Abstract
BACKGROUND:
In rodents, context specificity of Pavlovian extinction is attenuated by manipulations that impair hippocampal function, including systemic administration of scopolamine, a muscarinic-cholinergic receptor antagonist. Context renewal translates into return of fear following exposure therapy to feared situations. We evaluated the effectiveness of scopolamine for attenuating context renewal of phobic fear in humans.
METHODS:
A total of 60 participants (35 female, 22 male, 1 transgender, 2 undeclared) with social anxiety disorder and fear of public speaking were randomized to placebo, 0.5 mg scopolamine, or 0.6 mg scopolamine. They completed seven exposure sessions in an exposure context and subsequently tested in the exposure context (extinction retest) versus a different context (context renewal test), which were counterbalanced. Testing 1 month later occurred in the exposure context (long-term extinction retest). Fear measures included skin conductance and self-reported distress during speeches. Hippocampus-dependent cognitive tasks were completed as well.
RESULTS:
Scopolamine augmented extinction across exposure sessions on skin conductance response and skin conductance level. Lower skin conductance response at context renewal in scopolamine groups relative to the placebo group was constrained to simple effects and complicated by unexpected outcomes within placebo and on self-reported fear. Scopolamine led to lower skin conductance response at long-term extinction retest. Scopolamine impaired performance on a cognitive task of hippocampal function.
CONCLUSIONS:
Noninvasive and well-tolerated scopolamine impaired hippocampal processes and augmented extinction during exposure. Drug-free effects persisted 1 month later. Findings at context renewal were limited and suggestive only. Further investigation is warranted with varying scopolamine dosages.
Keywords: Context renewal, Extinction, Fear, Hippocampus, Public speaking, Scopolamine
Anxiety disorders are among the most common and costly mental health conditions. Exposure therapy, or repeated systematic exposure to feared stimuli, is the most empirically supported behavioral treatment for anxiety disorders (1,2). Yet, anywhere from 20% to 60% of people with anxiety disorders experience a return of fear when subsequently tested with the same object that was targeted during exposure therapy (3). Herein, we use the latest developments from translational neuroscience to investigate scopolamine as a pharmacological agent for “decontextualizing” exposure therapy and reducing return of fear.
Return of fear can be understood from the perspective of Pavlovian models of fear extinction that highlight inhibitory learning (4). In these models, the original conditioned stimulus–unconditioned stimulus (CS-US) association learned during fear conditioning is not erased during extinction, but rather is left intact as a new, secondary inhibitory learning about the CS-US relationship develops (4,5). The inhibitory association is dependent on the CS and the context in which the CS is presented. Retention of at least part of the original excitatory association can be uncovered following extinction training by changing the surrounding context from extinction to retest (6). The loss of context-dependent extinction memory results in “renewal” of previously extinguished fear.
Evidence for context renewal in animal fear conditioning studies has been replicated in human conditioning studies (7-11) and in fearful samples following exposure therapy (12-15). Such return of fear might easily trigger full relapse, especially if avoidance behavior blocks re-extinction. Consequently, any approach that renders extinction context independent would significantly advance both our understanding of extinction processes and optimization of exposure therapy.
The hippocampus is critical to processing of spatiotemporal contexts that elicit fear memories, and several studies have shown that invasive disruption of hippocampal function disrupts contextual fear [e.g., (16-19)]. This has led to an interest in downregulation of the hippocampus during extinction to eliminate context specificity, such that the learning that takes place is more likely to generalize to contexts other than extinction contexts. In rodents, Corcoran and Maren (20) and Ji and Maran (21) showed that hippocampal lesions or intra-hippocampal administration of muscimol prevented the renewal of fear that normally occurs when the context is changed between extinction and testing.
The invasive nature of the treatments used in animals, requiring brain surgery or engineered genetic mutation, makes them poor candidates for clinical translation. Scopolamine offers a readily translatable pharmacological approach to impair the hippocampus during extinction. Scopolamine is a muscarinic cholinergic receptor antagonist shown to have more pronounced effects on contextual than cued fear in adult rats, presumably mediated by a cholinergic blockade in the hippocampus (16,22,23). In our prior evaluation of scopolamine (0.01 to 100 mg/kg) in rodents, low systemic doses had selective effects on contextual learning and closely paralleled the behavioral effects of intrahippocampal scopolamine (16,17). Higher doses had a more general impact on learning and behavior. For example, the effective dose for hippocampus-independent tone fear conditioning was about 14 times higher than the corresponding dose for hippocampus-dependent context conditioning, even though this comparison was made off-drug, and tone and context learning occurred simultaneously. Hence, scopolamine, a systemic agent, appears to have specific effects on contextual and hippocampal processing relative to cue conditioning when administered at low doses.
We tested scopolamine effects on contextual renewal of auditory fear conditioning in rodents (24). Rats received tone-shock pairings in one context and then were extinguished in a second context. When extinction memory was tested in either the original training context or a novel context, previously extinguished fear returned (renewal). However, rats that received a 0.1-mg/kg dose of scopolamine showed no return of fear during a drug-free test. The low dose of scopolamine also slowed the formation of long-term extinction memory. Higher doses cause a more significant attenuation of extinction (25,26).
We aimed to evaluate doses of scopolamine that effectively reduced context renewal when tested in a context different than the exposure context, without mitigating fear reduction at extinction retest when tested in the same context in which exposure took place. We hypothesized that scopolamine would reduce context renewal relative to placebo and that the effects would be specific to context renewal relative to extinction retest.
METHODS AND MATERIALS
Participants
Sixty-six participants were randomly assigned to placebo, 0.5 mg scopolamine (SCOP 0.5 mg), or 0.6 mg scopolamine (SCOP 0.6 mg). Six participants were dropped before the test of context renewal; analyses are based on 60 participants (placebo: n = 21; SCOP 0.5 mg: n = 19; SCOP 0.6 mg: n = 20) (Supplemental Table S1).
Inclusion criteria included diagnosis of social anxiety disorder with a clinical severity 0-to 8-point rating of 3 or greater (mean = 4.22, SD = 0.86) and a score of 6 or greater on a 0 to 8 scale of self-reported fear of public speaking (mean = 7.05, SD = 0.74). Exclusion criteria included bipolar disorder, psychosis, substance use disorder, and medical conditions/medications contraindicated by scopolamine, as determined by study physicians.
Drug Condition
Scopolamine and placebo solutions were prepared by the University of California’s Investigational Drug Service, who maintained the study blind. Drug or placebo was delivered intranasally (see the Supplement).
Screening and Sample Description
Anxiety Disorder Interview Schedule for DSM-IV/5 (27) was administered by interviewers trained to reliability to determine diagnostic eligibility (see the Supplement). The 24-item Lei-bowitz Social Anxiety Scale: Self-Report (28) was used to measure fear and avoidance of social situations with strong reliability and validity.
Extinction/Exposure, Context Renewal, and Extinction Retest Measures
Skin Conductance Response.
Physiological responses were recorded using J&J Engineering I-330-C2 (Greenfield, IN) and PHYSIOLAB (PHYSIOLAB Technologies, Milton Keynes, UK) instruments (see the Supplement). The CS period began with a brief bell (or gong), indicating to begin speaking, followed by a 1-minute speech, and concluded with a second ringing of the same bell (or gong), indicating to stop speaking. The skin conductance response (SCR)-to-CS onset was the difference between maximum SC from 1 to 6 seconds after the beginning auditory cue minus mean SC for 2 seconds before the auditory cue. SCR-to-CS onset was our primary physiological measure. SCR-to-CS termination was the difference between maximum SC from 1 to 6 seconds after the ending auditory cue minus mean SC for 2 seconds before the ending auditory cue. Higher SCR-to-CS termination is associated with greater surprise that the US did not occur (i.e., US-omission response) and is posited to measure US expectancy (29). (US-omission parallels omission of humiliation/rejection in public speaking.) The hippocampus has been shown to track US expectancy in human studies (30). SCRs (microsiemens) were square-root transformed.1
Skin Conductance Level.
Mean skin conductance level (SCL) was calculated during a 30-second anticipation period (SCL anticipation) and first 30 seconds of the intertrial interval (SCL recovery) for every virtual reality (VR) speech. SCL (microsiemens) was our secondary physiological measure.
Subjective Units of Distress Scale.
The Subjective Units of Distress Scale (SUDS) ratings were obtained at the beginning and end of each VR speech, using a 0 to 100 scale, where 0 = no fear and 100 = extreme fear. SUDS was our subjective measure.
Hippocampal Target Engagement Measures
The Continuous Paired Associate Learning (CPAL) task is a hippocampal-dependent measure of cue-context learning (see the Supplement) that is affected by scopolamine (31). The Mnemonic Similarity Task (MST) (see the Supplement) reliably taxes the dentate gyrus/CA3 and measures processes including pattern separation and pattern completion (32).
Procedure
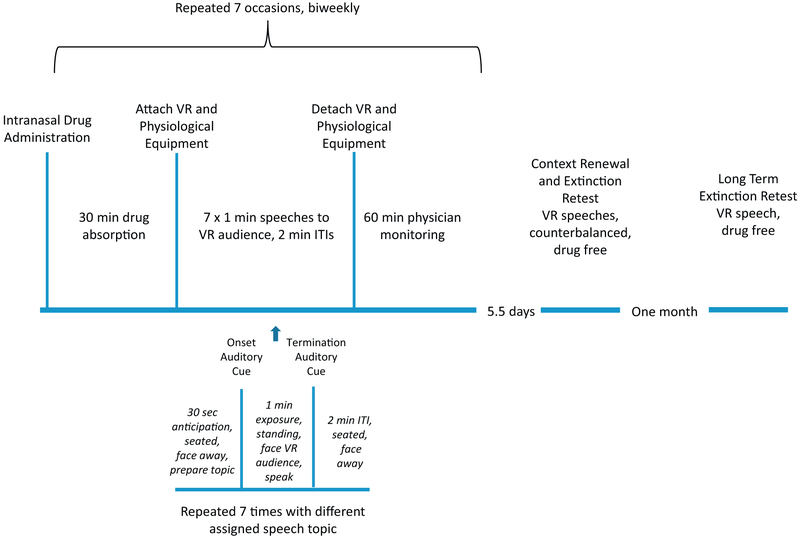
Following informed consent, eligibility was assessed using the Anxiety Disorder Interview Schedule for DSM-5 and a medical evaluation, baseline questionnaires, and CPAL task were completed. The first of seven VR exposure sessions began 19 days on average (range 5–60 days) after baseline. Participants completed two VR exposure sessions per week, averaging 4.22 days (range 2.5 to 7.5) between sessions (see Figure 1). Each session involved seven 1-minute speeches to the same VR scene, in the same physical room, with the same experimenter, and the same auditory and olfactory cues.
Figure 1.
Flow chart for seven exposure sessions, each including seven speeches to a virtual reality (VR) audience, context renewal and extinction retest, and long-term extinction retest. (Session 1 included a baseline speech before drug administration.) ITI, intertrial interval.
Participants returned for context renewal and extinction retest (counterbalanced) on average 5.5 days (range 1–14 days) following their seventh exposure session. Context renewal and extinction retest each included one VR speech using the exposure session format separated by approximately 30 minutes. Context renewal differed from exposure sessions in the following ways: VR audience scene, physical room, experimenter, olfactory cue (air freshener scent or not), and auditory cue (bell or gong) to indicate CS onset and CS termination (see the Supplement). Extinction retest replicated exposure sessions.
One month following context renewal/extinction retest participants completed another speech in the exposure VR context for long-term extinction retest. Given the context shift created by the temporal mismatch between the 5.5 days (average) that interceded between each exposure session versus the 1-month test of long-term extinction, we explored whether scopolamine attenuated long-term extinction retest.
Data Analytic Plan
Following analyses of variance to evaluate baseline differences between groups, the major analyses used multilevel modeling in Stata 14 (StataCorp LLC, College Station, TX) to examine the impact of scopolamine on SCR-to-CS onset, SCR-to-CS termination, SCL anticipation, SCL recovery, and initial SUDS. Multilevel modeling has several advantages over traditional repeated measure analysis of variance designs including accounting for missing data and uneven spacing between assessment points and is more appropriate for smaller sample sizes (33) (see the Supplement).
We first present results regarding extinction (first speech of first exposure session and last speech of exposure sessions 2–7), followed by context renewal and extinction retest, and finally long-term extinction retest. Extinction models contained all 60 participants, whereas models for context renewal, extinction retest, and long-term extinction retest excluded individuals who did not extinguish2 (see the Supplement).
RESULTS
Group Differences at Baseline
There were no significant group differences on Anxiety Disorder Interview Schedule for DSM-5 clinical severity rating, Lei-bowitz Social Anxiety Scale: Self-Report, CPAL task, or body mass index at baseline (ps > .54) (see Table 1) (34).
Table 1.
Baseline Characteristics by Group
| Placebo | SCOP 0.5 mg | SCOP 0.6 mg | |
|---|---|---|---|
| Age, Years | 23.05 (8.58) | 26.53 (10.21) | 25.47 (10.56) |
| Gender | Male, n = 4 Female, n = 17 |
Male, n = 8 Female, n = 10 Did not answer, n = 1 |
Male, n = 10 Female, n = 8 Transgendered, n = 1 Did not answer, n = 1 |
| ADIS CSR | 4.24 (0.89) | 4.17 (0.92) | 4.30 (0.86) |
| CPAL | 28.56 (36.37) | 45.81 (58.42) | 33.12 (37.41) |
| BMI | 23.5 (4.63) | 24.48 (4.31) | 25.05 (5.02) |
Values are mean (SD) unless otherwise indicated.
ADIS CSR, Anxiety Disorder Interview Schedule for DSM-5 clinical severity rating; BMI, body mass index; CPAL, Continuous Paired Associate Learning; SCOP, scopolamine.
Extinction
SCR-to-CS Onset.
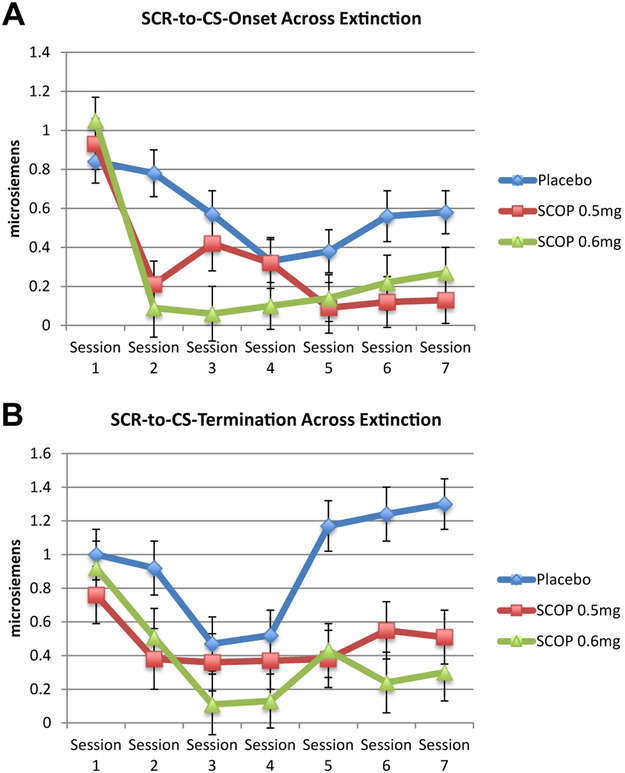
There was a main effect of group (χ2 = 15.86, p < .001), time (χ2 = 86.71, p < .001) and time × group interaction (χ2 = 26.48, p < .01) (Figure 2A).3 SCR-to-CS onset decreased over sessions (b = −0.62, 95% CI = −0.80 to −0.43, p < .001). SCR-to-CS onset was lower in SCOP 0.5 mg (b = −0.26, 95% CI = −0.42 to −0.10, p < .01) and SCOP 0.6 mg (b = −0.30, 95% CI = −0.47 to −0.14, p < .01) compared with placebo, with no difference between SCOP 0.5 mg and SCOP 0.6 mg (b = −0.04, 95% CI = −0.22 to 0.13, p = .64). Additional analyses are available in the Supplement.
Figure 2.
(A) Skin conductance response-to-conditional stimulus (SCR-to-CS) onset across extinction. Multilevel modeling time points included first speech of first exposure session (drug free) and last (seventh) speech of exposure sessions 2 through 7. Compared with placebo, both scopolamine 0.5 mg (SCOP 0.5 mg) and 0.6 mg (SCOP 0.6 mg) demonstrated significantly lower SCR-to-CS onset on average across extinction (ps < .01). There were no differences between SCOP 0.5 mg and SCOP 0.6 mg. (B) SCR-to-CS termination across extinction. Multilevel modeling time points included first speech of first exposure session (drug free) and last (seventh) speech of exposure sessions 2 through 7. Compared with placebo, both SCOP 0.5 mg and SCOP 0.6 mg demonstrated significantly lower SCR-to-CS termination on average across extinction (ps < .01). There were no differences between SCOP 0.5 mg and SCOP 0.6 mg.
SCR-to-CS Termination.
There was a main effect of group (χ2 = 17.08, p < .001), time (χ2 = 44.98, p < .001), and time × group interaction (χ2 = 30.51, p < .01) (Figure 2B). SCR-to-CS termination decreased over sessions (b = −0.19, 95% CI = −0.40 to 0.01, p = .07). SCR-to-CS termination was lower in SCOP 0.5 mg (b = −0.47, 95% CI = −0.77 to −0.18, p < .01) and SCOP 0.6 mg (b = −0.57, 95% CI = −0.87 to −0.28, p < .001) compared with placebo, with no difference between SCOP 0.5 mg and SCOP 0.6 mg (b = −0.10, 95% CI = −0.41 to 0.21, p = .54). Additional analyses are available in the Supplement.
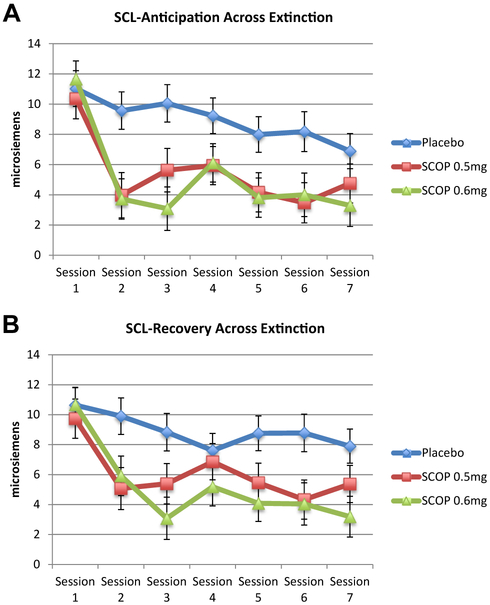
SCL Anticipation.
There was a main effect of group (χ2 = 16.38, p < .001) and time (χ2 = 68.03, p < .001), but no time × group interaction (χ2 = 17.39, p = .14) (Figure 3A). SCL anticipation decreased over sessions (b = −6.03, 95% CI = −7.78 to −4.29, p < .001). SCL anticipation was lower in SCOP 0.5 mg (b = −3.53, 95% CI = −5.63 to −1.42, p < .01) and SCOP 0.6 mg (b = −3.90, 95% CI = −6.02 to −1.78, p < .001) compared with placebo, with no difference between SCOP 0.5 mg and SCOP 0.6 mg (b =−0.37, 95% CI = −2.58 to 1.83, p = .74). Additional analyses are available in the Supplement.
Figure 3.
(A) Skin conductance level (SCL) anticipation across extinction. Multilevel modeling time points included first speech of first exposure session (drug free) and last (seventh) speech of exposure sessions 2 through 7. Compared with placebo, both scopolamine 0.5 mg (SCOP 0.5 mg) and 0.6 mg (SCOP 0.6 mg) demonstrated significantly lower SCL anticipation on average across extinction (ps < .01). There were no differences between SCOP 0.5 mg and SCOP 0.6 mg. (B) SCL recovery across extinction. Multilevel modeling time points included first speech of first exposure session (drug free) and last (seventh) speech of exposure sessions 2 through 7. Compared with placebo, both SCOP 0.5 mg and SCOP 0.6 mg demonstrated significantly lower SCL recovery on average across extinction (ps < .05). There were no differences between SCOP 0.5 mg and SCOP 0.6 mg.
SCL Recovery.
SCL recovery showed the same pattern of results as SCL anticipation (Figure 3B; see the Supplement).
SUDS.
There was a main effect of time (χ2 = 160, p < .001), but no main effect of group (χ2 = 0.26, p = .88) or time × group interaction (χ2 = 7.32, p = .84) (Supplemental Figure S1). SUDS decreased across sessions (b = −22.55, 95% CI = −26.73 to −18.38, p < .001).
Context Renewal and Extinction Retest
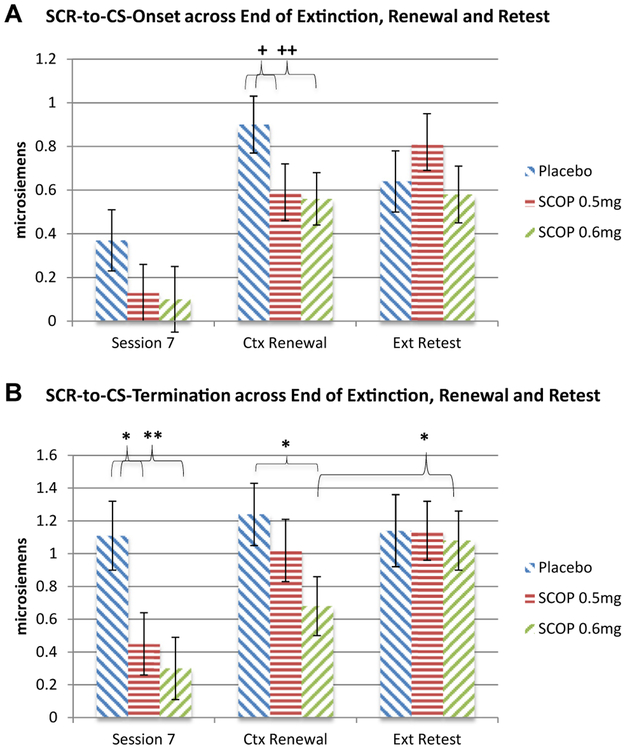
SCR-to-CS Onset.
There was a main effect of group (χ2 = 19.24, p < .001) and time (χ2 = 100.98, p < .001), but no time × group interaction (χ2 = 20.06, p = .22) (Figure 4A).4,5 Given a priori hypotheses, simple effects were analyzed. There were no group differences at end of extinction (ps > .19). SCR-to-CS onset trended to be lower at context renewal in SCOP 0.5 mg (b = −0.31, 95% CI = −0.67 to 0.06, p = .10) and SCOP 0.6 mg (b = −0.35, 95% CI = −0.70 to 0.01, p = .06) compared with placebo, with no difference between SCOP 0.5 mg and SCOP 0.6 mg (b = −0.04, 95% CI = −0.39 to 0.32, p = .85). There were no group differences at extinction retest (ps > .18). There were no significant differences between context renewal and extinction retest within any group (ps > .18).
Figure 4.
(A) Skin conductance response-to-conditional stimulus (SCR-to-CS) onset across end of extinction (Ext), renewal, and retest. Multilevel modeling time points included first speech of first exposure session (drug free) (not depicted), last (seventh) speech of exposure sessions 2 through 7, context (Ctx) renewal, and extinction retest (counterbalanced) in participants who extinguished. +p = .10, ++p = .06. There were no group differences at end of extinction (session 7). At context renewal, scopolamine 0.5 mg (SCOP 0.5 mg) (p = .10) and 0.6 mg (SCOP 0.6 mg) (p = .06) showed trends for lower SCR-to-CS onset than placebo did, and there were no differences between SCOP 0.5 mg and SCOP 0.6 mg. There were no group differences at extinction retest. There were no significant differences between context renewal and extinction retest within any group. (B) SCR-to-CS termination across end of extinction, renewal, and retest. Multilevel modeling time points included first speech of first exposure session (drug free) (not depicted), last (seventh) speech of exposure sessions 2 through 7, context renewal, and extinction retest (counterbalanced) in participants who extinguished. *p < .05, **p < .01. Compared with placebo, SCOP 0.5 mg (p < .05) and SCOP 0.6 mg (p < .01) demonstrated significantly lower SCR-to-CS termination at end of extinction (session 7). At context renewal, SCOP 0.6 mg, compared with placebo, demonstrated significantly lower SCR-to-CS termination (p < .05) with no differences between SCOP 0.5 mg and SCOP 0.6 mg. There were no group differences at extinction retest. SCOP 0.6 mg demonstrated higher scores at extinction retest than context renewal (p < .05), whereas there were no differences within either SCOP 0.5 mg or placebo.
SCR-to-CS Termination.
There was a main effect of group (χ2 = 9.14, p < .01) and time (χ2 = 61.97, p < .001), but no time × group interaction (χ2 = 14.92, p = .53) (Figure 4B).6,7 Given a priori hypotheses, simple effects were analyzed. SCR-to-CS termination was lower at end of extinction in SCOP 0.5 mg (b = −0.66, 95% Cl = −1.22 to −0.10, p < .05) and SCOP 0.6 mg (b = −0.81, 95% CI = −1.37 to −0.25, p < .001) compared with placebo, with no difference between SCOP 0.5 mg and SCOP 0.6 mg (b = −0.15, 95% CI = −0.68 to 0.37, p = .57). SCR-to-CS termination was lower at context renewal in SCOP 0.6 mg (b = −0.57, 95% CI = −1.08 to −0.06, p < .05) compared with placebo, with no difference between SCOP 0.5 mg and placebo (b = −.22, 95% CI = −0.75 to 0.31, p = .42) or between SCOP 0.5 mg and SCOP 0.6 mg (b = −0.35, 95% CI = −0.87 to 0.17, p = .18). There were no group differences at extinction retest (ps > .80). SCOP 0.6 mg demonstrated higher scores at extinction retest than at context renewal (b = 0.41, 95% CI = 0.04 to 0.80, p < .05), whereas there were no differences within either SCOP 0.5 mg or placebo (ps > .55).
SCL Anticipation.
There was a main effect of group (χ2 = 78.89, p < .05), time (χ2 = 94.71, p < .001), and time × group interaction (χ2 = 32.36, p < .01) (Supplemental Figure S2A).8,9 SCL anticipation trended to be lower at end of extinction in SCOP 0.6 mg compared with placebo (b = −3.51, 95% CI = −7.3 to 0.27, p = .07), with no difference between SCOP 0.5 mg and placebo or between SCOP 0.5 mg and SCOP 0.6 mg (ps > .15). There were no significant group differences at context renewal or extinction retest (ps > .29). Placebo demonstrated lower scores at extinction retest than context renewal (b = −2.61, 95% CI = −5.35 to 0.13, p = .06), but there were no differences within either SCOP 0.5 mg or SCOP 0.6 mg (ps > .19).
SCL Recovery.
There was a main effect of time (χ2 = 78.53, p < .001) and time × group interaction (χ2 = 34.13, p < .01), but no differences between groups at any time point or between time points within any group (see the Supplement and Supplemental Figure S2B).10,11
SUDS.
There was a main effect of time (χ2 = 176.85, p < .001) but no main effect of group (χ2 = 2.03, p = .36) or time × group interaction (χ2 = 9.61, p = .81) (Supplemental Figure S3).12,13
Long-Term Extinction Retest
See Supplemental Table S2 for means and SDs. See the Supplement for statistical results for SCR-to-CS onset, SCL anticipation, SCL recovery, and SUDs.14
SCR-to-CS Termination.
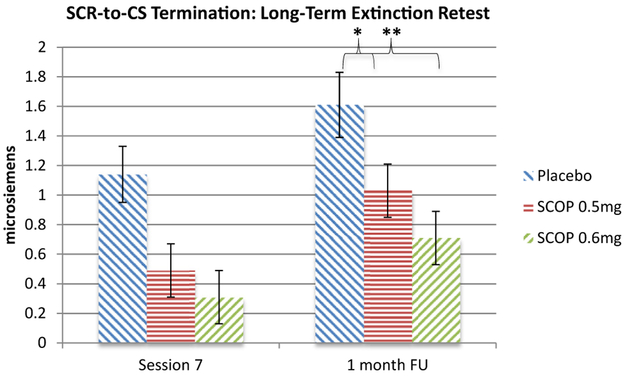
There was a main effect of group (χ2 = 13.58, p < .01) and time (χ2 = 58.80, p < .001) but no time × group interaction (χ2 = 12.87, p = .54). SCR-to-CS termination increased from end of extinction to long-term extinction retest (p < .001). Given a priori hypotheses, simple effects examined group differences at long-term extinction retest: compared with placebo, SCOP0.5 mg (b = −0.58, 95% CI = −1.15to −0.01, p < .05) and SCOP 0.6 mg (b = −0.91, 95% CI = −1.47 to −0.34, p < .01) showed lower SCR-to-CS termination, with no difference between SCOP 0.5 mg and SCOP 0.6 mg (b = −0.32, 95% CI = −0.82 to 0.28, p = .34) (Figure 5).
Figure 5.
Skin conductance response-to-conditional stimulus (SCR-to-CS) termination across end of extinction (session 7) and long-term extinction retest (1 month follow-up [FU]). Multilevel modeling time points included first speech of first exposure session (drug free), last (seventh) speech of exposure sessions 2 through 7, and long-term extinction retest. *p < .05, **p < .01. SCR-to-CS termination increased from end of extinction to long-term extinction retest (p < .001). Scopolamine 0.6 mg (SCOP 0.6 mg) and 0.5 mg (SCOP 0.5 mg), compared with placebo, demonstrated significantly lower SCR-to-CS termination at long-term extinction retest. There were no differences between SCOP 0.5 mg and SCOP 0.6 mg.
Testing Direct Physiological Effects of Scopolamine
There was no main effect of group or time × group interaction on any physiological index across speeches 2 through 8 within the first exposure session (ps > .23), before extinction learning accrued.
Hippocampal-Dependent Tasks
MST scores were analyzed in terms of errors (“similar” as “old,” “new” as “old,” and “new” as “similar”) and accuracy in discrimination (“similar as similar”) (Table 2). SCOP 0.5 mg and SCOP 0.6 mg were each associated with two errors (one overlapping and one different) relative to placebo, and placebo showed more accuracy than either scopolamine dose (statistical results in the Supplement).
Table 2.
Mean Rates on the MST and CPAL by Group
| Tasks | Placebo | SCOP 0.5 mg | SCOP 0.6 mg |
|---|---|---|---|
| MST Index | |||
| Similar/Old (error) | 0.38 (0.15) | 0.48 (0.12) | 0.40 (0.11) |
| New/Old (error) | 0.03 (0.03) | 0.09 (0.10) | 0.08 (0.06) |
| New/Similar (error) | 0.09 (0.06) | 0.11 (0.09) | 0.17 (0.12) |
| Similar/Similar (accuracy) | 0.41 (0.16) | 0.26 (0.14) | 0.29 (0.19) |
| CPAL 2 | 27.65 (32.87) | 45.67 (44.48) | 56.50 (58.06) |
| CPAL 6 | 22.95 (32.32) | 43.33 (41.30) | 40.50 (55.95) |
Values are mean (SD). MST means are proportion of responding in a given manner when presented with a stimulus type. CPAL performance is calculated as mean number of errors.
CPAL 2, Continuous Paired Associate Learning task during session 2; CPAL 6, Continuous Paired Associate Learning task during session 6; MST, Mnemonic Similarity Task; New/Old, classifying a new item as old on the MST; New/Similar, classifying a new item as similar on the MST; SCOP, scopolamine; Similar/Old, classifying a similar item as old on the MST; Similar/Similar, classifying a similar item as similar on the MST.
The groups did not differ in number of errors on the CPAL task at session 2 or session 6, nor on the average across sessions 2 and 6 (ps > .14).
DISCUSSION
In our double-blind, randomized controlled trial of nasal administration of scopolamine during public speaking exposure, our most robust finding was unexpected augmentation of extinction relative to placebo across exposure sessions. Simple effects showing less fear at context renewal (drug free) in SCOP 0.5 mg/SCOP 0.6 mg compared with placebo, in the absence of group differences at extinction retest (same context as exposure), are suggestive only and are complicated by other unexpected effects. There was some evidence for scopolamine to attenuate drug-free long-term extinction 1 month later in the exposure context. Finally, more errors occurred on a hippocampally dependent task for scopolamine groups versus placebo. The findings offer proof-of-concept for this noninvasive and translatable pharmacological approach for augmenting exposure therapy.
In a sample with clinically severe social anxiety disorder who feared public speaking, seven sessions of exposure to VR audience scenes substantially reduced subjective fear. This finding contributes to the growing body of literature supporting VR exposure (35). We selected VR because of its experimental control over context. All exposure sessions were in an identical VR-audience scene, the primary feared stimulus, combined with identical physical room locations, experimenters, olfactory cues, and auditory cues. Context renewal was tested in a different VR-audience scene in a different physical room location, with different experimenters, olfactory cues, and auditory cues. Our primary outcome was SCR to public speaking onset, as a proxy of conditional fear responding to conditional stimuli. We also measured SCR to the end of public speaking, as a proxy for reactivity to US absence, which is thought to index US expectancy (29). Additional measures included SCL in anticipation and recovery from public speaking and subjective distress. Scopolamine was well tolerated with no adverse incidents.
Given animal evidence that higher scopolamine doses attenuate extinction (24-26), we selected the lowest doses of scopolamine shown to influence hippocampal functioning in human studies (31). In contrast to our concerns, we observed beneficial effects from SCOP 0.5 mg and SCOP 0.6 mg on SCR to public speaking onset and termination. SCL in anticipation and recovery from public speaking was also lower overall across the seven exposure sessions in scopolamine groups relative to placebo. That the effects did not extend to self-reported fear is consistent with other translational research, such as pharmacological and behavioral disruptions of reconsolidation, which similarly has reported psychophysiological influences without impacting self-report (36,37). It is conceivable that effects of scopolamine on self-reported fear were mitigated by increased distress associated with side effects of scopolamine (see the Supplement).
Notably, beneficial effects of scopolamine on exposure/extinction cannot be solely attributed to direct physiological dampening effects of the drug, because no group differences were observed on any skin conductance index for any of the seven exposure trials in the first exposure session, when direct physiological effects should be apparent but extinction learning was still nascent. Attribution to the cognitive impact of scopolamine on associative learning is bolstered by our observation that scopolamine was associated with more errors, and placebo was associated with more accuracy, on the hippocampally dependent MST task. Although MST dosage effects were mixed (both SCOP 0.5 mg and SCOP 0.6 mg were associated with the same error, and each was associated with one different error), the effects consistently indicated more errors with scopolamine. Group differences were not replicated in the CPAL task for reasons that are not clear. Nevertheless, the results could suggest that scopolamine augmented extinction learning via effects associated with the hippocampus. One possible mechanism is that by interfering with processing of contextual cues (i.e., room and audience), the conditional stimulus (i.e., public speaking) became more salient, and that in turn facilitated prediction error learning (38). Another possibility is that temporal mismatch between massed exposure trials within each session and the passage of 3 to 4 days between exposure sessions created a context shift (39). Consequently, each session may have represented a new context, and by interfering with contextual processing, scopolamine attenuated session-dependent context renewal. Either way, the results argue for reverse-translation studies for basic animal research to examine the mechanism underlying enhanced extinction effects and the effect of scopolamine on retrieval/reconsolidation rather than extinction processes.
Only very limited support was found for our primary hypotheses regarding context renewal. SCOP 0.6 mg/SCOP .05 mg showed trends for dampened fear at drug-free context renewal relative to placebo in terms of SCR onset; in contrast, scopolamine did not influence counterbalanced extinction retest. Furthermore, SCOP 0.6 mg significantly dampened fear at context renewal relative to placebo in terms of SCR termination, again with no group differences at extinction retest. Although lower SCR termination at end of extinction within the scopolamine groups confounds group differences observed at test of context renewal, the end-of-extinction findings do not fully explain why group differences were observed only at context renewal and not at counterbalanced extinction retest. Moreover, SCOP 0.6 mg resulted in lower SCR termination at context renewal compared with at extinction retest.
But these findings are suggestive only, given reliance on simple effects in the absence of significant interactions. Interpretation is further complicated by lack of clear demonstration of context renewal, defined by higher fear at context renewal versus extinction retest, within placebo. Neither SCR onset nor SCR termination differed between context renewal and extinction retest within placebo: only SCL demonstrated context renewal within placebo. That neither scopolamine group showed differences between context renewal and extinction retest on anticipatory SCL could suggest that scopolamine attenuated context renewal, but the lack of differences between scopolamine and placebo groups at test of context renewal tempers this conclusion. Conceivably, our VR-audience scene, physical room, experimenter, olfactory cue (air freshener scent or not), and auditory cue (bell or gong) was insufficient to create distinctly different contexts. Alternatively, the VR equipment may have been such a prominent contextual feature in and of itself that it overrode other contextual feature differences. Lack of differences between context renewal and extinction retest could also reflect sensitization or spontaneous recovery, although context renewal effects have been clearly present in our prior studies with phobic samples that did not rely on VR (12-15). Thus, further investigation of scopolamine is warranted with conditions that more robustly induce context renewal. Another complication was unexpected higher self-reported fear in SCOP 0.6 mg at context renewal relative to extinction retest.
In terms of long-term extinction, SCOP 0.5 mg and SCOP 0.6 mg led to lower SCR to public speaking termination in the exposure context 1 month later, drug free. To the extent that 1 month represents another context shift (39), and given that SCR termination increased overall from end of extinction (session 7) to long-term extinction retest, the latter effects could be interpreted as dampening of context renewal. At the very least, the findings point to long-lasting benefits of scopolamine when tested 1 month later, drug free.
Limitations to our study include the lack of power to evaluate whether performance on the hippocampally dependent tasks mediated context renewal effects. The restriction to SCOP 0.5 mg and SCOP 0.6 mg limited our assessment of dosage effects, and further investigation is needed with higher dosages. Measures of startle eye blink may have proved more sensitive than those of skin conductance to context renewal. Generalizability of our findings to other anxiety disorders is unknown. Finally, the limited sample size after excluding participants who failed to extinguish undermined our power to detect effects.
In sum, this proof-of-concept study demonstrated that systemic administration of a noninvasive and well-tolerated muscarinic cholinergic receptor antagonist, scopolamine, augmented extinction during exposure therapy for clinically severe socially anxious individuals. The findings regarding context renewal following exposure therapy were limited to simple effects, complicated by other unexpected outcomes, and are suggestive only. Finally, scopolamine appeared to augment long-term extinction, with effects persisting for 1 month when tested drug free. The results highlight the importance of further investigation of this pharmacological approach to augmenting exposure therapy and reducing context fear renewal.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This research was supported by a National Institute of Mental Health Grant No. R34MH101359 “Cholinergic decontextualization of exposure therapy for anxiety” (to MGC and MF).
We thank the research coordinators, including Natalie Arbid, Gabriella Imbriano, Abigail Branch, and Richard Kim, as well as the participants in the study.
MGC, MT, and AB report no biomedical financial interests or potential conflicts of interest. MF is a founding board member and shareholder of Neurovation, Inc.; he receives no research support or salary from Neurovation.
ClinicalTrials.gov: Generalization of Extinction Learning; https://clinicaltrials.gov/ct2/show/NCT01900301?term=NCT01900301&rank=1; .
Footnotes
Startle eye blink reflex was our original primary psychophysiological response. However, owing to technical difficulties, our measurement of startle eye blink was not reliable and thus was not analyzed.
As used in other studies [Schiller et al. (34)], extinction was defined as a decrease of ≤0.01 microsiemens for SCR and SCL and ≤0 SUDS scores from first speech of first exposure session to the last (seventh) speech of the last (seventh) exposure session.
There was no main effect of type of VR equipment (VFX 3D Interactive Personal Display [Mindflux, Roseville, New South Wales, Australia] combined with a smartphone or a Vuzix Wrap 1200 [West Henrietta, NY]) on any dependent variable (ps > .29).
There was no main effect of type of VR equipment or main effect of order of renewal and extinction retest (ps > .70).
Six individuals (placebo = 5, SCOP .06 mg = 1) did not extinguish and were excluded from analyses.
Ten individuals (placebo = 8, SCOP 0.5 mg = 2) did not extinguish and were excluded from analyses.
There was no main effect of type of VR equipment or main effect of order of renewal and extinction retest (ps > .09).
Three individuals (placebo = 2, SCOP 0.5 mg = 1) did not extinguish and were excluded from analyses.
There was no main effect of type of VR equipment or main effect of order of renewal and extinction retest (ps > .41).
Four individuals (placebo = 3, SCOP 0.5 mg = 1) did not extinguish and were excluded from analyses.
There was no main effect of type of VR equipment or main effect of order of renewal and extinction retest (ps > .13)
There was no main effect of type of VR equipment or main effect of order of renewal and extinction retest (ps > .56).
Nine individuals (placebo = 4, SCOP 0.5 mg = 3, SCOP 0.6 mg = 2) did not extinguish and were excluded from analyses.
These analyses excluded the same participants who did not exhibit extinction and were excluded from analyses of context renewal and extinction retest.
Contributor Information
Michelle G. Craske, Staglin Center for Brain and Behavioral Health, University of California–Los Angeles, Los Angeles, California; Department of Psychology, and Department of Psychiatry and Biobehavioral Sciences, University of California–Los Angeles, Los Angeles, California.
Michael Fanselow, Staglin Center for Brain and Behavioral Health, University of California–Los Angeles, Los Angeles, California. Department of Psychology, and Department of Psychiatry and Biobehavioral Sciences, University of California–Los Angeles, Los Angeles, California..
Michael Treanor, Staglin Center for Brain and Behavioral Health, University of California–Los Angeles, Los Angeles, California. Department of Psychology, and Department of Psychiatry and Biobehavioral Sciences, University of California–Los Angeles, Los Angeles, California..
Alexander Bystritksy, Department of Psychology, and Department of Psychiatry and Biobehavioral Sciences, University of California–Los Angeles, Los Angeles, California..
REFERENCES
- 1.Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Huibers MJH (2016): How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry 15:245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolitzky-Taylor KB, Horowitz JD, Powers MB, Telch MJ (2008): Psychological approaches in the treatment of specific phobias: A metaanalysis. Clin Psychol Rev 28:1021–1037. [DOI] [PubMed] [Google Scholar]
- 3.Craske MG, Mystkowski JL (2006): Exposure therapy and extinction: Clinical studies In: Craske MG, Hermans D, Vansteenwegen D, editors. Fear and Learning: From Basic Processes to Clinical Implications. Washington, DC: American Psychological Association, 217–233. [Google Scholar]
- 4.Bouton ME (1993): Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull 114:80–99. [DOI] [PubMed] [Google Scholar]
- 5.Bouton ME, King DA (1983): Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. J Exp Psychol Anim Behav Process 9:248–265. [PubMed] [Google Scholar]
- 6.Miller RR, Matzel LD (1988): The comparator hypothesis: A response rule of the expression of associations In: Psychology of Learning and Motivation, vol 22 New York: Academic Press, 51–92. [Google Scholar]
- 7.Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Baeyens F, et al. (2005): Return of fear in a human differential conditioning paradigm caused by a return to the original acquisition context. Behav Res Ther 43:323–336. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C (2008): Contextual fear conditioning in humans: Cortical–hippocampal and amygdala contributions. J Neurosci 28:6211–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milad MR, Orr SP, Pitman RK, Rauch SL(2005): Context modulation of memory for fear extinction in humans. Psychophysiology 42:456–464. [DOI] [PubMed] [Google Scholar]
- 10.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007): Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62:446–454. [DOI] [PubMed] [Google Scholar]
- 11.Neumann DL, Waters AM, Westbury HR (2008): The use of an unpleasant sound as the unconditional stimulus in aversive Pavlovian conditioning experiments that involve children and adolescent participants. Behav Res Methods 40:622–625. [DOI] [PubMed] [Google Scholar]
- 12.Mystkowski JL, Craske MG, Echiverri AM (2002): Treatment context and return of fear in spider phobia. Behav Ther 33:399–416. [DOI] [PubMed] [Google Scholar]
- 13.Mystkowski JL, Mineka S, Vernon LL, Zinbarg RE (2003): Changes in caffeine states enhance return of fear in spider phobia. J Consult Clin Psychol 71:243–250. [DOI] [PubMed] [Google Scholar]
- 14.Mysktowski JL, Craske MG, Echiverri AM, Labus JS (2006): Mental reinstatement of context and return of fear in spider–fearful participants. Behav Ther 37:49–60. [DOI] [PubMed] [Google Scholar]
- 15.Culver NC, Stoyanova M, Craske MG (2011): Clinical relevance of retrieval cues for attenuating context renewal of fear. J Anxiety Disord 25:284–292. [DOI] [PubMed] [Google Scholar]
- 16.Anagnostaras SG, Maren S, Fanselow MS (1999): Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within–subjects examination. J Neurosci 19:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale GD, Anagnostaras SG, Fanselow MS (2001): Cholinergic modulation of Pavlovian fear conditioning: Effects of intrahippocampal scopolamine infusion. Hippocampus 11:371–376. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Fanselow MS (1992): Modality-specific retrograde amnesia of fear. Science 256:675–677. [DOI] [PubMed] [Google Scholar]
- 19.Fanselow MS (2000): Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res 110:73–81. [DOI] [PubMed] [Google Scholar]
- 20.Corcoran KA, Maren S (2004): Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem 11:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji J, Maren S (2008): Lesions of the entorhinal cortex or fornix disrupt the context dependence of fear extinction in rats. Behav Brain Res 194:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown KL, Kennard JA, Sherer DJ, Comalli DM, Woodruff-Pak DS (2011): The context preexposure facilitation effect in mice: A dose–response analysis of pretraining scopolamine administration. Behav Brain Res 225:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luyten L, Nuyts S, Beckers T (2017): Low-dose systemic scopolamine disrupts context conditioning in rats. J Psychopharmacol 31:667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelikowsky M, Hast TA, Bennett RZ, Merjanian M, Nocera NA, Ponnusamy R, et al. (2012): Cholinergic blockade frees fear extinction from its contextual dependency. Biol Psychiatry 73:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prado-Alcalá RA, Haiek M, Rivas S, Roldan–Roldan G, Quirarte GL (1994): Reversal of extinction by scopolamine. Physiol Behav 56:27–30. [DOI] [PubMed] [Google Scholar]
- 26.Santini E, Sepulveda-Orengo M, Porter JT (2012): Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology 37:2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown TA, Barlow DH (2014): Anxiety and Related Disorders Interview Schedule for DSM-5 (ADIS-5). Oxford, UK: Oxford University Press. [Google Scholar]
- 28.Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, Goetz D (2001): The Liebowitz Social Anxiety Scale: A comparison of the psychometric properties of self-report and clinician-administered formats. Psychol Med 31:1025–1035. [DOI] [PubMed] [Google Scholar]
- 29.Spoormaker VI, Blechert J, Goya-Maldonado R, Sämann PG, Wilhelm FH, Czisch M (2012): Additional support for the existence of skin conductance responses at unconditioned stimulus omission. Neuroimage 63:1404–1407. [DOI] [PubMed] [Google Scholar]
- 30.Knight DC (2004): Neural substrates mediating human delay and trace fear conditioning. J Neurosci 24:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harel BT, Pietrzak RH, Snyder PJ, Maruff P (2013): Effect of cholinergic neurotransmission modulation on visual spatial paired associate learning in healthy human adults. Psychopharmacology 228:673–683. [DOI] [PubMed] [Google Scholar]
- 32.Bakker A, Kirwan CB, Miller M, Stark CEL (2008): Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 319:1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tasca GA, Gallop R (2009): Multilevel modeling of longitudinal data for psychotherapy researchers: I. The basics. Psychother Res 19:429–437. [DOI] [PubMed] [Google Scholar]
- 34.Schiller D, Kanen JW, LeDoux JE, Monfils M-H, Phelps EA (2013): Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc Natl Acad Sci U S A 110:20040–20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botella C, Fernández-Álvarez J, Guillén V, García-Palacios A, Baños R (2017): Recent progress in virtual reality exposure therapy for phobias: A systematic review. Curr Psychiatry Rep 19:42. [DOI] [PubMed] [Google Scholar]
- 36.Kindt M, Soeter M (2014): Fear inhibition in high trait anxiety. PLoS One 9:e86462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soeter M, Kindt M (2010): Dissociating response systems: Erasing fear from memory. Neurobiol Learn Mem 94:30–41. [DOI] [PubMed] [Google Scholar]
- 38.Rescorla RA, Wagner AR (1972): A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York, NY: Appleton-Century-Crofts, 64–99. [Google Scholar]
- 39.Bouton ME (2004): Context and behavioral processes in extinction. Learn Mem 11:485–494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.