Abstract
Drug delivery strategies aim to maximize a drug’s therapeutic index by increasing the concentration of drug at target sites while minimizing delivery to off-target tissues. Because biological tissues are minimally responsive to magnetic fields, there has been a great deal of interest in using magnetic nanoparticles in combination with applied magnetic fields to selectively control the accumulation and release of drug in target tissues while minimizing the impact on surrounding tissue. In particular, spatially variant magnetic fields have been used to encourage accumulation of drug-loaded magnetic nanoparticles at target sites, while time variant magnetic fields have been used to induce drug release from thermally sensitive nanocarriers. In this review, we discuss nanoparticle formulations and approaches that have been developed for magnetic targeting and/or magnetically induced drug release, as well as ongoing challenges in using magnetism for therapeutic applications.
Graphical/Visual Abstract

Magnetic fields can be used in combination with magnetic nanoparticles to trigger drug release from thermally sensitive nanocarriers or increase drug accumulation at a target site via magnetic drug targeting.
Introduction
Effective drug delivery strategies must be able to achieve therapeutic drug concentrations in a particular region of interest, such as a tumor, while minimizing delivery to off-target tissues (Bae & Park, 2011; R. Singh & Lillard, 2009). Delivery of drug to non-target tissues can result in a range of complications, ranging from mild discomfort to life threating side effects (Kim, Lee, Lee, Park, & Lee, 2016). To solve this problem, nanoparticle-based drug delivery systems are being developed to improve the therapeutic index of drugs. Various nanocarriers have been used to increase the preferential accumulation of drug in tumors and impart control over drug biodistribution and release. These include polymer-drug conjugates (Duro-Castano, Movellan, & Vicent, 2015), dendrimers (A. K. Sharma et al., 2017), nanogels (Wu & Wang, 2016), metal nanoparticles (H. Sharma, Mishra, Talegaonkar, & Vaidya, 2015), mesoporous silica nanoparticles (Y. Li et al., 2017), virus-like particles (Rohovie, Nagasawa, & Swartz, 2017), lipid nanoparticles (Hörmann & Zimmer, 2016), and polymeric nanoparticles (Merino, Martín, Kostarelos, Prato, & Vázquez, 2015). Accumulation of the nanocarrier in the region of interest is achieved by direct injection (Cheng, Tietjen, Saucier-Sawyer, & Saltzman, 2015), passive targeting (enhanced permeability and retention) (Maeda, 2015), or active targeting methods including ligand targeting (Srinivasarao, Galliford, & Low, 2015), pH-dependent targeting (B. Chen et al., 2017), matrix metalloproteinases (Vandooren, Opdenakker, Loadman, & Edwards, 2016), radiotherapy (Haume et al., 2016), magnetic targeting (Ulbrich et al., 2016), and pharmacological methods such as TNF-alpha and VEGF inhibitors (Ojha et al., 2017). Drug release can then occur via leakage from the nanocarrier or as a result of nanoparticle degradation. Accelerated drug release can also be triggered using particles or linkages that are sensitive to the acidic tumor microenvironment (Kanamala, Wilson, Yang, Palmer, & Wu, 2016), hypoxia (Thambi, Park, & Lee, 2016), light (Linsley & Wu, 2017), ultrasound (Boissenot, Bordat, Fattal, & Tsapis, 2016), or temperature (Mura, Nicolas, & Couvreur, 2013; Sánchez-Moreno, de Vicente, Nardecchia, Marchal, & Boulaiz, 2018). Rapid/triggered drug release can lead to a transient spike in the local concentration of drug and result in improved therapeutic efficacy.
Strategies that rely on environmental and molecular signatures to enhance nanocarrier accumulation or to trigger drug release are often limited by heterogeneity within the target tissue and lack of selectivity, due to the presence of similar signatures in some non-target tissues (Muro, 2012; Tredan, Galmarini, Patel, & Tannock, 2007; Vaupel, Kallinowski, & Okunieff, 1989). Meanwhile, exogenous strategies that rely on light or ultrasound energy have the potential to be absorbed by, and therefore damage, adjacent and intervening tissue. One way to avoid these challenges is to use a bio-orthogonal trigger, such as magnetism. Biological tissue is nearly ‘transparent’ to magnetic energy, and magnetic fields pass through tissue without being significantly absorbed or distorted by body tissues. Magnetic nanoparticles introduced into the body can therefore allow for high local energy delivery to the particles, compared to the less magnetic surrounding tissue. As a result, magnetic fields and magnetically responsive particles can be harnessed for therapy and drug delivery, particularly in cancer (C. Sun, Lee, & Zhang, 2008). Two approaches are most common: (i) the use of magnetic particles to improve the accumulation of drugs in a desired region via magnetic targeting (Alexiou et al., 2000; Shapiro et al., 2015); and (ii) the use of magnetic fields to heat magnetic particles to directly induce hyperthermia in or ablation of diseased tissues (Hedayatnasab, Abnisa, & Daud, 2017) and/or to trigger the release of drugs from thermally-sensitive carriers (Moros et al., 2019; Yoo, Jeong, Noh, Lee, & Cheon, 2013) (Figure 1). As there are already a number of very comprehensive reviews on hyperthermia and thermal tissue ablation (Chang et al., 2018; Dewhirst, Lee, & Ashcraft, 2016; Périgo et al., 2015), these topics will not be discussed extensively here. Rather, we focus on approaches that have been developed for magnetic targeting of drug-loaded magnetic nanocarriers as well as magnetically induced drug release. We also discuss some of the challenges of using magnetism for therapeutic applications.
Figure 1. Magnetic fields and nanoparticles are used to improve drug delivery, particularly in cancer.
Time-variant magnetic fields can induce drug release from temperature sensitive drug carriers via magnetic heating (left). Space-variant magnetic fields can improve drug accumulation in target tissues via magnetic drug targeting (right).
MAGNETIC NANOPARTICLES
Magnetic drug delivery strategies rely on transferring externally applied magnetic energy to magnetic particles that have been introduced into the body (Sensenig, Sapir, MacDonald, Cohen, & Polyak, 2012). One of the most commonly used magnetic particles for drug delivery are superparamagnetic iron oxide nanoparticles (SPIONs). Unlike ferromagnetic materials, in which materials are permanently magnetized, paramagnetic materials are magnetized only when placed into an applied field. When the magnetic field is removed, the moments revert to a random orientation (Tipler, 1999). If the size and number of domains in a magnetic material is sufficiently small, generally less than 150 nm, these nanoparticles are considered to be superparamagnetic and exhibit significantly higher magnetic susceptibility than paramagnets (Andrews, Lipson, & Nann, 2019; Roch, Muller, & Gillis, 1999).
The use of magnetic nanoparticles for imaging, hyperthermia/thermal ablation, and magnetic targeting applications are all linked to the response of SPIONs to magnetic fields (Laurent, Dutz, Häfeli, & Mahmoudi, 2011). Therefore, there has been a continual desire to develop more effective SPIONs. The magnetic moment of a particle is typically proportional to its size (Rosensweig, 2002; Sutens et al., 2016); therefore, numerous strategies have been developed to increase SPION size. For example, seed growth is a well-studied method of gradually growing monodisperse SPIONs up to 20 nm in diameter (S. Sun et al., 2004); unfortunately, although this process produces very monodisperse particles, the gradual size increase in multiple steps lengthens synthesis time. Therefore, others have developed methods to synthesize large nanoparticles in a single step. For example, Hufschmid et al. have reported a large-scale synthesis of SPIONs 30 nm in diameter via thermal decomposition (Hufschmid et al., 2015).
Others have also studied the effect of transition element dopants such as cobalt, manganese, and zinc on SPIONs’ specific absorption rate (SAR) for heating. For example, Jang et al. found that by using Mn0.6Zn0.4Fe2O4 particles, they are able to demonstrate a 4× improvement in heating (Jang et al., 2009). Others have also doped cobalt into SPIONs at various proportions to make highly magnetic particles (R. Chen, Christiansen, & Anikeeva, 2013; Mahmoudi, Sant, Wang, Laurent, & Sen, 2011; S. Sun et al., 2004).
For spherical particles, the synthesis method can affect SPION properties and crystal structure. For example, Chen et al. found that syntheses using iron (III) acetylacetonate as a precursor produced particles with better magnetic saturation values than those that used iron oleate, though the resultant particles were typically smaller (R. Chen et al., 2013). Recently, Unni et al. have also suggested that typical SPION synthesis methods such as thermal decomposition can lead to particles with multiple magnetic domains due to defects in the crystal structure, resulting in an effective magnetic particle size that is smaller than the measured particle size. By controlled addition of 20% oxygen and 80% argon to the reaction, they were able to synthesize single-domain nanoparticles with a > 4× improvement in the magnetic saturation compared to particles synthesized via oxygen-free thermal decomposition (Figure 2) (Unni et al., 2017). Moreover, while typical SAR values for spherical, undoped, single core SPIONs synthesized by typical thermal decomposition and seed growth methods range from ~100–200 W/g (El-Dek, Ali, El-Zanaty, & Ahmed, 2018), SPIONs synthesized via the controlled oxygen method developed by Unni et al. had SARs three-fold higher (Unni et al., 2017).
Figure 2. Controlled addition of oxygen during thermal decomposition results in synthesis of particles with improved magnetic properties.
(a) In the absence of oxygen, the physical diameter of the nanoaparticles is well controlled. However, the magnetic diameter (Dm) of the particles is smaller and more polydisperse than the physical diameter (Dp). (b) When oxygen is added in a controlled manner to the synthesis, the physical and magnetic diameters of the particles is similar, leading to a three fold increase in the SAR of the particles. From Unni et al. (2017).
Another method of improving SPIONs’ magnetic properties is through the synthesis of geometrically complex particles such as core/shell SPIONs and other shapes. For example, nanoparticles with an iron core and an iron oxide shell exhibit improved hyperthermic properties because of the extremely magnetic iron core (Balivada et al., 2010; H. Lee, Yoon, & Weissleder, 2009). Others have also developed iron-cobalt core/cobalt iron oxide shell particles for improved hyperthermia (Habib, Ondeck, Chaudhary, Bockstaller, & McHenry, 2008) and iron core/magnesium oxide shell particles that modulate the dipole-dipole interactions between particles for improved hyperthermia (Martinez-Boubeta et al., 2012). In these formulations, the shell also typically helps improve magnetic properties by preventing oxidation of the core.
Other shapes have been synthesized to improve magnetization by minimizing surface anisotropy and maximizing exchange anisotropy. For example, Noh et al. have synthesized a 60 nm cubic structure comprising an zinc iron oxide core and cobalt iron oxide shell. This particle was demonstrated to have an extremely high SAR of >4000 W/g (Noh et al., 2012). Similarly, Guardia et al. synthesized a 19 nm iron oxide nanocube with a SAR of nearly 2500 W/g for magnetic hyperthermia treatments in vitro (Guardia et al., 2012). Iron oxide nanoflowers, have also been developed for magnetic heating applications. These particles comprise multiple magnetic cores stably encapsulated in a matrix. The small distance and strong interactions between cores allows for exchange coupling between cores (Lartigue et al., 2012), resulting in stronger magnetic moments than single core particles. Multicore particles have been shown to exhibit improved hyperthermia properties compared to single core particle (Guardia et al., 2012; Hemery et al., 2017). Others have shown that larger core sizes and larger complex sizes in multicore particles lead to improved heating properties (Blanco-Andujar, Ortega, Southern, Pankhurst, & Thanh, 2015). Iron oxide nanoflowers have also been modified in the same ways as traditional spherical iron oxide nanoparticles. For instance, Curcio et al. recently synthesized an iron oxide nanoflower core/copper sulfide shell particle (Curcio et al., 2019). Manganese-doped iron oxide nanoflowers have also been developed with improved heating properties compared to conventional nanoparticles (X. L. Liu et al., 2016).
USING MAGNETIC FIELDS TO IMPROVE ACCUMULATION OF MAGNETIC NANOPARTICLES IN TUMORS
Nanoparticle accumulation and penetration within tumors
Nanoparticles tend to exhibit fairly low levels of accumulation within tumors. In a review of the literature, Wilhelm et al. found that the median injected dose of nanoparticles accumulated in solid tumors was 0.6% using passive targeting, and only improved to 0.9% using active targeting. They propose that one of the major challenges to high nanoparticle accumulation in solid tumors may be the hydrostatic pressure, which can be as much as 10–40× greater in tumors than in normal tissues (Wilhelm et al., 2016). Drug delivery to solid tumors is also complicated by the heterogeneous tissue structure, vasculature, and microenvironment within the tumor (Baronzio, Parmar, & Baronzio, 2015; Sriraman, Aryasomayajula, & Torchilin, 2014). Nanocarriers that passively accumulate in tumors are often located perivascularly, with little penetration into the interstitium. Areas that are far away from blood vessels may not be exposed to drug because the interstitial hydrostatic pressure and extracellular matrix both interfere with diffusion of the drug carrier out of the vasculature (Baronzio et al., 2015; Goodman, Olive, & Pun, 2007; Sugahara et al., 2009; Wong et al., 2011). Therefore, there has been a great deal of interest in developing techniques to improve the accumulation and penetration of nanoparticles within tumors.
Magnetic Drug Targeting
Magnetic targeting, also referred to as magnetophoresis, has been proposed as a potential mechanism to improve the accumulation and penetration of magnetic drug carriers in tumors (C. Sun et al., 2008). For magnetic targeting, drug and magnetic nanoparticles are encapsulated into a nanocarrier, and a strong external static magnet is used to accumulate the drug carrier at a target near the static magnet. For example, Marie et al. used a static external magnet to encourage magnetoliposome accumulation in glioblastoma tumors in mice (Marie et al., 2015) (Figure 3), while Huang et al. have developed and tested doxorubicin loaded magnetic micelles in a squamous cell carcinoma model in rabbits (C. Huang et al., 2012). Magnetic targeting has even been validated in humans. In fact, as early as 1996, Lübbe et al. showed that magnetic drug targeting could be used to concentrate epirubicin-conjugated nanoparticles in sarcomas in phase I and II clinical trials (A S Lübbe et al., 1996; Andreas S. Lübbe, Alexiou, & Bergemann, 2001).
Figure 3. Magnetic drug targeting of magnetic nanoparticle loaded liposomes has been used to target glioblastoma in mice.
Upon exposure to a 0.4 T external magnet, the iron oxide nanoparticle-loaded liposomes localized to the tumor in an orthotopic U87 glioblastoma mouse model (left). In contrast, in the absence of the external magnetic targeting field, fewer nanocarriers accumulated in the tumor (right). Scale = 1mm. From Marie et al. (2015).
However, the primary challenge of magnetic targeting is that magnetic gradients drop off rapidly with distance from the surface of a magnet. As a result, magnetic capture strategies are generally only able to target surface tumors. For example, in Lübbe et al.’s clinical studies, magnetic drug targeting was limited to tumors within 5 mm of the body surface (Andreas S. Lübbe et al., 2001). Rotariu et al. have also calculated that using small (< 500nm) particles with typical magnetic properties combined with the fields generated using typical permanent rare earth element magnets, only tumors within 18 mm of the surface can be targeted. For large particles up to 5μm, targeting is achievable up to depths of 15 cm (Rotariu & Strachan, 2005). Unfortunately, intravenously injected particles are typically designed to be < 200 nm to improve circulation time in the body (Bertrand & Leroux, 2012), rendering magnetic capture ineffective for targeting deep tissues.
With an eye on improving magnetic nanocarrier targeting, recent work has focused on using nanocarriers loaded with many magnetic nanoparticles to improve magnetic drug targeting by improving both the particle circulation time and its response to magnetic fields. For instance, Al-Jamal et al. have encapsulated SPIONs in PLGA-PEG at increasing concentrations to optimize magnetic nanocarrier formulations for magnetic drug targeting (Al-Jamal et al., 2016). Interestingly, they found that initially, increasing the SPION concentration within the nanocarrier improved tumor accumulation. However, past a certain limit, there was no further improvement in magnetic accumulation, which they attributed to decreased stability of the carrier resulting in decreased circulation time. Meanwhile, others have loaded cell-derived vesicles with magnetic nanoparticles and drug for cancer therapy: Silva et al. have shown that magnetic nanoparticles can be loaded into macrophage-derived microvesicles to enhance drug accumulation via magnetophoresis (Silva et al., 2015), while Qi et al. have used SPION-tagged, doxorubicin-loaded exosomes to target a hepatoma in a mouse model (Qi et al., 2016).
Improving magnetic targeting with steering coils and multi-magnet systems
In addition to designing better nanocarriers for magnetic targeting, numerous attempts have also been made to utilize steering coils and/or develop multi-magnet systems with unique geometries that can create large field gradients at greater depths within a patient. In one study, Pouponneau et al. utilized a catheter-based method to release magnetic particles in the vicinity of a tumor blood vessel. Steering coils were then used to guide the particles into the tumor (Pouponneau, Leroux, & Martel, 2009; Pouponneau, Leroux, Soulez, Gaboury, & Martel, 2011). However, despite using an extremely strong magnetic field from a modified MRI, this method still required a 50 μm particle (Pouponneau et al., 2009, 2011). Similar catheter-aided approaches have used particles up to 1.5 mm in diameter. Because of their size, these particles cannot pass through pulmonary circulation (Bertrand & Leroux, 2012), and therefore cannot be injected intravenously. Rather, it is necessary to inject these particles into the arterial circulation near the target site and catheter-based methods are invasive (Martel et al., 2007).
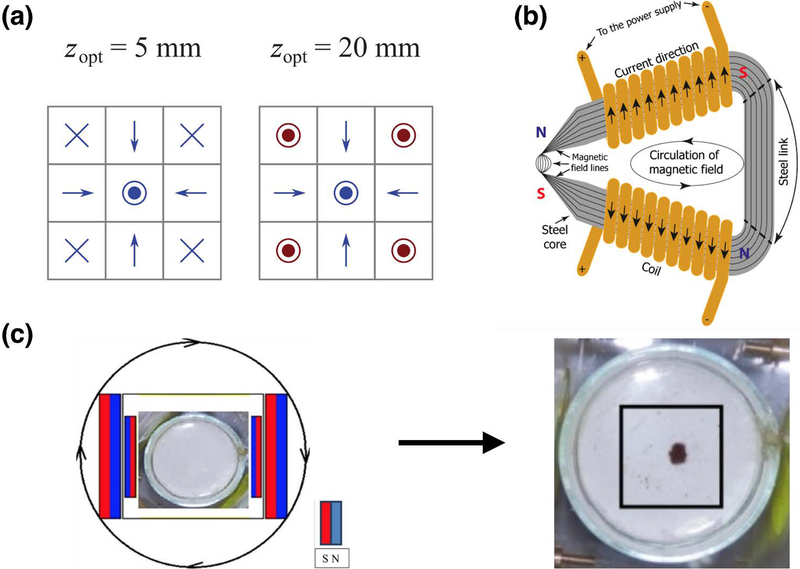
Recently, unique multi-magnet configurations have also been used to achieve higher targeting fields and “steer” particles through blood vessels to their target sites. For instance, multiple groups have used Halbach arrays (Figure 4a) to achieve strong local targeting fields (Barnsley, Carugo, Owen, & Stride, 2015; Shen et al., 2017). In one study, a linear Halbach array was used to trap magnetic microbubbles that were under a shear rate of 124.5 s−1 (Barnsley et al., 2015), while a cylindrical Halbach array has been used to enhance SPION-tagged neural progenitor cell accumulation in the brain (Shen et al., 2017). To achieve even higher magnetic gradients, some groups have also used steel yokes (Figure 4b) to “concentrate” the magnetic field and accumulate magnetic nanoparticles within a small region. Because magnetic fields preferentially pass through susceptible materials like steel, by applying a strong magnetic field to a steel yoke and tapering the steel, it is possible to generate strong gradients at the tip of the steel device for magnetic targeting (Voronin et al., 2017). As an alternative to permanent magnets, it is also possible to use electromagnetic coils that can be preferentially switched on, to generate complex patterned fields for greater spatial control of magnetic drug targeting (Hajiaghajani & Abdolali, 2018). However, while these methods are able to achieve strong magnetic gradients for targeting, they still tend to localize particles near the device, and therefore continue to be limited to use for drug targeting to surface regions. Interestingly, Krzyminiewski et al. have recently constructed a system of two sets of oppositely polarized magnets rotating about an axis that is able to drive magnetic nanoparticles toward the center of the device (Krzyminiewski, Dobosz, Schroeder, & Kurczewska, 2018). Although their approach has not yet been tested in tissue, the ability to accumulate magnetic particles away from the surface of a magnet would solve a long-standing problem in the field and could allow for non-invasive magnetic drug targeting to deep tissues (Figure 4c).
Figure 4. Novel magnetic targeting device designs.
(a) Halbach arrays can be used to achieve higher targeting fields compared to single magnets. Elements in the array can be manipulated (red) to optimize the magnitude of the magnetic flux at various distances from the array surface. From Barnsley et al. (2015). (b) A steel yoke design can be used to achieve extremely high magnetic fields within a small area by “concentrating” the magnetic field lines. From Voronin et al. (2017). (c) Recently, Krzyminiewski et al.have developed a system of rotating magnets to localize magnetic nanoparticles away from the surface of the device. From Krzyminiewski, Dobosz, Schroeder, & Kurczewska (2018).
MAGNETICALLY-INDUCED DRUG RELEASE
Once magnetic drug carriers accumulate within a tumor, an externally applied alternating magnetic field (AMF) can be coupled with SPIONs to generate heat. In the presence of an AMF, the SPIONs continually re-align their magnetic moment with the direction of the field, dissipating energy as heat in the process (Laurent et al., 2011). When the SPIONs are exposed to a magnetic field that changes rapidly, the SPIONs release sufficient heat to change their local temperature (Corot, Robert, Idée, & Port, 2006; Laurent et al., 2011). The heat can be harnessed for various applications, including induction of tumor cell death, either via hyperthermia (Hedayatnasab et al., 2017; Périgo et al., 2015) or ablation (Altanerova et al., 2017; Bobo, Robinson, Islam, Thurecht, & Corrie, 2016), as well as inducing release of tumor-targeting drugs from temperature-sensitive nanocarriers (Guisasola, Vallet-Regí, & Baeza, 2018; Moros et al., 2019).
A primary challenge of magnetic heating strategies for hyperthermia and ablation is that extremely high SPION concentrations are necessary to achieve therapeutic temperatures, typically in the range of 10–100 mg/mL (Laurent et al., 2011). As a result, current magnetic hyperthermia strategies often continue to rely on intratumoral injection to deliver a sufficient concentration of nanoparticles to the target site (Tay et al., 2018; Yoo et al., 2013). In contrast, with many thermally-responsive magnetic nanocarrier designs, an AMF can be used to trigger the release of drugs in the absence of high SPION concentrations, due to the strong temperature gradient at the surface of the magnetic nanoparticles. This is sometimes referred to as the “hot spot” effect. Various studies have shown experimentally that magnetic nanoparticles are able to generate extremely localized heating, with little to no effect on the global temperature (H. Huang, Delikanli, Zeng, Ferkey, & Pralle, 2010; Romero, Christiansen, Stocche Barbosa, Garcia, & Anikeeva, 2016). For example, in one study by Rühle et al., it was reported that the temperature 20nm from the SPION surface could be as high as 65°C, under conditions where there is no increase in the global temperature of the sample (Rühle, Datz, Argyo, Bein, & Zink, 2016). Others have reported similar findings, with temperatures reaching >43°C and triggering drug release at distance ~20nm from the SPION surface (Guisasola et al., 2015). However, some studies have reported more modest findings, with temperatures reaching only 45°C within 0.5nm of the SPION surface and dropping off exponentially with distance (Riedinger et al., 2013). In one study where DNA hybridization was used to assess temperature at the SPION surface, it was determined that the temperature only increased 8.3°C at a distance of 5nm from the nanoparticle surface. Of course, the change in temperature is highly dependent on the SAR of the SPION utilized, which could be at least partly responsible for the differences observed.
Thermally-responsive magnetic nanoparticles
Magnetic nanoparticles with thermally-sensitive bonds
Many different approaches have been taken to prepare thermally-responsive magnetic nanoparticles. Perhaps the most straight-forward approaches involve directly attaching drugs to magnetic nanoparticles via either covalent or non-covalent bonds that can be broken or destabilized when the temperature is increased (Moros et al., 2019). An advantage of non-covalent bonds is that the drug may not need to be modified (Mertz, Sandre, & Bégin-Colin, 2017; Moros et al., 2019). Non-covalent interactions can simply involve hydrogen bonding between the drug and SPION surface or polymer (Griffete et al., 2015; T.-J. Li et al., 2013), although this type of approach may be more prone to premature drug release. DNA hybridization has also been explored as an approach to non-covalently associate drugs with magnetic nanoparticles. The length and composition of the DNA strand can be tuned to precisely control the melting temperature and also allows for the use of multiple strands that can release different cargos at different temperatures (Banchelli et al., 2014; Derfus et al., 2007; Niemeyer, 2010).
Over the past decade, several thermally-sensitive covalent linkages have also been identified that cleave with an increase in temperature. One such bond can be formed via a thermally-reversible Diels-Alder reaction (N’Guyen et al., 2013; Xu, Zhu, Cui, Wojtas, & Zhang, 2013). Hammad et al. have used a Diels-Alder reaction to link doxorubicin to the surface of a core/shell particle, allowing for AMF-induced release of the drug to target HeLa cells in vitro (Hammad, Nica, & Hempelmann, 2017). Recently, Fan et al. have also synthesized SPION-containing micelles from a polymer containing a Diels-Alder bond. Upon exposure to an AMF, the SPIONs generated heat, cleaving the thermally labile bond to release cargo loaded within the micelle (Fan, Trant, Hemery, Sandre, & Gillies, 2017). An alternative type of bond involves the formation of thermally-labile azo linkages. For instance, Yoo et al. linked geldanamycin, a heat shock protein (HSP) inhibitor, to the surface of SPIONs via a thermally sensitive azo bond. The drug releasing particles were shown to be a more effective tumor therapy than non-drug releasing particles (Yoo et al., 2013).
Thermally labile bonds have also been used to “cap” drug-loaded mesoporous silica nanoparticles. One common strategy for doing so is to link a bulky group (e.g. small nanoparticle or polymer) to the surface of the mesoporous silica nanoparticle via a Diels-Alder reaction. When the nanocarrier is exposed to heat, the temperature sensitive bond is cleaved, releasing the cargo embedded in the carrier (Rühle et al., 2016). Others have also used thermally labile azo bonds for the same purpose (Saint-Cricq, Deshayes, Zink, & Kasko, 2015). Finally, a heat cleavable oligo (ethylene glycol) methyl ether methacrylate polymer has been used to coat a copper sulfide nanoparticle for thermally controlled release of the anesthetic bupivacaine, although this was demonstrated via near infrared irradiation, not magnetically-induced release (Ortiz de Solorzano et al., 2019).
Magnetic nanoparticles containing thermally-responsive materials
Both liposomes and micelles have been engineered to efficiently release their encapsulated cargo at elevated temperatures (Ganta, Devalapally, Shahiwala, & Amiji, 2008). Temperature sensitive micelles fall into two categories: lower critical solution temperature (LCST) formulations and upper critical solution temperature (UCST) formulations. In LCST micelles, the polymer becomes less water soluble as the solution temperature increases. Most LCST micelle formulations incorporate poly(N-isopropylacrylamide) (p(NIPAAm)) as their outer shell. Various groups have encapsulated magnetic nanoparticles in p(NIPAAm)-containing polymers for thermally controlled drug release (Patra et al., 2015; Pernia Leal et al., 2012; Yadavalli et al., 2015). Recently, Deng et al. have also developed a SPION-containing LCST micelle containing a novel star-block copolymer for AMF-triggered release of drug to target hepatocellular carcinoma cells in vitro (Deng et al., 2015). Similarly, Hervault et al. have coated an LCST polymer to the surface of a magnetic nanoparticle, allowing for magnetically triggered release of doxorubicin (Hervault et al., 2016).
UCST micelles disintegrate as the temperature is increased, releasing the encapsulated cargo (Jia, Chen, & Jiang, 2006; W. Li et al., 2015). Though UCST formulations are less well studied than LCST formulations, UCST micelles have begun to be applied for triggered drug release. In a recent study, magnetic nanoparticle loaded UCST micelles were used to deliver doxorubicin to tumors in vivo via microwave induced hyperthermia, and showed improved efficacy compared to controls (W.-S. Li et al., 2017). Although the thermal gradient in this study was not magnetically generated, the ability to encapsulated magnetic nanoparticles within the micelle indicates that it may be possible to use AMF-induced heating to trigger drug release from these nanocarrier formulations in the future.
In thermally-sensitive liposomes, the liposomal membrane usually becomes permeable as the temperature increases. Magnetoliposomes—liposomes containing magnetic particles—have been designed to co-deliver SPIONs with drug. For instance, Guo et al. have recently developed a doxorubicin-loaded dipalmoylphosphatidylcholine (DPPC)-based thermally sensitive liposomes with magnetic nanoparticles embedded in the lipid membrane for combined imaging, magnetic targeting, and magnetically triggered drug release in an in vivo mouse HeLa cell model (Guo et al., 2018). Similarly, Ferreira et al. have encapsulated hydrophilic magnetic nanoparticles within the lumen of a thermally sensitive liposome to magnetically trigger gemcitabine release from the nanocarrier (Ferreira et al., 2016).
Several thermally sensitive polymersome formulations have been developed that similarly rely on increasing membrane porosity with increasing temperature to allow for triggered drug release. Various groups have used polymersomes with membrane-embedded SPIONs for AMF-induced release of doxorubicin and fluorescent dyes (Bixner, Kurzhals, Virk, & Reimhult, 2016; Oliveira et al., 2013; Sanson et al., 2011). Though less common, the same strategy has been employed with hydrophobic SPIONs embedded in liposomal membranes (Y. Chen, Bose, & Bothun, 2010; Y. Chen et al., 2014).
Finally, although most studies use Néel relaxation to generate heat for thermally triggered drug release because it is independent of the viscosity of the medium, some groups have also reported that Brownian relaxation may be harnessed for drug release by mechanically disrupting the liposomal membrane. For example, Peiris et al. have developed a nanoparticle comprising three 10nm SPIONs linked in series to a 30nm liposome. This unique structure can be disrupted with a low-frequency AMF for drug delivery to glioblastoma tumors (Peiris et al., 2015). Others have also suggested that low-frequency AMFs can induce oscillations in SPIONs encapsulated in the lumen of liposomes to mechanically trigger drug release from the nanocarriers (Nappini, Bombelli, Bonini, Nordèn, & Baglioni, 2010; Spera et al., 2015).
Focusing of the alternating magnetic field
One challenge associated with AMF-induced magnetic hyperthermia and drug release is that SPIONs are typically most responsive to frequencies on the order of 100–500 kHz (R. Chen et al., 2013; Jang et al., 2009; Laurent et al., 2011; Mehdaoui et al., 2011). As electromagnetic waves, AMFs are limited by diffraction. Therefore, it is difficult to target AMFs for magnetic heating to resolutions of better than ~1 meter. As a result, it continues to be common practice to introduce particles to target tissues by direct injection before applying the AMF in order to avoid off-target effects (Laurent et al., 2011; Yoo et al., 2013), which negates the advantages of using magnetism and nanoparticles as a non-invasive therapeutic strategy. To improve the specificity of magnetically induced drug delivery, most strategies continue to rely on traditional biomolecular targeting strategies, such as small molecule (Pradhan et al., 2010) and antibody (Yang et al., 2016) targeting. Unfortunately, these methods are limited by the same challenges as typical biological strategies, including heterogeneous expression of ligands and receptors, and off-target binding (Muro, 2012; Tredan et al., 2007; Vaupel et al., 1989).
Unlike alternating fields, static fields have no inherent length scale and are not subject to diffraction. Therefore, one way to “focus” alternating magnetic fields is to superimpose a strong static field containing a field free region onto the AMF. SPIONs within the field free region will respond to the AMF, whereas particles outside of the field free region will have their magnetic moment pinned, preventing their response to the AMF (Figure 5a). This concept has been applied for magnetic particle imaging as well as magnetic hyperthermia. For example, Gleich and Weizenecker have used a static field containing a field-free point generated using two oppositely polarized static magnets to improve the resolution of magnetic particle imaging. By scanning the field-free region over the sample to be imaged, they were able to achieve an imaging resolution of 1 mm (Gleich & Weizenecker, 2005). Others have also applied the concept of a field free region to hyperthermia. For example, Ma et al. demonstrated spatially targeted magnetic hyperthermia in phantoms using a static field containing a field free region (Ma et al., 2015), while Tay et al. have recently demonstrated spatially specific, tumor targeted hyperthermia with 7 mm resolution in small animal models (Figure 5b) (Tay et al., 2018).
Figure 5. Strong static fields containing field-free regions can be used to target alternating magnetic fields for magnetic heating and drug release.
(a) In the low-field region, magnetic nanoparticles respond to the applied AMF to generate heat. Outside of the field-free region, the nanoparticles are “pinned” in the direction of the static field, preventing their response to the AMF and thereby suppressing heating. (b) Static fields containing field-free regions have been used for high-resolution magnetic particle imaging and targeted tissue ablation via magnetic hyperthermia. From Tay et al. (2018). (c) By adding a third magnet, it is possible to independently control the size and location of the field-free targeting region. From J. F. Liu et al. (2018).
Recently, we have expanded the use of static gating fields for spatial targeting of AMFs to trigger drug release from thermally sensitive liposomes using a three-magnet system (J. F. Liu et al., 2018). The presence of a third magnet “tethers” the field lines to allow for independent control of the size and location of the field free region (Figure 5c). Future work will focus on combining this targeting system with membrane-decorated liposomes and polymersomes for extremely localized drug release without requiring bulk hyperthermia.
BIOLOGICAL INTERACTIONS OF MAGNETIC NANOPARTICLES
Iron oxide has long been considered relatively safe(Arami, Khandhar, Liggitt, & Krishnan, 2015; Weissleder et al., 1989). However, in the last two decades, it has become increasingly clear that they can induce cytotoxicity and oxidative stress at high concentrations and with chronic exposure (Naqvi et al., 2010; Nemmar et al., 2015; N. Singh, Jenkins, Asadi, & Doak, 2010). Furthermore, the addition of dopants to improve heating properties may trade magnetic response for toxicity. To reduce toxicity, SPIONs are often coated with biocompatible polymers such as dextran, PEG, and PEI (Muthiah, Park, & Cho, 2013). There have also been attempts to encapsulate nanoparticles in lipids (H.-C. Huang et al., 2009; Liang, Zhang, Miao, Li, & Gan, 2017) and peptides (Chee et al., 2018) to reduce toxicity and improve circulation time. Others have also found that cytotoxicity is both coating and size dependent (Feng et al., 2018). Finally, although there have been many toxicity studies of SPIONs (with various coatings) in animal models, the safety in humans of these various formulations is less well established (Arami et al., 2015). To achieve high concentrations of SPIONs in target tissues without causing unintended local or systemic toxicity, further work is necessary to synthesize more biocompatible particle cores and coatings, and to establish the clinical safety of existing formulations.
Magnetic nanoparticles and nanocarriers have also been shown to interact with tumor associated immune cells. For instance, macrophages associated with breast cancer have been shown to selectively uptake ultrasmall SPIONs (Daldrup-Link et al., 2011). In another study, SPIONs were also tagged with glucan to specifically target tumor-associated macrophages to image liver metastases (Vu-Quang et al., 2012). Because tumor associated macrophages are generally considered a poor prognostic sign, both of these methods may allow for non-invasive predictions of outcome via imaging. Once taken up by tumor associated macrophages, SPIONs have also been shown to increase the inflammatory response by the macrophages, potentially harnessing the body’s immune system to improve tumor cell killing (Reichel, Tripathi, & Perez, 2019; Zanganeh et al., 2016). Finally, in preliminary studies, others have also attempted to drive immune cells (microglia) towards regions of interest by functionalizing them with magnetic nanoparticles (White et al., 2015). Future work may focus on modulating the SPION/immune system interaction to more effectively target tumor cells.
FUTURE DIRECTIONS
Challenges in nanoparticle synthesis
As described above, there has been significant recent advances in synthesizing magnetic nanoparticles with improved imaging and heating properties. The use of these particles promises to improve drug release from thermally sensitive nanocarriers. By decreasing both the field strengths and particle concentrations necessary to induce a temperature gradient, these developments put the goal of non-invasive, magnetically triggered drug release closer within reach. However, by virtue of being newer geometries, the synthesis methods for these novel particles are less well developed, less well understood, and less consistent than for traditional spherical iron oxide nanoparticles (Cotin et al., 2018). Answering these questions will be an important future step in making these particles easily usable for downstream applications.
Maximum amplitude of an alternating magnetic field
Because alternating magnetic fields can induce eddy currents in electrically conductive materials (such as human tissue), it has been well-established that the product of the amplitude of the alternating field, its frequency, and the square of the diameter of the coil used to apply the field should not exceed approximately 4 × 10^7 A⋅m/s (Atkinson, Brezovich, & Chakraborty, 1984; Laurent et al., 2011) for 1 hour. Because the frequency of the field is often set to match the particles’ optimum heating frequency, and the diameter of the coil is limited by the patient, the most easily tunable parameter affecting this limit is the amplitude of the field applied. Therefore, the development of more magnetically responsive particles will also allow for better heating, and therefore drug release, without encroaching upon this limit.
Developments in thermally-responsive nanocarriers
Thermally-sensitive SPION-loaded micelles are an attractive nanocarrier for triggered release of hydrophobic drugs via magnetic heating. In addition to having high encapsulation efficiencies compared to liposome and polymersome formulations, the high density of particles inside the micelle core would be more likely to rapidly generate the heat necessary to disrupt the membrane. Because hydrophobic SPIONs have already been encapsulated in micelles (McQuade et al., 2015; Nasongkla et al., 2006), the main barrier to AMF-induced release from magnetic micelles is the polymer formulation of the carrier. Unfortunately, many of these polymers have broad transition points (Roy, Brooks, & Sumerlin, 2013), which hinders rapid drug release from the micelles. Polymers with higher transition temperatures and sharper transition points would enable further development of thermally-sensitive drug-loaded magnetic micelles.
System scale-up
Another challenge for all magnetic systems is in scale-up of the system. Because static magnetic fields have no inherent length scale, the geometry of the magnetic fields does not change. However, achieving sufficiently high fields for targeted drug release and nanocarrier delivery requires replacing the static permanent magnets used in prototypes with costly high-power electromagnets. Fortunately, significant progress has been made in this area: Matter et al. have demonstrated that copper coils can be used as a resource-efficient means of generating strong fields over short timescales (Matter et al., 2006). Further work may help make scale-up more achievable and resource-efficient.
Conclusion
Magnetic fields and nanoparticles have been harnessed for therapy and drug delivery in cancer by increasing drug accumulation in tumor tissues, ablating diseased tissues, and triggering drug release in tissues. Recent developments have aimed to increase the spatial specificity of triggered drug release, as well as improve magnetic drug targeting to deep tissues. Future work is needed to continue to improve the properties of magnetic particles and drug carriers, as well as developing technologies for cost-effective scale-up of magnetic systems.
Vision and Challenges of Thermally Sensitive Nanocarriers.
Thermally sensitive nanocarriers are used in conjunction with alternating magnetic fields (AMFs) for magnetically triggered drug release. Thermally sensitive micelles can be modified to incorporate hydrophobic iron oxide nanoparticles into their core, while thermally sensitive liposomes and polymersomes can have hydrophilic or hydrophobic magnetic nanoparticles incorporated in the lumen or embedded in the lipid/polymer bilayer, respectively. When these nanoparticles are exposed to an alternating magnetic field, the rapid change in the direction of the field causes them to generate heat, which increases the temperature in the vicinity of the thermally sensitive nanocarrier, leading to drug release. Unfortunately, many magnetically triggered drug release strategies can be limited by the difficulty in focusing the alternating magnetic field to trigger spatially localized drug release.
A Scale-Up Challenge.
Magnetic fields are bio-orthogonal. Therefore, magnetic nanoparticles introduced into the body allow for high-contrast delivery of magnetic energy for drug delivery. Magnetic fields and nanoparticles are commonly used to trigger drug release from nanocarriers via magnetic hyperthermia, and to encourage drug accumulation at target sites via magnetic drug targeting. However, one major challenge that remains is difficulty in system scale-up. While small animal-sized device prototypes can be built using permanent magnets and benchtop amplifiers, building larger systems will likely require a shift to high-power electromagnets. Developments in coil technology are needed to make resource-efficient system scale-up possible.
Funding Information
This work was supported by the NIH/NIBIB (R21 EB023989; DI), the NIH/NCI (R01 CA181429; AT), NIH/NINDS (T32 NS091006; JFL), the Brain Research Foundation (AT), and the Brain and Behavior Research Foundation (AT).
Footnotes
Conflicts of Interest: None
Contributor Information
Jessica F. Liu, Department of Bioengineering, University of Pennsylvania, Philadelphia, PA 19104.
Bian Jang, Department of Bioengineering, University of Pennsylvania, Philadelphia, PA 19104.
David Issadore, Department of Bioengineering, University of Pennsylvania, Philadelphia, PA 19104.
Andrew Tsourkas, Department of Bioengineering, University of Pennsylvania, Philadelphia, PA 19104.
References
- Al-Jamal KT, Bai J, Wang JT-W, Protti A, Southern P, Bogart L, … Pankhurst QA (2016). Magnetic Drug Targeting: Preclinical in Vivo Studies, Mathematical Modeling, and Extrapolation to Humans. Nano Letters, 16(9), 5652–5660. 10.1021/acs.nanolett.6b02261 [DOI] [PubMed] [Google Scholar]
- Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, … Lübbe AS (2000). Locoregional cancer treatment with magnetic drug targeting. Cancer Research, 60(23), 6641–6648. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11118047 [PubMed] [Google Scholar]
- Altanerova U, Babincova M, Babinec P, Benejova K, Jakubechova J, Altanerova V, … Altaner C (2017). Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. International Journal of Nanomedicine, 12, 7923 10.2147/IJN.S145096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DL, Lipson RH (Robert H, & Nann T (2019). Comprehensive Nanoscience and Nanotechnology. [Google Scholar]
- Arami H, Khandhar A, Liggitt D, & Krishnan KM (2015). In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chemical Society Reviews, 44(23), 8576–8607. 10.1039/C5CS00541H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson WJ, Brezovich IA, & Chakraborty DP (1984). Usable frequencies in hyperthermia with thermal seeds. IEEE Transactions on Bio-Medical Engineering, 31(1), 70–75. 10.1109/TBME.1984.325372 [DOI] [PubMed] [Google Scholar]
- Bae YH, & Park K (2011). Targeted drug delivery to tumors: myths, reality and possibility. Journal of Controlled Release : Official Journal of the Controlled Release Society, 153(3), 198–205. 10.1016/j.jconrel.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchelli M, Nappini S, Montis C, Bonini M, Canton P, Berti D, & Baglioni P (2014). Magnetic nanoparticle clusters as actuators of ssDNA release. Physical Chemistry Chemical Physics, 16(21), 10023 10.1039/c3cp55470h [DOI] [PubMed] [Google Scholar]
- Barnsley LC, Carugo D, Owen J, & Stride E (2015). Halbach arrays consisting of cubic elements optimised for high field gradients in magnetic drug targeting applications. Physics in Medicine and Biology, 60(21), 8303–8327. 10.1088/0031-9155/60/21/8303 [DOI] [PubMed] [Google Scholar]
- Baronzio G, Parmar G, & Baronzio M (2015). Overview of Methods for Overcoming Hindrance to Drug Delivery to Tumors, with Special Attention to Tumor Interstitial Fluid. Frontiers in Oncology, 5, 165 10.3389/fonc.2015.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, & Leroux J-C (2012). The journey of a drug-carrier in the body: An anatomo-physiological perspective. Journal of Controlled Release, 161(2), 152–163. 10.1016/J.JCONREL.2011.09.098 [DOI] [PubMed] [Google Scholar]
- Bixner O, Kurzhals S, Virk M, & Reimhult E (2016). Triggered Release from Thermoresponsive Polymersomes with Superparamagnetic Membranes. Materials, 9(1), 29 Retrieved from http://www.mdpi.com/1996-1944/9/1/29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Andujar C, Ortega D, Southern P, Pankhurst QA, & Thanh NTK (2015). High performance multi-core iron oxide nanoparticles for magnetic hyperthermia: microwave synthesis, and the role of core-to-core interactions. Nanoscale, 7(5), 1768–1775. 10.1039/C4NR06239F [DOI] [PubMed] [Google Scholar]
- Bobo D, Robinson KJ, Islam J, Thurecht KJ, & Corrie SR (2016). Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharmaceutical Research, 33(10), 2373–2387. 10.1007/s11095-016-1958-5 [DOI] [PubMed] [Google Scholar]
- Boissenot T, Bordat A, Fattal E, & Tsapis N (2016). Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: From theoretical considerations to practical applications. Journal of Controlled Release, 241, 144–163. 10.1016/J.JCONREL.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Chang D, Lim M, Goos JACM, Qiao R, Ng YY, Mansfeld FM, … Kavallaris M (2018). Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Frontiers in Pharmacology, 9, 831 10.3389/fphar.2018.00831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee HL, Gan CRR, Ng M, Low L, Fernig DG, Bhakoo KK, & Paramelle D (2018). Biocompatible Peptide-Coated Ultrasmall Superparamagnetic Iron Oxide Nanoparticles for In Vivo Contrast-Enhanced Magnetic Resonance Imaging. ACS Nano, 12(7), 6480–6491. 10.1021/acsnano.7b07572 [DOI] [PubMed] [Google Scholar]
- Chen B, Dai W, He B, Zhang H, Wang X, Wang Y, & Zhang Q (2017). Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment. Theranostics, 7(3), 538–558. 10.7150/thno.16684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Christiansen MG, & Anikeeva P (2013). Maximizing hysteretic losses in magnetic ferrite nanoparticles via model-driven synthesis and materials optimization. ACS Nano, 7(10), 8990–9000. 10.1021/nn4035266 [DOI] [PubMed] [Google Scholar]
- Chen Y, Bose A, & Bothun GD (2010). Controlled Release from Bilayer-Decorated Magnetoliposomes via Electromagnetic Heating. ACS Nano, 4(6), 3215–3221. 10.1021/nn100274v [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen Y, Xiao D, Bose A, Deng R, & Bothun GD (2014). Low-dose chemotherapy of hepatocellular carcinoma through triggered-release from bilayer-decorated magnetoliposomes. Colloids and Surfaces. B, Biointerfaces, 116, 452–458. 10.1016/j.colsurfb.2014.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CJ, Tietjen GT, Saucier-Sawyer JK, & Saltzman WM (2015). A holistic approach to targeting disease with polymeric nanoparticles. Nature Reviews Drug Discovery, 14(4), 239–247. 10.1038/nrd4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corot C, Robert P, Idée J-M, & Port M (2006). Recent advances in iron oxide nanocrystal technology for medical imaging. Advanced Drug Delivery Reviews, 58(14), 1471–1504. 10.1016/j.addr.2006.09.013 [DOI] [PubMed] [Google Scholar]
- Cotin G, Kiefer C, Perton F, Ihiawakrim D, Blanco-Andujar C, Moldovan S, … Bégin-Colin S (2018). Unravelling the Thermal Decomposition Parameters for The Synthesis of Anisotropic Iron Oxide Nanoparticles. Nanomaterials (Basel, Switzerland), 8(11). 10.3390/nano8110881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio A, Silva AKA, Cabana S, Espinosa A, Baptiste B, Menguy N, … Abou-Hassan A (2019). Iron Oxide Nanoflowers @ CuS Hybrids for Cancer Tri-Therapy: Interplay of Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy. Theranostics, 9(5), 1288–1302. 10.7150/thno.30238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldrup-Link HE, Golovko D, Ruffell B, Denardo DG, Castaneda R, Ansari C, … Coussens LM (2011). MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research, 17(17), 5695–5704. 10.1158/1078-0432.CCR-10-3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Ren J, Li J, Leng J, Qu Y, Lin C, & Shi D (2015). Magnetothermally responsive star-block copolymeric micelles for controlled drug delivery and enhanced thermo-chemotherapy. Nanoscale, 7(21), 9655–9663. 10.1039/C5NR00642B [DOI] [PubMed] [Google Scholar]
- Derfus AM, von Maltzahn G, Harris TJ, Duza T, Vecchio KS, Ruoslahti E, & Bhatia SN (2007). Remotely Triggered Release from Magnetic Nanoparticles. Advanced Materials, 19(22), 3932–3936. 10.1002/adma.200700091 [DOI] [Google Scholar]
- Dewhirst MW, Lee C-T, & Ashcraft KA (2016). The future of biology in driving the field of hyperthermia. International Journal of Hyperthermia, 32(1), 4–13. 10.3109/02656736.2015.1091093 [DOI] [PubMed] [Google Scholar]
- Duro-Castano A, Movellan J, & Vicent MJ (2015). Smart branched polymer drug conjugates as nano-sized drug delivery systems. Biomaterials Science, 3(10), 1321–1334. 10.1039/C5BM00166H [DOI] [PubMed] [Google Scholar]
- El-Dek SI, Ali MA, El-Zanaty SM, & Ahmed SE (2018). Comparative investigations on ferrite nanocomposites for magnetic hyperthermia applications. Journal of Magnetism and Magnetic Materials, 458, 147–155. 10.1016/J.JMMM.2018.02.052 [DOI] [Google Scholar]
- Fan B, Trant JF, Hemery G, Sandre O, & Gillies ER (2017). Thermo-responsive self-immolative nanoassemblies: direct and indirect triggering. Chemical Communications, 53(89), 12068–12071. 10.1039/C7CC06410A [DOI] [PubMed] [Google Scholar]
- Feng Q, Liu Y, Huang J, Chen K, Huang J, & Xiao K (2018). Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Scientific Reports, 8(1), 2082 10.1038/s41598-018-19628-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira RV, Martins TM da M, Goes AM, Fabris JD, Cavalcante LCD, Outon LEF, & Domingues RZ (2016). Thermosensitive gemcitabine-magnetoliposomes for combined hyperthermia and chemotherapy. Nanotechnology, 27(8), 085105 10.1088/0957-4484/27/8/085105 [DOI] [PubMed] [Google Scholar]
- Ganta S, Devalapally H, Shahiwala A, & Amiji M (2008). A review of stimuli-responsive nanocarriers for drug and gene delivery. Journal of Controlled Release, 126(3), 187–204. 10.1016/J.JCONREL.2007.12.017 [DOI] [PubMed] [Google Scholar]
- Gleich B, & Weizenecker J (2005). Tomographic imaging using the nonlinear response of magnetic particles. Nature, 435(7046), 1214–1217. 10.1038/nature03808 [DOI] [PubMed] [Google Scholar]
- Goodman TT, Olive PL, & Pun SH (2007). Increased nanoparticle penetration in collagenase-treated multicellular spheroids. International Journal of Nanomedicine, 2(2), 265–274. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17722554 [PMC free article] [PubMed] [Google Scholar]
- Griffete N, Fresnais J, Espinosa A, Wilhelm C, Bée A, & Ménager C (2015). Design of magnetic molecularly imprinted polymer nanoparticles for controlled release of doxorubicin under an alternative magnetic field in athermal conditions. Nanoscale, 7(45), 18891–18896. 10.1039/C5NR06133D [DOI] [PubMed] [Google Scholar]
- Guardia P, Di Corato R, Lartigue L, Wilhelm C, Espinosa A, Garcia-Hernandez M, … Pellegrino T (2012). Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano, 6(4), 3080–3091. 10.1021/nn2048137 [DOI] [PubMed] [Google Scholar]
- Guisasola E, Baeza A, Talelli M, Arcos D, Moros M, de la Fuente JM, & Vallet-Regí M (2015). Magnetic-Responsive Release Controlled by Hot Spot Effect. Langmuir, 31(46), 12777–12782. 10.1021/acs.langmuir.5b03470 [DOI] [PubMed] [Google Scholar]
- Guisasola E, Vallet-Regí M, & Baeza A (2018). Magnetically responsive polymers for drug delivery applications. Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications , Volume 1, 143–168. 10.1016/B978-0-08-101997-9.00008-4 [DOI] [Google Scholar]
- Guo Y, Zhang Y, Ma J, Li Q, Li Y, Zhou X, … Zhu X (2018). Light/magnetic hyperthermia triggered drug released from multi-functional thermo-sensitive magnetoliposomes for precise cancer synergetic theranostics. Journal of Controlled Release, 272, 145–158. 10.1016/J.JCONREL.2017.04.028 [DOI] [PubMed] [Google Scholar]
- Hajiaghajani A, & Abdolali A (2018). Magnetic field pattern synthesis and its application in targeted drug delivery: Design and implementation. Bioelectromagnetics, 39(4), 325–338. 10.1002/bem.22107 [DOI] [PubMed] [Google Scholar]
- Hammad M, Nica V, & Hempelmann R (2017). On-command controlled drug release by diels-Alder reaction using Bi-magnetic core/shell nano-carriers. Colloids and Surfaces B: Biointerfaces, 150, 15–22. 10.1016/j.colsurfb.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Haume K, Rosa S, Grellet S, Śmiałek MA, Butterworth KT, Solov’yov AV, … Mason NJ (2016). Gold nanoparticles for cancer radiotherapy: a review. Cancer Nanotechnology, 7(1), 8 10.1186/s12645-016-0021-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedayatnasab Z, Abnisa F, & Daud WMAW (2017). Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Materials & Design, 123, 174–196. 10.1016/J.MATDES.2017.03.036 [DOI] [Google Scholar]
- Hemery G, Keyes AC, Garaio E, Rodrigo I, Garcia JA, Plazaola F, … Sandre O (2017). Tuning Sizes, Morphologies, and Magnetic Properties of Monocore Versus Multicore Iron Oxide Nanoparticles through the Controlled Addition of Water in the Polyol Synthesis. Inorganic Chemistry, 56(14), 8232–8243. 10.1021/acs.inorgchem.7b00956 [DOI] [PubMed] [Google Scholar]
- Hervault A, Dunn AE, Lim M, Boyer C, Mott D, Maenosono S, & Thanh NTK (2016). Doxorubicin loaded dual pH- and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale, 8(24), 12152–12161. 10.1039/C5NR07773G [DOI] [PubMed] [Google Scholar]
- Hörmann K, & Zimmer A (2016). Drug delivery and drug targeting with parenteral lipid nanoemulsions — A review. Journal of Controlled Release, 223, 85–98. 10.1016/J.JCONREL.2015.12.016 [DOI] [PubMed] [Google Scholar]
- Huang C, Tang Z, Zhou Y, Zhou X, Jin Y, Li D, … Zhou S (2012). Magnetic micelles as a potential platform for dual targeted drug delivery in cancer therapy. International Journal of Pharmaceutics, 429(1–2), 113–122. 10.1016/J.IJPHARM.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Huang H-C, Chang P-Y, Chang K, Chen C-Y, Lin C-W, Chen J-H, … Chang F-H (2009). Formulation of novel lipid-coated magnetic nanoparticles as the probe for in vivo imaging. Journal of Biomedical Science, 16(1), 86 10.1186/1423-0127-16-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Delikanli S, Zeng H, Ferkey DM, & Pralle A (2010). Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nature Nanotechnology, 5(8), 602–606. 10.1038/nnano.2010.125 [DOI] [PubMed] [Google Scholar]
- Hufschmid R, Arami H, Ferguson RM, Gonzales M, Teeman E, Brush LN, … Krishnan KM (2015). Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale, 7(25), 11142–11154. 10.1039/C5NR01651G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J, Nah H, Lee J-H, Moon SHH, Kim MGG, & Cheon J (2009). Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angewandte Chemie (International Ed. in English), 48(7), 1234–1238. 10.1002/anie.200805149 [DOI] [PubMed] [Google Scholar]
- Jia X, Chen D, & Jiang M (2006). Preparation of PEO-b-P2VPH + –S 2 O 8 2− micelles in water and their reversible UCST and redox-responsive behavior. Chem. Commun, 0(16), 1736–1738. 10.1039/B600859C [DOI] [PubMed] [Google Scholar]
- Kanamala M, Wilson WR, Yang M, Palmer BD, & Wu Z (2016). Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials, 85, 152–167. 10.1016/J.BIOMATERIALS.2016.01.061 [DOI] [PubMed] [Google Scholar]
- Kim D, Lee J, Lee S, Park J, & Lee D (2016). Predicting unintended effects of drugs based on off-target tissue effects. Biochemical and Biophysical Research Communications, 469(3), 399–404. 10.1016/J.BBRC.2015.11.095 [DOI] [PubMed] [Google Scholar]
- Krzyminiewski R, Dobosz B, Schroeder G, & Kurczewska J (2018). Focusing of Fe3O4 nanoparticles using a rotating magnetic field in various environments. Physics Letters A, 382(44), 3192–3196. 10.1016/J.PHYSLETA.2018.07.051 [DOI] [Google Scholar]
- Lartigue L, Hugounenq P, Alloyeau D, Clarke SP, Lévy M, Bacri J-C, … Gazeau F (2012). Cooperative Organization in Iron Oxide Multi-Core Nanoparticles Potentiates Their Efficiency as Heating Mediators and MRI Contrast Agents. ACS Nano, 6(12), 10935–10949. 10.1021/nn304477s [DOI] [PubMed] [Google Scholar]
- Laurent S, Dutz S, Häfeli UO, & Mahmoudi M (2011). Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Advances in Colloid and Interface Science, 166(1–2), 8–23. 10.1016/j.cis.2011.04.003 [DOI] [PubMed] [Google Scholar]
- Li T-J, Huang C-C, Ruan P-W, Chuang K-Y, Huang K-J, Shieh D-B, & Yeh C-S (2013). In vivo anti-cancer efficacy of magnetite nanocrystal - based system using locoregional hyperthermia combined with 5-fluorouracil chemotherapy. Biomaterials, 34(32), 7873–7883. 10.1016/J.BIOMATERIALS.2013.07.012 [DOI] [PubMed] [Google Scholar]
- Li W-S, Wang X-J, Zhang S, Hu J-B, Du Y-L, Kang X-Q, … Du Y-Z (2017). Mild microwave activated, chemo-thermal combinational tumor therapy based on a targeted, thermal-sensitive and magnetic micelle. Biomaterials, 131, 36–46. 10.1016/j.biomaterials.2017.03.048 [DOI] [PubMed] [Google Scholar]
- Li W, Huang L, Ying X, Jian Y, Hong Y, Hu F, & Du Y (2015). Antitumor Drug Delivery Modulated by A Polymeric Micelle with an Upper Critical Solution Temperature. Angewandte Chemie International Edition, 54(10), 3126–3131. 10.1002/anie.201411524 [DOI] [PubMed] [Google Scholar]
- Li Y, Li N, Pan W, Yu Z, Yang L, & Tang B (2017). Hollow Mesoporous Silica Nanoparticles with Tunable Structures for Controlled Drug Delivery. ACS Applied Materials & Interfaces, 9(3), 2123–2129. 10.1021/acsami.6b13876 [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang X, Miao Y, Li J, & Gan Y (2017). Lipid-coated iron oxide nanoparticles for dual-modal imaging of hepatocellular carcinoma. International Journal of Nanomedicine, 12, 2033–2044. 10.2147/IJN.S128525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley CS, & Wu BM (2017). Recent advances in light-responsive on-demand drug-delivery systems. Therapeutic Delivery, 8(2), 89–107. 10.4155/tde-2016-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JF, Neel N, Dang P, Lamb M, McKenna J, Rodgers L, … Issadore D (2018). Radiofrequency-Triggered Drug Release from Nanoliposomes with Millimeter-Scale Resolution Using a Superimposed Static Gating Field. Small (Weinheim an Der Bergstrasse, Germany), e1802563 10.1002/smll.201802563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Ng CT, Chandrasekharan P, Yang HT, Zhao LY, Peng E, … Fan HM (2016). Synthesis of Ferromagnetic Fe 0.6 Mn 0.4 O Nanoflowers as a New Class of Magnetic Theranostic Platform for In Vivo T 1 -T 2 Dual-Mode Magnetic Resonance Imaging and Magnetic Hyperthermia Therapy. Advanced Healthcare Materials, 5(16), 2092–2104. 10.1002/adhm.201600357 [DOI] [PubMed] [Google Scholar]
- Lübbe AS, Bergemann C, Riess H, Schriever F, Reichardt P, Possinger K, … Huhn D (1996). Clinical experiences with magnetic drug targeting: a phase I study with 4’-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Research, 56(20), 4686–4693. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8840985 [PubMed] [Google Scholar]
- Lübbe Andreas S., Alexiou C, & Bergemann C (2001). Clinical Applications of Magnetic Drug Targeting. Journal of Surgical Research, 95(2), 200–206. 10.1006/JSRE.2000.6030 [DOI] [PubMed] [Google Scholar]
- Ma M, Zhang Y, Shen X, Xie J, Li Y, & Gu N (2015). Targeted inductive heating of nanomagnets by a combination of alternating current (AC) and static magnetic fields. Nano Research, 8(2), 600–610. 10.1007/s12274-015-0729-7 [DOI] [Google Scholar]
- Maeda H (2015). Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Advanced Drug Delivery Reviews, 91, 3–6. 10.1016/J.ADDR.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Sant S, Wang B, Laurent S, & Sen T (2011). Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Advanced Drug Delivery Reviews, 63(1–2), 24–46. 10.1016/J.ADDR.2010.05.006 [DOI] [PubMed] [Google Scholar]
- Marie H, Lemaire L, Franconi F, Lajnef S, Frapart Y-M, Nicolas V, … Lesieur S (2015). Superparamagnetic Liposomes for MRI Monitoring and External Magnetic Field-Induced Selective Targeting of Malignant Brain Tumors. Advanced Functional Materials, 25(8), 1258–1269. 10.1002/adfm.201402289 [DOI] [Google Scholar]
- Martel S, Mathieu J-B, Felfoul O, Chanu A, Aboussouan E, Tamaz S, … Mankiewicz M (2007). Automatic navigation of an untethered device in the artery of a living animal using a conventional clinical magnetic resonance imaging system. Applied Physics Letters, 90(11), 114105 10.1063/1.2713229 [DOI] [Google Scholar]
- Matter NI, Scott GC, Venook RD, Ungersma SE, Grafendorfer T, Macovski A, & Conolly SM (2006). Three-dimensional prepolarized magnetic resonance imaging using rapid acquisition with relaxation enhancement. Magnetic Resonance in Medicine, 56(5), 1085–1095. 10.1002/mrm.21065 [DOI] [PubMed] [Google Scholar]
- McQuade C, Al Zaki A, Desai Y, Vido M, Sakhuja T, Cheng Z, … Tsourkas A (2015). A Multifunctional Nanoplatform for Imaging, Radiotherapy, and the Prediction of Therapeutic Response. Small, 11(7), 834–843. 10.1002/smll.201401927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdaoui B, Meffre A, Carrey J, Lachaize S, Lacroix L-M, Gougeon M, … Respaud M (2011). Optimal Size of Nanoparticles for Magnetic Hyperthermia: A Combined Theoretical and Experimental Study. Advanced Functional Materials, 21(23), 4573–4581. 10.1002/adfm.201101243 [DOI] [Google Scholar]
- Merino S, Martín C, Kostarelos K, Prato M, & Vázquez E (2015). Nanocomposite Hydrogels: 3D Polymer–Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano, 9(5), 4686–4697. 10.1021/acsnano.5b01433 [DOI] [PubMed] [Google Scholar]
- Mertz D, Sandre O, & Bégin-Colin S (2017). Drug releasing nanoplatforms activated by alternating magnetic fields. Biochimica et Biophysica Acta (BBA) - General Subjects, 1861(6), 1617–1641. 10.1016/J.BBAGEN.2017.02.025 [DOI] [PubMed] [Google Scholar]
- Moros M, Idiago-López J, Asín L, Moreno-Antolín E, Beola L, Grazú V, … de la Fuente JM (2019). Triggering antitumoural drug release and gene expression by magnetic hyperthermia. Advanced Drug Delivery Reviews, 138, 326–343. 10.1016/J.ADDR.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Mura S, Nicolas J, & Couvreur P (2013). Stimuli-responsive nanocarriers for drug delivery. Nature Materials, 12(11), 991–1003. 10.1038/nmat3776 [DOI] [PubMed] [Google Scholar]
- Muro S (2012). Challenges in design and characterization of ligand-targeted drug delivery systems. Journal of Controlled Release, 164(2), 125–137. 10.1016/J.JCONREL.2012.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthiah M, Park I-K, & Cho C-S (2013). Surface modification of iron oxide nanoparticles by biocompatible polymers for tissue imaging and targeting. Biotechnology Advances, 31(8), 1224–1236. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23528431 [DOI] [PubMed] [Google Scholar]
- N’Guyen TTT, Duong HTT, Basuki J, Montembault V, Pascual S, Guibert C, … Fontaine L (2013). Functional Iron Oxide Magnetic Nanoparticles with Hyperthermia-Induced Drug Release Ability by Using a Combination of Orthogonal Click Reactions. Angewandte Chemie International Edition, 52(52), 14152–14156. 10.1002/anie.201306724 [DOI] [PubMed] [Google Scholar]
- Nappini S, Bombelli FB, Bonini M, Nordèn B, & Baglioni P (2010). Magnetoliposomes for controlled drug release in the presence of low-frequency magnetic field. Soft Matter, 6(1), 154–162. 10.1039/B915651H [DOI] [Google Scholar]
- Naqvi S, Samim M, Abdin M, Ahmed FJ, Maitra A, Prashant C, & Dinda AK (2010). Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. International Journal of Nanomedicine, 5, 983–989. 10.2147/IJN.S13244 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, … Gao J (2006). Multifunctional Polymeric Micelles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems. Nano Letters, 6(11), 2427–2430. 10.1021/NL061412U [DOI] [PubMed] [Google Scholar]
- Nemmar A, Beegam S, Yuvaraju P, Yasin J, Tariq S, Attoub S, & Ali BH (2015). Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Particle and Fibre Toxicology, 13(1), 22 10.1186/s12989-016-0132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer CM (2010). Semisynthetic DNA-Protein Conjugates for Biosensing and Nanofabrication. Angewandte Chemie International Edition, 49(7), 1200–1216. 10.1002/anie.200904930 [DOI] [PubMed] [Google Scholar]
- Noh S-H, Na W, Jang J-T, Lee J-H, Lee EJ, Moon SH, … Cheon J (2012). Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Letters, 12(7), 3716–3721. 10.1021/nl301499u [DOI] [PubMed] [Google Scholar]
- Ojha T, Pathak V, Shi Y, Hennink WE, Moonen CTW, Storm G, … Lammers T (2017). Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Advanced Drug Delivery Reviews, 119, 44–60. 10.1016/j.addr.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira H, Pérez-Andrés E, Thevenot J, Sandre O, Berra E, & Lecommandoux S (2013). Magnetic field triggered drug release from polymersomes for cancer therapeutics. Journal of Controlled Release, 169(3), 165–170. 10.1016/J.JCONREL.2013.01.013 [DOI] [PubMed] [Google Scholar]
- Ortiz de Solorzano I, Alejo T, Abad M, Bueno-Alejo C, Mendoza G, Andreu V, … Arruebo M (2019). Cleavable and thermo-responsive hybrid nanoparticles for on-demand drug delivery. Journal of Colloid and Interface Science, 533, 171–181. 10.1016/j.jcis.2018.08.069 [DOI] [PubMed] [Google Scholar]
- Patra S, Roy E, Karfa P, Kumar S, Madhuri R, & Sharma PK (2015). Dual-Responsive Polymer Coated Superparamagnetic Nanoparticle for Targeted Drug Delivery and Hyperthermia Treatment. ACS Applied Materials & Interfaces, 7(17), 9235–9246. 10.1021/acsami.5b01786 [DOI] [PubMed] [Google Scholar]
- Peiris PM, Abramowski A, Mcginnity J, Doolittle E, Toy R, Gopalakrishnan R, … Karathanasis E (2015). Treatment of Invasive Brain Tumors Using a Chain-like Nanoparticle. Cancer Research, 75(7), 1356–1365. 10.1158/0008-5472.CAN-14-1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périgo EA, Hemery G, Sandre O, Ortega D, Garaio E, Plazaola F, & Teran FJ (2015). Fundamentals and advances in magnetic hyperthermia. Applied Physics Reviews, 2(4), 041302 10.1063/1.4935688 [DOI] [Google Scholar]
- Pernia Leal M, Torti A, Riedinger A, La Fleur R, Petti D, Cingolani R, … Pellegrino T (2012). Controlled Release of Doxorubicin Loaded within Magnetic Thermo-responsive Nanocarriers under Magnetic and Thermal Actuation in a Microfluidic Channel. ACS Nano, 6(12), 10535–10545. 10.1021/nn3028425 [DOI] [PubMed] [Google Scholar]
- Pouponneau P, Leroux J-C, & Martel S (2009). Magnetic nanoparticles encapsulated into biodegradable microparticles steered with an upgraded magnetic resonance imaging system for tumor chemoembolization. Biomaterials, 30(31), 6327–6332. 10.1016/J.BIOMATERIALS.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Pouponneau P, Leroux J-C, Soulez G, Gaboury L, & Martel S (2011). Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials, 32(13), 3481–3486. 10.1016/J.BIOMATERIALS.2010.12.059 [DOI] [PubMed] [Google Scholar]
- Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Döblinger M, … Plank C (2010). Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. Journal of Controlled Release : Official Journal of the Controlled Release Society, 142(1), 108–121. 10.1016/j.jconrel.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, … Kang C (2016). Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano, 10(3), 3323–3333. 10.1021/acsnano.5b06939 [DOI] [PubMed] [Google Scholar]
- Reichel D, Tripathi M, & Perez JM (2019). Biological Effects of Nanoparticles on Macrophage Polarization in the Tumor Microenvironment. Nanotheranostics, 3(1), 66–88. 10.7150/ntno.30052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedinger A, Guardia P, Curcio A, Garcia MA, Cingolani R, Manna L, & Pellegrino T (2013). Subnanometer Local Temperature Probing and Remotely Controlled Drug Release Based on Azo-Functionalized Iron Oxide Nanoparticles. Nano Letters, 13(6), 2399–2406. 10.1021/nl400188q [DOI] [PubMed] [Google Scholar]
- Roch A, Muller RN, & Gillis P (1999). Theory of proton relaxation induced by superparamagnetic particles. The Journal of Chemical Physics, 110(11), 5403 10.1063/1.478435 [DOI] [Google Scholar]
- Rohovie MJ, Nagasawa M, & Swartz JR (2017). Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioengineering & Translational Medicine, 2(1), 43–57. 10.1002/btm2.10049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero G, Christiansen MG, Stocche Barbosa L, Garcia F, & Anikeeva P (2016). Localized Excitation of Neural Activity via Rapid Magnetothermal Drug Release. Advanced Functional Materials, 26(35), 6471–6478. 10.1002/adfm.201602189 [DOI] [Google Scholar]
- Rosensweig RE (2002). Heating magnetic fluid with alternating magnetic field. Journal of Magnetism and Magnetic Materials, 252, 370–374. 10.1016/S0304-8853(02)00706-0 [DOI] [Google Scholar]
- Rotariu O, & Strachan NJC (2005). Modelling magnetic carrier particle targeting in the tumor microvasculature for cancer treatment. Journal of Magnetism and Magnetic Materials, 293(1), 639–646. 10.1016/J.JMMM.2005.01.081 [DOI] [Google Scholar]
- Roy D, Brooks WLA, & Sumerlin BS (2013). New directions in thermoresponsive polymers. Chemical Society Reviews, 42(17), 7214 10.1039/c3cs35499g [DOI] [PubMed] [Google Scholar]
- Rühle B, Datz S, Argyo C, Bein T, & Zink JI (2016). A molecular nanocap activated by superparamagnetic heating for externally stimulated cargo release. Chemical Communications, 52(9), 1843–1846. 10.1039/C5CC08636A [DOI] [PubMed] [Google Scholar]
- Saint-Cricq P, Deshayes S, Zink JI, & Kasko AM (2015). Magnetic field activated drug delivery using thermodegradable azo-functionalised PEG-coated core–shell mesoporous silica nanoparticles. Nanoscale, 7(31), 13168–13172. 10.1039/C5NR03777H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Moreno P, de Vicente J, Nardecchia S, Marchal JA, & Boulaiz H (2018). Thermo-Sensitive Nanomaterials: Recent Advance in Synthesis and Biomedical Applications. Nanomaterials (Basel, Switzerland), 8(11). 10.3390/nano8110935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson C, Diou O, Thévenot J, Ibarboure E, Soum A, Brûlet A, … Lecommandoux S (2011). Doxorubicin Loaded Magnetic Polymersomes: Theranostic Nanocarriers for MR Imaging and Magneto-Chemotherapy. ACS Nano, 5(2), 1122–1140. 10.1021/nn102762f [DOI] [PubMed] [Google Scholar]
- Sensenig R, Sapir Y, MacDonald C, Cohen S, & Polyak B (2012). Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine (London, England), 7(9), 1425–1442. 10.2217/nnm.12.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B, Kulkarni S, Nacev A, Muro S, Stepanov PY, & Weinberg IN (2015). Open challenges in magnetic drug targeting. Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology, 7(3), 446–457. 10.1002/wnan.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Gothwal A, Kesharwani P, Alsaab H, Iyer AK, & Gupta U (2017). Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discovery Today, 22(2), 314–326. 10.1016/J.DRUDIS.2016.09.013 [DOI] [PubMed] [Google Scholar]
- Sharma H, Mishra PK, Talegaonkar S, & Vaidya B (2015). Metal nanoparticles: a theranostic nanotool against cancer. Drug Discovery Today, 20(9), 1143–1151. 10.1016/J.DRUDIS.2015.05.009 [DOI] [PubMed] [Google Scholar]
- Shen W-B, Anastasiadis P, Nguyen B, Yarnell D, Yarowsky PJ, Frenkel V, & Fishman PS (2017). Magnetic Enhancement of Stem Cell–Targeted Delivery into the Brain Following MR-Guided Focused Ultrasound for Opening the Blood–Brain Barrier. Cell Transplantation, 26(7), 1235–1246. 10.1177/0963689717715824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AKA, Luciani N, Gazeau F, Aubertin K, Bonneau S, Chauvierre C, … Wilhelm C (2015). Combining magnetic nanoparticles with cell derived microvesicles for drug loading and targeting. Nanomedicine : Nanotechnology, Biology, and Medicine, 11(3), 645–655. 10.1016/j.nano.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Singh N, Jenkins GJS, Asadi R, & Doak SH (2010). Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Reviews, 1(1), 5358 10.3402/nano.v1i0.5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, & Lillard JW (2009). Nanoparticle-based targeted drug delivery. Experimental and Molecular Pathology, 86(3), 215–223. 10.1016/J.YEXMP.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spera R, Apollonio F, Liberti M, Paffi A, Merla C, Pinto R, & Petralito S (2015). Controllable release from high-transition temperature magnetoliposomes by low-level magnetic stimulation. Colloids and Surfaces B: Biointerfaces, 131, 136–140. 10.1016/J.COLSURFB.2015.04.030 [DOI] [PubMed] [Google Scholar]
- Srinivasarao M, Galliford CV, & Low PS (2015). Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nature Reviews Drug Discovery, 14(3), 203–219. 10.1038/nrd4519 [DOI] [PubMed] [Google Scholar]
- Sriraman SK, Aryasomayajula B, & Torchilin VP (2014). Barriers to drug delivery in solid tumors. Tissue Barriers, 2, e29528 10.4161/tisb.29528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, … Ruoslahti E (2009). Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell, 16(6), 510–520. 10.1016/J.CCR.2009.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Lee JSH, & Zhang M (2008). Magnetic nanoparticles in MR imaging and drug delivery. Advanced Drug Delivery Reviews, 60(11), 1252–1265. 10.1016/j.addr.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, & Li G (2004). Monodisperse MFe 2 O 4 (M = Fe, Co, Mn) Nanoparticles. Journal of the American Chemical Society, 126(1), 273–279. 10.1021/ja0380852 [DOI] [PubMed] [Google Scholar]
- Sutens B, Swusten T, Zhong K, Jochum JK, Van Bael MJ, Van der Eycken EV, … Verbiest T (2016). Tunability of Size and Magnetic Moment of Iron Oxide Nanoparticles Synthesized by Forced Hydrolysis. Materials (Basel, Switzerland), 9(7). 10.3390/ma9070554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay ZW, Chandrasekharan P, Chiu-Lam A, Hensley DW, Dhavalikar R, Zhou XY, … Conolly SM (2018). Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano, 12(4), 3699–3713. 10.1021/acsnano.8b00893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambi T, Park JH, & Lee DS (2016). Hypoxia-responsive nanocarriers for cancer imaging and therapy: recent approaches and future perspectives. Chemical Communications, 52(55), 8492–8500. 10.1039/C6CC02972H [DOI] [PubMed] [Google Scholar]
- Tipler P (1999). Physics for Scientists and Engineers (4th ed.). W. H. Freeman. [Google Scholar]
- Tredan O, Galmarini CM, Patel K, & Tannock IF (2007). Drug Resistance and the Solid Tumor Microenvironment. JNCI Journal of the National Cancer Institute, 99(19), 1441–1454. 10.1093/jnci/djm135 [DOI] [PubMed] [Google Scholar]
- Ulbrich K, Holá K, Šubr V, Bakandritsos A, Tuček J, & Zbořil R (2016). Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chemical Reviews, 116(9), 5338–5431. 10.1021/acs.chemrev.5b00589 [DOI] [PubMed] [Google Scholar]
- Unni M, Uhl AM, Savliwala S, Savitzky BH, Dhavalikar R, Garraud N, … Rinaldi C (2017). Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano, 11(2), 2284–2303. 10.1021/acsnano.7b00609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandooren J, Opdenakker G, Loadman PM, & Edwards DR (2016). Proteases in cancer drug delivery. Advanced Drug Delivery Reviews, 97, 144–155. 10.1016/J.ADDR.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Vaupel P, Kallinowski F, & Okunieff P (1989). Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Research, 49(23), 6449–6465. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2684393 [PubMed] [Google Scholar]
- Voronin DV, Sindeeva OA, Kurochkin MA, Mayorova O, Fedosov IV, Semyachkina-Glushkovskaya O, … Sukhorukov GB (2017). In Vitro and in Vivo Visualization and Trapping of Fluorescent Magnetic Microcapsules in a Bloodstream. ACS Applied Materials & Interfaces, 9(8), 6885–6893. 10.1021/acsami.6b15811 [DOI] [PubMed] [Google Scholar]
- Vu-Quang H, Muthiah M, Lee HJ, Kim Y-K, Rhee JH, Lee J-H, … Park I-K (2012). Immune cell-specific delivery of beta-glucan-coated iron oxide nanoparticles for diagnosing liver metastasis by MR imaging. Carbohydrate Polymers, 87(2), 1159–1168. 10.1016/J.CARBPOL.2011.08.091 [DOI] [Google Scholar]
- Weissleder R, Stark DD, Engelstad BL, Bacon BR, Compton CC, White DL, … Lewis J (1989). Superparamagnetic iron oxide: pharmacokinetics and toxicity. AJR. American Journal of Roentgenology, 152(1), 167–173. 10.2214/ajr.152.1.167 [DOI] [PubMed] [Google Scholar]
- White EE, Pai A, Weng Y, Suresh AK, Van Haute D, Pailevanian T, … Berlin JM (2015). Functionalized iron oxide nanoparticles for controlling the movement of immune cells. Nanoscale, 7(17), 7780–7789. 10.1039/C3NR04421A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, & Chan WCW (2016). Analysis of nanoparticle delivery to tumours. Nature Reviews Materials, 1(5), 16014 10.1038/natrevmats.2016.14 [DOI] [Google Scholar]
- Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, … Fukumura D (2011). Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proceedings of the National Academy of Sciences of the United States of America, 108(6), 2426–2431. 10.1073/pnas.1018382108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-Q, & Wang C-C (2016). Biodegradable Smart Nanogels: A New Platform for Targeting Drug Delivery and Biomedical Diagnostics. Langmuir, 32(25), 6211–6225. 10.1021/acs.langmuir.6b00842 [DOI] [PubMed] [Google Scholar]
- Xu X, Zhu S, Cui X, Wojtas L, & Zhang XP (2013). Cobalt(II)-Catalyzed Asymmetric Olefin Cyclopropanation with α-Ketodiazoacetates. Angewandte Chemie International Edition, 52(45), 11857–11861. 10.1002/anie.201305883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadavalli T, Ramasamy S, Chandrasekaran G, Michael I, Therese HA, & Chennakesavulu R (2015). Dual responsive PNIPAM–chitosan targeted magnetic nanopolymers for targeted drug delivery. Journal of Magnetism and Magnetic Materials, 380, 315–320. 10.1016/J.JMMM.2014.09.035 [DOI] [Google Scholar]
- Yang R, An LY, Miao QF, Li FM, Han Y, Wang HX, … Tang SQ (2016). Effective elimination of liver cancer stem-like cells by CD90 antibody targeted thermosensitive magnetoliposomes. Oncotarget, 7(24), 35894–35916. 10.18632/oncotarget.9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo D, Jeong H, Noh S-H, Lee J-H, & Cheon J (2013). Magnetically triggered dual functional nanoparticles for resistance-free apoptotic hyperthermia. Angewandte Chemie (International Ed. in English), 52(49), 13047–13051. 10.1002/anie.201306557 [DOI] [PubMed] [Google Scholar]
- Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, … Daldrup-Link HE (2016). Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nature Nanotechnology, 11(11), 986–994. 10.1038/nnano.2016.168 [DOI] [PMC free article] [PubMed] [Google Scholar]