Abstract
Background.
In 2016, the World Health Organization (WHO) recommended a shorter (9–12 month) multidrug-resistant tuberculosis (MDR-TB) treatment regimen (as compared to the conventional 18–24 month regimen) for patients without extrapulmonary TB, pregnancy, previous second-line TB medication exposure, or drug resistance to pyrazinamide, ethambutol, kanamycin, moxifloxacin, ethionamide, or clofazimine. The recommendation was based on successful clinical trials conducted in Asia and Africa, but studies, using mainly European data, have shown few patients in higher resource settings would meet WHO eligibility criteria.
Methods.
We assessed eligibility for the shorter regimen among U.S. MDR-TB cases that had full drug susceptibility testing (DST) results and were reported during 2011–2016 to the U.S. National TB Surveillance System. We estimated costs by applying the eligibility criteria for the shorter regimen, and proportional inpatient/outpatient costs from a previous population-based study to all MDR-TB patients reported to NTSS.
Results.
Of 586 reported MDR cases, 10% (59) were eligible for the shorter regimen. Of 527 ineligible patients, 386 had full DST, of which 246 were resistant to ethambutol and 217 resistant to pyrazinamide. Compared with conventional MDR-TB treatment, implementing the shorter regimen would reduce the U.S. annual societal MDR-TB cost burden by 4%, but the cost burden for eligible individuals would be reduced by 37–46%.
Conclusions.
Relying on full DST use, our analysis found a minority of U.S. MDR-TB patients would be eligible for the shorter regimen. Cost reductions would be minimal for society, but large for eligible individuals.
Keywords: Tuberculosis, multidrug-resistant, treatment, cost
summary:
Only 10% of U.S. multidrug-resistant TB cases during 2011–2016 would have been eligible for the WHO-recommended shorter treatment regimen. This regimen would greatly reduce individual patient costs, but there would be only a small reduction in overall societal cost.
Background
From 2011–2016, an annual mean of 99 multidrug-resistant tuberculosis (MDR-TB) cases were reported in the United States [1]. In 2016, the World Health Organization (WHO) recommended a standardized shorter (9–12 month) regimen to treat MDR TB [2–4] (Figure 1). The regimen was recommended based on observational clinical studies in Bangladesh [5] and other Asian and African countries [6], with intended use in low/middle income countries (LMIC). LMICs have limited capacity and resources to manage MDR-TB treatment for the standard 18–24 months and conduct routine drug susceptibility testing (DST) [7]. Studies where laboratory DST are performed routinely have found limited eligibility for the regimen: 4% in the International Carbapenems Study (ICS) (Europe/South America) [8], 11% in the European Union [9], 8% in Europe [10], 4%–6% in Eastern Europe [11], and 15%–20% in California [12]. In California, investigators examined genotype data to enhance eligibility from 15% to 20% if the shorter regimen allowed for ethionamide (ETA) resistance in patients with low-level isoniazid (INH) resistance. Data from 17 European countries found 92% ineligibility because 67% of patients had strains resistant to pyrazinamide (PZA), 64% prothionamide/ETA, 59% ethambutol (EMB), 37% second-line injectable drugs (SLIDs), and 33% fluoroquinolones [10]. The ICS noted high proportions of patients’ isolates resistant to EMB/PZA (>60%), PRO (55%), kanamycin (44%), and fluoroquinolones (41%) [8]. Clinical trials examining shorter MDR/XDR regimens are ongoing, including STREAM (Evaluation of a Standard Treatment Regimen of Anti-TB Drugs for Patients with MDR-TB) and NIX (New Investigational Drugs for Extensively Drug-Resistant TB) [13, 14] (See Supplementary Material).
Figure 1.
Features and Conditions of the Shorter Multidrug-resistant Tuberculosis (MDR-TB) Treatment Regimen
a Kanamycin is not used, and prothionamide is not available in the United States; ethionamide (ETA) is considered bio-equivalent by many, but could have some differences.
b Gatifloxacin was used instead of moxifloxacin in the initial clinical studies.
In addition to minimizing toxicity and shortening the duration of therapy, shorter MDR-TB regimens could provide economic benefits to patients, the healthcare system, and society. A previous U.S. study showed direct costs per MDR-TB patient, updated to 2016 dollars [15, 16], averaged $160,000, of which outpatient medications made up ~40% of direct costs and averaged ~$64,000. Direct-plus-productivity-loss costs averaged $295,000 per MDR-TB patient [17]. A modeling study using data representative of a southeast Asian setting illustrated minimal effects of implementing a shorter regimen, with sensitivity to assumptions of long-term efficacy, ability to scale-up treatment access, and impact of additional drug resistance [18].
We analyzed 2011–2016 U.S. MDR-TB data to assess patient eligibility for the WHO-recommended shorter regimen and to estimate economic impact of implementation in the United States.
Methods
We analyzed data reported from the 50 United States and the District of Columbia from 2011–2016 to the Centers for Disease Control and Prevention (CDC) through the National TB Surveillance System (NTSS), including demographics, laboratory results, clinical characteristics, site of disease, prior TB, and risk factors.
A MDR-TB case was defined as having a culture positive for Mycobacterium tuberculosis (Mtb) with initial phenotypic (or growth-based) DST results with INH and rifampicin (RIF) resistance. Phenotypic resistance to INH includes low- and high-level INH resistance; these are not distinguished in DST results nor are molecular DST results reported to NTSS. We examined eligibility criteria among MDR-TB cases that were diagnosed while alive. They were ineligible for the shorter regimen if they had any extrapulmonary TB (EPTB), prior TB, or initial drug resistance to the following medications: PZA, EMB, ETA, moxifloxacin, or any SLID (provided that DST was performed for PZA, EMB, ETA, ≥one fluoroquinolone, and ≥one SLID). We used prior TB and initial drug resistance based on DST to apply the WHO exclusion of confirmed or suspected resistance to any medication in the regimen [4]. Because of partial cross-resistance among moxifloxacin and other fluoroquinolones [19], DST for any fluoroquinolone was assessed. Ineligibility was determined on whether any of the SLIDs were resistant. Clofazimine susceptibility could not be determined as it is not collected in NTSS. Thus, having full DST was defined as DST results for PZA, EMB, ETA, any fluoroquinolone, and any SLID. NTSS does not collect data on prior exposure to second-line TB drugs, nor is length of treatment for specific drugs recorded; it only captures drugs in the initial regimen, and not subsequent regimen changes. In order to exclude possible prior exposure to second-line drugs, we excluded MDR-TB patients with prior TB from eligibility. NTSS does not collect pregnancy data, so pregnancy status was not considered.
We performed sensitivity analyses to include cases with the following characteristics as being eligible for the shorter regimen:
prior TB
resistance to PZA or EMB
- meeting major STREAM trial stage 1 eligibility criteria
- excluded MDR cases with excessive alcohol or non-injecting drugs use (NIDU) in the past year, or TB infection of only meninges, bone or joint or having resistance to a fluoroquinolone or SLID
Univariate analyses were conducted on eligibility. Bivariate analyses examined statistically significant (i.e., odds ratio estimates within 95% confidence intervals (CI)) associations of patient characteristics, including race/ethnicity and HIV infection, with eligibility. Adjusted odds ratios (aOR) and 95% CIs were calculated using a multivariable logistic regression model, with backward selection of main effect variables and all 2-way interactions* to assess associations between characteristics of MDR-TB patients and eligibility (SAS version 9.3; SAS Institute, Cary, NC, USA).
Cost analysis
We estimated the economic impact of implementing the shorter regimen by applying our WHO shorter regimen eligibility criteria to a previous study of a population-based sample of 134 U.S. MDR-TB patients, which collected data on treatment practices, outcomes, and costs [17]. In this previous study, medication costs were obtained using Micromedex Red Book wholesale acquisition costs. For our study, we identified patients in the previous study who would have been eligible for the shorter regimen and applied a ratio of average treatment length for the shorter regimen (11 months) to the average length found in the previous study (23 months) to adjust other costs (inpatient, outpatient, and productivity losses). We computed direct costs and direct-plus-productivity-loss costs, and updated to 2016 U.S. dollars [15, 16]. We compared the U.S. direct-plus-productivity cost burden in the previous study without implementation of the shorter regimen to the cost burden with implementation of the shorter regimen to those eligible. We estimated average costs per patient, including direct inpatient and outpatient costs plus patient productivity losses due to illness. We also estimated the economic impact of implementing a shorter regimen by applying the STREAM eligibility criteria.
Results
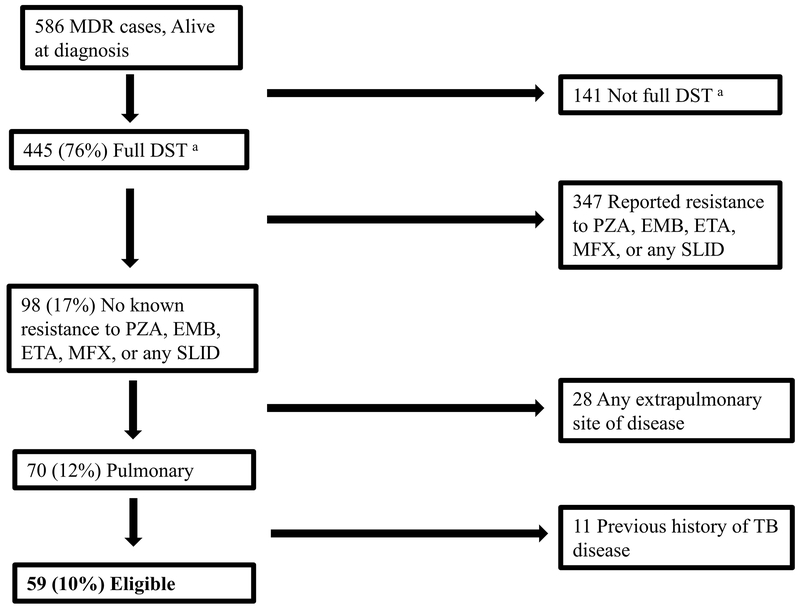
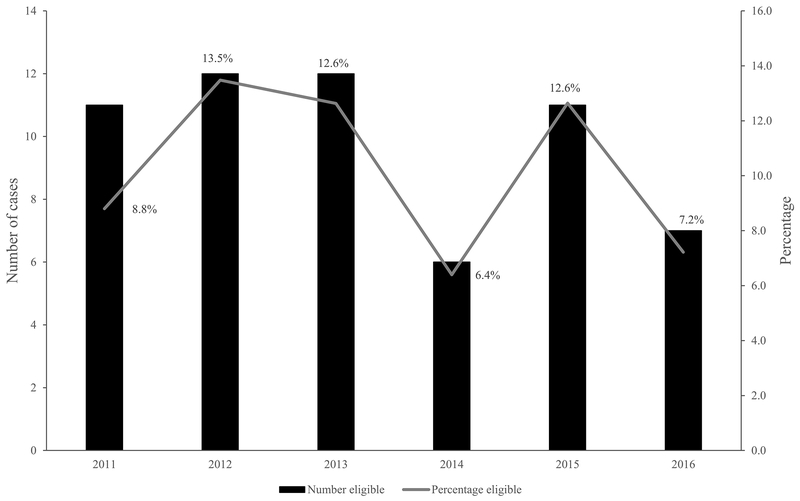
During 2011–2016, there were 586 MDR-TB cases in persons who were alive at diagnosis in the United States; 59 (10%) were eligible for the shorter regimen (6–13% by year) (Figure 2 and 3). Of the 527 ineligible cases, 141 (27%) did not have full DST results: DST results were missing for ETA in 101 (72%) of the 141 cases, fluoroquinolone in 77 (55%), SLID in 51 (36%), PZA in 41 (29%), and EMB in 8 (6%). Of the 527 ineligible cases, 347 (66%) had reported resistance to a medication in the regimen. An additional 5% had EPTB and the remaining 2% had prior TB (Figure 2). Among the 386 ineligible having full DST, most were resistant to EMB (64%) and/or PZA (56%) often in combination with other drug resistance (Table 1). These 386 cases had resistance to other medications: 37% to ETA, 13% to ≥1 fluoroquinolone, and 11% to ≥1 SLID. Figure 4 displays a percentage breakdown of reasons for ineligibility.
Figure 2.
Diagram of Classifying Eligible Multidrug-resistant Tuberculosis (MDR TB) Cases for the WHO-recommended Shorter Regimen, United States, 2011–2016.
a Full Drug Susceptibility Testing (DST)= reported drug susceptibility testing to pyrazinamide (PZA), ethambutol (EMB), ethionamide (ETA), to any fluoroquinolone, and to any second line injectable drug (SLID)
MFX = moxifloxacin
Figure 3.
Eligibility for a Shorter Treatment Regimen for Multidrug-resistant Tuberculosis (MDR TB) in the United States by Year, 2011–2016
Table 1.
Bivariate Analysis of Characteristics among Multidrug-resistant Tuberculosis (MDR-TB) Cases who are Eligible for MDR-TB Shorter Course Regimen, United States, 2011–2016, N = 586
| Eligible for WHO short-term regimen | Ineligible for WHO short-term regimen | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | No. of cases (n = 59) | % | No. of cases (n = 527) | % | P-value | Unadjusted odds ratio | 95% Confidence Interval |
| Age group | |||||||
| 0–14 | 1 | 12.5 | 7 | 87.5 | 0.5a | 1.7 | 0.2–14.1 |
| 15–24 | 14 | 13.3 | 91 | 86.7 | 0.1 | 1.8 | 0.9–3.6 |
| 25–44 | 21 | 8.0 | 243 | 92.0 | Ref. | ||
| 45–64 | 16 | 11.6 | 122 | 88.4 | 0.2 | 1.5 | 0.8–3.0 |
| ≥65 | 7 | 10.0 | 63 | 90.0 | 0.6 | 1.3 | 0.5–3.2 |
| Unknown | 0 | 0.0 | 1 | 100.0 | N/A | ||
| Sex | |||||||
| Male | 30 | 9.7 | 279 | 90.3 | 0.8 | 0.9 | 0.5–1.6 |
| Female | 29 | 10.5 | 248 | 89.5 | Ref. | ||
| Race/ethnicityb | |||||||
| Black | 10 | 12.5 | 70 | 87.5 | 0.5 | 1.4 | 0.5–4.2 |
| Asian | 23 | 7.5 | 285 | 92.5 | 0.7 | 0.8 | 0.3–2.1 |
| Hispanic | 18 | 15.3 | 100 | 84.7 | 0.2 | 1.8 | 0.7–4.8 |
| White | 6 | 9.1 | 60 | 90.9 | Ref. | ||
| Other | 2 | 14.3 | 12 | 85.7 | 0.6a | 1.7 | 0.3–9.3 |
| Origin of birth | |||||||
| U.S.-born | 12 | 17.1 | 58 | 82.9 | 0.04 | 2.1 | 1.0–4.1§ |
| Non-U.S.–bornc | 47 | 9.1 | 469 | 90.9 | Ref. | ||
| Previous Diagnosis of TB Disease | 0 | 0.0 | 109 | 100.0 | N/A | ||
| Extrapulmonary Site of TB Disease | 0 | 0.0 | 125 | 100.0 | N/A | ||
| Homeless | 1 | 9.1 | 10 | 90.9 | 1.0a | 0.9 | 0.1–7.1 |
| Resident of correctional facility at time of diagnosis | 3 | 16.7 | 15 | 83.3 | 0.4a | 1.8 | 0.5–6.5 |
| Long-term care facility resident at time of diagnosis | 0 | 0.0 | 5 | 100.0 | 1.0a | N/A | |
| Excess Alcohol Use | 3 | 9.4 | 29 | 90.6 | 1.0a | 0.9 | 0.3–3.1 |
| HIV Seropositivity | 3 | 10.7 | 25 | 89.3 | 0.8a | 1.1 | 0.3–3.7 |
| Injecting Drug Use (IDU) or Non-IDU | 3 | 14.3 | 18 | 85.7 | 0.5a | 1.5 | 0.4–5.3 |
| Diabetes mellitus | 12 | 14.6 | 70 | 85.4 | 0.1 | 1.7 | 0.8–3.3 |
| Immunosuppressive conditiond | 2 | 8.0 | 23 | 92.0 | 1.0a | 0.8 | 0.2–3.3 |
| Sputum smear positive | 42 | 12.8 | 285 | 87.2 | 0.01 | 2.1 | 1.2–3.8 |
| Cavitary lesion (CXR or CT) | 31 | 13.2 | 204 | 86.8 | 0.04 | 1.8 | 1.0–3.0 |
| Primary Healthcare Providere | |||||||
| Private/Other | 15 | 9.0 | 151 | 91.0 | 0.6 | 0.8 | 0.5–1.6 |
| Health Department | 39 | 9.9 | 356 | 90.1 | 0.8 | 0.9 | 0.5–1.7 |
| Institutional/Correctional | 3 | 18.8 | 13 | 81.3 | 0.2a | 2.1 | 0.6–7.7 |
| Inpatient Care Only | 3 | 10.0 | 27 | 90.0 | 1.0a | 1.0 | 0.3–3.4 |
| Other | 1 | 6.7 | 14 | 93.3 | 1.0a | 0.6 | 0.1–4.9 |
| Private Outpatient | 8 | 7.3 | 102 | 92.7 | 0.3 | 0.7 | 0.3–1.4 |
| Individual Resistancef | |||||||
| Ethambutol | 0 | 0.0 | 246 | 100.0 | <.0001a | N/A | |
| Pyrazinamide | 0 | 0.0 | 217 | 100.0 | <.0001a | N/A | |
| Ethionamide | 0 | 0.0 | 144 | 100.0 | <.0001a | N/A | |
| Kanamycin | 0 | 0.0 | 32 | 100.0 | 0.008a | N/A | |
| Amikacin | 0 | 0.0 | 24 | 100.0 | 0.06a | N/A | |
| Capreomycin | 0 | 0.0 | 24 | 100.0 | 0.06a | N/A | |
| Streptomycin | 31 | 11.0 | 250 | 89.0 | 0.03 | 0.5 | 0.3–0.9 |
| Moxifloxacin | 0 | 0.0 | 24 | 100.0 | 0.4a | N/A | |
| Ofloxacin | 2 | 5.3 | 36 | 94.7 | 0.1a | 0.3 | 0.1–1.3 |
| Levofloxacin | 0 | 0.0 | 15 | 100.0 | 0.3a | N/A | |
| Ciprofloxacin | 2 | 8.3 | 22 | 91.7 | 0.5a | 0.5 | 0.1–2.3 |
| Other Quinolones | 0 | 0.0 | 3 | 100.0 | 1.0a | N/A | |
| Pattern Resistancef | |||||||
| XDRg | 0 | 0.0 | 14 | 100.0 | 0.2a | N/A | |
| Pre-XDRh | 2 | 3.0 | 64 | 97.0 | 0.003a | 0.2 | 0.0–0.7 |
| All firstlinei | 0 | 0.0 | 152 | 100.0 | <.0001a | N/A | |
| Any SLID | 0 | 0.0 | 41 | 100.0 | 0.003a | N/A | |
| Any FQ | 2 | 3.8 | 51 | 96.2 | 0.03a | 0.2 | 0.1–1.0 |
IDU, injecting drug use; CXR, chest x-ray; CT, cat scan; SLID, second-line injectable drug; FQ, fluoroquinolone
Fisher’s Exact Test p-value due to small cell-size
Persons of Hispanic ethnicity might be of any race; non-Hispanic persons are categorized as Asian, black, white, American Indian/Alaska. Other includes American Indian/Alaska Native, Native Hawaiian or Other Pacific Islander, multiple race or unknown race. Native, Native Hawaiian or other Pacific Islander, or of multiple races.
Non-U.S.–born is defined as persons born outside of the United States, U.S. territories (American Samoa, Guam, Puerto Rico, U.S. Virgin Islands, and Commonwealth of the Northern Mariana Islands) (except persons born abroad to a U.S. citizen parent) but include persons born in the freely associated states (Federated States of Micronesia, Republic of the Marshall Islands, and Republic of Palau) unless one or both parents are U.S. citizens.
Immunosuppressive conditions include end-stage renal disease, TNF-α antagonist therapy, previous organ transplantation, and immunosuppression due to either a medical condition or medication, or immunosuppressive therapy (not HIV/AIDS).
NTSS categorizes types of healthcare providers as those in which the primary responsibility for clinical decision making was within TB programs or health clinics associated with a health department, private outpatient care, in-patient hospital care only, or a correctional facility.
No. of cases with full Drug Susceptibility Testing results was 445; 386 ineligible cases had full DST results
XDR (extensive drug-resistance) is defined as being resistant to at least isoniazid and rifampin and at least one second-line injectable drug and to a fluoroquinolone.
Pre-XDR is defined as being resistant to at least isoniazid and rifampin and at least one second-line injectable drug or a fluoroquinolone
All firstline means resistance to all first-line drugs (isoniazid, rifampin, ethambutol, pyrazinamide).
Figure 4.
Percentage Distribution of Multidrug-resistant Tuberculosis (MDR TB) Cases Ineligible for the Shorter Treatment Regimen, United States, 2011–2016, N = 527
a Other combinations for ineligibility for the WHO-recommended shorter treatment regimen. In addition to the options presented in the chart, 61 other combinations of either being resistant to ethambutol and/or pyrazinamide and/or ethionamide and/or second-line injectable drug (SLID) and/or moxifloxacin (MFX) and/or having a history of previous TB, and/or having extrapulmonary disease. There were no instances where patients had resistance to MFX and SLIDs only.
When examining eligibility criteria by including those with prior TB and no other exclusion criteria (11 cases), the percentage eligible increased to 12% (8–16% by year). If providers in the United States prescribed the shorter regimen in spite of known PZA/EMB resistance (since there are challenges with DST for PZA/EMB), then the percent eligible would increase to 31%. When applying the STREAM exclusion criteria, 49% of MDR-TB cases would be eligible. U.S.-born persons had a greater adjusted odds (aOR=2.1, CI=1.0–4.1) of being eligible for the shorter regimen.†
Cost analysis
Eighteen percent of the previous U.S. MDR-TB population-based study [17] would have been eligible. The shorter regimen medication costs would average $24,821 (IQR $17,509–$31,499), varying by treatment length. Applying the shorter regimen to those eligible and comparing IQRs of costs among those eligible, direct costs would average $12,000–$46,000 less with the shorter regimen, 25%–41% less than the conventional regimen among those eligible (direct costs: $54,207, IQR $37,430–$66,252 vs. $86,193, IQR $49,726–$112,451). Direct plus patient productivity losses would average 37%–46% less with application of the shorter regimen to those eligible ($88,121, IQR $67,808–$101,188 vs. $157,104, IQR $107,354–$187,232) (Table 2). While implementing the shorter regimen would greatly reduce costs for those eligible, the small proportion of eligible patients, who also have less complex disease, results in a small reduction (4%) in overall societal cost burden of U.S. MDR-TB treatment. When using the STREAM eligibility criteria, total direct cost burden also decreases by 4%, but societal costs might be reduced 22% (Table 3).
Table 2.
Multidrug-resistant tuberculosis (MDR-TB) Cost Comparisons using our Modified WHO-recommended Shorter Regimen Criteria.
| 2016 Dollars | |||||||
|---|---|---|---|---|---|---|---|
| Direct Costs | Societal Costsb | ||||||
| Regimen | na | Total costs in sample | Average | IQR | Total costs in sample | Average | IQR |
| Conventional Regimens | |||||||
| Not Eligible for WHO Shorter Regimen Shorter Regimen | 110 | $ 18,938,362 | $ 172,167 | ($73,763-$204,889) | $ 36,528,866 | $ 332,081 | ($162,963-$392,953) |
| Eligible for WHO Shorter Regimen | 24 | $ 2,068,644 | $ 86,193 | ($49,726-$112,451) | $ 3,770,504 | $ 157,104 | ($107,354-187,232) |
| Total Cost of Conventional Regimens | 134 | $ 21,007,005 | $ 40,299,593 | ||||
| WHO Shorter Regimen | |||||||
| Not Eligible for WHO Shorter Regimen | 110 | $ 18,938,362 | $ 172,167 | ($73,763-$204,889) | $ 36,528,866 | $ 332,081 | ($162,963-$392,953) |
| Eligible for WHO Shorter Regimen | 24 | $ 1,300,978 | $ 54,207 | ($37,430-$66,252) | $ 2,114,911 | $ 88,121 | ($67,808-$101,188) |
| Total Cost of WHO Shorter Regimen | 134 | $ 20,239,340 | $ 38,643,777 | ||||
| Cost of Conventional Regimen Minus WHO Shorter Regimen | $ 767,665 | $ 1,655,816 | |||||
| Cost Savings of the WHO Shorter Regimen | 4% | 4% | |||||
IQR, interquartile range
n is the number of MDR-TB patients alive at TB diagnosis in the 2005–2007 cohort
Societal costs is the direct costs plus patient productivity losses due to illness
Table 3.
Multidrug-resistant Tuberculosis (MDR-TB) Cost Comparisons using STREAM Eligibility Criteria.
| 2016 Dollars | |||||||
|---|---|---|---|---|---|---|---|
| Direct Costs | Societal Costsb | ||||||
| Regimen | na | Total costs in sample | Average | IQR | Total costs in sample | Average | IQR |
| Conventional Regimens | |||||||
| Not Eligible for STREAM1 Shorter Regimen | 50 | $ 102,267,483 | $ 205,350 | ($72,268-$252,631) | $ 20,147,312 | $ 402,946 | ($135,606-$485,122) |
| Eligible for STREAM1 Shorter Regimen | 84 | $ 10,739,523 | $ 127,852 | ($68,910-$157,193) | $ 20,152,057 | $ 239,905 | ($140,442-$295,168) |
| Total Cost of Conventional Regimens | 134 | $ 113,007,006 | $ 40,299,369 | ||||
| STREAM1 Shorter Regimen | |||||||
| Not Eligible for STREAM1 Shorter Regimen | 50 | $ 102,267,483 | $ 205,350 | ($72,268-$252,631) | $ 20,147,312 | $ 402,946 | ($135,606-$485,122) |
| Eligible for STREAM1 Shorter Regimen | 84 | $ 5,684,469 | $ 67,672 | ($45,264-$74,850) | $ 11,329,556 | $ 134,876 | ($73,913-$137,845) |
| Total Cost of STREAM1 Shorter Regimen | 134 | $ 107,951,952 | $ 31,476,868 | ||||
| Cost of Conventional Regimen Minus STREAM1 Shorter Regimen | $ 5,055,054 | $ 8,822,501 | |||||
| Cost Savings of the STREAM1 Shorter Regimen | 4% | 22% | |||||
IQR = interquartile range
n = the number of MDR-TB patients alive at TB diagnosis in the 2005–2007 cohort
Societal costs = direct costs plus patient productivity losses due to illness
Discussion
Our assessment showed that 10% of MDR-TB cases from 2011–2016 were eligible for the shorter regimen; this proportion would increase to 12% if those with prior TB were not excluded. Because few U.S. MDR patients were eligible, implementing the shorter regimen in the United States would have minimal impact on lowering the societal MDR-TB cost burden.
Our eligibility finding falls within the range from Europe and South America (4–11%) [8–10] but on the higher end. When adjusting criteria to allow the inclusion of those with prior TB, our results are slightly below California findings [12] of 15%–20% eligibility. U.S. levels of resistance to fluoroquinolones and SLIDs were much lower than in countries from the ICS [8].
The WHO has documents to support recommendations for the MDR shorter regimen [2, 4, 20]. Our definition for eligibility was more strict than WHO’s because we excluded prior TB and resistance to PZA/EMB; however, we assessed the impact of including these cases in sensitivity analyses. Figure 4 shows that 60% of those excluded were ineligible for ≥one reason. Twenty-one percent of ineligible patients were excluded because of resistance to EMB and/or PZA only. In the United States, molecular DST results are available in many settings; routine use of such results could improve the speed and accuracy of DST results. Although CDC and some local agencies offer molecular testing for the identification of drug resistance associated mutations in isolates of Mtb, testing for EMB/PZA resistance is challenging and the ability to identify relevant resistance to these drugs is limited [21–23]. Studies are ongoing to increase understanding of the molecular mechanisms underlying resistance to second-line anti-TB drugs.
If our eligibility criteria were similar to that in STREAM then more MDR patients in the United States would be eligible (49%) for the shorter regimen. Preliminary findings from STREAM showed 78% of participants on the shorter regimen had favorable outcomes compared to 81% on the standard regimen, with similar incidence of adverse events (46% vs. 45%). However, the shorter regimen group had more deaths (9% vs. 6%) [24]. If the NIX regimen could be applied more broadly (e.g., for cases other than those with XDR TB), it might replace all MDR-TB regimens. However, best use of the NIX regimen has not been determined. The WHO continues to make new recommendations as trials continue, including replacing kanamycin with amikacin and ensuring drug resistance is excluded (at least to fluoroquinolones and SLIDs) [25].
In our study, U.S.-born persons were more likely to be eligible for the shorter regimen than non-U.S.–born. Studies have shown that EPTB is more common in non-U.S.–born persons than in U.S.-born; in our study, EPTB was an exclusion criterion for eligibility [26, 27]. Among persons with MDR TB, a higher proportion of non-U.S.–born persons have prior TB compared to U.S.-born [1] and those with prior TB were also excluded from being eligible in our study.
The main reason for ineligibility in our study was resistance to EMB (64%) or PZA (56%) among the 386 ineligible having full DST results. Previous studies have demonstrated that PZA/EMB resistance were major limiting factors in eligibility [8, 10]. Despite this, high treatment completion rates (80–95%) have been achieved in trials of the short regimen conducted in countries in Asia and Africa, compared to typical rates for the long regimen [28, 29]; however, side effects and adverse drug reactions were quite frequent [5, 6, 30] and deaths were higher [24, 30]. A study conducted in nine African countries showed 82% treatment success among persons on the shorter regimen with minimal effects due to initial resistance to PZA/EMB [31]. In our study, 37% of patients ineligible for the shorter regimen had resistance to ETA. A prevalence study done in South Africa showed a high proportion of resistance to ETA and PZA (45% and 59%, respectively), indicating the need for routine DST at initiation of MDR therapy [32, 33]. In the United States where DST is widely available, providers individualize treatment according to DST results and would not prescribe medications with known resistance that are likely to be ineffective and could cause adverse reactions. A recent study documented poorer outcomes if MDR treatment relies on a drug having known resistance [34]. However, in many international settings, initial and retreatment regimens are often standardized because of limited DST capabilities [20].
Despite high MDR-TB treatment completion with conventional regimens in the United States (80% from NTSS data), treatment is much longer and more expensive than it would be with the shorter regimen among those eligible (average 23 months and direct-plus-productivity cost of $157,000 in 2016 dollars, versus an average 11 months and cost of $88,000). Because of the small percentage of MDR-TB patients eligible, implementation of the shorter regimen in eligible U.S. patients would reduce the overall annual societal MDR-TB cost burden by only 4%. However, per patient direct-plus-productivity costs would be reduced by 37%–46%; this impact is large for eligible individual patients, both in terms of cost and time (including lost work) burden. Our sensitivity analysis showed that eligibility varies depending on criteria used. Although clinicians in the United States likely would not prescribe STREAM medications to which there is known resistance, implementation of the STREAM regimen is estimated to result in similar (to the WHO regimen) direct cost savings of 4%, but higher cost savings of 22% with productivity losses. While shorter, less toxic regimens are needed, we believe that effective shortening of MDR-TB treatment will require novel drugs.
Our investigation has some limitations. NTSS only collects data from initial and final DST, and not multiple DSTs during the course of treatment nor does it collect molecular DST results. Using initial DST reports might have underestimated or overestimated drug resistance. In addition, 27% (141/527) of ineligible patients did not have full DST results. Not every laboratory tests for ETA or a fluoroquinolone, and tests might be sent to another commercial or reference laboratory. Thus, these test results may not get reported to CDC. One WHO exclusion criterion is prior treatment with any of the short regimen’s second-line TB medications for ≥one month. Since a large proportion of U.S. MDR-TB patients are non-U.S.–born, from whom records of past treatment are unavailable, and since MDR-TB patients in high burden settings develop disease from primary infection with drug-resistant strains [35] and there is limited capacity for DST internationally, it is difficult to know which medications were received in the past and prior resistance to individual medications. In practice, providers ask patients about prior TB (including exposure to second-line medications) at diagnosis and either prescribe medications empirically based on initial assessments (adjusting them later when DSTs are available) or wait to prescribe medications based on DSTs when they are available. Generally, providers rely on DSTs, and do not rely on patient history. While some might think it is overly restrictive to exclude all patients with prior TB from eligibility, we also conducted a sensitivity analysis to assess eligibility by including those with prior TB.
Since NTSS is a surveillance system focused on epidemiologic data rather than clinical issues, there are further limitations in its use. Medication side effects are not collected (unless they result in treatment discontinuation); thus, we could not address toxicity and its effects on costs. Although WHO includes pregnancy as an exclusion criterion for the shorter regimen, NTSS does not currently collect standardized data on pregnancy or DST for clofazimine. Another limitation is not knowing the level of INH resistance among MDR-TB patients. Genotyping data on INH resistance associated mutations (e.g., gene mutations in inhA and katG) are not collected in NTSS. Patients with isolates having inhA mutations are likely to have ETA resistance, but their low-level INH resistance might be overcome with high-dose isoniazid (HDH) [36]. KatG mutations are common causes of high-level INH resistance and are less likely to be associated with ETA resistance. Patients with isolates having inhA mutations that are ETA sensitive could be effectively treated with the shorter regimen that has both HDH and ETA. In some instances, HDH can be offered to those with mutation in katG [37]. Among INH resistance isolates, prevalence of katG mutations is ~50% and prevalence of inhA mutations is ~25% [38]. The capacity to collect gene mutations at a national level would provide further insight into eligibility. Another limitation is that since we did not study the regimen’s impact on hospitalization, we made assumptions based on hospitalization of patients having similar low resistance patterns from the previous study [17]. Thus, since eligible patients may have a shorter hospitalization duration, we may have underestimated the benefits of implementing the WHO-recommended regimen.
In conclusion, 10% of MDR-TB cases from 2011–2016 in the United States would have been eligible for the WHO shorter regimen under our revised eligibility criteria. The majority of ineligible patients were excluded because of resistance to EMB or PZA. Because of the low level of eligibility, the shorter regimen would have a minimal impact on the overall societal cost burden of MDR TB in the United States, but would greatly reduce the burden on eligible individual patients. Trials are ongoing of new short MDR-TB regimens [13, 14]; these may support additional shorter MDR-TB regimens for use with a greater eligible U.S. MDR-TB population.
Supplementary Material
Acknowledgments
The authors acknowledge Robert Pratt and J. Steve Kammerer at the Centers for Disease Control and Prevention (CDC), Division of Tuberculosis Elimination (DTBE) for providing insight to data analysis and DTBE, Laboratory Branch for providing recommendations to the discussion: Angela Starks, Tracy Dalton, and Melisa Willby.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding. The authors received no specific funding for this work.
Footnotes
2-way interactions assessed in the multivariable logistic regression model: sex, race/ethnicity, age, origin of birth, homeless, corrections, alcohol, HIV status, injecting drug use, NIDU, diabetes, renal disease, immunocompromising conditions, primary occupation, smear positive, and cavitary lesion.
after adjusting for sex, race/ethnicity, age, HIV status, correctional facility inmate, long-term care facility resident, diabetes, immunocompromising conditions, healthcare provider type, and past year history of homelessness, excess alcohol use, and drug use.
Potential conflicts of interest. All authors have no potential conflicts of interest.
References
- 1.CDC. Reported Tuberculosis in the United States, 2016. Atlanta, GA: US Department of Health and Human Services, CDC; 2017. [Google Scholar]
- 2.WHO. WHO treatment guidelines for drug-resistant tuberculosis (2016 update). Geneva: World Health Organization, 2016. [PubMed] [Google Scholar]
- 3.Scardigli A, Caminero JA, Sotgiu G, et al. Efficacy and tolerability of ethionamide versus prothionamide: a systematic review. Eur Respir J, 2016; 48(3):946–952. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Frequently asked questions about the implementation of the new WHO recommendation on the use of the shorter MDR-TB regimen under programmatic conditions. Geneva: World Health Organization, 2016. Available at: http://www.who.int/tb/areas-of-work/drug-resistant-tb/treatment/FAQshorter_MDR_regimen.pdf. Accessed 6 November 2018. [Google Scholar]
- 5.Van Deun A, Maug AK, Salim MA, et al. Short, Highly Effective, and Inexpensive Standardized Treatment of Multidrug-resistant Tuberculosis. Am J Respir Crit Care, 2010; 182(5):684–692. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad Khan F, Salim MA, du Cros P, et al. Effectiveness and safety of standardized shorter regimens for multidrug-resistant tuberculosis: individual patient data and aggregate data meta-analyses. Eur Respir J, 2017; 50:1–13. [DOI] [PubMed] [Google Scholar]
- 7.Curry International Tuberculosis Center and California Department of Public Health, 2016: Drug-Resistant Tuberculosis: A Survival Guide for Clinicians, Third Edition. Available at: http://www.currytbcenter.ucsf.edu/sites/default/files/tb_sg3_book.pdf. Accessed 6 November 2018.
- 8.Sotgiu G, Tiberi S, D’Amgrosio L, et al. Faster for less: The new “shorter” regimen for multidrug-resistant tuberculosis. Eur Respir J, 2016; 48:1503–1507. [DOI] [PubMed] [Google Scholar]
- 9.Van der Werf MJ, Hollo V, Kodmon C, Dara M, Catchpole M. Eligibility for shorter treatment of multidrug-resistant tuberculosis in the European Union. Eur Respir J, 2017; 49(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange C, Duarte R, Frechet-Jachym M, et al. Limited benefit of the new shorter multidrug-resistant tuberculosis regimen in Europe. Am J Respir Crit Care, 2016; 194(8). [DOI] [PubMed] [Google Scholar]
- 11.Balabanova Y, Fiebig L, Ignatyeva O, et al. Multidrug-resistant TB in Eastern region of the EU: is the shorter regimen an exception or a rule? Thorax, 2017; 72(9):850–852. [DOI] [PubMed] [Google Scholar]
- 12.Barry PM, Lowenthal P, True L, et al. Benefit of the Shorter Multidrug-Resistant Tuberculosis Treatment Regimen in California and Modified Eligibility Criteria. Am J Respir Crit Care Med, 2017; 196 (11):1488–1489. [DOI] [PubMed] [Google Scholar]
- 13.The Evaluation of a Standard Treatment Regimen of Anti-tuberculosis Drugs for Patients With MDR-TB (STREAM); updated 2018 Jan 26 Available at: https://clinicaltrials.gov/ct2/show/NCT02409290?term=NCT02409290&rank=1. Accessed 15 August 2018.
- 14.A Phase 3 Study Assessing the Safety and Efficacy of Bedaquiline Plus PA-824 Plus Linezolid in Subjects With Drug Resistant Pulmonary Tuberculosis; updated 2018 May 18 Available at: https://clinicaltrials.gov/ct2/show/NCT02333799. Accessed 15 August 2018.
- 15.Bureau of Labor Statistics, U.S. Department of Labor, Consumer Price Index for Medical Care-All Urban Consumers [Internet]. Washington, DC: Bureau of Labor Statistics; cited 2017 Oct 25 Available at: https://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths. Accessed 25 August 2018. [Google Scholar]
- 16.Bureau of Labor Statistics, U.S. Department of Labor, The Economics Daily, Real average hourly earnings for production and nonsupervisory employees up 0.5 percent in 2016 [Internet]. Washington, DC: Bureau of Labor Statistics; updated 2017 Jan 24 Available at: https://www.bls.gov/opub/ted/2017/real-average-hourly-earnings-for-production-and-nonsupervisory-employees-up-0-point-5-percent-in-2016.htm. Accessed 6 November 2018. [Google Scholar]
- 17.Marks SM, Flood J, Seaworth B, et al. Treatment Practices, Outcomes, and Costs of Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis, United States, 2005–2007. Emerg Infect Dis, 2014; 20(5): 812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kendall EA, Fojo AT, Dowdy DW. Expected effects of adopting a 9-month regimen for multidrug-resistant tuberculosis: a population modelling analysis. Lancet Respir Med, 2017; 5: 191–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah NS, Grace Lin SY, Barry PM, Cheng YN, Schecter G, Desmond E. Clinical Impact on Tuberculosis Treatment Outcomes of Discordance Between Molecular and Growth-Based Assays for Rifampin Resistance, California 2003–2013. Open Forum Infect Dis, 2016; 3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falzon D, Schünemann HJ, Harausz E, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J, 2017; 49(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chedore P, Bertucci L, Wolfe J, Sharma M, Jamieson F. Potential for erroneous results indicating resistance when using the Bactec MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Clin Microbiol, 2010; 48(1):300–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouzouita I, Cabibbe AM, Trovato A, Draoui H, et al. , Is sequencing better than phenotypic tests for the detection of pyrazinamide resistance? Int J Tuberc Lung Dis, 2018; 22(6):661–666. [DOI] [PubMed] [Google Scholar]
- 23.Madison B, Robinson-Dunn B, George I, Gross W, et al. , Multicenter Evaluation of Ethambutol Susceptibility Testing of Mycobacterium tuberculosis by Agar Proportion and Radiometric Methods. J Clin Microbiol, 2002; 40(11): 3976–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akkermans R 48th Union World Conference on Lung Health. Lancet Respir Med, 2017; 5(12):926–927. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Rapid Communication: Key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB), August 2018.
- 26.Asghar RJ, Pratt RH, Kammerer JS, Navin TR. Tuberculosis in South Asians Living in the United States, 1993-2004. Arch Intern Med, 2008; 168 (9): 936–42. [DOI] [PubMed] [Google Scholar]
- 27.Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin Infect Dis, 2009; 49(9):1350–1357. [DOI] [PubMed] [Google Scholar]
- 28.Aung KJ, Van Deun A, Declercq E, Sarker MR, et al. , Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis, 2014; 18(10): 1180–1187. [DOI] [PubMed] [Google Scholar]
- 29.Kuaban C, Noeske J, Rieder HL, Aït-Khaled N, Abena Foe JL, Trѐbucq A. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis, 2015; 19(5):517–24. [DOI] [PubMed] [Google Scholar]
- 30.Piubello A, Harouna SH, Souleymane MB, et al. High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis, 2014; 18(10): 1188–1194. [DOI] [PubMed] [Google Scholar]
- 31.Trébucq A, Schwoebel V, Kashongwe Z, et al. , Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis, 2018; 22(1):17–25. [DOI] [PubMed] [Google Scholar]
- 32.Ismail NA, Mvusi L, Nanoo A, Dreyer A, et al. , Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: a national and sub-national cross-sectional survey. Lancet Infect Dis, 2018; 18(7):779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Variava E, Martinson N. Drug-resistant tuberculosis: the rise of the monos. Lancet Infect Dis, 2018; 18(7):705–706. [DOI] [PubMed] [Google Scholar]
- 34.The Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment-2017, Ahmad N, Ahuja SD, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. The Lancet, 2018; 392(10150):821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kendall EA, Fofana MO, Dowdy DW. Burden of transmitted multidrug resistance in epidemics of tuberculosis: a transmission modelling analysis. Lancet Respir Med, 2015; 3(12):963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katiyar SK, Bihari S, Prakash S, Mamtani M, Kulkarni H. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis, 2008; 12:139–145. [PubMed] [Google Scholar]
- 37.Rieder HL, Van Deun A. Rationale for high-dose isoniazid in the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis, 2017; 21(1):123–124. [DOI] [PubMed] [Google Scholar]
- 38.Iseman MD. A Clinician’s Guide to Tuberculosis. Philadelphia: Lippincott Williams & Wilkins, 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.