Abstract
Objectives:
to estimate linkage to HIV care and ART initiation after the introduction of home-based HIV counselling and testing (HBHCT) and telephone-facilitated support for linkage in rural South Africa.
Methods:
Population-based prospective cohort study in KwaZulu Natal. All residents aged ≥15 years (y) were eligible for HBHCT. Those who tested positive and were not in care were referred for ART at one of 11 public-sector clinics. Individuals who did not attend the clinic within 2 weeks were sent an SMS reminder; those who had not attended after a further 2 weeks were telephoned by a nurse counsellor, to discuss concerns and encourage linkage. Kaplan-Meier methods were used to estimate the proportion of newly-diagnosed individuals linking to care and initiating ART.
Results:
Among 38,827 individuals visited, 26% accepted HBHCT. Uptake was higher in women than men (30% vs 20%), but similar in people aged <30y and ≥30y (28% vs 26%). 784 (8%) tested HIV positive, of whom 427 (54%) were newly diagnosed. Within 6 months, 31% of women and 18% of men <30y had linked to care, and 29% and 16%, respectively, had started ART. Among those ≥30y, 41% of women and 38% of men had linked to care within 6 months, and 41% and 35% had started ART.
Conclusions:
Despite facilitated linkage, timely linkage to care and ART initiation after HBHCT was very low, particularly among young men. Innovations are needed to provide effective HIV care and prevention interventions to young people, and thus maximise the benefits of universal test-and-treat.
Keywords: linkage to care, testing, treatment, prevention, universal test-and-treat, South Africa
Introduction
Universal test-and-treat (UTT) has the potential to improve the health of HIV-positive individuals and reduce HIV transmission. The effectiveness of UTT relies on high uptake of HIV testing and timely initiation of antiretroviral treatment (ART) among those testing positive. South Africa has the largest HIV treatment programme globally and rolled out UTT in September 2016. However, a 2017 national survey estimated that only 40% of HIV-positive young people aged 15–25 years were on ART, and viral suppression was lowest among young men, suggesting that reaching young people with UTT should be prioritised[1].
Home-based HIV counselling and testing (HBHCT) facilitates early HIV diagnosis and could promote prompt linkage to care[2]. However, the ANRS-12249 Treatment-as-Prevention (TasP) trial in KwaZulu-Natal, South Africa showed that only 30% of those newly diagnosed through HBHCT linked to care within 6 months, and linkage was lower in young people[3]. Lower rates of linkage to HIV care have been reported among young people elsewhere in sub-Saharan Africa (SSA)[4,5]. These findings suggest that rolling out UTT without interventions to link young people to treatment will not realise the full individual and population-level benefits of UTT.
In 2017, we introduced a programme combining HBHCT and enhanced telephone-facilitated support for linkage to care within a demographic surveillance area (DSA) in rural KwaZulu-Natal. We report on uptake of testing and linkage to care in the first year of the programme.
Methods
Setting
This study was conducted in the Africa Health Research Institute (AHRI) DSA in rural uMkhanyakude District, KwaZulu-Natal[6]. Triannual household-based surveys are used to collect demographic data from approximately 150,000 people in an 845 km2 area. Additionally, all residents aged ≥15 years are invited to participate in an annual individual-level survey, which includes collection of dried blood spots (DBS) for anonymised HIV testing. HIV prevalence in the 2017 survey was 30% (unpublished data) and HIV incidence, although declining, remains extraordinarily high[7,8].
HIV counselling and testing
To maximise the benefits of UTT, AHRI rolled out HBHCT in January 2017, offering rapid point of care tests for all residents aged ≥15 years during the annual survey. Individuals opting out of the survey are still eligible for HBHCT. Individuals are asked if they have ever tested for HIV previously; those who report a previous positive test are asked if they are on ART and, if so, discouraged from testing again.
A finger-prick blood sample is tested using two parallel HIV rapid tests: Abon™ HIV 1/2/O Tri-Line (Abon Biopharm, China) and Advanced Quality™ Rapid Anti-HIV(1&2) (InTec, China). Participants who test positive and are not on ART are referred for care at one of the 11 Department of Health (DoH) primary health care clinics in the DSA; they are given an appointment and asked for consent for facilitated linkage. Consenting individuals who do not attend the clinic within 2 weeks are sent an SMS reminder with a message of their choice. Individuals who have not attended within a further 2 weeks are contacted by telephone by an AHRI nurse, to discuss concerns and encourage them to attend the clinic.
Clinic attendance
Attendance at the 11 DoH clinics in the DSA is captured by an AHRI research assistant stationed at each clinic, using the AHRI ClinicLink system. All individuals visiting a clinic are asked for consent to record the date and reason for their visit, and are linked to their surveillance identification number at the time of the visit. If an individual who tested HIV-positive through HBHCT attends the clinic for any reason and has not yet linked to care, the ClinicLink system notifies the AHRI nurse stationed at the clinic.
AHRI has a memorandum of agreement with the DoH to receive data transfers from an HIV care electronic patient records system (TIER.net) used in government clinics. The TIER.net database includes ART dispensing records for all individuals on ART. AHRI receives TIER.net data from 17 clinics in the district, including those in the DSA. Individuals in TIER.net who are members of the DSA are retrospectively linked with their surveillance identification number, using deterministic record linkage algorithms.
Statistical methods
Data were collected electronically using REDCap tools[9], and analysed using Stata14 (College Station, USA). Uptake of HBHCT was assessed among individuals who were eligible to participate (aged ≥15 years and resident in the DSA in 2017) and did not report being on ART. Linkage to care was examined among individuals who were newly diagnosed through HBHCT between January-December 2017, and used ClinicLink data from 1st January 2017 through 30th May 2018. Individuals were considered newly diagnosed if: 1) at the time of the HBHCT visit, they did not report having previously tested HIV-positive; 2) they had no record in ClinicLink of having attended a clinic for ART before the HBHCT visit; 3) they had no record of ART in the TIER.net database before the HBHCT visit. The proportion linking to HIV care was estimated using Kaplan Meier methods, and compared between groups using log rank tests. Person-time was calculated from the date of HBHCT (taken as the date of HIV diagnosis) until the earliest of date of attending a clinic for HIV referral, out-migration from the DSA, or death. Individuals who had not attended a clinic, and were not known to have died or out-migrated, were administratively censored on 30th May 2018. Individuals were considered to have linked to care if they attended the clinic and reported HIV referral as the reason. ART initiation was ascertained using data from both ClinicLink and TIER.net. As a sensitivity analysis, linkage was defined as attending the clinic for any reason, to account for the possibility that some individuals may not have reported their true reason for attendance.
Ethics
Ethical approval for the demographic surveillance, ClinicLink, and linkage to government ART records (TIER.net) was granted by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal, South Africa. Individuals provided separate written informed consent for HBHCT, facilitated linkage, and recording visits in ClinicLink.
Results
Overall, 41,815 of 51,380 (81%) eligible individuals were contacted for the 2017 survey (Supplementary Figure S1). Among those contacted, 2988 (7%) reported being on ART and so were excluded from analysis of HBHCT uptake. 4265 (28%) individuals <30 years and 5992 (26%) ≥30 years consented to HBHCT (Table 1). Women were more likely to accept HBHCT than men (30% vs 20%, p<0.001). In both men and women, HBHCT uptake was lowest among those aged 25–44 years, and highest among those ≥50 years. HBHCT uptake was also lower among people who were married, who had higher levels of education, who lived in urban areas and who were employed. Over 95% of those accepting HBHCT consented to facilitated linkage, with no evidence of a difference by age or sex.
Table 1.
Uptake of home-based HIV counselling and testing (HBHCT) among individuals who were contacted
| Contacted and eligible (col %)1 | Accepted HBHCT(row %) | |
|---|---|---|
| 38,827 | 10,257 | |
| Age group (years) | P<0.001 | |
| <30 | 15,509 (39.9%) | 4265 (27.5%) |
| 30–39 | 6591 (17.0%) | 1164 (17.7%) |
| 40–49 | 4849 (12.5%) | 896 (18.5%) |
| ≥50 | 11,878 (30.6%) | 3932 (33.1%) |
| Sex | P<0.001 | |
| Male | 15,042 (38.7%) | 3009 (20.0%) |
| Female | 23,785 (61.3%) | 7248 (30.5%) |
| Education | P<0.001 | |
| None | 7413 (26.6%) | 2325 (31.4%) |
| Primary/incomplete secondary | 8327 (29.9%) | 2511 (30.2%) |
| Complete secondary/above | 12,126 (43.5%) | 2111 (17.4%) |
| Missing | 10,961 | 3310 |
| Marital status | P<0.001 | |
| Single | 8234 (23.4%) | 2171 (26.4%) |
| Married/informal union | 22,997 (65.3%) | 5734 (24.9%) |
| Sep/divorced/widowed | 4013 (11.4%) | 1380 (34.4%) |
| Missing | 3583 | 972 |
| Employed? | P<0.001 | |
| No | 17,203 (71.5%) | 4924 (28.6%) |
| Yes | 6846 (28.5%) | 867 (12.7%) |
| Missing | 11,625 | 4466 |
| Residence location | P<0.001 | |
| Urban | 2002 (7.6 %) | 339 (16.9%) |
| Peri-urban | 8968 (33.9%) | 2242 (25.0%) |
| Rural | 15469 (58.5%) | 3886 (25.1%) |
| Missing | 12,388 | 3790 |
| SES tertile | P=0.36 | |
| Low | 9213 (33.7%) | 2327 (25.3%) |
| Middle | 9398 (34.3%) | 2358 (25.1%) |
| High | 8756 (32.0%) | 2135 (24.4%) |
| Missing | 11,460 | 3437 |
| Distance to nearest clinic (quartiles) | P<0.001 | |
| 0– <1.5 km | 9344 (25.0%) | 2201 (23.6%) |
| 1.5–2.5 km | 9388 (25.1%) | 2352 (25.1%) |
| >2.5–3.9 km | 9321 (24.9%) | 2460 (26.4%) |
| >3.9 km | 9318 (24.9%) | 2792 (30.0%) |
| Missing | 1456 | 452 |
Excludes 2988 individuals who reported being on ART and were therefore discouraged from testing again.
P-value from Chi-squared test (excluding individuals with missing values).
Among those accepting HBHCT, 784 (8%) tested HIV-positive, of whom 357 (46%) had been previously diagnosed (88 self-report, 269 with ART records). Overall, 427 (4%) were newly diagnosed (209 aged <30 years and 218 aged ≥30 years), and included in the analysis of linkage to care. Among women <30 years, an estimated 11%, 21% and 31% had linked to care within 1, 3 and 6 months, respectively (Figure 1). Linkage was slower in men of the same age, with 8%, 16% and 18% linking to care in the same time frame. Within 6 months, 29% of women and 16% of men <30 years had started ART. Linkage among individuals ≥30 years was higher, with 18%, 34% and 41% of women and 14%, 31%, and 38% of men linking to care within 1, 3 and 6 months, respectively. By 6 months, 41% of women and 35% of men ≥30 years had started ART. There was some evidence of a difference in linkage at 6 months between the four age/sex groups (p=0.08). Overall, 34% and 48% of individuals had linked to care within 6 and 12 months, respectively.
Figure 1.
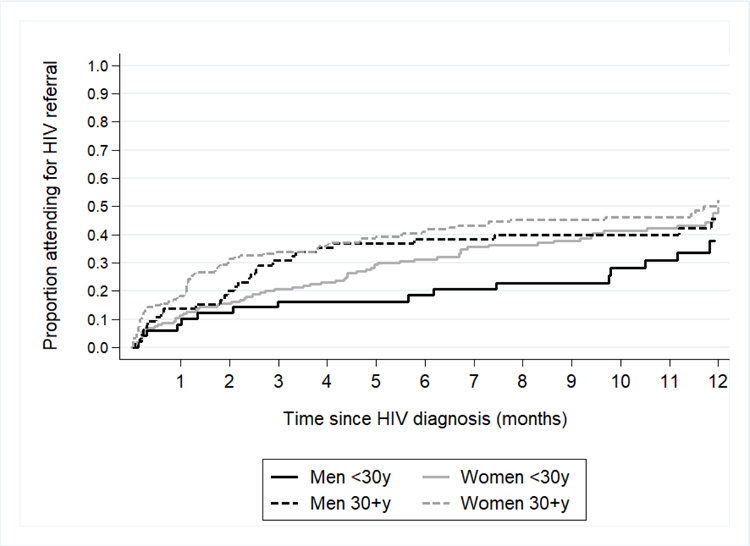
Time from HIV diagnosis until attending the clinic for referral to HIV care among 427 individuals who had not been previously diagnosed as HIV-positive
In the sensitivity analysis, 44% of all individuals (44% of women and 20% of men <30 years, and 50% of women and 46% of men ≥30 years) had attended a clinic for any reason within 6 months, and 59% had attended within 12 months (Supplementary Figure S2).
SMS reminders were successfully transmitted in over 90% of cases, and 82% of individuals received a phone call. Only 4% of calls turned out to be to an incorrect number; 65% of calls were answered, and the others went over to voicemail (26%) or were not answered (5%).
Discussion
We found that, even with a programme to facilitate linkage, including SMS reminders and nurse-led telephone calls for those not linked within a month, less than a third of newly diagnosed young adults <30 years in this hyper-endemic HIV setting had linked to care within 6 months. Linkage was particularly low in young men, with only 16% starting ART within 6 months. Our results suggest that HBHCT, early SMS reminders and telephone support for linkage are not sufficient to eliminate barriers to timely ART initiation among young adults, particularly young men, in this population.
Our estimates of linkage to care are lower than those reported by several trials evaluating UTT interventions in SSA, although they also report lower linkage among younger adults[5,10]. In Year 2 of the HPTN-071 PopART trial in South Africa, 50% of women and 42% of men initiated ART within 6 months[10]. However, the TasP trial, conducted in the same area as our study, found similar low rates of linkage within 6 months, with lowest linkage in young people, particularly young men, and in those who had never been on ART[3]. Our estimates of linkage are also comparable to South Africa national estimates[11].
Barriers to linkage are multifactorial and include individual and health-systems factors such as stigma, fear of disclosure, distance, and cost of travel to clinic[12,13]. We observed poor linkage despite interventions to link those who did not attend a clinic within 2 weeks and having dedicated AHRI nurses in each clinic to overcome some of the facility-level barriers. This raises concern as to whether primary care clinics in their current format are able to attract young people, particularly men, to early HIV treatment, and whether alternative modes of care are required.
We also found low HBHCT uptake, and the proportion of individuals testing HIV-positive through HBHCT was low compared with prevalence estimates from anonymous HIV serosurveys in the same population. Also of note was the large proportion testing positive who already knew their status, and were already in care. This suggests that many HIV-positive people who are potentially unaware of their status might not accept HBHCT. Other interventions, such as HIV self-testing, multi-disease screening, work-based or mobile-clinic based testing may be needed to overcome barriers to testing.
Limitations include that our primary definition of linkage was based on clinic attendance for a self-reported reason of HIV referral or ART, or a record in TIER.net. This may have underestimated linkage if people are unwilling to report their true reason for attendance. A sensitivity analysis assuming that all who attended the clinic for any reason had linked to care yielded somewhat higher estimates, although linkage was still very low among young men. We did not collect data from clinics outside the DSA, however TIER.net contains ART records for all 17 government clinics in or near the area.
In summary, despite facilitated linkage, timely ART initiation after HBHCT was very low among young men. Home-based HIV testing and telephone-supported linkage to care may be insufficient to obtain the desired impact of UTT on improving health outcomes among HIV-positive people or reducing HIV transmission. Innovations are needed to provide effective HIV care and prevention interventions to young people, particularly young men, and thus maximise the individual and population benefits of a UTT approach.
Supplementary Material
Acknowledgments
We are grateful to the study participants and members of the communities where the study was conducted. We would also like to acknowledge the study teams, especially the nurse counsellors and the data management team.
Sources of support:
Financial support for this research was provided by the Wellcome Trust through core funding to the Africa Health Research Institute (082384/Z/07/Z). MS also receives support from the UK Medical Research Council (MRC), and KB and JS receive support through joint funding by the UK MRC and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement which is also part of the EDCTP2 programme supported by the European Union (MR/K012126/1). MJS receives support from the US National Institutes of Health (K23 MH099916; P30 30AI060354). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Human Sciences Research Council. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey. 2017 http://www.hsrc.ac.za/en/departments/hiv-aids-stis-and-tb/HAST_National_HIV_Survey.
- 2.Ruzagira E, Baisley K, Kamali A, Biraro S, Grosskurth H; Working Group on Linkage to HIV Care. Linkage to HIV care after home-based HIV counselling and testing in sub-Saharan Africa: a systematic review. Trop Med Int Health 2017; 22:807–821. [DOI] [PubMed] [Google Scholar]
- 3.Iwuji CC, Orne-Gliemann J, Larmarange J et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV 2018; 5:e116–e125. [DOI] [PubMed] [Google Scholar]
- 4.Mavegam BO, Pharr JR, Cruz P, Ezeanolue EE. Effective interventions to improve young adults’ linkage to HIV care in Sub-Saharan Africa: a systematic review. AIDS Care 2017; 29:1198–1204. [DOI] [PubMed] [Google Scholar]
- 5.Petersen M, Balzer L, Kwarsiima D et al. Association of implementation of a universal testing and treatment intervention with HIV diagnosis, receipt of antiretroviral therapy, and viral suppression in East Africa. JAMA 2017; 317:2196–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanser F, Hosegood V, Bärnighausen T et al. Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol 2008; 37:956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013; 339:966–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chimbindi N, Mthiyane N, Birdthistle I et al. Persistently high incidence of HIV and poor service uptake in adolescent girls and young women in rural KwaZulu-Natal, South Africa prior to DREAMS. PLoS One 2018;13:e0203193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seeley J, Bond V, Yang B et al. Understanding the time needed to link to care and start ART in seven HPTN 071 (PopART) study communities in Zambia and South Africa. AIDS Beh 2018; in press. [DOI] [PMC free article] [PubMed]
- 11.Takuva S, Brown AE, Pillay Y, Delpech V, Puren AJ. The continuum of HIV care in South Africa: implications for achieving the second and third UNAIDS 90–90-90 targets. AIDS 2017; 31:545–52. [DOI] [PubMed] [Google Scholar]
- 12.MacPherson P, MacPherson EE, Mwale D et al. Barriers and facilitators to linkage to ART in primary care: a qualitative study of patients and providers in Blantyre, Malawi. J Int AIDS Soc 2012;15:18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS 2012; 26:2059–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


