned up to 25 000 rubles a month (which is much lower than the 48 684 rubles monthly average wage in St. Petersburg) (4). 15% had been homeless at some time of their lives. Most (76%) assigned themselves to middle-class while growing up. The overwhelming majority (92%) of the respondents had never been incarcerated.
Table 1.
Sociodemographic characteristics, HIV testing and drug use behaviors among 39 young people who use drugs in St. Petersburg, Russia, 2016
| Total (n=39) % |
PWID (n=29) % |
NIDU (n=10) % |
p-value1 | |
|---|---|---|---|---|
| Median age in years (IQR)2 | 22(20–25) | 23(20–25) | 21(20–25) | 0.1043 |
| Gender | ||||
| Male | 56 | 55 | 60 | 1.000 |
| Female | 44 | 45 | 40 | |
| St. Petersburg resident registration | 0.364 | |||
| Permanent | 56 | 59 | 50 | |
| Temporary | 23 | 17 | 40 | |
| Registration outside of St. Petersburg | 21 | 24 | 10 | |
| Ethnic group | 0.600 | |||
| Russian | 91 | 93 | 90 | |
| German | 3 | 0 | 10 | |
| Sloveni | 2 | 3 | 0 | |
| None | 4 | 4 | 0 | |
| Highest level of education | 0.018 | |||
| Full secondary | 15 | 21 | 0 | |
| Vocational Education | 10 | 10 | 10 | |
| University student | 31 | 17 | 70 | |
| Did not complete higher education | 23 | 24 | 20 | |
| Higher education | 21 | 28 | 0 | |
| Marital status | 0.664 | |||
| Married | 5 | 7 | 0 | |
| Civil cohabitation | 25 | 24 | 30 | |
| Separated | 3 | 0 | 10 | |
| Never married | 67 | 69 | 60 | |
| Living situation | 0.954 | |||
| With parents and/or other family | 18 | 17 | 20 | |
| With partner without children | 51 | 52 | 50 | |
| With partner with children | 3 | 3 | 0 | |
| With roommates | 23 | 21 | 30 | |
| I live alone | 3 | 3 | 0 | |
| No stable arrangements/homeless | 2 | 4 | 0 | |
| Employment status | 0.899 | |||
| Full time | 33 | 31 | 40 | |
| Part time | 39 | 41 | 30 | |
| Unemployed | 28 | 28 | 30 | |
| Total income (rubles) in past 12 months | 0.179 | |||
| 0 – 25,000 | 59 | 62 | 50 | |
| 26,000 – 50,000 | 28 | 31 | 20 | |
| 51,000 – 100,000 | 13 | 7 | 30 | |
| Ever been homeless | 0.308 | |||
| No | 85 | 79 | 100 | |
| Yes | 15 | 21 | 0 | |
| Socioeconomic class while growing up | 0.355 | |||
| Upper middle class | 10 | 7 | 20 | |
| Middle class | 33 | 31 | 40 | |
| Lower middle class | 33 | 38 | 20 | |
| Working class | 18 | 21 | 10 | |
| Poor | 3 | 3 | 0 | |
| Missing | 3 | 0 | 10 | |
| Ever incarcerated | 1.000 | |||
| No | 92 | 93 | 90 | |
| Yes | 5 | 7 | 0 | |
| Missing | 1 | 0 | 10 | |
| Ever tested for HIV | 0.056 | |||
| No | 33 | 24 | 60 | |
| Yes | 67 | 76 | 40 | |
| Ever tested for HCV | 0.071 | |||
| No | 44 | 35 | 70 | |
| Yes | 56 | 65 | 30 | |
| OraQuick HIV test result | -- | |||
| Negative | 100 | 100 | 100 | |
| Positive | 0 | 0 | 0 | |
| OraQuick HCV test result | 1.000 | |||
| Negative | 92 | 90 | 100 | |
| Positive | 5 | 7 | 0 | |
| Failed | 3 | 3 | 0 | |
| Drugs use, past 30 days | 0.111 | |||
| Amphetamines only | 49 | 38 | 80 | |
| Amphetamines and mephedrone | 21 | 28 | 0 | |
| Methadone | 3 | 3 | 0 | |
| Polydrug use4 | 27 | 31 | 20 | |
| Opiate use, past 30 days | 0.653 | |||
| No | 82 | 79 | 90 | |
| Yes | 18 | 21 | 10 | |
| Ever experienced physical dependence on opiates | -- | |||
| No | 100 | 100 | 100 | |
| Yes | 0 | 0 | 0 | |
| Number of people known to overdose | <0.001 | |||
| 0 | 23 | 7 | 70 | |
| 1 or more | 74 | 90 | 30 | |
| Missing | 3 | 3 | 0 | |
| Number of people known to die of overdose | 0.011 | |||
| 0 | 44 | 31 | 80 | |
| 1 or more | 56 | 69 | 20 | |
| Ever overdosed5 | 1.000 | |||
| No | 69 | 69 | 70 | |
| Yes | 31 | 31 | 30 | |
| Ever been in drug treatment | 1.000 | |||
| No | 95 | 97 | 90 | |
| Yes | 2 | 3 | 0 | |
| Missing | 3 | 0 | 10 | |
Fisher’s exact test unless otherwise specified
IQR = interquartile range
Mann-Whitney test; U=95.00, z=−1.63, r=−0.26
Methadone, heroin, amphetamines, mephedrone, cocaine, psychedelics, and/or cannabinoids
Lost consciousness, stopped breathing, or were unresponsive
More PWID reported that they had ever been tested for HIV (76%) and HCV (65%) as compared to NIDU (40% for HIV (p=0.056) and 30% (p=0.071) for HCV). None of the respondents were HIV positive and 7% (two people) were HCV positive; for one person the result of HCV test was indeterminate.
Substances used in the past month:
Nearly half (49%) of the sample used amphetamines, 21% used amphetamines and mephedrone – also a stimulant (thus, 70% of the sample used only stimulants), 3% used methadone, 27% reported polydrug use (defined as combination of at least two among stimulants, opiates, or psychedelics). Exclusive use of amphetamine was more prevalent among NIDU, none of whom used mephedrone. Of seven respondents (18%) who had used opioids in the past month, none reported ever having physical dependence on opiates. We can see from this data that opiates are much less popular among young hard drug users compared to their popularity among older drug using cohorts.
Nine out of ten (90%) PWID personally knew someone who had had a drug overdose and 69% of this group had known someone who died from a drug overdose, compared to 30% (p < 0.001) and 20% (p=0.011) respectively among the NIDU subsample. Somewhat unexpectedly, the share of those who reported ever having experienced a drug overdose is roughly the same in both subsamples – around 30%. The number of those who ever had been in treatment for drug use was very low in the sample – 2%.
Injecting practices of the PWID subsample are of particular importance as they are directly related to HIV and HCV transmission (table 2). Seventy-two percent reported injecting in the past 90 days. The median injecting duration was 4 years (IQR=2–6, range=1–9). The median age of injecting drug use initiation was 18 years (IQR=17–20, range=12–24). For 83% of the subsample, first injection occurred with a person who was in the age range 18 – 26 years; 10% injected for the first time with a person younger than 18, none with a person older than 26. The data on injection risk practices correspond to the serological data – none of the participants reported syringe sharing for the last 12 months, while about half the subsample (52%) reported using new syringes only.
Table 2.
Injection practices among 29 young people who inject drugs in St. Petersburg, Russia, 2016
| % | |
|---|---|
| Median years of injection drug use (IQR)1 | 4(2–6) |
| Median age at first injection in years (IQR) | 18(17–20) |
| Injected in the past 90 days | |
| No | 28 |
| Yes | 72 |
| Age of person injected with the first time | |
| < 18 | 10 |
| 18–26 | 83 |
| > 26 | 0 |
| Injected by themselves | 7 |
| Syringe use in the past 12 months | |
| Used new only | 52 |
| Used new and reused his/her own | 27 |
| Used others’ | 0 |
| Did not inject in past 12 months | 21 |
| Shared paraphernalia in the past 12 months | |
| Never | 28 |
| Rarely | 21 |
| Sometimes | 21 |
| Often | 6 |
| All the time | 3 |
| Did not inject in past 12 months | 21 |
| Spoke with people inject with about the need to inject safely in the past 6 months | |
| Never | 24 |
| Rarely | 21 |
| Some of the time | 17 |
| Most of the time | 17 |
| All the time | 0 |
| Did not inject in past 6 months or missing | 21 |
| Supplied sterile syringes to people inject with in the past 6 months | |
| Never | 17 |
| Rarely | 17 |
| Some of the time | 17 |
| Most of the time | 28 |
| All the time | 0 |
| Did not inject in past 6 months or missing | 21 |
| Made sure had enough sterile syringes so can provide to others in the past 6 months | |
| Never | 24 |
| Rarely | 17 |
| Some of the time | 17 |
| Most of the time | 14 |
| All the time | 7 |
| Did not inject in past 6 months or missing | 21 |
| When someone uses or is about to use someone else’s syringe and no one has a clean syringe | |
| We will not let them do it | 38 |
| They will not be invited into our company again | 3 |
| There will be sarcastic jokes, etc. | 38 |
| Everything will continue as if nothing happened | 3 |
| Such people do not come into our company | 18 |
IQR = interquartile range
In the qualitative interviews participants told us of their fear of contracting HIV and HCV, and also that non-sharing of syringes was to a large extent a matter of personal hygiene. The following exchange illustrates this point:
Interviewer: Was it [non-sharing of syringe] because of the fear of HIV, Hepatitis C, or anything else?
Informant: Yes, I think so, naturally. But it was discussed as a question of elementary hygiene (Alexey, male, 22, PWID)
However, the data on sharing other drug preparation paraphernalia (i.e., water, cookers, and filters) are more disturbing – only 28% reported that they never had shared drug paraphernalia in the past year, while 9% reported having shared drug paraphernalia often or all the time. This may be partly due to informants’ limited knowledge about the ways HCV is transmitted, especially early in their injection careers. Ksenia, who got tested and learned that she was HCV-positive during her first several months after she started to inject and later underwent HCV treatment, explains:
“I didn’t know that you should watch that everything should be yours – a spoon, a cotton. Before I was told that you just have to watch that you always have your own syringe. Junkies told me this, and I thought so... [then after learning about HCV transmission] Yes, I have everything my own now, I started to watch it, of course” (Ksenia, 20, female, PWID)
The qualitative data show that in addition to not sharing syringes, many participants took care of themselves in multiple ways. They sniffed drugs if they did not have their own syringe. They tried to maintain nutrition even when they were not feeling hungry, took vitamins, mineral supplements, used medicines for vein treatment, and visited a doctor when they were sick.
In the past 6 months, 34% of the PWID subsample had talked with some regularity to their peers about the need to inject safely (table 2). Nearly half (45%) of PWID reported giving sterile syringes to people whom they injected with some or most of the time, and one-fifth (21%) reported having enough sterile syringes with them that they could provide anyone who needed one most or all the time. Over a third (38%) of the participants reported that they would not allow somebody to use someone else’s syringe if it happened among their friends. The following quote illustrates this point of safety embedding:
“People...tried, if somebody didn’t have a syringe, people gave him syringes, clean I mean, that is, helped him in order to exclude a possibility for someone to degrade...Sometimes, somebody would say: “Why? Don’t do it! A pharmacy is just five minutes away, go, I can give you the money” (Roman, 20, male, PWID)
Sergey (aged 20, PWID) carried naloxone with him because he could find himself in a company where opiates were used, despite the fact that he did not use opiates himself. Ksenia describes how safety norms can even be enforced:
“And they, if they’re present, and if you just use a syringe a second time [even if your own], they can just throw you out, or won’t let you do it, even if it’s happening at your own house” (Ksenia, 20, female, PWID)
Most (95%) of the sample were sexually active in the past 90 days, with the median number of partners being 1 for both men (IQR=1–3, range=1–4) and women ((IQR=1–2, range=1–4); table 3).
Table 3.
Sexual practices in past 90 days among 39 young people who use drugs in St. Petersburg, Russia, 2016, by sex
| Past 90 day sexual practices | Total (n=39) % |
Male (n=22) % |
Female (n=17) % |
p-value1 |
|---|---|---|---|---|
| Median number of vaginal, anal, or oral sex partners (IQR)2 | 1(1–2) | 1(1–3) | 1(1–2) | 0.9833 |
| Had sex in past 90 days | 0.495 | |||
| No | 5 | 9 | 0 | |
| Yes | 95 | 91 | 100 | |
| Number of partners that were regular drug users | 1.000 | |||
| 0 | 31 | 32 | 29 | |
| 1 or more | 64 | 58 | 71 | |
| Did not have sex | 5 | 9 | 100 | |
| Number of partners that were drug injectors in past 90 days | 0.498 | |||
| 0 | 59 | 50 | 71 | |
| 1 or more | 36 | 41 | 29 | |
| Did not have sex | 5 | 9 | 100 | |
| Used male condoms when had sex | 0.126 | |||
| Always | 10 | 18 | 0 | |
| Not always | 77 | 73 | 82 | |
| Did not have sex | 5 | 9 | 8 | |
| Only had sex with another woman | 8 | -- | 8 | |
Fisher’s exact test unless otherwise specified
IQR = interquartile range
Mann-Whitney test; U=168.50, z=−0.574, r=−0.09
Almost two thirds of the respondents (64%) reported having had at least one sexual partner who was a regular drug user in the past 90 days, and 36% had sex with at least one PWID. There is evidence of low condom use among this sample of young hard drug users – just 10% of respondents reported always using condoms over the past three months and about a third (36%) never used condoms during the period. Although there was no statistically significant difference in condom use during the past three months between male and female respondents, the percentage of women who reported consistent condom use was alarmingly low – 0%.
We then explored affective and interactive distances that exist between different categories of drug users with respect to types of drugs, patterns of their use as well as behaviors and outcomes associated with drug use (e.g. blood-borne viral infections). As mentioned above, we interpret affective distance in terms of attitudes and emotions toward a particular group and interactive distance - as a frequency of interaction with members of the group. The data suggest that opiate use is controversial. For 58% of those with a history of opiate use (“opiate users”), making sure that their non-using friends will not find out about their opiate use was important or very important (data not shown). As for the general sample, less than third (28%) felt strong or very strong aversion to opiate users, whereas around 36% did not feel any aversion at all. Also, age does not seem to be a factor in attitudes to opiate users as roughly the same shares of respondents felt strong to very strong aversion towards older (30+) opiate users (28%) and did not feel it at all (33%).
Our subjects are considerably separated from opiate users; 62% of the sample never or rarely interacted with opiate users, whereas 33% did it more often. The interactive social distance from older 30+ opiate users is even larger – 89% of participants never or rarely communicated with them and just 6% reported communicating with them more often.
The qualitative data support the quantitative on this dual status of opiates. On the one hand, heroin is a heavily stigmatized drug associated with HIV and HCV, as Vika describes it:
“You know, heroin, Hepatitis C and HIV – it is one direct line” (Vika, 21, female, non-PWID)
Anton narrated how he had to hide his heroin use from his non-opiate using friends.
“They [his friends] shouldn’t have known that I use drugs [heroin]...So therefore, I hid it from them...It is heroin, heroin remains heroin, doesn’t matter how you use it – a fearful word” (Anton, 25, male, PWID)
On the other hand, for some young drug users, heroin was romanticized as the “best high” and they felt attracted to it. However, several of them were disillusioned after trying it. As Lena tells:
“No, on the contrary, everybody had long wanted to try heroin. Well, one time. We thought that it was something special, as people say, it is the best of all drugs. But, unfortunately, everybody got disappointed” (Lena, 23, female, PWID).
She continues:
“After trying I went out and threw up and felt very dizzy. I read in the Internet that first time it is always like that, I’ll try a second time and it’s going to be better. So I tried several times and every time I expected that the high is going to be better, that it’s going to be something marvelous but in the end I never experienced something outstanding” (Lena, 23, female, PWID)
Other participants, while clearly describing symptoms of opiate intoxication – nausea, scratching - thought they were sold “fake” opioids because they did not experience the high they expected.
Sharing of syringes was highly stigmatized in PWID subsample - 72% of PWID felt very strong or strong aversion for people who, when they do not have their own syringe, used their friends’ syringes; and 86% for those who reuse used strangers’ syringes (table 5), thus suggesting that a substantial affective distance may exist between these two groups. The interactive distance between PWID respondents and sharers also seems to be large – 87% of PWID never or rarely communicated with those who share their friends’ syringes, 10% did it some of the time, and none - most of the time or all the time. As for those who share strangers’ syringes, PWID either never interacted with them (72%) or did it rarely (21%).
Table 5.
Social distance from people who share syringes or are HCV/HIV positive among 29 young people who inject drugs in St. Petersburg, Russia, 2016
| % | |
|---|---|
| Aversion to people who, when they don’t have their own syringe, use syringes of their friends | |
| Very strong | 38 |
| Strong | 35 |
| Not very strong | 21 |
| A little bit | 0 |
| Not at all | 6 |
| Interact with people who, when they don’t have their own syringe, use syringes of their friends | |
| Never | 35 |
| Rarely | 52 |
| Some of the time | 10 |
| Most of the time | 0 |
| All the time | 0 |
| Missing | 3 |
| Aversion to people who, when they don’t have their own syringe, use syringes of people they don’t know | |
| Very strong | 45 |
| Strong | 41 |
| Not very strong | 7 |
| A little bit | 0 |
| Not at all | 0 |
| Missing | 7 |
| Interact with people who, when they don’t have their own syringe, use syringes of people they don’t know | |
| Never | 72 |
| Rarely | 21 |
| Some of the time | 0 |
| Most of the time | 0 |
| All the time | 0 |
| Missing | 7 |
| Aversion to HCV positive people | |
| Very strong | 3 |
| Strong | 21 |
| Not very strong | 24 |
| A little bit | 24 |
| Not at all | 28 |
| Interact with HCV positive people | |
| Never | 48 |
| Rarely | 31 |
| Some of the time | 10 |
| Most of the time | 3 |
| All the time | 5 |
| Missing | 3 |
| Aversion to HIV positive people | |
| Very strong | 0 |
| Strong | 7 |
| Not very strong | 14 |
| A little bit | 28 |
| Not at all | 41 |
| Missing | 10 |
| Interact with HIV positive people | |
| Never | 69 |
| Rarely | 21 |
| Some of the time | 7 |
| Most of the time | 0 |
| All the time | 0 |
| Missing | 3 |
When discussing syringe sharing in semi-structured interviews informants often used a metaphor – “it’s like taking another person’s toothbrush.” “Sharer” was also a morally-laden category: non-sharers were described as “decent people” and sharers as “degraded”, “asocial”, “low-down”, or “dirty” (more on stigmatization of sharing below). Non-sharing also was taken as something taken-for-granted, “normal”, or “natural.”
HCV status turned out to be highly stigmatized – almost three quarters (72%) of PWID respondents felt some form of aversion towards HCV-infected people (table 5). Interestingly, HIV-positives were less stigmatized than HCV-positives – “only” 49% reported some form of aversion towards HIV-positives. Almost half (48%) of the PWID subsample never communicated with HCV-positives, and only 8% interacted with them frequently. More than two thirds (69%) of the PWID subsample never interacted with HIV-positives. Taking these data on aversion and communication as a whole, there appears to be a considerable social distance between our PWID subsample and people living with either HCV or HIV.
DISCUSSION
These pilot data suggest that a new generation of hard drug users, that is quite different in their drug use practices from older generations of PWUD in St. Petersburg, may have emerged. Although our sample was small, the discrepancies between the pilot data and earlier reported data with respect to HIV and HCV prevalence (7,13,17) — 0% vs. 59% for HIV and 7% vs. 90% for HCV—can hardly be attributed to mere chance. However, Kornilova et al. (25) suggest that street PWID in St Petersburg may have more similarity to, or connection with, the older generation than did the young PWID we studied. Obviously, a larger study or studies are needed to confirm or refute this statement.
Following Mannheim (42) and Golub, Johnson and Dunlap (18), we call these new cohorts of young hard drug users part of a “generation” because they shared the “same formative experiences” in adolescence that were quite distinct from those that the previous “heroin generation” had to live through – social stability vs. anomie, relative prosperity vs. abject poverty, functioning of social institutions (though very imperfect) vs. collapse of established social institutions, mature drug markets and recent history of widespread drug use vs. unsaturated drug markets and lack of recent drug use epidemics, and, importantly, high prevalence of HIV/AIDS among older PWID and awareness thereof vs. almost total absence of HIV and lack of understanding of its importance for PWID in Russia in the beginning of “heroin generation” drug careers (44). Further studies must explicate these and other aspects of different social contexts for different generations of drug users in Russia.
Nearly half of the participants used only amphetamines, and a substantial number used amphetamines and novel psychoactive substances (NPS) such as mephedrone (also a stimulant), whereas opioids were used by a much smaller number of participants and almost always episodically. Thus, this generation of drug users may constitute an “amphetamine generation” or given the rapid spread and popularity of stimulant-type NPS, a “stimulants generation.” Future studies should explore if this is indeed the case.
Both serological and behavioral data indicate that this generation seems to be much safer in its injection practices than older PWID cohorts - none of the participants reported syringe sharing in the past 12 months, and about half the subsample reported using new syringes only. Lower rates of syringe sharing among young PWID are observed not only in Russia but in countries with similar histories of drug epidemics, such as Estonia and Ukraine where younger PWID inject more safely than their older peers (45,46). The results of the study indicate that participants not only avoid syringe sharing but take care of themselves in multiple ways, for example, preventing their veins from collapsing, and seeking medical help when feeling sick and therefore are more similar to recent generations of “staying safe” New York PWID than to older cohorts of Russian PWID (47,48).
This raises an intriguing and important question: how did these new pro-safety norms among young hard drug users in St. Petersburg emerge, especially in the context of a Russian drug policy that neglects and often opposes harm reduction among PWID? The answer clearly does not reside in the fact that new generation switched to stimulants since a number of studies report that injection of amphetamine-type stimulants is associated with more needle sharing compared to opiates (49–52). Also, to add complexity to the question, a number of studies have found that young injectors are particularly vulnerable to HIV and HCV infections (19,20) but our study data do not show this. We can learn from this pilot study that relationships between drug and HIV policies and behavioral and serological outcomes cannot be reduced to policies per se and involves much more complex social and cultural processes. Also, broader policies and socioeconomic conditions are very important. In Russia syringes are widely available without prescription and are very cheap – 3 rubles or 4–5 US cents per syringe – and the number of 24/7 pharmacies is also very large. These conditions appeared not as governmental AIDS response but were formed by market forces after the collapse of the Soviet Union.
Sound HIV and drug policies are extremely important. This is shown by numerous successful implementations of syringe exchange programs throughout the world (53,54). However, programs and policies are not everything. Collective representations of acceptable and unacceptable behaviors (such as syringe sharing or lack thereof) seem to be quite resilient and independent of policies. This parallels the relative lack of influence of drug policy on the prevalence of drug use and drug addiction. As Peter Reuter writes: “There is no research showing that tougher enforcement, more prevention or even increased treatment has reduced substantially the number of users or addicts in a nation. Numerous other cultural and social factors appear to be much more important” (55). Similarly, Friedman et al. (56,57) showed that drug arrest rates in large US metropolitan areas had no association with subsequent rates of or changes in levels of injection drug use in those metropolitan areas. These data suggest that such independence between policy and enforcement and behaviors exists for injection risk behavior as well as for the number of people who use drugs.
The results of this pilot study indicate a possible norm change regarding injection safety among young hard drug users in St. Petersburg, Russia. Previous studies (58–60) have suggested an important part of the norm change process: stigmatization of the previous cohort, the substance(s) they use, the way they administer these substances, and behavioral norms around their consumption. This parallels the way that the derogatory term “crackhead” helped to form new “blunt generation” and dissociate this new generation from the previous “crack generation” in New York City (60,61). Our pilot study found a strong stigmatization among younger users of the syringe sharing that was typical of the “heroin generation.” Almost three quarters of PWID were strongly averse to those who share their friends’ syringes, and 86% felt that way about those who share strangers’ syringes. It is also revealing that many of the participants framed their unwillingness to share syringes as a matter of personal hygiene, comparing sharing needles to using other person’s toothbrush; sharing was a moral category: non-sharers were constructed as “decent people” and sharers as “degraded”, “asocial”, “low-down”, “dirty.” The sociocultural theory of risk developed by Mary Douglas – “Purity and Danger” (62), “Risk and Blame” (63), that says that people tend to avoid things that are culturally defined as “polluted” and morally disgusting – might help explain the cultural foundations behind the new PWID generation’s injection practices.
Health statuses that can result from sharing, such as being infected with HIV or HCV, were also severely stigmatized. The greater stigmatization of HCV-positives may be related to their greater presence in our participants’ injection networks; thus, HIV-positives may be perceived as a more abstract and distant danger, while HCV-positives may be perceived as a clear and present danger for our informants. The data show that indeed the participants interact much more frequently with HCV-positives than HIV-positives. Stigmatization of opiate users and particularly of old opiate users was also observed in both qualitative and quantitative data. Aversion to the older opiate users may be related to a stigmatized image of a heavy opioid user or may be a psychological strategy against greater involvement in opiate use or both. More research is needed on this topic.
The new generation of young hard drug users seems to be very much separated from the older (30+) “opiate generation.” This can be seen from our failure to recruit young hard drug users from older PWID and from the reported data – 83% of younger users with a history of opioid use rarely or never socialized with the older opiate users (data not shown), and other respondents almost never socialized with them. Such a separation would supply the new cohorts of young hard drug users with “network protection”, as scarcity of interactions translates into small probabilities of sharing syringes, drug paraphernalia, or having sex with older PWID networks heavily burdened with HIV and HCV. It also potentially guards them from absorbing norms supportive of syringe sharing that are widespread in the older PWID injection networks. Separateness from older opioid cohorts along with safer injection behaviors found in our sample may make them resilient to parenteral HIV-infection. This is epidemiologically parallel to Friedman et al.’s similar discussion of new injectors’ network protection from long-term injectors (64,65).
We found that the cultural status of opioids among our sample is contradictory – on the one hand, as was mentioned above, opioid use is stigmatized, while on the other, popular discourses picturing heroin as the “best high” attract young hard drug users to them. It appears that the heroin high was oversold in these discourses, as some users expected “something marvelous” but did not experience “something outstanding.” Other informants attributed their lack of proper high to having been cheated and sold “fake” opioids. Similar findings regarding negative interpretation of effects of heroin and cocaine by inexperienced users were reported by Bancroft et al. (66). As a result, many participants discontinued opiate use. More research is needed to clarify whether this phenomenon is related to poor heroin and methadone quality in the St. Petersburg drug market, the subjective and social nature of the drug high described in the literature (67–69), or both.
Though the low prevalence of HIV and HCV detected in the sample is encouraging, considerable risks exist with regards to HCV- and HIV-infection via indirect sharing and sexual transmission, since few participants reported that they never shared drug paraphernalia and only a tenth reported consistent condom use. Also worrisome is that over two thirds of the respondents reported having known someone who died of overdose. Indeed, in qualitative interviews several informants told us that they themselves had had opiate overdoses while using opiates episodically, and knew episodic opiate users who had died of opiate overdose. The spread in Russia of fentanyl and fentanyl-type substances renders the opiate overdose problem, perhaps particularly among inexperienced users, especially critical.
While we did not collect data on respondents’ harm reduction service use or willingness to use (there are several syringe-exchange mobile service points operating in St. Petersburg), at the end of a number of interviews we asked the respondents whether they would be willing to exchange syringes at syringe exchange programs. The answer was always negative. When probed why, informants named financial well-being and the cheap price of syringes. These findings are in accordance with those that report an unwillingness of young drug injectors to access harm reduction services in other settings (70). It may turn out that SEPs are not attractive to young hard drug users. However, this study shows that participants are often involved in providing their peers with clean injecting equipment, supporting safe injecting practices and discouraging unsafe ones – actions that Friedman et. al. called “intraventions” (71). Intraventions can be a powerful vehicle for reduction of various risks among PWID, especially in the settings where sufficient harm reduction services are lacking, like in Russia (72). The potential of intraventions should be fully exploited among young hard drug users in Russia, including but not limited to syringe exchange, naloxone distribution, online interventions, and consulting on drug users’ forums (especially on HCV and overdoses). High digital literacy (every participant used the Internet on a daily basis) and youth civic potential can also be capitalized upon – HIV and drug user online and offline activism may be attractive to this group of highly educated young hard drug users.
This study has several limitations. First, due to the pilot nature of the data and small sample size, data analysis was performed in an exploratory manner so all p-values should be interpreted as indicators that there might be some relationships, which should be verified in further studies. Second, the non-random nature of the sample resulted in our respondents’ being highly educated and mostly from middle class background. Third, one of the primary reasons for using RDS procedures in this study, despite the small sample size, was to demonstrate feasibility of an RDS study of young hard drug users in St. Petersburg, Russia; clearly for attaining equilibrium, and making population level inferences, a much larger, “proper” RDS study is needed. Previous research (25) showed that HIV prevalence was very high among street youth who injected drugs in recent years in St. Petersburg, but we were unable to recruit this group of PWID. Thus, more data are needed on respondents from working and lower class backgrounds—and particularly for those who are “street” users—for a more complete picture of St. Petersburg young hard drug users. Substance consumption was self-reported without confirmatory toxicology. Behavioral data can be inaccurate due to recall and social desirability bias. Thus, participants may have over-reported the use of sterile needles. However, the fact that the behavioral data correspond to the serological data, and that participants reported low condom use and high drug paraphernalia sharing despite knowing that these behaviors were “undesirable,” renders these findings largely credible.
Table 4.
Social distance from opiate users among 39 young people who use drugs in St. Petersburg, Russia, 2016
| % | |
|---|---|
| Aversion to people who use opiates | |
| Very strong | 10 |
| Strong | 18 |
| Not very strong | 18 |
| A little bit | 13 |
| Not at all | 36 |
| Missing | 5 |
| Interact with people who use opiates | |
| Never | 23 |
| Rarely | 39 |
| Some of the time | 21 |
| Most of the time | 12 |
| All the time | 0 |
| Missing | 5 |
| Aversion to older people (> 30 years) who use opiates | |
| Very strong | 15 |
| Strong | 13 |
| Not very strong | 18 |
| A little bit | 13 |
| Not at all | 33 |
| Missing | 8 |
| Interact with older people (> 30 years) who use opiates | |
| Never | 56 |
| Rarely | 33 |
| Some of the time | 3 |
| Most of the time | 3 |
| All the time | 0 |
| Missing | 5 |
ACKNOWLEDGMENTS
We would like to acknowledge The Center for Drug Use and HIV Research (CDUHR) at New York University (Grant # P30DA011041). The article was prepared as a result of a research project “Complex Research on Evaluation of Quality of Life Related with Russian People’s Health and Behavior Patterns” supported by the National Research University Higher School of Economics, St. Petersburg in 2019.
REFERENCES
- 1.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2015. Stockholm: ECDC; 2016. [Google Scholar]
- 2.Burchell AN, Calzavara LM, Orekhovsky V, Ladnaya NN, Russian HIV Response Network. Characterization of an emerging heterosexual HIV epidemic in Russia. Sex Transm Dis. 2008. September;35(9):807–13. [DOI] [PubMed] [Google Scholar]
- 3.Kozlov AP, Skochilov RV, Toussova OV, Verevochkin SV, Krasnoselskikh TV, Malov SV, et al. HIV incidence and behavioral correlates of HIV acquisition in a cohort of injection drug users in St Petersburg, Russia. Medicine (Baltimore). 2016. November 4;95(44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrostat. Statistical bulletin “Wage in St. Petersburg and Leningrad oblast in January-December, 2016.” Saint-Petersburg: The Federal Service for State Statistics in St. Petersburg and Leningrad Oblast; 2017. [Google Scholar]
- 5.Heimer R, White E. Estimation of the number of injection drug users in St. Petersburg, Russia. Drug Alcohol Depend. 2010. June 1;109(1–3):79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes T, Platt L, Maximova S, Koshkina E, Latishevskaya N, Hickman M, et al. Prevalence of HIV, hepatitis C and syphilis among injecting drug users in Russia: a multi-city study. Addiction. 2006;101(2):252–266. [DOI] [PubMed] [Google Scholar]
- 7.Eritsyan K, Heimer R, Barbour R, Odinokova V, White E, Rusakova MM, et al. Individual-level, network-level and city-level factors associated with HIV prevalence among people who inject drugs in eight Russian cities: a cross-sectional study. BMJ Open. 2013. June 14;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruse GR, Barbour R, Heimer R, Shaboltas AV, Toussova OV, Hoffman IF, et al. Drug choice, spatial distribution, HIV risk, and HIV prevalence among injection drug users in St. Petersburg, Russia. Harm Reduct J. 2009. July 31;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niccolai LM, Verevochkin SV, Toussova OV, White E, Barbour R, Kozlov AP, et al. Estimates of HIV incidence among drug users in St. Petersburg, Russia: continued growth of a rapidly expanding epidemic. Eur J Public Health. 2011. October;21(5):613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toussova O, Shcherbakova I, Volkova G, Niccolai L, Heimer R, Kozlov A. Potential bridges of heterosexual HIV transmission from drug users to the general population in St. Petersburg, Russia: is it easy to be a young female? J Urban Health Bull N Y Acad Med. 2009. July;86 Suppl 1:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heimer R, Lyubimova A, Barbour R, Levina OS. Emergence of Methadone as a Street Drug in St. Petersburg, Russia. Int J Drug Policy. 2016. January;27:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uusküla A, Raag M, Vorobjov S, Rüütel K, Lyubimova A, Levina OS, et al. Non-fatal overdoses and related risk factors among people who inject drugs in St. Petersburg, Russia and Kohtla-Järve, Estonia. BMC Public Health. 2015. December 18;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cepeda JA, Niccolai LM, Lyubimova A, Kershaw T, Levina O, Heimer R. High-risk behaviors after release from incarceration among people who inject drugs in St. Petersburg, Russia. Drug Alcohol Depend. 2015. February 1;147:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdala N, Carney JM, Durante AJ, Klimov N, Ostrovski D, Somlai AM, et al. Estimating the prevalence of syringe-borne and sexually transmitted diseases among injection drug users in St Petersburg, Russia. Int J STD AIDS. 2003. October 1;14(10):697–703. [DOI] [PubMed] [Google Scholar]
- 15.Shaboltas AV, Toussova OV, Hoffman IF, Heimer R, Verevochkin SV, Ryder RW, et al. HIV prevalence, sociodemographic, and behavioral correlates and recruitment methods among injection drug users in St. Petersburg, Russia. J Acquir Immune Defic Syndr 1999. 2006. April 15;41(5):657–63. [DOI] [PubMed] [Google Scholar]
- 16.Niccolai LM, Toussova OV, Verevochkin SV, Barbour R, Heimer R, Kozlov AP. High HIV Prevalence, Suboptimal HIV Testing, and Low Knowledge of HIV-Positive Serostatus Among Injection Drug Users in St. Petersburg, Russia. AIDS Behav. 2010. August;14(4):932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heimer R, Eritsyan K, Barbour R, Levina OS. Hepatitis C virus seroprevalence among people who inject drugs and factors associated with infection in eight Russian cities. BMC Infect Dis. 2014. September 19;14(Suppl 6):S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golub A, Johnson BD, Dunlap E. Subcultural evolution and illicit drug use. Addict Res Theory. 2005;13(3):217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekker L-G, Hosek S. HIV and adolescents: focus on young key populations. J Int AIDS Soc. 2015. February 26;18(2Suppl 1). [Google Scholar]
- 20.Fennema JSA, Van Ameijden EJC, van Den Hoek A, Coutinho RA. Young and recent-onset injecting drug users are at higher risk for HIV. Addiction. 1997. November 1;92(11):1457–65. [PubMed] [Google Scholar]
- 21.WHO. HIV and young people who inject drugs: a technical brief. Geneva: Inter-Agency Working Group on Key Populations; 2015. p. 34. [Google Scholar]
- 22.Results from the HIV/STI Integrated Biolgoical and Behavioral Surveillance (IBBS) in Viet Nam 2005–2006. Ministry of Health - Viet Nam. Hanoi, Viet Nam; 2006.
- 23.Atkinson J, McCurdy S, Williams M, Mbwambo J, Kilonzo G. HIV risk behaviors, perceived severity of drug use problems, and prior treatment experience in a sample of young heroin injectors in Dar es Salaam, Tanzania. Afr J Drug Alcohol Stud. 2011;10(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Kissin DM, Zapata L, Yorick R, Vinogradova EN, Volkova GV, Cherkassova E, et al. HIV seroprevalence in street youth, St Petersburg, Russia. AIDS. 2007. November;21(17):2333–40. [DOI] [PubMed] [Google Scholar]
- 25.Kornilova MS, Batluk JV, Yorick RV, Baughman AL, Hillis SD, Vitek CR. Decline in HIV seroprevalence in street youth 2006–2012, St. Petersburg, Russia: moving toward an AIDS- free generation. Int J STD AIDS. 2017. March 1;28(4):345–56. [DOI] [PubMed] [Google Scholar]
- 26.EHRN. Young people and injecting drug use in selected countries of Central and Eastern Europe. Vilnius: Eurasian Harm Reduction Network; 2009. [Google Scholar]
- 27.Broz D, Pham H, Spiller M, Wejnert C, Le B, Neaigus A, et al. Prevalence of HIV Infection and Risk Behaviors Among Younger and Older Injecting Drug Users in the United States, 2009. AIDS Behav. 2014. April 1;18(3):284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cedarbaum ER, Banta-Green CJ. Health behaviors of young adult heroin injectors in the Seattle area. Drug Alcohol Depend. 2016. January 1;158:102–9. [DOI] [PubMed] [Google Scholar]
- 29.Kral AH, Lorvick J, Edlin BR. Sex-and drug-related risk among populations of younger and older injection drug users in adjacent neighborhoods in San Francisco. J Acquir Immune Defic Syndr 1999. 2000. June;24(2):162–7. [DOI] [PubMed] [Google Scholar]
- 30.Des Jarlais DC, Diaz T, Perlis T, Vlahov D, Maslow C, Latka M, et al. Variability in the Incidence of Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus Infection among Young Injecting Drug Users in New York City. Am J Epidemiol. 2003. March 1;157(5):467–71. [DOI] [PubMed] [Google Scholar]
- 31.UNICEF. Biological and behavioural survey among injection drug users. Bosnia and Herzegovina: UNICEF Bosnia and Herzegovina; 2007. [Google Scholar]
- 32.UNICEF Serbia. The estimation of the prevalence of HIV and viral hepatitis C infection, risk factors, risk behavior and the use of services among the population of young injecting drug users in Belgrade, Novi Sad and Nis. 2009. [Google Scholar]
- 33.Amundsen EJ, Bretteville-Jensen AL. Hard drug use in Norway. Nord Stud Alcohol Drugs.2010. February 1;27(1):87–94. [Google Scholar]
- 34.Creswell JW, Clark VLP. Designing and conducting mixed methods research. Sage publications; 2017. [Google Scholar]
- 35.Greene JC, Caracelli VJ, Graham WF. Toward a Conceptual Framework for Mixed-Method Evaluation Designs. Educ Eval Policy Anal. 1989;11(3):255–74. [Google Scholar]
- 36.Patton MQ. Qualitative evaluation and research methods. Thousands Oaks. CA: Sage Publication; 2002. [Google Scholar]
- 37.Heckathorn DD, Semaan S, Broadhead RS, Hughes JJ. Extensions of Respondent-Driven Sampling: A New Approach to the Study of Injection Drug Users Aged 18–25. AIDS Behav. 2002. March 1;6(1):55–67. [Google Scholar]
- 38.Abdul-Quader AS, Heckathorn DD, McKnight C, Bramson H, Nemeth C, Sabin K, et al. Effectiveness of respondent-driven sampling for recruiting drug users in New York City: findings from a pilot study. J Urban Health Bull N Y Acad Med. 2006. May;83(3):459–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karakayali N Social Distance and Affective Orientations. Sociol Forum. 2009;24(3):538–62. [Google Scholar]
- 40.Guest G, MacQueen KM, Namey EE. Applied the matic analysis. sage; 2011. [Google Scholar]
- 41.IBM Corp. IBM SPSS Statistics for Windows, version 22.0. Armonk, NY: IBM Corp.; 2013. [Google Scholar]
- 42.Mannheim K The problem of generations In: Kecskemeti P (Ed), Essays in the sociology of knowledge (pp 276–322). Boston: Routledge & Kegan Paul.; 1952. [Google Scholar]
- 43.Meylakhs P, Aasland A, Grønningsæter A “Until people start dying in droves, no actions will be taken”: perception and experience of HIV-preventive measures among people who inject drugs in northwestern Russia. Harm Reduct J. 2017. June 5;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uusküla A, Des Jarlais DC, Kals M, Rüütel K, Abel-Ollo K, Talu A, et al. Expanded syringe exchange programs and reduced HIV infection among new injection drug users in Tallinn, Estonia. BMC Public Health. 2011. June 30;11:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitek CR, Cakalo J-I, Kruglov YV, Dumchev KV, Salyuk TO, Božičević I, et al. Slowing of the HIV epidemic in Ukraine: evidence from case reporting and key population surveys, 2005–2012. PloS One. 2014;9(9):e103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman SR, Sandoval M, Mateu-Gelabert P, Meylakhs P, Des Jarlais DC. Symbiotic goals and the prevention of blood-borne viruses among injection drug users. Subst Use Misuse. 2011;46(2–3):307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meylakhs P, Friedman SR, Mateu-Gelabert P, Sandoval M, Meylakhs N. Taking care of themselves: how long-term injection drug users remain HIV and Hepatitis C free. Sociol Health Illn. 2015. May;37(4):626–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall W, Darke S, Ross M, Wodak A. Patterns of drug use and risk-taking among injecting amphetamine and opioid drug users in Sydney, Australia. Addict Abingdon Engl. 1993. April;88(4):509–16. [DOI] [PubMed] [Google Scholar]
- 49.Kozlov AP, Shaboltas AV, Toussova OV, Verevochkin SV, Masse BR, Perdue T, et al. HIV incidence and factors associated with HIV acquisition among injection drug users in St Petersburg, Russia. AIDS Lond Engl. 2006. April 4;20(6):901–6. [DOI] [PubMed] [Google Scholar]
- 50.Lorvick J, Martinez A, Gee L, Kral AH. Sexual and injection risk among women who inject methamphetamine in San Francisco. J Urban Health Bull N Y Acad Med. 2006. May;83(3):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braine N, Des Jarlais DC, Goldblatt C, Zadoretzky C, Turner C. HIV risk behavior among amphetamine injectors at U.S. syringe exchange programs. AIDS Educ Prev Off Publ Int Soc AIDS Educ. 2005. December;17(6):515–24. [DOI] [PubMed] [Google Scholar]
- 52.Van den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007. September 1;102(9):1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wodak A, Cooney A. Do needle syringe programs reduce HIV infection among injecting drug users: a comprehensive review of the international evidence. Subst Use Misuse. 2006;41(6–7):777–813. [DOI] [PubMed] [Google Scholar]
- 54.Reuter P Ten years after the United Nations General Assembly Special Session (UNGASS): assessing drug problems, policies and reform proposals. Addict Abingdon Engl. 2009. April;104(4):510–7. [DOI] [PubMed] [Google Scholar]
- 55.Friedman SR, Pouget ER, Chatterjee S, Cleland CM, Tempalski B, Brady JE, et al. Drug Arrests and Injection Drug Deterrence. Am J Public Health. 2011. February 1;101(2):344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedman SR, Tempalski B, Brady JE, West BS, Pouget ER, Williams LD, et al. Income inequality, drug-related arrests, and the health of people who inject drugs: Reflections on seventeen years of research. Int J Drug Policy. 2016. June 1;32:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Golub A, Brownstein HH. Drug Generations in the 2000s: An Analysis of Arrestee Data. J Drug Issues. 2013. July 1;43(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curtis R The Improbable Transformation of Inner-City Neighborhoods: Crime, Violence, Drugs, and Youth in the 1990s. J Crim Law Criminol. 1997 1998;88:1233. [Google Scholar]
- 59.Furst RTD Johnson B, Dunlap E, Curtis R the stigmatized image of the “crack head”: a sociocultural exploration of a barrier to cocaine smoking among a cohort of youth in New York City. Deviant Behav. 1999;20(2):153–181. [Google Scholar]
- 60.Golub AL, Johnson BD. Cohort changes in illegal drug use among arrestees in Manhattan: from the Heroin Injection Generation to the Blunts Generation. Subst Use Misuse. 1999. November;34(13):1733–63. [DOI] [PubMed] [Google Scholar]
- 61.Douglas M Purity and Danger. 1966. N Y Routedge; 2002; [Google Scholar]
- 62.Douglas M Risk and blame. Routledge; 2013. [Google Scholar]
- 63.Friedman SR, Des Jarlais DC, Jose B, Neaigus A, Goldstein M. Seroprevalence, Seroconversion, and the History of the HIV Epidemic among Drug Injectors In: HIV Epidemiology: Models and Methods. A. Nicolosi. New York: Raven Press; 1994. p. 137–50. [Google Scholar]
- 64.Friedman SR, Friedmann P, Telles P, Bastos F, Bueno R, Mesquita F, et al. New Injectors and HIV-1 risk In: Drug Injecting and HIV Infection: Global Dimensions and Local Responses. London: UCL Press; 1998. p. 76–90. [Google Scholar]
- 65.Bancroft A, Reid PS. Concepts of illicit drug quality among darknet market users: Purity, embodied experience, craft and chemical knowledge. Int J Drug Policy. 2016;35:42–9. [DOI] [PubMed] [Google Scholar]
- 66.Becker HS. Becoming a marihuana user. Am J Sociol. 1953;59(3):235–242. [Google Scholar]
- 67.Zinberg NE. Drug, set, and setting: The basis for controlled intoxicant use. Yale University Press; 1986. [Google Scholar]
- 68.Bourgois P Anthropology and epidemiology on drugs: the challenges of cross-methodological and theoretical dialogue. Int J Drug Policy. 2002. October 1;13(4):259–69. [Google Scholar]
- 69.Krug A, Hildebrand M, Sun N. “We don’t need services. We have no problems”: exploring the experiences of young people who inject drugs in accessing harm reduction services. J Int AIDS Soc. 2015. February 1;18:n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friedman SR, Maslow C, Bolyard M, Sandoval M, Mateu-Gelabert P, Neaigus A. Urging others to be healthy: “intravention” by injection drug users as a community prevention goal. AIDS Educ Prev Off Publ Int Soc AIDS Educ. 2004. June;16(3):250–63. [DOI] [PubMed] [Google Scholar]
- 71.Mateu-Gelabert P, Guarino H, Quinn K, Meylakhs P, Campos S, Meylakhs A, et al. Young Drug Users: a Vulnerable Population and an Underutilized Resource in HIV/HCV Prevention. Curr HIV/AIDS Rep. 2018. June 22; [DOI] [PMC free article] [PubMed] [Google Scholar]


