Abstract
Problem:
Preterm birth is commonly preceded by preterm labor, a syndrome that is causally linked to both intra-amniotic infection and intra-amniotic inflammation. However, the stereotypical cellular immune responses in these two clinical conditions are poorly understood.
Method of Study:
Amniotic fluid samples (n = 26) were collected from women diagnosed with preterm labor and intra-amniotic infection (amniotic fluid IL-6 concentrations ≥ 2.6 ng/mL and culturable microorganisms, n = 10) or intra-amniotic inflammation (amniotic fluid IL-6 concentrations ≥ 2.6 ng/mL without culturable microorganisms, n = 16). Flow cytometry was performed to evaluate the phenotype and number of amniotic fluid leukocytes. Amniotic fluid concentrations of classical pro-inflammatory cytokines, type 1 and type 2 cytokines, and T-cell chemokines were determined using immunoassays.
Results:
Women with spontaneous preterm labor and intra-amniotic infection had: 1) a greater number of total leukocytes including neutrophils and monocytes/macrophages in amniotic fluid, 2) a higher number of total T cells and CD4+ T cells, but not CD8+ T cells or B cells, in amniotic fluid, and 3) increased amniotic fluid concentrations of IL-6, IL-1β, and IL-10, compared to those with intra-amniotic inflammation. However, no differences in amniotic fluid concentrations of T-cell cytokines and chemokines were observed between these two clinical conditions.
Conclusions:
The cellular immune responses observed in women with preterm labor and intra-amniotic infection are more severe than in those with intra-amniotic inflammation, and neutrophils, monocyte/macrophages, and CD4+ T cells are the main immune cells responding to microorganisms invading the amniotic cavity. These findings provide insights into the intra-amniotic immune mechanisms underlying the human syndrome of preterm labor.
Keywords: Chorioamnionitis, funisitis, fetal inflammatory response syndrome, prematurity, microbial invasion of the amniotic cavity, MIAC, pregnancy, placental inflammation
Graphical Abstract
INTRODUCTION
Preterm birth remains one of the most common obstetrical syndromes today, and is a primary cause of perinatal morbidity and mortality worldwide1–5. On average, two-thirds of all preterm births are preceded by spontaneous preterm labor6, a syndrome of multiple etiologies7. Of all the proposed causes of preterm labor, intra-amniotic inflammation/infection has been causally linked to preterm birth8–17. Intra-amniotic inflammation can result from microbial invasion of the amniotic cavity, which is referred to as intra-amniotic infection9,10,12,18–29. Yet, inflammation in the amniotic cavity can also occur in the absence of culturable microorganisms, which is simply known as intra-amniotic inflammation16,27,30. More recently, we showed that a subset of patients with preterm labor and intra-amniotic inflammation do not have detectable bacteria using molecular microbiology techniques, which we termed sterile intra-amniotic inflammation31–34. This condition is associated with elevated concentrations of endogenous danger signals or alarmins (molecules released upon cellular stress or damage35–37) in amniotic fluid38–43 and, although of interest, it is not yet a clinical diagnosis since the use of molecular microbiological techniques is not common practice in obstetrics. Therefore, patients with preterm labor are either diagnosed with intra-amniotic infection or intra-amniotic inflammation. Although both clinical conditions are associated with preterm labor and adverse neonatal outcomes32,44, their management is different (intra-amniotic infection is treated with antibiotics45), and only intra-amniotic infection is linked to maternal morbidity and mortality46. Therefore, elucidating the stereotypical immune responses in intra-amniotic infection and intra-amniotic inflammation is essential for understanding these clinical conditions.
Flow cytometry has emerged as a cutting-edge technique for the evaluation of immune cells in small volumes of biological fluids such as cerebrospinal fluid47,48, urine49–51, ascitic fluid52, and sputum53 in the clinical setting. Indeed, flow cytometry has been utilized to identify specific immune cell types, as well as their expressed mediators, in amniotic fluid of women with intra-amniotic inflammation/infection and clinical chorioamnionitis at term54,55. This technique also allowed for the identification of the newly described innate lymphoid cells in the amniotic cavity of women during the second trimester55,56. Herein, we utilized flow cytometry to characterize the cellular immune responses in the amniotic cavity of women diagnosed with preterm labor and intra-amniotic infection or intra-amniotic inflammation.
MATERIALS AND METHODS
Study population and characteristics
This was a cross-sectional study including patients who underwent transabdominal amniocentesis due to clinical indications. The collection of samples was approved by the Institutional Review Boards of the Detroit Medical Center (Detroit, MI, USA), Wayne State University, and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS). All women provided written informed consent prior to the collection of amniotic fluid. This study included 26 amniotic fluid samples (collected from 2013 to 2016) from women classified into the following groups: 1) women with spontaneous preterm labor who delivered preterm with intra-amniotic inflammation (n = 16) and 2) women with spontaneous preterm labor who delivered preterm with intra-amniotic infection (n = 10) (see diagnostic criteria below). For all patients who delivered preterm, the time between the collection of the amniotic fluid sample and delivery was ≤ 7 days. Demographic and clinical characteristics of the study population are shown in Table 1.
Table 1.
Clinical and demographic characteristics of women who underwent spontaneous preterm labor
| Preterm labor and birth with intra-amniotic infection (n=10) | Preterm labor and birth with intra-amniotic inflammation (n=16) | p-value | |
|---|---|---|---|
| Maternal age (years; median [IQR])a | 27.5 (22.8–35.8) | 25.5 (22–28.3) | 0.3 |
| Body mass index (kg/m2; median [IQR])a | 28.3 (27–36.5)d | 26.3 (24.2–30.7) | 0.1 |
| Primiparityb | 0% (0/10) | 12.5% (2/16) | 0.5 |
| Raceb | 1 | ||
| African-American | 80% (8/10) | 81.3% (13/16) | |
| Caucasian | 20% (2/10) | 12.5% (2/16) | |
| Other | 0% (0/10) | 6.3% (1/16) | |
| Gestational age at amniocentesis (weeks; median [IQR])a | 25.9 (22.5–29.8) | 27 (22.9–31.2) | 0.4 |
| IL-6 (ng/mL; median [IQR])a | 128.7 (78–186.2) | 47.8 (16.7–122.9) | 0.04 |
| White blood cell count, cells/mm3,a | 307 (89–1254.5) | 2 (0–11.8) | 0.003 |
| Amniotic fluid glucose, mg/dLa | 3.5 (1–9.8) | 12.5 (6–26) | 0.053 |
| Gestational age at delivery (weeks; median [IQR])a | 25.9 (23–30) | 27.6 (23.4–31.3) | 0.4 |
| Cesarean sectionb | 0% (0/10) | 31.3% (5/16) | 0.1 |
| Birthweight (grams)a | 935 (533.3–1428.8) | 1037.5 (697.5–1778.8) | 0.6 |
| Acute maternal inflammatory response | |||
| Stage 1 (acute subchorionitis)b | 0% (0/8)c | 23.1% (3/13)d | 0.2 |
| Stage 2 (acute chorioamnionitis)b | 37.5% (3/8)c | 30.8% (4/13)d | 1 |
| Stage 3 (acute necrotizing chorioamnionitis)b | 50% (4/8)c | 30.8% (4/13)d | 0.6 |
| Acute fetal inflammatory response | |||
| Stage 1 (acute phlebitis/chronic vasculitis)b | 50% (4/8)c | 53.8% (7/13)d | 1 |
| Stage 2 (acute arteritis)b | 25% (2/8)c | 0% (0/13)d | 0.1 |
| Stage 3 (necrotizing funisitis)b | 12.5% (1/8)c | 0% (0/13)d | 0.3 |
Data are given as median (interquartile range, IQR) and percentage (n/N).
Mann-Whitney U-test.
Fisher’s exact test.
Two missing data.
Three missing data.
Clinical Definitions
Gestational age was determined by the date of the last menstrual period and confirmed by ultrasound examination. The gestational age derived from sonographic fetal biometry was used if the estimation was inconsistent with menstrual dating. Spontaneous preterm labor was diagnosed by the presence of regular uterine contractions (at least two contractions every ten minutes) associated with cervical changes in patients < 37 weeks of gestation. Microbial invasion of the amniotic cavity (MIAC) was defined as a positive amniotic fluid culture, including genital mycoplasmas9,10,22,57,58. Intra-amniotic inflammation was defined as an amniotic fluid IL-6 concentration ≥ 2.6 ng/mL27,59–62 in the absence of culturable bacteria63–65. Intra-amniotic infection was defined as the presence of MIAC together with intra-amniotic inflammation31–33,66–75.
Placental histopathological examination
Placentas were examined histologically by perinatal pathologists blinded to clinical diagnoses and obstetrical outcomes according to standardized Perinatology Research Branch protocols76,77. Briefly, three to nine sections of the placenta were examined, and at least one full-thickness section was taken from the center of the placenta; others were taken randomly from the placental disc. Acute inflammatory lesions of the placenta (maternal inflammatory response and fetal inflammatory response) were diagnosed according to established criteria, including staging and grading76,78. The proportions of patients whose placentas presented acute maternal and/or fetal inflammatory responses are displayed in Table 1.
Amniotic fluid sample collection
Amniotic fluid samples were obtained by transabdominal amniocentesis under antiseptic conditions and monitored by ultrasound in order to detect intra-amniotic inflammation and/or infection in patients with preterm labor. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and immunophenotyping was performed immediately. The rest of the sample was centrifuged at 1,300 × g for 10 min at 4°C, and the supernatant was stored at −80°C until use. Also, an aliquot of amniotic fluid was transported to the clinical laboratory for culture of aerobic/anaerobic bacteria and genital mycoplasmas. The clinical tests also included the determination of amniotic fluid white blood cell count79, Gram stain examination80, glucose concentration81, and IL‐6 concentration27.
Determination of IL-6 in amniotic fluid
Amniotic fluid concentrations of IL‐6 were determined by using a sensitive and specific enzyme immunoassay obtained from R&D Systems (Minneapolis, MN). The IL‐6 concentrations were determined by interpolation from the standard curve. The inter‐ and intra‐assay coefficients of variation for IL‐6 were 8.7% and 4.6%, respectively. The detection limit of the IL‐6 assay was 0.09 pg/mL. The IL‐6 concentrations in amniotic fluid were determined for clinical purposes.
Immunophenotyping by flow cytometry
Amniotic fluid samples (0.5–1 mL) were centrifuged at 300 × g for 5 minutes at room temperature. The resulting amniotic fluid pellet was re-suspended in 1 mL of 1X phosphate-buffered saline (PBS) (Life Technologies, Grand Island, NY, USA) and stained with the BD Horizon Fixable Viability Stain 510 dye (BD Biosciences, San Jose, CA, USA). Cells were washed in 1X PBS and incubated with 20 μL of human FcR blocking reagent (Miltenyi Biotec, San Diego, CA, USA) in 80 μL of stain buffer (BD Biosciences) for 10 min at 4°C. Next, cells were incubated with extracellular fluorochrome-conjugated anti-human monoclonal antibodies for 30 min at 4°C in the dark (Supplementary Table 1). Stained cells were then washed with 1X PBS, re-suspended in 0.5 mL of stain buffer, and acquired using the BD LSR II or LSRFortessa Flow Cytometer (BD Bioscience) and BD FACSDiva 6.0 software (BD Bioscience). The analysis was performed and the figures were generated using the FlowJo version 10 software (FlowJo, Ashland, OR, USA). The absolute number of cells was determined using CountBright absolute counting beads (Molecular Probes, Eugene, OR, USA).
Amniotic fluid cytokine/chemokine concentrations
Amniotic fluid samples were assessed using the V-PLEX Proinflammatory Panel 1 kit (Meso Scale Discovery, Rockville, MD, USA) to measure amniotic fluid concentrations of IFNγ, TNFα, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, and IL-13, according to the manufacturer’s instructions. Plates were read using the SECTOR 2400 Imager (Meso Scale Discovery). Standard curves were generated and the assay values of the samples were interpolated from the curves. The detection limits of the assays were 0.37 pg/mL (IFNγ), 0.04 pg/mL (TNFα), 0.05 pg/mL (IL-1β), 0.09 pg/mL (IL-2), 0.02 pg/mL (IL-4), 0.06 pg/mL (IL-6), 0.07 pg/mL (IL-8), 0.04 pg/mL (IL-10), 0.11 pg/mL (IL-12p70), and 0.24 pg/mL (IL-13). Inter-assay and intra-assay coefficients of variation were less than 10.5%.
Amniotic fluid concentrations of CXCL10 (Cat#DIP100, R&D Systems, Minneapolis, MN, USA) and CXCL11 (Cat#K151UWK-1, Meso Scale Discovery) were determined using individual sensitive and specific immunoassays, according to the manufacturer’s instructions. The concentrations of CXCL10 and CXCL11 were determined by interpolation from the standard curve. The detection limits of the assays were 1.67 pg/mL (CXCL10) and 1.5 pg/mL (CXCL11). The inter-assay and intra-assay coefficients of variation were less than 9.8% for CXCL10 and less than 16.8% for CXCL11.
Statistical analysis
Statistical analyses were conducted using SPSS software version 19.0 (IBM Corporation, Armonk, NY, USA). For patient demographics, the Mann-Whitney U-test was performed for continuous variables and the Fisher’s exact test for nominal variables. The Mann-Whitney U-test was also performed when comparing cell numbers and cytokine/chemokine concentrations between study groups. A p‐value < 0.05 was considered statistically significant.
RESULTS
Characteristics of the study population
The demographic and clinical characteristics of the study population are shown in Table 1. A total of 26 amniotic fluid samples were collected from women who underwent spontaneous preterm labor and birth either with intra-amniotic infection (n = 10) or intra-amniotic inflammation (n = 16). Amniotic fluid concentrations of IL-6 and white blood cell counts were higher in women with preterm labor and intra-amniotic infection compared to those with intra-amniotic inflammation (Table 1). Glucose concentrations tended to be lower in women with intra-amniotic infection compared to those with intra-amniotic inflammation (Table 1). Both women with intra-amniotic inflammation and those with intra-amniotic infection presented acute maternal and fetal inflammatory responses in the placenta (Table 1). The following microorganisms were detected in women diagnosed with intra-amniotic infection: Ureaplasma urealyticum, Mycoplasma hominis, Fusobacterium spp., Candida spp., Gardnerella vaginalis, Peptostreptococcus, Streptococcus serogroup C, and Enterococcus faecalis.
Leukocyte populations in amniotic fluid of women with preterm labor and intra-amniotic inflammation or intra-amniotic infection
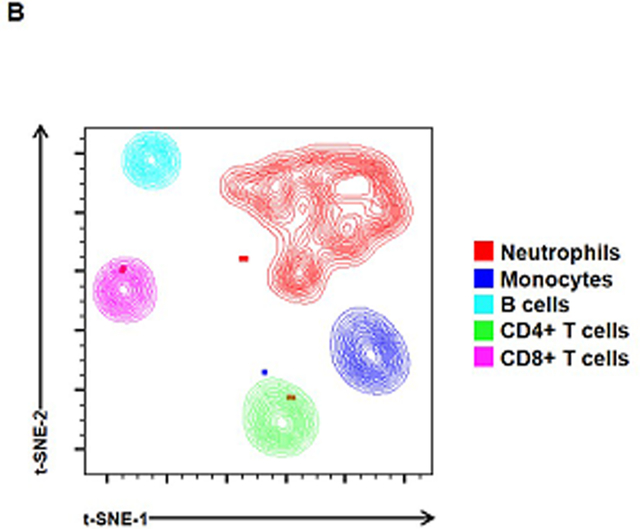
Figures 1A&C show representative images of the flow cytometry gating strategy used to detect leukocytes in amniotic fluid from women with preterm labor and intra-amniotic inflammation (Figure 1A) or intra-amniotic infection (Figure 1C). Representative t-SNE plots illustrate the amniotic fluid leukocyte populations found in the two study groups (Figures 1B&D). Notably, more women with preterm labor and intra-amniotic infection (80%) displayed a high proportion of neutrophils and monocytes/macrophages in amniotic fluid compared to those with intra-amniotic inflammation without detectable microorganisms (50%) (Figure 1D vs. 1B). Indeed, the abundant neutrophils and monocytes/macrophages in amniotic fluid of women with intra-amniotic infection masked the other immune cell types (e.g. T cells and B cells) that are clearly identified in women with intra-amniotic inflammation (Figure 1D vs. 1B).
Figure 1. Leukocyte populations in amniotic fluid.
Representative flow cytometry gating strategies and t-distributed stochastic neighbor embedding (t-SNE) plots showing leukocyte populations in amniotic fluid from women who underwent spontaneous labor and birth with intra-amniotic inflammation (A-B) or with intra-amniotic infection (C-D). Immune cells were initially gated within the viability gate and CD45+ gate followed by lineage gating for neutrophils (CD45+CD15+ cells), monocytes/macrophages (CD45+CD14+ cells), T cells (CD45+CD3+ cells) that were subsequently gated for CD4+ T cells (CD45+CD3+CD4+ cells) and CD8+ T cells (CD45+CD3+CD8+ cells), and B cells (CD45+CD19+ cells). Plots are representative of 10 – 16 samples per group.
We then quantified the numbers of total leukocytes in amniotic fluid from the two study groups. Women with preterm labor and intra-amniotic infection had greater total numbers of leukocytes in amniotic fluid compared to those with intra-amniotic inflammation (Figure 2A). Quantification of neutrophils (CD15+ leukocytes) and monocytes/macrophages (CD14+ leukocytes) in amniotic fluid showed that the numbers of these innate immune cells were greater in women with intra-amniotic infection compared to those with intra-amniotic inflammation (Figure 2B&C), mirroring the relative differences observed between the t-SNE plots in Figure 1.
Figure 2. Total leukocytes and innate immune cells in amniotic fluid.
Numbers of (A) total leukocytes (CD45+ cells/mL), (B) neutrophils (CD15+ cells/mL), and (C) monocytes/macrophages (CD14+ cells/mL) in amniotic fluid from women who underwent spontaneous preterm labor and birth with intra-amniotic inflammation or with intra-amniotic infection. N = 10 – 16 per group. Midlines = medians, boxes = interquartile ranges, and whiskers = minimum/maximum ranges.
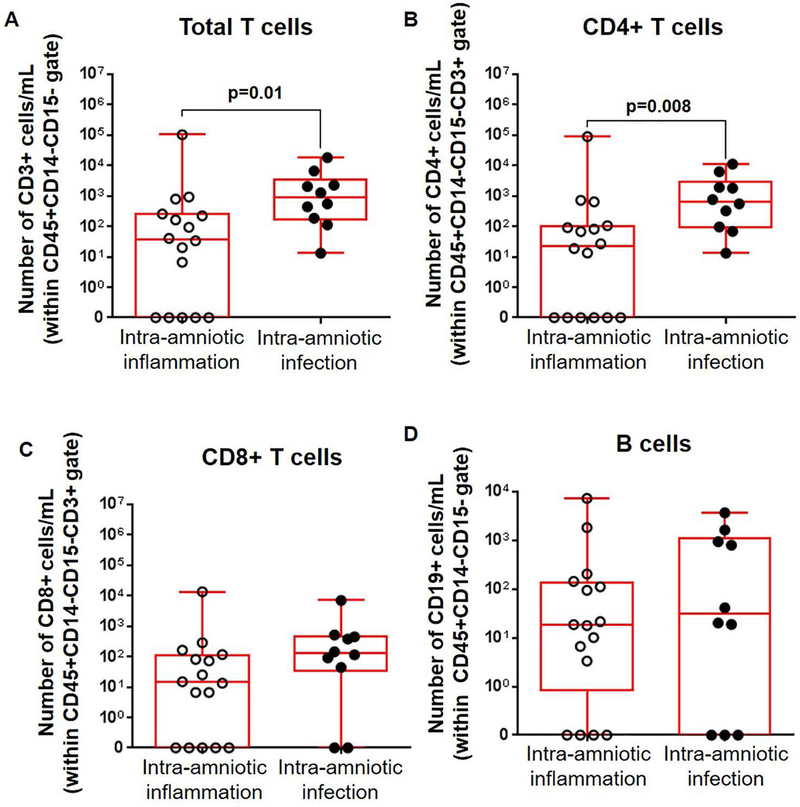
We previously demonstrated that adaptive immune cells (i.e. T cells and B cells) are also present in amniotic fluid during normal pregnancy55. Therefore, we then determined whether the numbers of such cells were altered in amniotic fluid from our two study groups. We found that the total T-cell population (CD3+ leukocytes) as well as CD4+ T cells were significantly increased in women with preterm labor and intra-amniotic infection compared to those with intra-amniotic inflammation (Figures 3A&B). The numbers of CD8+ T cells were also higher in women with preterm labor and intra-amniotic infection compared to those with intra-amniotic inflammation, although this was not significant (Figure 3C). Lastly, we determined the numbers of B cells (CD19+ leukocytes) and found no statistical differences between the two study groups (Figure 3D).
Figure 3. Adaptive immune cells in amniotic fluid.
Numbers of (A) total T cells (CD3+ cells/mL), (B) CD4+ T cells (CD3+CD4+ cells/mL), (C) CD8+ T cells (CD3+CD8+ cells/mL), and (D) B cells (CD19+ cells/mL) in amniotic fluid from women who underwent spontaneous preterm labor and birth with intra-amniotic inflammation or with intra-amniotic infection. N = 10 – 16 per group. Midlines = medians, boxes = interquartile ranges, and whiskers = minimum/maximum ranges.
Together, these results indicate that total leukocytes, as well as specific leukocyte subsets, namely neutrophils, monocytes/macrophages, and CD4+ T cells, are increased in amniotic fluid of women with preterm labor and intra-amniotic infection compared to women with preterm labor and intra-amniotic inflammation.
Cytokine and chemokine concentrations in amniotic fluid of women with preterm labor and intra-amniotic inflammation or intra-amniotic infection
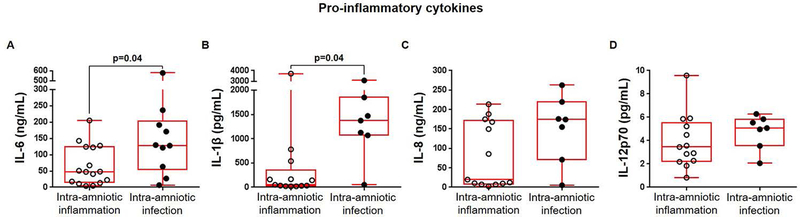
Next, we investigated whether the increased numbers of amniotic fluid leukocytes in women with preterm labor and intra-amniotic infection were associated with an increase in cytokine concentrations. We found that the concentrations of the pro-inflammatory cytokines IL-6 and IL-1β were both significantly elevated in amniotic fluid of women with preterm labor and intra-amniotic infection compared to those with intra-amniotic inflammation (Figures 4A&B). In contrast, concentrations of IL-8 and IL-12p70 were not significantly different between women with preterm labor and intra-amniotic infection and those with intra-amniotic inflammation (Figures 4C&D).
Figure 4. Pro-inflammatory cytokines in amniotic fluid.
Concentrations of (A) IL-6 (ng/mL), (B) IL-1β (pg/mL), (C) IL-8 (ng/mL), and (D) IL-12p70 (pg/mL) in amniotic fluid from women who underwent spontaneous preterm labor and birth with intra-amniotic inflammation or with intra-amniotic infection. N = 7 – 16 per group. Midlines = medians, boxes = interquartile ranges, and whiskers = minimum/maximum ranges.
Since the numbers of amniotic fluid total T cells and CD4+ T cells were increased in women with intra-amniotic infection, the concentrations of type 1 and 2 cytokines, as well as T-cell chemokines, were determined. The type 1 cytokines IL-2, IFNγ, and TNFα were not significantly different between women with intra-amniotic infection and those with intra-amniotic inflammation (Figures 5A–C). While IL-4 and IL-13 did not change, the amniotic fluid concentration of the type 2 cytokine IL-10 tended to increase in women with intra-amniotic infection compared to those with intra-amniotic inflammation (Figures 5D–F). No differences were observed in amniotic fluid concentrations of the T-cell chemokines CXCL10 and CXCL11 between the study groups (Figures 5G&H).
Figure 5. Type 1 and 2 cytokines and T-cell chemokines in amniotic fluid.
Concentrations of the type 1 cytokines (A) IL-2 (pg/mL), (B) IFNγ (pg/mL), and (C) TNFα (pg/mL), type 2 cytokines (D) IL-4 (pg/mL), (E) IL-13 (pg/mL), and (F) IL-10 (pg/mL), and T-cell chemokines (G) CXCL10 (pg/mL) and (H) CXCL11 (pg/mL) in amniotic fluid from women who underwent spontaneous preterm labor and birth with intra-amniotic inflammation or with intra-amniotic infection. N = 7 – 13 per group. Midlines = medians, boxes = interquartile ranges, and whiskers = minimum/maximum ranges.
DISCUSSION
Principal Findings
Herein, we report that women with spontaneous preterm labor and intra-amniotic infection had: 1) a greater number of total leukocytes including neutrophils and monocytes/macrophages in amniotic fluid, 2) a higher number of total T cells and CD4+ T cells, but not CD8+ T cells or B cells, in amniotic fluid, and 3) increased amniotic fluid concentrations of IL-6, IL-1β, and IL-10, compared to those with intra-amniotic inflammation. However, no differences in amniotic fluid concentrations of T-cell cytokines and chemokines were observed between these two clinical conditions. Collectively, these results indicate that the cellular immune responses observed in women with preterm labor and intra-amniotic infection are more severe than in those with intra-amniotic inflammation, and are characterized by an increased number of neutrophils, monocyte/macrophages, and CD4+ T cells.
Amniotic fluid neutrophils in women with preterm labor and intra-amniotic infection or intra-amniotic inflammation
It is well known that neutrophils are the most abundant immune cell type in the amniotic cavity of women with intra-amniotic infection and/or inflammation54,79,82,83. Yet, whether the number of amniotic fluid neutrophils is different between intra-amniotic inflammatory processes with and without culturable microorganisms has until now not been shown. Herein, we showed that the number of amniotic fluid neutrophils is higher in women with preterm labor and intra-amniotic infection than in those with intra-amniotic inflammation without culturable microorganisms, indicating that different thresholds in the number of these immune cells may allow for the differentiation of these two clinical conditions.
In women with preterm labor and intra-amniotic infection, as well as in other pathogen-mediated immune responses84,85, neutrophils participate in the main mechanisms of microbial killing: degranulation, phagocytosis, and neutrophil extracellular trap (NET) formation. For example, amniotic fluid neutrophils can produce reactive oxygen species86 and release anti-microbial molecules such as alpha-defensins87–90, myeloperoxidase29,90,91, cathepsin G90,92, elastase90,93,94, lactoferrin95, pentraxin-396, and cathelicidin29,90, all of which are found in the intra-amniotic space. Amniotic fluid neutrophils of fetal or maternal origin83 can also actively participate in killing microbes invading the amniotic cavity by performing phagocytosis97 and forming NETs98. Indeed, NETs are also formed by maternal neutrophils invading the amniotic cavity98 and chorioamniotic membranes99,100 in cases with intra-amniotic infection. Besides killing microbes, amniotic fluid neutrophils can release pro-inflammatory cytokines such as interleukin (IL)-8, tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-1α, MIP-1β, IL-1α, and IL-1β into the intra-amniotic space in cases with MIAC and clinical chorioamnionitis at term54. These cytokines have been implicated in the pathogenesis of preterm labor in the context of intra-amniotic infection38,39,43,82,101–110. Specifically, IL-1β is a central mediator in the pathogenesis of preterm labor since the systemic111,112 and intra-amniotic11,113–119 administration of this cytokine in pregnant animals induces preterm birth. The mechanisms whereby IL-1β induces preterm labor and birth, in the context of intra-amniotic infection, involve the Nucleotide-Binding Oligomerization Domain, Leucine Rich Repeat and Pyrin Domain Containing 3 (NLRP3) inflammasome, an intracellular multi-protein complex that can be activated in the fetal membranes by microbial products in mice (e.g. lipopolysaccharide; LPS)120. Consistently, women with preterm labor and acute histologic chorioamnionitis (a placental lesion associated with intra-amniotic infection121–123) also display inflammasome activation in the amniotic fluid34 and chorioamniotic membranes124. Together, these data indicate that amniotic fluid neutrophils participate in both the host defense and inflammatory mechanisms implicated in the pathogenesis of preterm labor and birth in women with intra-amniotic infection.
Women with intra-amniotic inflammation also had neutrophils in their amniotic fluid; however, the numbers were lower than in those with intra-amniotic infection. This finding suggests that the mechanisms of inflammation occurring in women without culturable microorganisms in amniotic fluid are distinct from and less severe than in those with intra-amniotic infection. One possibility is that a subset of women who underwent preterm labor with intra-amniotic inflammation had elevated amniotic fluid concentrations of alarmins43 (e.g. IL-1α39, HMGB142,125, HSP7041, and S100B40), referred to as sterile intra-amniotic inflammation32–34. Human studies have provided evidence that preterm labor with sterile intra-amniotic inflammation is less severe than preterm labor with microbial-induced intra-amniotic inflammation34,43,126. Indeed, only ~50% of pregnant mice intra-amniotically injected with physiologically-relevant concentrations of alarmins undergo preterm birth127,128, whereas almost all of those injected with a microbial product (LPS) deliver preterm129,130. The mechanisms whereby alarmins induced preterm labor and birth also involved the NLRP3 inflammasome128,131; yet, these will be discussed below since sterile inflammation is mainly mediated by monocytes/macrophages132,133. Taken together, these data consistently show that women with preterm labor and intra-amniotic inflammation without culturable microorganisms had a milder intra-amniotic inflammatory response, including a lower number of amniotic fluid neutrophils, than those with intra-amniotic infection.
It is worth mentioning that women with preterm labor and intra-amniotic inflammation may have been infected by non-culturable microorganisms. Microorganisms such as Sneathia spp.31,33,134, Neisseria spp.33,134, and Fusobacterium nucleatum31,135 have proven difficult to culture from amniotic fluid using traditional clinical methods. However, whether such non-culturable microorganisms can lead to a stronger intra-amniotic inflammatory response than that mediated by alarmins requires further investigation.
Amniotic fluid monocytes/macrophages in women with preterm labor and intra-amniotic infection or intra-amniotic inflammation
We also demonstrated that the number of monocytes/macrophages is increased in women with preterm labor and intra-amniotic infection compared to those with intra-amniotic inflammation. Monocytes/macrophages are commonly found together with neutrophils in amniotic fluid of women with intra-amniotic infection and/or inflammation54,79. Since neutrophils typically represent the dominant immune cell population in such women54,79, the functions of monocytes/macrophages in the context of intra-amniotic infection or inflammation have been less investigated.
Monocytes are chemoattracted to sites of inflammation, where they attain one of several different activation states depending on the microenvironment136. Stimulation of innate sensors such as Toll-like receptors through detection of bacterial products activates the production of reactive oxygen species and pro-inflammatory cytokines such as TNFα, IL-1β, and IL-12 by monocytes136, which are mediators found in amniotic fluid of women with preterm labor and intra-amniotic infection38,39,54,105–107,137. A recent study demonstrated that placental macrophages can respond to microbes by releasing extracellular traps (METs)138, suggesting that monocytes/macrophages in the amniotic cavity may have other functions in addition to cytokine release. Yet, the question of whether amniotic fluid monocytes/macrophages are predominantly of maternal or fetal origin, especially in the context of intra-amniotic infection, remains unanswered.
Monocytes/macrophages were present, albeit in lesser numbers, in amniotic fluid of women with preterm labor and intra-amniotic inflammation, providing further confirmation that intra-amniotic inflammation is less severe in the absence of culturable microorganisms34,43,126. In tissues, resident macrophages act as sentinels, orchestrating the clearance of damaged cells in order to maintain homeostasis139. This sentinel-like function relies, in part, on the variety of pattern recognition receptors (e.g. Toll-like receptors) and cytosolic receptors (e.g. NLRP3) expressed by macrophages140–142. Macrophages are therefore considered to be the first to detect danger signals or alarmins released by damaged cells133. This concept was demonstrated by a murine study showing that sterile inflammation in response to cell death is driven by macrophages through the release of IL-1α and IL-1β132. Moreover, neutrophilic influx to the site of injury was dependent on macrophage-released cytokines, as deficient mice lacked such infiltration132. These findings provide a model in which macrophages are the initiators of sterile inflammation that is followed by neutrophil recruitment133. Similarly, amniotic fluid monocytes/macrophages may be acting as sentinels, responding to alarmins released by placental/fetal tissues143 which, in turn, will induce pro-inflammatory immune responses and recruit more immune cells (e.g. neutrophils) into the amniotic cavity (i.e. sterile intra-amniotic inflammation). Yet, further research is required to test this hypothesis. In support of this concept, it has been shown that surfactant protein A released by the fetal lung can trigger migration and IL-1β secretion by amniotic fluid macrophages in mice144,145.
The molecular mechanisms of sterile inflammation in the amniotic cavity32–34 are thought to involve the NLRP3 inflammasome128,131. Inflammasome complexes assemble to provide a scaffold for activation of caspase-1146–164, which in turn cleaves pro-IL-1β and pro-IL-18 into their mature and active forms165–173. Inflammasome activation can result in an inflammatory type of cell death, referred to as pyroptosis174–177, in which the molecule gasdermin D forms pores in the host cell membrane175,178–181 allowing for the release of cytosolic proteins such as IL-1β167,170. Pyroptosis was originally described in macrophages174,175,182, and we recently demonstrated that the effector molecule of pyroptosis, gasdermin D, is present in amniotic fluid of women with preterm labor and sterile intra-amniotic inflammation126. Herein, we propose that amniotic fluid monocytes/macrophages undergo inflammasome-mediated pyroptosis, a potential mechanism for sterile intra-amniotic inflammation in women with preterm labor.
Collectively, these findings indicate that amniotic fluid monocytes/macrophages play different roles in subsets of women with preterm labor: while cytokine release and MET formation are central for intra-amniotic infection, inflammasome-mediated pyroptosis occurs in the setting of sterile intra-amniotic inflammation.
Amniotic fluid CD4+ T cells in women with preterm labor and intra-amniotic infection or intra-amniotic inflammation
In the current study, we found that CD4+ T cells, but not CD8+ T cells, are increased in women with preterm labor and intra-amniotic infection compared to those with intra-amniotic inflammation. This is consistent with our previous report showing that T cells are one of the dominant immune cell populations present in the amniotic fluid of women in preterm gestation55. These adaptive cells are likely derived from the fetus since amniotic fluid neutrophils in preterm gestation are predominantly of fetal origin83; however, their origin has yet to be elucidated. Our findings are in line with previous reports showing that fetal immune activation occurs in preterm labor183,184 and that a population of central memory CD4+ T cells is increased in the cord blood of preterm neonates born to women with preterm labor185. Such a fetal T-cell response could be initiated by in utero exposure to pathogens186–188 and/or maternal antigens185,189. The mechanisms whereby fetal T cells could initiate preterm parturition involve the secretion of pro-inflammatory mediators, such as IFNγ and TNFα, and the activation of myometrial contractility185. Whether fetal T-cell activation is implicated in the mechanisms leading to preterm labor and birth in the absence of intra-amniotic infection/inflammation is still unknown.
CONCLUSION
In the current study, we report that women with spontaneous preterm labor and intra-amniotic infection had increased numbers of amniotic fluid neutrophils, monocytes/macrophages, and CD4+ T cells compared to those with intra-amniotic inflammation. Such cellular immune responses were accompanied by elevated amniotic fluid concentrations of IL-6, IL-1β, and IL-10. These results provide evidence that the cellular immune responses observed in women with preterm labor and intra-amniotic infection are more severe than in those with intra-amniotic inflammation, and that neutrophils, monocyte/macrophages, and CD4+ T cells are the main immune cells responding to microorganisms invading the amniotic cavity.
Supplementary Material
Acknowledgements:
We gratefully acknowledge the PRB Translational Research Laboratory for their contributions to the execution of this study. We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit for their help in collecting human samples. The authors also thank the staff members of the PRB Clinical Laboratory and the PRB Histology/Pathology Unit for the processing and examination of the pathological sections.
Funding: This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No. HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health.
Footnotes
Disclosure statement: The authors report no conflicts of interest.
REFERENCES
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. [DOI] [PubMed] [Google Scholar]
- 2.Monier I, Ancel PY, Ego A, et al. Fetal and neonatal outcomes of preterm infants born before 32 weeks of gestation according to antenatal vs postnatal assessments of restricted growth. Am J Obstet Gynecol. 2017;216(5):516.e511–516.e510. [DOI] [PubMed] [Google Scholar]
- 3.Joseph RM, Korzeniewski SJ, Allred EN, et al. Extremely low gestational age and very low birthweight for gestational age are risk factors for autism spectrum disorder in a large cohort study of 10-year-old children born at 23–27 weeks’ gestation. Am J Obstet Gynecol. 2017;216(3):304 e301–304 e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travers CP, Carlo WA, McDonald SA, et al. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol. 2018;218(1):130.e131–130.e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol. 1986;67(2):229–237. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12(4):262–279. [PubMed] [Google Scholar]
- 10.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161(3):817–824. [DOI] [PubMed] [Google Scholar]
- 11.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171(6):1660–1667. [DOI] [PubMed] [Google Scholar]
- 12.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11(1):135–176. [DOI] [PubMed] [Google Scholar]
- 13.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;280(3):L527–536. [DOI] [PubMed] [Google Scholar]
- 14.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16(1):56–70. [DOI] [PubMed] [Google Scholar]
- 15.Whidbey C, Harrell MI, Burnside K, et al. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J Exp Med. 2013;210(6):1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e121–125.e115. [DOI] [PubMed] [Google Scholar]
- 17.Cobo T, Kacerovsky M, Jacobsson B. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;211(6):708. [DOI] [PubMed] [Google Scholar]
- 18.Bobitt JR, Ledger WJ. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med. 1977;19(1):8–12. [PubMed] [Google Scholar]
- 19.Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol. 1981;140(8):947–952. [DOI] [PubMed] [Google Scholar]
- 20.Wallace RL, Herrick CN. Amniocentesis in the evaluation of premature labor. Obstet Gynecol. 1981;57(4):483–486. [PubMed] [Google Scholar]
- 21.Wahbeh CJ, Hill GB, Eden RD, Gall SA. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol. 1984;148(6):739–743. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31(3):553–584. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci. 1991;622:355–375. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166(5):1515–1528. [DOI] [PubMed] [Google Scholar]
- 25.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79(3):351–357. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15 Suppl 2:41–56. [DOI] [PubMed] [Google Scholar]
- 27.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185(5):1130–1136. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7(4):259–274. [DOI] [PubMed] [Google Scholar]
- 29.Gravett MG, Novy MJ, Rosenfeld RG, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292(4):462–469. [DOI] [PubMed] [Google Scholar]
- 30.Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191(4):1339–1345. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71(4):330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28(12):1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez-Lopez N, Romero R, Panaitescu B, et al. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol. 2018;80(5):e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matzinger P An innate sense of danger. Semin Immunol. 1998;10(5):399–415. [DOI] [PubMed] [Google Scholar]
- 36.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–365. [DOI] [PubMed] [Google Scholar]
- 37.Lotze MT, Deisseroth A, Rubartelli A. Damage associated molecular pattern molecules. Clin Immunol. 2007;124(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160(5 Pt 1):1117–1123. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27(3–4):117–123. [DOI] [PubMed] [Google Scholar]
- 40.Friel LA, Romero R, Edwin S, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35(5):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaiworapongsa T, Erez O, Kusanovic JP, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008;21(7):449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24(12):1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213(6):836 e831–836 e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh KJ, Kim SM, Hong JS, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol. 2017;216(6):604 e601–604 e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon BH, Romero R, Park JY, et al. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neggers YH. Trends in maternal mortality in the United States. Reprod Toxicol. 2016;64:72–76. [DOI] [PubMed] [Google Scholar]
- 47.de Graaf MT, de Jongste AH, Kraan J, Boonstra JG, Sillevis Smitt PA, Gratama JW. Flow cytometric characterization of cerebrospinal fluid cells. Cytometry B Clin Cytom. 2011;80(5):271–281. [DOI] [PubMed] [Google Scholar]
- 48.Jaime-Perez JC, Borrego-Lopez MF, Jimenez-Castillo RA, et al. Comparison of conventional cytomorphology, flow cytometry immunophenotyping, and automated cell counting of CSF for detection of CNS involvement in acute lymphoblastic leukemia. Int J Lab Hematol. 2018;40(2):169–174. [DOI] [PubMed] [Google Scholar]
- 49.Duong HP, Wissing KM, Tram N, Mascart G, Lepage P, Ismaili K. Accuracy of Automated Flow Cytometry-Based Leukocyte Counts To Rule Out Urinary Tract Infection in Febrile Children: a Prospective Cross-Sectional Study. J Clin Microbiol. 2016;54(12):2975–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Coca M, Gadea I, Esteban J. Relationship between conventional culture and flow cytometry for the diagnosis of urinary tract infection. J Microbiol Methods. 2017;137:14–18. [DOI] [PubMed] [Google Scholar]
- 51.Broeren M, Nowacki R, Halbertsma F, Arents N, Zegers S. Urine flow cytometry is an adequate screening tool for urinary tract infections in children. Eur J Pediatr. 2018. [DOI] [PubMed] [Google Scholar]
- 52.van de Geijn GM, van Gent M, van Pul-Bom N, Beunis MH, van Tilburg AJ, Njo TL. A new flow cytometric method for differential cell counting in ascitic fluid. Cytometry B Clin Cytom. 2016;90(6):506–511. [DOI] [PubMed] [Google Scholar]
- 53.Brooks CR, van Dalen CJ, Hermans IF, Douwes J. Identifying leukocyte populations in fresh and cryopreserved sputum using flow cytometry. Cytometry B Clin Cytom. 2013;84(2):104–113. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Varea A, Romero R, Xu Y, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med. 2017;45(5):523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomez-Lopez N, Romero R, Xu Y, et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol. 2018;79(4):e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marquardt N, Ivarsson MA, Sundstrom E, et al. Fetal CD103+ IL-17-Producing Group 3 Innate Lymphoid Cells Represent the Dominant Lymphocyte Subset in Human Amniotic Fluid. J Immunol. 2016;197(8):3069–3075. [DOI] [PubMed] [Google Scholar]
- 57.Romero R, Shamma F, Avila C, et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol. 1990;163(3):757–761. [DOI] [PubMed] [Google Scholar]
- 58.Romero R, Ghidini A, Mazor M, Behnke E. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991;34(4):769–778. [DOI] [PubMed] [Google Scholar]
- 59.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med. 2016;29(3):349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2016;29(3):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romero R, Chaemsaithong P, Chaiyasit N, et al. CXCL10 and IL-6: Markers of two different forms of intra-amniotic inflammation in preterm labor. Am J Reprod Immunol. 2017;78(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaiyasit N, Romero R, Chaemsaithong P, et al. Clinical chorioamnionitis at term VIII: a rapid MMP-8 test for the identification of intra-amniotic inflammation. J Perinat Med. 2017;45(5):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varrey A, Romero R, Panaitescu B, et al. Human beta-defensin-1: A natural antimicrobial peptide present in amniotic fluid that is increased in spontaneous preterm labor with intra-amniotic infection. Am J Reprod Immunol. 2018;80(4):e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomez-Lopez N, Romero R, Maymon E, et al. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. J Perinat Med. 2019;47(3):276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Para R, Romero R, Miller D, et al. Human beta-defensin-3 participates in intra-amniotic host defense in women with labor at term, spontaneous preterm labor and intact membranes, and preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2019:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med. 2016;44(1):33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med. 2016;44(1):53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med. 2016;44(1):77–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med. 2016;44(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016;44(1):5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaemsaithong P, Romero R, Docheva N, et al. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes(). J Matern Fetal Neonatal Med. 2018;31(2):228–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kusanovic JP, Romero R, Martinovic C, et al. Transabdominal collection of amniotic fluid “sludge” and identification of Candida albicans intra-amniotic infection. J Matern Fetal Neonatal Med. 2018;31(10):1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oh KJ, Romero R, Park JY, Kang J, Hong JS, Yoon BH. A high concentration of fetal fibronectin in cervical secretions increases the risk of intra-amniotic infection and inflammation in patients with preterm labor and intact membranes. J Perinat Med. 2019;47(3):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pacora P, Romero R, Erez O, et al. The diagnostic performance of the beta-glucan assay in the detection of intra-amniotic infection with Candida species. J Matern Fetal Neonatal Med. 2019;32(10):1703–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romero R, Kim YM, Pacora P, et al. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J Perinat Med. 2018;46(6):613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213(4 Suppl):S21–28. [DOI] [PubMed] [Google Scholar]
- 79.Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165(4 Pt 1):821–830. [DOI] [PubMed] [Google Scholar]
- 80.Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159(1):114–119. [DOI] [PubMed] [Google Scholar]
- 81.Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163(3):968–974. [DOI] [PubMed] [Google Scholar]
- 82.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165(4 Pt 1):813–820. [DOI] [PubMed] [Google Scholar]
- 83.Gomez-Lopez N, Romero R, Xu Y, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol. 2017;217(6):693 e691–693 e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liew PX, Kubes P. The Neutrophil’s Role During Health and Disease. Physiol Rev. 2019;99(2):1223–1248. [DOI] [PubMed] [Google Scholar]
- 86.Novakovic TR, Dolicanin ZC, Djordjevic NZ. Effects of maternal subclinical hypothyroidism on amniotic fluid cells oxidative status. Reprod Toxicol. 2018;78:97–101. [DOI] [PubMed] [Google Scholar]
- 87.Heine RP, Wiesenfeld H, Mortimer L, Greig PC. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin Infect Dis. 1998;27(3):513–518. [DOI] [PubMed] [Google Scholar]
- 88.Espinoza J, Chaiworapongsa T, Romero R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13(1):2–21. [DOI] [PubMed] [Google Scholar]
- 89.Akinbi HT, Narendran V, Pass AK, Markart P, Hoath SB. Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol. 2004;191(6):2090–2096. [DOI] [PubMed] [Google Scholar]
- 90.Romero R, Kusanovic JP, Gotsch F, et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23(4):261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Myntti T, Rahkonen L, Nupponen I, et al. Amniotic Fluid Infection in Preterm Pregnancies with Intact Membranes. Dis Markers. 2017;2017:8167276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Musilova I, Andrys C, Drahosova M, et al. Amniotic fluid cathepsin-G in pregnancies complicated by the preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2017;30(17):2097–2104. [DOI] [PubMed] [Google Scholar]
- 93.Rivero-Marcotegui A, Larranaga-Azcarate C, Ceres-Ruiz R, Garcia-Merlo S. Polymorphonuclear elastase and interleukin-6 in amniotic fluid in preterm labor. Clin Chem. 1997;43(5):857–859. [PubMed] [Google Scholar]
- 94.Helmig BR, Romero R, Espinoza J, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12(4):237–246. [DOI] [PubMed] [Google Scholar]
- 95.Pacora P, Maymon E, Gervasi MT, et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol. 2000;183(4):904–910. [DOI] [PubMed] [Google Scholar]
- 96.Musilova I, Andrys C, Krejsek J, et al. Amniotic fluid pentraxins: Potential early markers for identifying intra-amniotic inflammatory complications in preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2018;79(5):e12789. [DOI] [PubMed] [Google Scholar]
- 97.Gomez-Lopez N, Romero R, Garcia-Flores V, et al. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol. 2017;78(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gomez-Lopez N, Romero R, Xu Y, et al. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod Sci. 2017;24(8):1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gomez-Lopez N, Romero R, Leng Y, et al. Neutrophil extracellular traps in acute chorioamnionitis: A mechanism of host defense. Am J Reprod Immunol. 2017;77(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boldenow E, Gendrin C, Ngo L, et al. Group B Streptococcus circumvents neutrophils and neutrophil extracellular traps during amniotic cavity invasion and preterm labor. Sci Immunol. 2016;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169(5):1299–1303. [DOI] [PubMed] [Google Scholar]
- 102.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86(2):223–229. [DOI] [PubMed] [Google Scholar]
- 103.Elliott CL, Loudon JA, Brown N, Slater DM, Bennett PR, Sullivan MH. IL-1beta and IL-8 in human fetal membranes: changes with gestational age, labor, and culture conditions. Am J Reprod Immunol. 2001;46(4):260–267. [DOI] [PubMed] [Google Scholar]
- 104.Gomez-Lopez N, Tong WC, Arenas-Hernandez M, et al. Chemotactic activity of gestational tissues through late pregnancy, term labor, and RU486-induced preterm labor in Guinea pigs. Am J Reprod Immunol. 2015;73(4):341–352. [DOI] [PubMed] [Google Scholar]
- 105.Romero R, Manogue KR, Mitchell MD, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161(2):336–341. [DOI] [PubMed] [Google Scholar]
- 106.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166(5):1576–1587. [DOI] [PubMed] [Google Scholar]
- 107.Thomakos N, Daskalakis G, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Amniotic fluid interleukin-6 and tumor necrosis factor-alpha at mid-trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2010;148(2):147–151. [DOI] [PubMed] [Google Scholar]
- 108.Romero R, Gomez R, Galasso M, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32(2):108–113. [DOI] [PubMed] [Google Scholar]
- 109.Dudley DJ, Hunter C, Mitchell MD, Varner MW. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstet Gynecol. 1996;87(1):94–98. [DOI] [PubMed] [Google Scholar]
- 110.Kacerovsky M, Celec P, Vlkova B, et al. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One. 2013;8(3):e60399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165(4 Pt 1):969–971. [DOI] [PubMed] [Google Scholar]
- 112.Romero R, Sepulveda W, Mazor M, et al. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol. 1992;167(4 Pt 1):863–872. [DOI] [PubMed] [Google Scholar]
- 113.Witkin SS, Gravett MG, Haluska GJ, Novy MJ. Induction of interleukin-1 receptor antagonist in rhesus monkeys after intraamniotic infection with group B streptococci or interleukin-1 infusion. Am J Obstet Gynecol. 1994;171(6):1668–1672. [DOI] [PubMed] [Google Scholar]
- 114.Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3(3):121–126. [DOI] [PubMed] [Google Scholar]
- 115.Vadillo-Ortega F, Sadowsky DW, Haluska GJ, et al. Identification of matrix metalloproteinase-9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin-1 beta infusion in pregnant rhesus monkeys. Am J Obstet Gynecol. 2002;186(1):128–138. [DOI] [PubMed] [Google Scholar]
- 116.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195(6):1578–1589. [DOI] [PubMed] [Google Scholar]
- 117.Aagard K, Ganu R, Ma J, et al. Intraamniotic interleukin-1 (IL1β) induces histologic chorioamnionitis and alters the microbiome in a primate model of inflammatory preterm birth. Am J Obstet Gynecol. 2013;208(1):S218. [Google Scholar]
- 118.Prince A, Ma J, Miller L, et al. Chorioamnionitis induced by intraamniotic injection of IL1, LPS, or Ureaplasma parvum is associated with an altered microbiome in a primate model of inflammatory preterm birth. Am J Obstet Gynecol. 2015;212(1):S153. [Google Scholar]
- 119.Presicce P, Senthamaraikannan P, Alvarez M, et al. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biol Reprod. 2015;92(2):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Faro J, Romero R, Schwenkel G, et al. Intra-amniotic inflammation induces preterm birth by Activating the NLRP3 inflammasome. Biol Reprod. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Russel P Inflammatory lesions of the human placenta. I. Clinical significance of acute chorioamnionitis. Am J Diagn Gynecol Obstet. 1978;2:127–137. [Google Scholar]
- 122.Guzick DS, Winn K. The association of chorioamnionitis with preterm delivery. Obstet Gynecol. 1985;65(1):11–16. [PubMed] [Google Scholar]
- 123.van Hoeven KH, Anyaegbunam A, Hochster H, et al. Clinical significance of increasing histologic severity of acute inflammation in the fetal membranes and umbilical cord. Pediatr Pathol Lab Med. 1996;16(5):731–744. [PubMed] [Google Scholar]
- 124.Gomez-Lopez N, Romero R, Xu Y, et al. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod Sci. 2017;24(10):1382–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Romero R, Chaiworapongsa T, Savasan ZA, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25(6):558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gomez-Lopez N, Romero R, Tarca AL, et al. Gasdermin D: Evidence of Pyroptosis in Spontaneous Preterm Labor with Sterile Intra-amniotic Inflammation or Intra-amniotic Infection. Submitted. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gomez-Lopez N, Romero R, Plazyo O, et al. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol. 2016;75(1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gomez-Lopez N, Romero R, Garcia-Flores V, et al. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth and adverse neonatal outcomes. Biol Reprod. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gomez-Lopez N, Romero R, Arenas-Hernandez M, et al. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J Matern Fetal Neonatal Med. 2018;31(4):439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garcia-Flores V, Romero R, Miller D, et al. Inflammation-Induced Adverse Pregnancy and Neonatal Outcomes Can Be Improved by the Immunomodulatory Peptide Exendin-4. Front Immunol. 2018;9:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Plazyo O, Romero R, Unkel R, et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod. 2016;95(6):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J Immunol. 2010;184(8):4470–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peiseler M, Kubes P. Macrophages play an essential role in trauma-induced sterile inflammation and tissue repair. Eur J Trauma Emerg Surg. 2018;44(3):335–349. [DOI] [PubMed] [Google Scholar]
- 134.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol. 2004;21(6):319–323. [DOI] [PubMed] [Google Scholar]
- 136.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. [DOI] [PubMed] [Google Scholar]
- 137.Revello R, Alcaide MJ, Dudzik D, Abehsera D, Bartha JL. Differential amniotic fluid cytokine profile in women with chorioamnionitis with and without funisitis. J Matern Fetal Neonatal Med. 2016;29(13):2161–2165. [DOI] [PubMed] [Google Scholar]
- 138.Doster RS, Sutton JA, Rogers LM, Aronoff DM, Gaddy JA. Streptococcus agalactiae Induces Placental Macrophages To Release Extracellular Traps Loaded with Tissue Remodeling Enzymes via an Oxidative Burst-Dependent Mechanism. MBio. 2018;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. [DOI] [PubMed] [Google Scholar]
- 141.Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3(5):371–382. [DOI] [PubMed] [Google Scholar]
- 142.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4(2):95–104. [DOI] [PubMed] [Google Scholar]
- 143.Gomez-Lopez N, Romero R, Plazyo O, et al. Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol. 2017;217(5):592 e591–592 e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A. 2004;101(14):4978–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mendelson CR, Condon JC. New insights into the molecular endocrinology of parturition. J Steroid Biochem Mol Biol. 2005;93(2–5):113–119. [DOI] [PubMed] [Google Scholar]
- 146.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. [DOI] [PubMed] [Google Scholar]
- 147.Petrilli V, Papin S, Tschopp J. The inflammasome. Curr Biol. 2005;15(15):R581. [DOI] [PubMed] [Google Scholar]
- 148.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126(4):659–662. [DOI] [PubMed] [Google Scholar]
- 149.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7(1):31–40. [DOI] [PubMed] [Google Scholar]
- 150.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119(12):3502–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10(3):241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jha S, Ting JP. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol. 2009;183(12):7623–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. [DOI] [PubMed] [Google Scholar]
- 154.Franchi L, Munoz-Planillo R, Reimer T, Eigenbrod T, Nunez G. Inflammasomes as microbial sensors. Eur J Immunol. 2010;40(3):611–615. [DOI] [PubMed] [Google Scholar]
- 155.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev. 2011;243(1):136–151. [DOI] [PubMed] [Google Scholar]
- 156.Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol. 2011;187(2):597–602. [DOI] [PubMed] [Google Scholar]
- 157.Horvath GL, Schrum JE, De Nardo CM, Latz E. Intracellular sensing of microbes and danger signals by the inflammasomes. Immunol Rev. 2011;243(1):119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32(3):110–116. [DOI] [PubMed] [Google Scholar]
- 159.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13(4):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Franchi L, Nunez G. Immunology. Orchestrating inflammasomes. Science. 2012;337(6100):1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Henao-Mejia J, Elinav E, Strowig T, Flavell RA. Inflammasomes: far beyond inflammation. Nat Immunol. 2012;13(4):321–324. [DOI] [PubMed] [Google Scholar]
- 162.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. [DOI] [PubMed] [Google Scholar]
- 164.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Black RA, Kronheim SR, Merriam JE, March CJ, Hopp TP. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J Biol Chem. 1989;264(10):5323–5326. [PubMed] [Google Scholar]
- 166.Kostura MJ, Tocci MJ, Limjuco G, et al. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989;86(14):5227–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thornberry NA, Bull HG, Calaycay JR, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356(6372):768–774. [DOI] [PubMed] [Google Scholar]
- 168.Cerretti DP, Kozlosky CJ, Mosley B, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256(5053):97–100. [DOI] [PubMed] [Google Scholar]
- 169.Gu Y, Kuida K, Tsutsui H, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275(5297):206–209. [DOI] [PubMed] [Google Scholar]
- 170.Ghayur T, Banerjee S, Hugunin M, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386(6625):619–623. [DOI] [PubMed] [Google Scholar]
- 171.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. [DOI] [PubMed] [Google Scholar]
- 172.Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J Clin Immunol. 1999;19(1):1–11. [DOI] [PubMed] [Google Scholar]
- 173.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33:49–77. [DOI] [PubMed] [Google Scholar]
- 174.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–114. [DOI] [PubMed] [Google Scholar]
- 175.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243(1):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22(4):526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gaidt MM, Hornung V. Pore formation by GSDMD is the effector mechanism of pyroptosis. Embo j. 2016;35(20):2167–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sborgi L, Ruhl S, Mulvihill E, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. Embo j. 2016;35(16):1766–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aglietti RA, Dueber EC. Recent Insights into the Molecular Mechanisms Underlying Pyroptosis and Gasdermin Family Functions. Trends Immunol. 2017;38(4):261–271. [DOI] [PubMed] [Google Scholar]
- 181.Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42(4):245–254. [DOI] [PubMed] [Google Scholar]
- 182.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Berry SM, Romero R, Gomez R, et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol. 1995;173(4):1315–1320. [DOI] [PubMed] [Google Scholar]
- 184.Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179(1):186–193. [DOI] [PubMed] [Google Scholar]
- 185.Frascoli M, Coniglio L, Witt R, et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci Transl Med. 2018;10(438). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Melville JM, Bischof RJ, Meeusen EN, Westover AJ, Moss TJ. Changes in fetal thymic immune cell populations in a sheep model of intrauterine inflammation. Reprod Sci. 2012;19(7):740–747. [DOI] [PubMed] [Google Scholar]
- 187.Rueda CM, Presicce P, Jackson CM, et al. Lipopolysaccharide-Induced Chorioamnionitis Promotes IL-1-Dependent Inflammatory FOXP3+ CD4+ T Cells in the Fetal Rhesus Macaque. J Immunol. 2016;196(9):3706–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Odorizzi PM, Jagannathan P, McIntyre TI, et al. In utero priming of highly functional effector T cell responses to human malaria. Sci Transl Med. 2018;10(463). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.