Abstract
Objectives
Peak beryllium inhalation exposures and exposure to the skin may be relevant for developing beryllium sensitization (BeS). The objective of this study was to identify risk factors associated with BeS to inform the prevention of sensitization, and the development of chronic beryllium disease (CBD).
Methods
In a survey of short-term workers employed at a primary beryllium manufacturing facility between the years 1994–1999, 264 participants completed a questionnaire and were tested for BeS. A range of qualitative and quantitative peak inhalation metrics and skin exposure indices were created using: personal full-shift beryllium exposure measurements, 15 min to 24 h process-specific task and area exposure measurements, glove measurements as indicator of skin exposure, process-upset information gleaned from historical reports, and self-reported information on exposure events. Hierarchical clustering was conducted to systematically group participants based on similarity of patterns of 16 exposure variables. The associations of the exposure metrics with BeS and self-reported skin symptoms (in work areas processing beryllium salts as well as in other work areas) were evaluated using correlation analysis, log-binomial and logistic regression models with splines.
Results
Metrics of peak inhalation exposure, indices of skin exposure, and using material containing beryllium salts were significantly associated with skin symptoms and BeS; skin symptoms were a strong predictor of BeS. However, in this cohort, we could not tease apart the independent effects of skin exposure from inhalation exposure, as these exposures occurred simultaneously and were highly correlated. Hierarchical clustering identified groups of participants with unique patterns of exposure characteristics resulting in different prevalence of BeS and skin symptoms. A cluster with high skin exposure index and use of material containing beryllium salts had the highest prevalence of BeS and self-reported skin symptoms, followed by a cluster with high inhalation and skin exposure index and a very small fraction of jobs in which beryllium salts were used. A cluster with low inhalation and skin exposure and no workers using beryllium salts had no cases of BeS.
Conclusion
Multiple pathways and types of exposure were associated with BeS and may be important for informing BeS prevention. Prevention efforts should focus on controlling airborne beryllium exposures with attention to peaks, use of process characteristics (e.g. the likelihood of upset conditions to design interventions) minimize skin exposure to beryllium particles, and in particular, eliminate skin contact with beryllium salts to interrupt potential exposure pathways for BeS risk.
Keywords: beryllium, peak exposure, risk management, sensitization, skin exposure
Introduction
Beryllium sensitization (BeS) is caused by exposure to beryllium, and elevated risk of BeS and chronic beryllium disease (CBD) has been reported for specific work processes in beryllium manufacturing and downstream processing facilities (Kreiss et al., 2007). While exposure–response relationships have been inconsistent, some studies have observed associations of BeS with metrics of average, highest-ever job, or task exposure (Balmes et al., 2014). The Occupational Safety and Health Administration (OSHA) recently promulgated a comprehensive occupational exposure standard for beryllium that lowered the permissible exposure limit to 0.2 µg/m3 and established a short-term exposure limit of 2 µg/m3 (DoL, 2017). For BeS, peak exposures may be particularly important because they may be sufficient to initiate immune sensitization which may induce lung inflammation ultimately resulting in CBD (NAS, 2008). While true short-duration exposure measurements are not widely available, studies have shown associations between BeS and surrogates of peak exposure such as highest-annual maximum task exposure (Henneberger et al., 2001), highest annual average full-shift job exposure (Schuler et al., 2012), or the 95th percentile of full-shift measurements in a year (Madl et al., 2007). Skin exposure to soluble beryllium salts is relevant for BeS and dermatitis (Curtis, 1951). In addition, skin exposure to poorly soluble particles has been hypothesized as relevant for BeS (Tinkle et al., 2003; Cummings et al., 2007), but formal epidemiological analyses with metrics of skin exposure have not been conducted as measurements are sparse and can be difficult to interpret (Day et al., 2006; Day et al., 2007; Kreiss et al., 2007; Armstrong et al., 2014).
A major challenge in exposure assessment for epidemiological studies is the conceptualization of biologically relevant exposure metric(s) and obtaining appropriate exposure data to construct these metric(s) (de Vocht et al., 2015). For BeS, the exposure metric is ideally based on the amount of beryllium available to interact with immune cells in the lungs or in the epidermis. In a simple model, beryllium particles deposited in the lungs or on the skin may undergo chemical dissolution in the fluid lining the lungs/phagocyte cells and in sweat respectively. In models using simulated lung fluids or sweat solution, it is estimated that over 1010 ions may be released within 24 h in the lungs (Stefaniak et al., 2003; Stefaniak et al., 2011b; Stefaniak et al., 2012), and 107–1014 ions per hour on the skin (Stefaniak et al., 2011b) depending on the form of beryllium. Beryllium ions and particles in contact with damaged or compromised skin (e.g. cuts, abrasions) may cross the skin barrier (Day et al., 2006). Once in the viable epidermis, beryllium ions may interact with the major histocompatibility complex class II molecules of the antigen-presenting cells such as dendritic cells (lung) or Langerhans cells (skin), with the appropriate form of the antigen being displayed for recognition by naïve CD4+ T-lymphocytes. The rate at which beryllium ions are produced would need to be sufficient to form ample antigen to activate the T-lymphocyte-mediated immune response (Viola and Lanzavecchia, 1996). The recognition by CD4+ T-lymphocytes leads to their activation, proliferation, and differentiation into beryllium-specific memory CD4+ T-cells, and to induction of BeS. In addition to the exposure and immune factors, genetic characteristics confer an increased susceptibility for BeS (Samuel and Maier, 2008). The foregoing suggests that short-duration beryllium exposure in the air or on the skin could be relevant measures for BeS; however, personal samples that capture these exposures are likely not available. Quantitative metrics based on full-shift measurements, or qualitative metrics based on events such as accidental exposures, summarized over longer time scales such as months or years may be relevant if they are correlated with the short-duration exposures.
Peak metrics are not commonly or consistently used in epidemiologic studies in part because the relevant characteristics of peaks vary depending on the kinetics of the exposure agent and the dynamics of the disease mechanism (Rappaport, 1985). Peaks can be thought of as arising from normal process variation (regular peaks) or from process upset conditions and non-routine operations (irregular peaks) (Bullock and Ignacio, 2006; Deubner, 2013). Regular peaks are more likely assessed using exposure data from routine monitoring, whereas irregular peaks from unplanned events or non-routine operations will likely be missed unless continuous monitoring of the workplace is conducted. Irregular peaks are more frequently assessed qualitatively from questionnaire surveys or during the investigation of an occupational illness. Quantitative peak metrics are ideally obtained from real-time measurements, however, often only full-shift time-integrated measurements are available to construct peak metrics. Skin exposure to chemicals is not commonly or consistently assessed and skin assessment methodology is not well developed (Stefaniak et al., 2011a). Hence, novel metrics that attempt to account for biological mechanisms must be created from available exposure data/information for use in epidemiologic studies.
In our previous work, we observed associations of BeS with average and highest ‘total’ (measurements collected using a 37-mm closed-face cassette) exposure metrics but not with cumulative exposure, and of CBD with cumulative respirable and ‘total’ exposure metrics, but not with the average exposure metric (Schuler et al., 2012). In the present study, we investigate the associations of peak inhalation metrics and skin exposure indices with BeS and self-reported skin symptoms. We focus on BeS as sensitization is prerequisite for the development of CBD, and identifying factors associated with BeS will enable improved prevention of BeS (and thus also CBD). The objectives of the study were to: (i) assess correlations among metrics of regular and irregular peak inhalation exposure and skin indices obtained from different types of data such as quantitative measurements, self-reports, and historical records of upset conditions; (ii) explore groupings of exposure metrics and workers based on common underlying characteristics; (iii) establish the association of BeS with exposure metrics and understand the shape of the exposure–response curve; and (iv) identify exposure and workplace factors that may be useful for managing exposures and BeS risk.
Methods
A cross-sectional epidemiologic survey for BeS was conducted in 1999 at a primary beryllium production facility processing beryllium salts, beryllium metal and alloys, and beryllium oxide. A sub-cohort of 264 short-term workers hired after 1 January 1994 was defined, which minimized exposure misclassification errors due to imprecise understanding of the timing of BeS onset. The survey included blood samples drawn to test for BeS, and a worker questionnaire with self-reported items on: start and end dates of each job held at the facility, including the processes and tasks performed and their location, frequency and timing of skin rashes and ulcers (in work areas processing beryllium salts as well as in other work areas), performance of shut-down maintenance, decontamination work, spill cleanups, and accidental exposure to high levels of airborne beryllium. Qualitative and quantitative exposure measures were summarized by job and location-task/process and were assigned to each participant based on their work history. The overall strategy was to select from each individual’s work history the maximum values of exposure metrics as indicators of peak exposure, which were then used in epidemiologic analysis. Details of the study population and survey methods have been previously described (Schuler et al., 2012; Virji et al., 2012). The study protocol was reviewed by the Institutional Review Board of the National Institute for Occupational Safety and Health (NIOSH), and written informed consent was obtained from each study participant.
Exposure metrics from quantitative measurements
A brief description of the exposure metrics created is described here, details of which are provided in Supplementary Material (available at Annals of Occupational Hygiene online). Quantitative exposure data used to create the peak inhalation metrics and skin indices came from three sources: (i) a targeted comprehensive (baseline) air sampling campaign conducted by the company over three months in 1999 which collected 4022 full-shift (typically 6–8 h) personal samples representative of standard work (Virji et al., 2011); (ii) ongoing surveillance (historical) air monitoring conducted by the company throughout the study period (1994–1999) which collected short-duration (mostly <60 min) task samples (n = 2593 or 3.3% of the historical data) from locations known to have higher or variable exposure, and longer-duration (mostly <24 h) general-area process samples (n = 77,046 or 96.7%) from all locations (Kolanz et al., 2001); and (iii) a sampling campaign conducted jointly by NIOSH and the company in 2007 that collected 323 cotton glove samples over multiple 2-h periods from 138 workers as a relative index of historical skin exposure (Armstrong et al., 2014). The baseline data are suitable for describing the typical exposure distribution for jobs, but are less likely to capture any irregular events or upset conditions because of infrequency of sample collection (Deubner, 2013).
Data from baseline sampling were summarized for each job (denoted with a subscript ‘b’) and are described in Supplementary Table S1 (available at Annals of Occupational Hygiene online), e.g. the 95th percentile point estimate (P95b). These peak exposure metrics were selected for the highest exposure over the entire work history and over the first year only, as well as exposure intensity associated with the longest duration jobs. In addition, exceedance metrics (nominal 0/1 variables) were created for summary measures exceeding 2 µg/m3 or the fraction of measurements above 2 µg/m3 exceeding 5%. Metrics reflecting the duration of time (in years) in jobs with highest exposures were also calculated to evaluate the role of time-related metrics in predicting BeS.
The historical air sampling data (surveillance data) are more likely to capture exposures associated with process upset conditions or non-routine tasks. Historical air sampling data for tasks and general-area were summarized separately for location-task/process for each quarter (denoted with a subscript ‘h’) and are described in Supplementary Table S1 (available at Annals of Occupational Hygiene online), e.g. the maximum measured concentration (Maxh). These data were summarized in a similar manner as described above for the baseline data.
Glove sampling data were summarized by selecting the mean and the maximum associated with a job as indices of skin exposure (Supplementary Table S1, available at Annals of Occupational Hygiene online). Historical jobs that were no longer performed in 2007 were assigned glove loading values of similar jobs by a panel of experts which included the company industrial hygienist (IH) and occupational medicine physician, and in consultation with four NIOSH IHs familiar with the processes. In addition, jobs were assigned a solubility characteristic (nominal 0/1 variable) that noted the presence or absence of beryllium salts (i.e. beryllium fluoride or ammonium beryllium fluoride) in the materials used (Virji et al., 2011). Note, the analytical method for quantifying beryllium in the air or on gloves measures total beryllium and cannot distinguish among the forms of beryllium that may be present in the air or on surfaces.
Metrics based on company records and self-reports of upset conditions
The company maintained monthly reports and/or quarterly summaries of exposure measurements and events which were available for production locations throughout the study period. Information was extracted for each location and quarter on the number of occurrences of specific events such as: leaks and upset conditions, ventilation or equipment failure and reportable spills, and instances of evacuations, and the sum of the number of events over the duration of employment were assigned to the work histories based on location, year and quarter (Supplementary Table S1, available at Annals of Occupational Hygiene online). Workers also provided responses to a survey questionnaire on the number of times they participated in shutdown maintenance, experienced high exposure due to unexpected events, and whether they ever performed decontamination of work areas, materials and products leaving the plant, or cleanup after spills and their self-reported exposure score (Supplementary Table S1, available at Annals of Occupational Hygiene online). The relevant questions from the questionnaire are presented in Supplementary Table S2 (available at Annals of Occupational Hygiene online).
Statistical analysis
Statistical analyses were conducted using SAS software version 9.3 and JMP software version 11.2.0 (SAS Institute, Inc., Cary, NC), and plots were prepared in SigmaPlot 12.5 (Systat Software Inc., San Jose, CA). Summary statistics and correlation coefficients (Spearman rho - ρ) were calculated, and distributions were explored via histograms and probability plots for the quantitative metrics. Hierarchical clustering was done to partition workers into groups that share a set of exposure characteristics that may impart different risk of BeS, such as different combinations of high or low inhalation or skin exposure, handling soluble versus poorly soluble beryllium, or having experienced certain very high-exposure events. Hierarchical clustering creates a single variable of groups of participants who share such similar exposure profiles across the different input variables. In this case, 16 variables including quantitative and qualitative inhalation and skin exposure metrics and use of materials containing beryllium salts, were offered as input variables. The inputs were standardized to minimize the effect of scale and outliers. Ward’s linkage method and Euclidean distance measure were used to estimate the similarity of input variables between clusters, and then to combine two most similar clusters at each step until all clusters belonged to one single cluster, forming a cluster tree (Friesen et al., 2015). A plot of the dendrogram (tree diagram) and constellation plots were used to visually select the number of clusters. Seven clusters were extracted from the cluster tree, which were summarized by worker, process, and exposure characteristics to aid in the interpretation of the clusters, which in turn may inform risk management. These characteristics included the 16 input variables in the clustering as well as additional variables not used in clustering such as prevalence of BeS and skin symptoms, the most common form of soluble beryllium material used (e.g. beryllium fluoride or ammonium beryllium fluoride), exceedance metrics (e.g. ExPcntb>2), company recorded or self-reported events, and the process in which most workers had their highest exposure.
Log-binomial regression models were used to explore exposure–response relationships for BeS and skin symptoms with log-transformed quantitative and qualitative metrics. As there are no known risk factors for BeS other than genetic factors, and our previous work did not find associations of BeS with age, sex, tenure, smoking status, or race, models were not adjusted for these factors (Schuler et al., 2012). Prevalence ratios (PR) with corresponding 95% confidence intervals (CI) were calculated using the GENMOD procedure in SAS (Coutinho et al., 2008). To assess any non-linearity in the relationships between BeS and quantitative peak metrics, a published SAS macro was used which fit logistic regression models with restricted cubic spline terms (Desquilbet and Mariotti, 2010). These models were specified with three to five knots (which join adjacent polynomials) based on the minimum Akaike Information Criteria (AIC) value, and the 5th percentile value for the exposure metrics was used as the reference value. The log-odds were plotted against exposure values to inspect for non-linear curves. To examine the contribution of the various exposure metrics (regular or irregular, inhalation, or skin exposure) on the risk of BeS, multiple log-binomial regression models were developed using two exposure metrics as predictor variables at a time. Binary variables for inhalation (>5% measurements exceeding 2 µg/m3), skin exposure (average glove measurement greater than median), and using material containing beryllium salts were combined to generate one composite variable with eight levels to explore their interaction. The composite variable and the cluster variable were also evaluated in the log-binomial regression models for BeS. Finally, to compare risk across processes in a normalized way, rates of BeS were calculated for processes as number of workers experiencing the event while ever having worked in a process per person-years of follow-up in that process (Cummings et al., 2007).
Results
As previously reported, the study population was mostly male (77.3%) and white (98.1%) with median age at hire of 31.4 years (range: 19.3–60 years) and median tenure at the time of survey of 20.9 months (range: 0.2–72.7 months) (Schuler et al., 2012). Of the 264 participants, 26 (9.8%) were sensitized and 84 (31.8%) reported any skin symptoms (skin rashes 31.1% and skin ulcers 8.3%). Prevalence of BeS was 19.1% among those who reported any skin symptoms (BeS prevalence values were 19.5% and 13.6% among those reporting rash and ulcers, respectively) compared to 5.6% among those who did not report any skin symptoms. Note that 6 of the 26 sensitized were also diagnosed with CBD; we did not exclude them from the analysis because all who are diagnosed with CBD are sensitized, by definition and blood test.
Histograms and probability plots of the quantitative metrics show lognormal distributions. The distributions of the historical metrics were more right-skewed and up to orders of magnitude greater than their corresponding baseline metrics (Table 1). Ranges of correlations within and across the different types of data are shown in Supplementary Table S3 (available at Annals of Occupational Hygiene online). There was a high degree of correlation (ρ s) within the baseline intensity metrics (ρ s: 0.73–0.96) especially among P95b, Pcntb>2 and Avgb, and between GSDb and Maxb. Intensity metrics from historical data were also highly correlated (ρ s: 0.62–0.94), especially among P95h, Pcnth>2 and Avgh, and between GSDh and P95h. Moderate correlations were observed across the baseline and historical datasets (ρ s: 0.47–0.72). Self-reported events were generally poorly correlated with all other metrics. Historical events had moderate correlation with historical intensity metrics, and less so with baseline intensity metrics, while glove measurements were moderately correlated with baseline intensity metrics. The longest job and the first year exposure intensity metrics followed a pattern similar to the baseline intensity metrics. All of the duration metrics were negatively correlated with the intensity metrics.
Table 1.
Summary statistics of selected exposure metrics for the participants.
| Peak Metric | Mean | SD | Median | P95 | Max | IQR |
|---|---|---|---|---|---|---|
| Baseline Metrics | ||||||
| P95b (µg/m3) | 8.5 | 26.9 | 1.6 | 15.7 | 141.4 | 0.3–5.6 |
| Pcntb>2 µg/m3 (%) | 12.6 | 16.8 | 3.1 | 46.2 | 69.2 | 0.6–17.6 |
| Avgb (µg/m3) | 2.1 | 6 | 0.6 | 3 | 31.7 | 0.1–1.9 |
| Maxb (µg/m3) | 19.2 | 50.3 | 5.1 | 48.1 | 254.2 | 2.2–14.6 |
| GSDb | 3.2 | 1.4 | 2.9 | 5.8 | 9.8 | 2.1–3.7 |
| Historical Metrics | ||||||
| P95h (µg/m3) | 114.2 | 297.7 | 4.2 | 726.4 | 1552.5 | 2.1–40.7 |
| Pcnth>2 µg/m3 (%) | 30.1 | 34.8 | 14.0 | 100 | 100 | 0–52.5 |
| Avgh (µg/m3) | 10.3 | 22.5 | 1.4 | 67.1 | 187.9 | 0.5–6.4 |
| Maxh (µg/m3) | 111.7 | 128.5 | 40.1 | 375 | 377.9 | 0.9–250.4 |
| GSDh | 7.3 | 5.5 | 4.5 | 18.2 | 32.8 | 4.4–7.8 |
| Skin Metrics (µg/h) | ||||||
| GloveMax | 8839 | 13,557 | 546 | 26,500 | 67,500 | 135–17,500 |
| GloveAvg | 1715 | 3066 | 318 | 6798 | 17,224 | 57–2649 |
| Historical Events (total # events) | ||||||
| Evacuations | 41.9 | 64.3 | 3 | 171 | 348 | 0–74.5 |
| Leaks | 28.7 | 45.4 | 7 | 123 | 284 | 0–40 |
| Spills | 3 | 4.6 | 0 | 13 | 25 | 0–5 |
| Any Events | 31.8 | 48.9 | 7.5 | 134 | 305 | 0–45 |
| Self-Reports (# times during tenure) | ||||||
| Any Skin | 7.3 | 41.5 | 0 | 15 | 440 | 0–1 |
| Shutdown Maintenance | 1.4 | 2.5 | 1 | 5 | 30 | 0–2 |
| Accidental Exposure | 4.9 | 20.8 | 0 | 24 | 250 | 0–1 |
IQR = interquartile range; Max = maximum value; P95 = lognormal based 95th percentile point estimate; SD = standard deviation.
Hierarchical cluster analysis generated a dendrogram and a constellation plot displaying the formation of the seven extracted clusters (Supplementary Fig. S1, available at Annals of Occupational Hygiene online). The characteristics of the clusters with regard to the input variables and other variables not used in clustering are displayed in Table 2, and a simplified table is presented in Supplementary Table S4 (available at Annals of Occupational Hygiene online). Cluster 1 is characterized by workers in administration, who had the lowest exposure levels (e.g. GloveAvg, P95b), the lowest prevalence of skin symptoms, jobs with no salt exposure, and with no cases of BeS. Cluster 2 is similar to cluster 1 with slightly higher levels for the exposure metrics, no jobs with salt exposure with moderate prevalence of skin symptoms and some BeS. Clusters 3 and 5 were very similar and had mid-range prevalence of BeS and skin symptoms, no jobs with salt exposure and mid-range peak inhalation exposure. Cluster 3 had higher value for the index of skin exposure and higher prevalence of skin symptoms, while cluster 5 had higher number of historical events. Cluster 4 had the highest prevalence of skin symptoms, BeS and jobs with salt exposure (from metal extraction), the highest value for the index of skin exposure but moderate levels of inhalation exposure and moderate to high historical events. Cluster 6 had the third highest prevalence of skin symptoms with low BeS, moderate skin exposure index, and high levels of historical exposures and events. Cluster 7 had the second highest prevalence of BeS and skin symptoms and almost no jobs with salt exposure (in powder metal production), the highest peak inhalation exposures, and the second highest value for the index of skin exposure. Clusters exhibited different exposure characteristics leading to different prevalence of BeS and skin symptoms.
Table 2.
Summary of the clusters by input and other variables not used in clustering.
| Variable | C1: Ref. (n = 43) |
C2: Low exposure (n = 58) |
C3: Moderate exposure (n = 74) |
C4: Salt and skin exposure (n = 31) |
C5: Moderate exposure (n = 20) |
C6: Historic events (n = 26) |
C7: High air and skin (n = 12) |
|---|---|---|---|---|---|---|---|
| Variables included in clustering | |||||||
| Beryllium Salt Material (0/1) | 0.00 | 0.00 | 0.00 | 0.90 | 0.00 | 0.19 | 0.08 |
| GloveAvg (µg/h) | 217 | 423 | 1733 | 6835 | 542 | 1453 | 2505 |
| P95b (µg/m3) | 0.18 | 1.13 | 3.82 | 5.19 | 2.84 | 6.23 | 127 |
| Pcntb>2 µg/m3 (%) | 0.28 | 3.58 | 14.6 | 15.0 | 8.89 | 22.4 | 66.6 |
| Maxb (µg/m3) | 1.84 | 5.32 | 9.89 | 18.1 | 7.71 | 11.6 | 244 |
| GSDb | 1.9 | 2.6 | 3.2 | 4.0 | 2.8 | 3.5 | 7.8 |
| P95h (µg/m3) | 2.30 | 7.36 | 22.0 | 49.4 | 45.7 | 940 | 93.7 |
| Pcnth>2 µg/m3 (%) | 1.35 | 9.47 | 27.5 | 45.7 | 45.6 | 87.3 | 59.3 |
| Maxh (µg/m3) | 9.48 | 9.91 | 138 | 145 | 225 | 281 | 169 |
| GSDh | 4.2 | 4.8 | 5.5 | 7.9 | 6.3 | 21 | 11 |
| Evacuations (# events) | 3.91 | 0.66 | 29.3 | 55.8 | 170 | 102 | 74.8 |
| Leaks (# events) | 1.14 | 0.50 | 18.5 | 62.8 | 84.7 | 69.3 | 57.8 |
| Spills (# events) | 0.14 | 0.10 | 3.03 | 4.23 | 10.7 | 6.38 | 4.83 |
| Shutdown Maintenance (# events) | 0.23 | 0.93 | 1.27 | 2.29 | 2.30 | 2.81 | 1.25 |
| Decontaminate Area (0/1) | 0.00 | 1.00 | 0.93 | 0.90 | 1.00 | 0.92 | 1.00 |
| Decontaminate Products (0/1) | 0.00 | 0.00 | 0.55 | 0.55 | 0.30 | 0.42 | 0.25 |
| Variables not included in clustering | |||||||
| BeS (%) | 0.00 | 8.62 | 10.8 | 22.6 | 10.0 | 7.69 | 16.7 |
| Skin Symptoms (%) | 6.98 | 15.5 | 34.4 | 77.4 | 25.0 | 42.3 | 66.7 |
| GloveAvg (% >median) | 9.3 | 24.1 | 66.2 | 100 | 60.0 | 42.3 | 100 |
| Pcntb>2 µg/m3 (% >5) | 0.0 | 22.4 | 55.4 | 93.5 | 50.0 | 96.2 | 100 |
| Pcnth>2 µg/m3 (% >5) | 7.0 | 32.8 | 70.3 | 90.3 | 100 | 100 | 100 |
| Shutdown Maintenance (%) | 7.0 | 51.7 | 54.1 | 80.6 | 90.0 | 69.2 | 58.3 |
| Any Events (# events) | 1.3 | 0.6 | 22 | 67 | 95 | 76 | 63 |
| Most Common Be Material | Be PF | Be MWF | Be PF | BeF2/NH42BeF4 | Be MWF | BeF2 | BeF2/NH42BeF4 |
| Most Common Process for Highest Air and Glove Metric | Admin. | P. Ops. | P. Ops./R.R. | Pebbles | P. Ops. | P. Ops. | PMP |
Admin. = administration; Be PF = beryllium in process fluids; Be MWF = beryllium in metalworking fluids; BeF2 = beryllium fluoride; NH42BeF4 = ammonium beryllium fluoride; P.Ops. = primary operations; R.R. = resource recovery; Pebbles = beryllium extraction; PMP = powder metal production.
The associations of each of the metrics with BeS and skin symptoms were evaluated in single variable log-binomial regression models and the PR and their 95% CI are presented in Fig. 1 and Supplementary Table S5 (available at Annals of Occupational Hygiene online). Baseline intensity metrics representing peaks from normal process variations were significantly associated with BeS (except Pcntb>2, GSDb) and skin symptoms (except Pcntb>2) with narrow confidence intervals and a strong signal to noise ratio (β/SE(β)). Only Maxh of the historical intensity metrics representing irregular peaks was significantly associated with BeS with a strong signal to noise ratio and the smallest AIC value; historical intensity metrics were significantly associated with skin symptoms (except Pcnth>2, GSDh). Using process material containing beryllium salts and average glove loading were significantly associated with both BeS and skin symptoms. Historical events and self-reported events had elevated PRs, which were significantly associated with skin symptoms but not with BeS; the lower CI for some of these metrics approached 1 and the signal to noise ratios were close to those observed for the intensity metrics. The exposure intensity metrics for jobs in the first-year and the longest duration jobs were not associated with BeS, had poorer fit (larger AIC values) and weaker signal to noise ratios compared to their corresponding highest exposure jobs with full tenure metrics (Supplementary Tables S6 and S7 (available at Annals of Occupational Hygiene online)). The duration metrics associated with the highest exposure jobs were inversely associated with BeS, albeit non-significant with poorer fit and weaker signal to noise ratios (Supplementary Table S8, available at Annals of Occupational Hygiene online).
Figure 1.
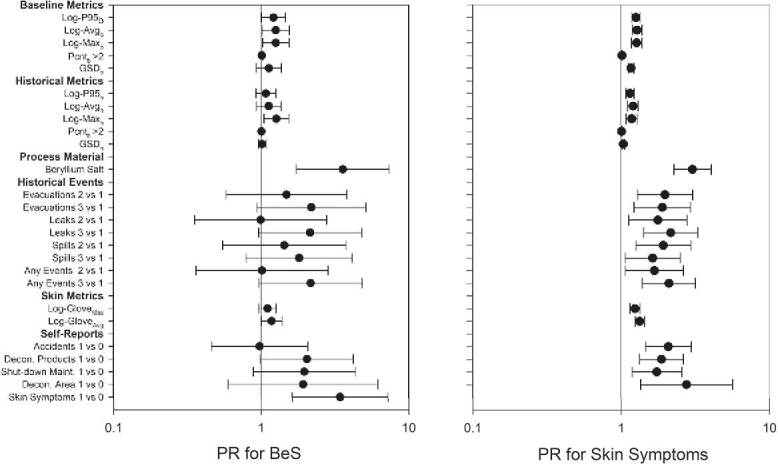
Prevalence ratios for BeS and skin symptoms with metrics of peak and skin exposures from multiple types of data and sources of information.
Non-linear exposure–response relationships were examined for quantitative baseline (P95b, Avgb, Maxb), historical (P95h, Avgh, Maxh), and skin indices (GloveAvg, GloveMax) for BeS. Only the splines for Avgb and P95b showed significant or marginally significant non-linear associations with BeS, as displayed in Supplementary Figs S2 and S3 (available at Annals of Occupational Hygiene online); however, both metrics had similar AIC values (168.8 and 170.6), which were similar to linear models using log-transformed exposure variables.
In multiple log-binomial regression models for BeS with two variables, i.e. regular and irregular peak metrics, inhalation and skin exposure metric, or exposure metrics and jobs with salt exposure, only one or none of the two metrics remained significant, in part due to correlation among metrics (Table 3). The models with salt exposure and historical inhalation exposure had the best fit and signal to noise ratio of all models, with the CI excluding 1 for the salt variable, and almost at 1 for the historical exposure variable. The combination of the inhalation (historical or baseline), skin and salt exposure variables yielded a composite variable with values in six out of the possible eight levels, with the prevalence of BeS ranging from 6 to 25% (excluding one level with only two workers, one of whom was sensitized) (Supplementary Table S9, available at Annals of Occupational Hygiene online). The six levels were further combined into three levels reflecting a low exposure group, a group with high inhalation exposure and variable glove/salt exposure, and a final group with salt and high glove exposure and variable inhalation exposure (Supplementary Table S9, available at Annals of Occupational Hygiene online). In the log-binomial model of the three-level combination variable, compared to level one, the PRs for level three (i.e. high glove and salt exposure) were significantly elevated, whereas the PRs for level two were not in models using the historical or baseline inhalation exposures. The log-binomial model for BeS with the cluster variable showed the highest PR of 4.561 (CI: 1.557–13.36) for cluster 4, and elevated PRs for the rest of the clusters albeit not significant, compared to the combined clusters 1 and 2 as reference. Note that cluster 1 had no cases of BeS, and was thus combined with cluster 2 as the reference group.
Table 3.
Prevalence ratios for BeS associated with multiple exposure metrics.
| Exposure Metric | PR | 95% CI | β/SE(β) | AIC | Correlation |
|---|---|---|---|---|---|
| Regular and Irregular Metrics | |||||
| Maxb (µg/m3) | 1.126 | 0.874–1.119 | 0.92 | 167.9 | 0.53 |
| Maxh (µg/m3) | 1.211 | 0.973–1.507 | 1.71 | ||
| Maxb (µg/m3) | 1.221 | 0.969–1.534 | 1.69 | 171.9 | 0.44 |
| Evacuations 2 vs. 1 | 1.053 | 0.384–2.892 | 0.10 | ||
| 3 vs. 1 | 1.592 | 0.638–3.974 | 1.00 | ||
| Maxh (µg/m3) | 1.337 | 1.030–1.737 | 2.17 | 169.7 | 0.72 |
| Evacuations 2 vs. 1 | 0.600 | 0.193–1.875 | −0.89 | ||
| 3 vs. 1 | 0.840 | 0.283–2.492 | −0.31 | ||
| Maxb (µg/m3) | 1.218 | 0.977–1.518 | 1.76 | 169.3 | 0.29 |
| Decontaminate Products | 1.690 | 0.805–3.545 | 1.39 | ||
| Maxh (µg/m3) | 1.232 | 1.009–1.505 | 2.05 | 167.3 | 0.33 |
| Decontaminate Products | 1.558 | 0.740–3.281 | 1.17 | ||
| Maxb (µg/m3) | 1.225 | 0.982–1.528 | 1.82 | 169.7 | 0.34 |
| Shutdown Maintenance | 1.614 | 0.718–3.626 | 1.16 | ||
| Maxh (µg/m3) | 1.235 | 1.011–1.508 | 2.06 | 167.8 | 0.31 |
| Shutdown Maintenance | 1.481 | 0.652–3.366 | 0.94 | ||
| Inhalation and Skin Metrics | |||||
| Maxb (µg/m3) | 1.171 | 0.901–1.524 | 1.18 | 170.9 | 0.67 |
| GloveAvg (µg/h) | 1.100 | 0.898–1.348 | 0.92 | ||
| Maxh (µg/m3) | 1.226 | 0.996–1.510 | 1.92 | 167.5 | 0.44 |
| GloveAvg (µg/h) | 1.107 | 0.923–1.327 | 1.10 | ||
| GloveAvg (µg/h) | 1.160 | 0.970–1.388 | 1.63 | 171.9 | 0.39 |
| Evacuations 2 vs. 1 | 1.096 | 0.408–2.949 | 0.18 | ||
| 3 vs. 1 | 1.729 | 0.721–4.143 | 1.23 | ||
| GloveAvg (µg/h) | 1.137 | 0.955–1.353 | 1.44 | 170.1 | 0.36 |
| Decontaminate Products | 1.627 | 0.752–3.523 | 1.23 | ||
| GloveAvg (µg/h) | 1.148 | 0.965–1.367 | 1.57 | 170.4 | 0.36 |
| Shutdown Maintenance | 1.575 | 0.691–3.591 | 1.08 | ||
| Inhalation or Skin Metrics and Salt | |||||
| Maxb (µg/m3) | 1.161 | 0.914–1.475 | 1.23 | 165.1 | 0.40 |
| Be Salt Material | 2.860 | 1.304–6.270 | 2.62 | ||
| Maxh (µg/m3) | 1.212 | 0.988–1.487 | 1.85 | 162.7 | 0.26 |
| Be Salt Material | 2.745 | 1.305–5.773 | 2.66 | ||
| GloveAvg (µg/h) | 1.049 | 0.869–1.267 | 0.50 | 166.3 | 0.47 |
| Be Salt Material | 3.057 | 1.197–7.810 | 2.34 | ||
| Combination Variable For Air*, Skin and Salt | |||||
| High Baseline Air: 2 vs. 1 | 1.061 | 0.419–2.688 | 0.12 | 166.6 | - |
| High Skin, Salt: 3 vs. 1 | 3.706 | 1.496–9.183 | 2.83 | ||
| High Historical Air: 2 vs. 1 | 1.451 | 0.490–4.296 | 0.67 | 166.1 | - |
| High Skin, Salt: 3 vs. 1 | 4.699 | 1.557–14.18 | 2.75 | ||
| Hierarchical Clustering Variable | |||||
| Cluster 1 and 2 (Ref) | - | - | - | 173.5 | - |
| Cluster 3 | 2.184 | 0.774–6.407 | 1.42 | ||
| Cluster 4 | 4.561 | 1.557–13.36 | 2.77 | ||
| Cluster 5 | 2.020 | 0.421–9.691 | 0.88 | ||
| Cluster 6 | 1.554 | 0.319–7.560 | 0.55 | ||
| Cluster 7 | 3.367 | 0.731–15.50 | 1.56 | ||
Spearman correlation; *Exceedance for historical and baseline air.AIC = Akaike Information Criteria value; 95% CI = 95% confidence intervals; β/SE(β) = signal to noise ratio; PR = prevalence ratio.
The rates (#events/person-years) of BeS are displayed in Table 4, and were similar across the processes; the order of processes is different from the previously reported order based on prevalence not accounting for person-year of follow-up in each process (Schuler et al., 2012). The highest rate of BeS was observed in maintenance workers followed by metal production in pebbles plant.
Table 4.
Rate of BeS by process.
| Process | Person-years | Rate (95% CI) |
|---|---|---|
| All Maintenance | 52.1 | 0.154 (0.081–0.291) |
| Pebbles Plant | 49.7 | 0.141 (0.071–0.280) |
| Bulk Products | 31.1 | 0.129 (0.052–0.322) |
| Strip Operations | 31.5 | 0.127 (0.051–0.317) |
| All Facilities | 34.3 | 0.117 (0.047–0.294) |
| Powder Metal Products | 28.5 | 0.105 (0.036–0.306) |
| Resource Recovery | 18.3 | 0.109 (0.029–0.403) |
| Primary Operations and Extrusion | 168.6 | 0.095 (0.060–0.151) |
| Machine Shop | 9.6 | - |
| Administration with Time in Plant | 65.2 | - |
| Quality-Assurance/Control and Research and Development | 23.6 | - |
Rate = number of workers experiencing an event per person years; 95% CI = 95% confidence interval.
Discussion
The data reported here represent historical workplace conditions, before the implementation of a redesigned comprehensive prevention program starting in 2000, which included targeted measures to reduce inhalation and skin exposure through enhanced engineering controls and greater use of personal protective equipment (PPE) and clothing, improved housekeeping, minimizing the migration of beryllium from work areas and additional health and safety and work practice training (Deubner and Kent, 2007). As such, the exposure–response relationships are assumed to represent the historical exposures received by workers, and for the most part are not affected by PPE use. While PPE use was required and documented for the performance of some short-term high-exposure tasks, PPE use was not systematically documented for all other instances of being in a process area or performing a job, and was thus not used to adjust worker exposure estimates.
These data presented a unique opportunity to explore the different types of peak metrics and examine their utility in relation to BeS and skin symptoms. Significant linear associations for BeS and skin symptoms were observed with multiple peak metrics representing both regular and irregular peaks, skin exposure index, and use of material containing beryllium salts. The company recorded historical event metrics and some of the self-reported event metrics also showed elevated PRs, and the lower confidence limit approached 1 for some of these metrics, with the signal to noise ratio for the effect measure close to 2. The moderate to high correlation among these metrics suggests that multiple types of metrics may be useful in predicting BeS or skin symptoms. With a larger population size, these types of peak metrics would be useful predictors of BeS. However no one single metric stood out as the best predictor of BeS. Hierarchical clustering revealed multiple exposure characteristics dominating in the different clusters. Cluster 4 had the highest prevalence of BeS and skin symptoms, and exposure characteristics included high skin exposure index, high prevalence of jobs with salt exposure, and moderate levels of inhalation exposure, thus implicating the role of skin pathway for salt exposure in BeS. Cluster 7 had the second highest prevalence of BeS and skin symptoms, with exposures characterized by the high peak inhalation and skin exposures and very low prevalence of jobs with of salt exposure, suggesting a role of inhalation/skin exposures in absence of jobs with salt exposure. However, no cluster reflected high skin exposure, low inhalation exposure, and jobs with no salt exposure, which would have enabled the separation of the skin from inhalation pathway. Thus the risk of BeS in this cohort is characterized by the combination of inhalation and skin exposure, and the influence of jobs with salt exposure and upset events, which cannot be disentangled to evaluate their independent effects. Risk management may be best achieved by simultaneously addressing all relevant aspects of exposure.
The importance of skin exposure to beryllium salts in initiating sensitization has been known for decades (Curtis, 1951) and the potential for poorly soluble beryllium particles to initiate sensitization has been demonstrated in an animal study (Tinkle et al., 2003) and hypothesized in case descriptions of workers (Cummings et al., 2007). A range of skin lesions including allergic or irritant contact dermatitis, chemical ulcers and ulcerating granulomas can occur as result of skin exposure to soluble beryllium salts. Dermal granulomas can result from subcutaneous instillation of beryllium metal or oxide, or (historically) beryllium-containing phosphor in fluorescent lamps (Epstein, 1991). However, epidemiological studies of the association between skin exposure and BeS or skin symptoms are lacking largely because of the absence of clearly recognized skin symptoms for forms other than beryllium salts and the related paucity of skin exposure measurements. In this study, we used glove measurements as a crude relative index of potential for skin exposure. Skin exposure was significantly associated with self-reported skin symptoms and self-reported skin symptoms were a significant predictor of BeS, supporting the relevance of the dermal pathway for BeS. However, multiple log-binomial regression models including both inhalation and skin exposure metrics were not able to tease apart the independent effects of skin exposure from inhalation exposure, as these exposures often occurred simultaneously and are correlated (Armstrong et al., 2014). In recognition of the potential relevance of all pathways of exposure, the company implemented an enhanced comprehensive preventive program after the reduction of inhalation exposures through engineering controls in the mid-late 1990s failed to lower the prevalence of sensitization; the comprehensive preventive program included controlling both inhalation and skin exposures along with minimizing the migration of beryllium from contaminated surfaces (Deubner and Kent, 2007; Knudson and Kolanz, 2009). Surveys of workers hired after the implementation of the enhanced program in 2000 at a beryllium metal, alloy and oxide manufacturing facility, a ceramics oxide facility and a copper-beryllium alloy processing facility showed a marked reduction in prevalence of sensitization when compared with workers hired before the enhanced program (Cummings et al., 2007; Thomas et al., 2009; Bailey et al., 2010).
Consistent with the previously observed non-linear trend in quartiles of exposure categories (Schuler et al., 2012), the association between BeS and baseline metrics showed significant non-linear relationships (via spline regression) represented by a rapid increase in log-prevalence odds for BeS starting from the lowest exposure levels, and then flattening out or rising slowly. This shape may have resulted from confounding by beryllium salt exposure or effect modification by genetic predisposition. The shape of these curves also suggests that log-transforming exposure metrics, which stretches out the lower end and draws in the higher end of the curves, can result in linear associations, which is desirable as it facilitates ease of interpretation and prediction of outcomes. In log-binomial models, log-transformed inhalation metrics showed significant linear association with BeS. On the other hand, log-transformation can dampen the steep rise in the risk of BeS at lower concentrations leading to possible overestimation of the no-effect or low-effect levels.
Both the study design and selection of exposure metrics have strengths and limitations. The time from hire to the date BeS was identified (survey/test date) includes some irrelevant exposure time, as we do not know when the individual actually became sensitized. By limiting the study population to short-term workers (i.e. those with 6 years or less since first exposure; median tenure of 20.9 months), we were able to limit exposure misclassification and increase the plausibility of the exposure–response relationships we identified. However, limiting work tenure also necessarily limited the number of study participants. Since our intent was to understand risk factors associated with BeS, we retained in our sensitized group those who had also been diagnosed with CBD. BeS is an immune response and is necessary for development of CBD; that is, all with CBD were first sensitized. Thus, it is unnecessary to separate those workers who had progressed to develop CBD from the BeS group in order evaluate the relationship between exposure and BeS.
Another challenge was addressing peak exposure in the absence of real-time exposure data. Metrics based on baseline exposure measurements were personal measurements with a large sample size per job title (n = 15) to adequately characterize personal exposure, but were collected for a full-shift which does not capture short-duration exposures, were not corrected for changes in exposure over time, and were collected and summarized over the 3-month sampling period. Metrics from the historical data included short-duration task samples collected near the source or the breathing zone but were very sparse (3.3%), and the longer-duration process samples were plentiful (n = 77,046) but do not represent personal exposure and were not collected equally across all locations. These measurements were summarized over a quarter and incorporated changes in exposure over time, but only 20% of the location-task/process and quarter combinations could be assigned a specific summary value. For the remaining 80% of the cells, higher-level summaries (i.e. annual or overall for a process) were successively applied until all the cells had estimates as described in the Supplementary Material (available at Annals of Occupational Hygiene online), which likely resulted in errors with each successive higher-level summary assignment. Mobile workers such maintenance workers were assigned general-area exposures which likely underestimated their exposures; however, maintenance workers were most likely to wear respiratory protection during higher exposure tasks. Glove measurements collected in a 2007 survey were used as crude indices to approximate skin exposure in the 1990s and were imprecise or inaccurate due to limitations of the sampling method, and may not reflect conditions at the time employees developed BeS. The company records of historical events were assigned to a worker if their work history indicated a job conducted in an area and during a quarter in which an event occurred. This does not account for the fact that a worker may have been absent during the event, that workers in adjacent processes may also have been exposed during the event, or that maintenance workers may have been called in to fix the problem. In calculating the rate of BeS, a case was attributed to all the processes worked by the affected worker because the timing of sensitization was unknown, leading to errors in the rate calculated for each process. Given the known role of skin exposure to beryllium salts in development of rashes and ulcers, we attributed these self-reported symptoms to beryllium exposure based on the modeling results, but cannot rule out that other industrial exposures as the putative agent.
Conclusion
Our results show that multiple peak inhalation metrics were important for understanding BeS. Multiple pathways and types of exposure such as regular or irregular peak inhalation, skin exposure, upset conditions, and exposure to beryllium salts were associated with BeS, but these factors occur together and it was not possible to distinguish the effect of each factor based on the characteristics of this cohort. Hierarchical clustering identified groups of participants with unique patterns of exposure characteristics resulting in different prevalence of BeS and skin symptoms. The cluster with high prevalence of jobs with salt exposure, high skin exposure index and moderate inhalation exposure had the highest prevalence of BeS and skin symptoms. The cluster with the second highest prevalence of BeS and skin symptoms had high inhalation and skin exposure index and very few jobs with salt exposure. Cluster 1, comprising mostly administrative workers, had the lowest average skin and inhalation exposures, no jobs with salt exposure and no sensitization. These results suggest that interventions should focus on reducing exposures to the lowest technically feasible level through engineering controls, changing work practices, and the use of PPE when required. Maintenance work was associated with the highest rate of BeS; however, the nature of this work is highly variable and necessitates the use of PPE as an integral part of an exposure control strategy, which starts with consideration of engineering and administrative controls. To focus prevention efforts, risk management should minimize skin exposure to beryllium particles, in particular eliminate skin contact with beryllium salts, control airborne beryllium exposure with attention to peaks, and use process characteristics, such as the likelihood of upset conditions or presence of salt, to design interventions to interrupt potential exposure pathways for BeS risk.
Disclaimer
Mention of a specific product or company does not constitute endorsement by the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention. One author Michael S Kent is employed by Materion Brush Inc., the owner of the beryllium manufacturing facility described in this report. The rest of the authors declare no conflict of interest relating to the material presented in this Article.
Funding
This work was funded through the National Institute for Occupational Safety and Health intramural research grant.
Supplementary Material
Acknowledgements
The authors thank Dr. Douglas Johns from NIOSH and Dr. David Kriebel from the University of Massachusetts, Lowell for their review of this manuscript, Dr. Gregory Day for conducting the 2007 exposure survey and Nicole Edwards for data management.
References
- Armstrong JL, Day GA, Park JYet al. (2014) Migration of beryllium via multiple exposure pathways among work processes in four different facilities. J Occup Environ Hyg; 11: 781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RL, Thomas CA, Deubner DCet al. (2010) Evaluation of a preventive program to reduce sensitization at a beryllium metal, oxide, and alloy production plant. J Occup Environ Med; 52: 505–12. [DOI] [PubMed] [Google Scholar]
- Balmes JR, Abraham JL, Dweik RAet al. (2014) An official American Thoracic Society statement: diagnosis and management of beryllium sensitivity and chronic beryllium disease. Am J Respir Crit Care Med; 190: e34-59. [DOI] [PubMed] [Google Scholar]
- Bullock WH, Ignacio JS. (2006) A strategy for assessing and managing occupational exposures. 3rd edn. Fairfax, VA: AIHA Press. ISBN: 1-931504-69-5.
- Coutinho LM, Scazufca M, Menezes PR. (2008) Methods for estimating prevalence ratios in cross-sectional studies. Rev Saude Publica; 42: 992–8. [PubMed] [Google Scholar]
- Cummings KJ, Deubner DC, Day GAet al. (2007) Enhanced preventive programme at a beryllium oxide ceramics facility reduces beryllium sensitisation among new workers. Occup Environ Med; 64: 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis GH. (1951) Cutaneous hypersensitivity due to beryllium; a study of thirteen cases. AMA Arch Derm Syphilol; 64: 470–82. [DOI] [PubMed] [Google Scholar]
- Day GA, Dufresne A, Stefaniak ABet al. (2007) Exposure pathway assessment at a copper-beryllium alloy facility. Ann Occup Hyg; 51: 67–80. [DOI] [PubMed] [Google Scholar]
- Day GA, Stefaniak AB, Weston Aet al. (2006) Beryllium exposure: dermal and immunological considerations. Int Arch Occup Environ Health; 79: 161–4. [DOI] [PubMed] [Google Scholar]
- Desquilbet L, Mariotti F. (2010) Dose-response analyses using restricted cubic spline functions in public health research. Stat Med; 29: 1037–57. [DOI] [PubMed] [Google Scholar]
- Deubner D, Kent M. (2007) Keeping beryllium workers safe: an enhanced preventive model. J Occup Environ Hyg; 4: D23–30. [DOI] [PubMed] [Google Scholar]
- Deubner DC. (2013) Implications of common and special variation for occupational health: examples from beryllium manufacturing. J Occup Environ Med; 55: 839–45. [DOI] [PubMed] [Google Scholar]
- DoL (2017) Occupational exposure to beryllium: final rule - 29 CFR parts 1910, 1915, 1926. Fed Regist; 82: 2470–757. [PubMed] [Google Scholar]
- Epstein WL. (1991) Cutaneous effects of beryllium. In Rossman MD, Preuss OP, Powers MD, editors. Beryllium biomedical and environmental aspects. Baltimore, MD: Williams and Wilkins. pp. 113-7. ISBN: 0-683-07387-7. [Google Scholar]
- Friesen MC, Shortreed SM, Wheeler DCet al. (2015) Using hierarchical cluster models to systematically identify groups of jobs with similar occupational questionnaire response patterns to assist rule-based expert exposure assessment in population-based studies. Ann Occup Hyg; 59: 455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger PK, Cumro D, Deubner DDet al. (2001) Beryllium sensitization and disease among long-term and short-term workers in a beryllium ceramics plant. Int Arch Occup Environ Health; 74: 167–76. [DOI] [PubMed] [Google Scholar]
- Knudson TL, Kolanz ME. (2009) An innovative safety model and e-learning guide to working safely with beryllium throughout the industrial supply chain. J Occup Environ Hyg; 6: 758–61. [DOI] [PubMed] [Google Scholar]
- Kolanz ME, Madl AK, Kelsh MAet al. (2001) A comparison and critique of historical and current exposure assessment methods for beryllium: implications for evaluating risk of chronic beryllium disease. Appl Occup Environ Hyg; 16: 593–614. [DOI] [PubMed] [Google Scholar]
- Kreiss K, Day GA, Schuler CR. (2007) Beryllium: a modern industrial hazard. Annu Rev Public Health; 28: 259–77. [DOI] [PubMed] [Google Scholar]
- Madl AK, Unice K, Brown JLet al. (2007) Exposure-response analysis for beryllium sensitization and chronic beryllium disease among workers in a beryllium metal machining plant. J Occup Environ Hyg; 4: 448–66. [DOI] [PubMed] [Google Scholar]
- NAS . (2008) Managing health effects of beryllium exposure. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Rappaport SM. (1985) Smoothing of exposure variability at the receptor: implications for health standards. Ann Occup Hyg; 29: 201–14. [DOI] [PubMed] [Google Scholar]
- Samuel G, Maier LA. (2008) Immunology of chronic beryllium disease. Curr Opin Allergy Clin Immunol; 8: 126–34. [DOI] [PubMed] [Google Scholar]
- Schuler CR, Virji MA, Deubner DCet al. (2012) Sensitization and chronic beryllium disease at a primary manufacturing facility, part 3: exposure-response among short-term workers. Scand J Work Environ Health; 38: 270–81. [DOI] [PubMed] [Google Scholar]
- Stefaniak A, Day G, Virji MAet al. (2011a) The skin and the work environment. In Anna DH, editor. The occupational environment: its evaluation, control, and management, Fairfax, VA: American Industrial Hygiene Association. [Google Scholar]
- Stefaniak AB, Hoover MD, Dickerson RMet al. (2003) Surface area of respirable beryllium metal, oxide, and copper alloy aerosols and implications for assessment of exposure risk of chronic beryllium disease. AIHA J (Fairfax, Va); 64: 297–305. [DOI] [PubMed] [Google Scholar]
- Stefaniak AB, Virji MA, Day GA. (2011b) Dissolution of beryllium in artificial lung alveolar macrophage phagolysosomal fluid. Chemosphere; 83: 1181–7. [DOI] [PubMed] [Google Scholar]
- Stefaniak AB, Virji MA, Day GA. (2011c) Release of beryllium from beryllium-containing materials in artificial skin surface film liquids. Ann Occup Hyg; 55: 57–69. [DOI] [PubMed] [Google Scholar]
- Stefaniak AB, Virji MA, Day GA. (2012) Release of beryllium into artificial airway epithelial lining fluid. Arch Environ Occup Health; 67: 219–28. [DOI] [PubMed] [Google Scholar]
- Thomas CA, Bailey RL, Kent MSet al. (2009) Efficacy of a program to prevent beryllium sensitization among new employees at a copper-beryllium alloy processing facility. Public Health Rep; 124(Suppl 1): 112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle SS, Antonini JM, Rich BAet al. (2003) Skin as a route of exposure and sensitization in chronic beryllium disease. Environ Health Perspect; 111: 1202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A, Lanzavecchia A. (1996) T cell activation determined by T cell receptor number and tunable thresholds. Science; 273: 104–6. [DOI] [PubMed] [Google Scholar]
- Virji MA, Park JY, Stefaniak ABet al. (2012) Sensitization and chronic beryllium disease at a primary manufacturing facility, part 1: historical exposure reconstruction. Scand J Work Environ Health; 38: 247–58. [DOI] [PubMed] [Google Scholar]
- Virji MA, Stefaniak AB, Day GAet al. (2011) Characteristics of beryllium exposure to small particles at a beryllium production facility. Ann Occup Hyg; 55: 70–85. [DOI] [PubMed] [Google Scholar]
- de Vocht F, Burstyn I, Sanguanchaiyakrit N. (2015) Rethinking cumulative exposure in epidemiology, again. J Expo Sci Environ Epidemiol; 25: 467–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


