Abstract
Background.
Activated microglia, which can be detected in vivo by 11C-PBR28 positron emission tomography (PET), represent a main component of MS pathology in the brain. Their role in the cerebellum is still unexplored, although cerebellar involvement in MS is frequent and accounts for disability progression.
Objectives.
We aimed at characterizing cerebellar neuroinflammation in MS patients compared to healthy subjects by combining 11C-PBR28 MR-PET with 7 Tesla MRI and assessing its relationship with brain neuroinflammation and clinical outcome measures.
Methods.
Twenty-eight MS patients and 16 healthy controls underwent 11C-PBR28 MR-PET to measure microglia activation in normal-appearing cerebellum and lesions segmented from 7 Tesla scans. Patients were evaluated using the Expanded Disability Status Scale and Symbol Digit Modalities Test. 11C-PBR28 binding was assessed in regions of interest using 60-90 minutes standardized uptake values normalized by a pseudo-reference region in the brain normal-appearing white matter. Multilinear regression was used to compare tracer uptake in MS and healthy controls and assess correlations with clinical scores.
Results.
In all cerebellar regions examined, MS patients showed abnormally increased tracer uptake, which correlated with cognitive and neurological disability.
Conclusions.
Neuroinflammation is widespread in the cerebellum of patients with MS and related to neurological disability and cognitive impairment.
Keywords: cerebellum, neuroinflammation, MR-PET, microglia
INTRODUCTION
Cerebellar involvement occurs early in MS, where it contributes to disease progression and predicts poor outcome1,2. Neurological dysfunction related to cerebellar pathology in MS is generally highly disabling, since both motor and cognitive function are affected3. Cerebellar pathology in MS is characterized by extensive cortical and white matter (WM) demyelination, and neuronal loss3. In the MS brain, structural cortical and WM pathology closely associate with neuroinflammation4–7 Apart from scattered histological examinations in progressive disease that reported microglia activation in both the cerebellar WM and cortex8, the in vivo presence of neuroinflammation in the MS cerebellum is still to be characterized.
We combined 11C-PBR28 imaging on an integrated 3T MRI-Positron Emission Tomography (MR-PET) system with 7T MRI to assess neuroinflammation in lesioned and normal appearing cerebellar tissue in patients at different stages of MS. 11C-PBR28 is a second-generation radiotracer with high specificity and reproducibility that binds to the 18kDa translocator protein (TSPO), which is overexpressed in activated microglia, a main component of neuroinflammation in MS9–11. Cerebellar lesions were visualized on ultra-high resolution 7T MRI, which demonstrated high sensitivity to focal lesion detection in MS12,13. We aimed at verifying the hypothesis that activated microglia represent a main component of cerebellar pathology in MS, and at investigating whether this activation is focal or diffuse, and whether it correlates with neuroinflammation in the brain. Finally, we investigated the association between cerebellar TSPO expression and clinical measures of neurological disability and information processing speed.
MATERIALS AND METHODS
Standard Protocol Approval, Patient Consents and Confidentiality
The Institutional Ethics Committee and the Radioactive Drug Research Committee approved all study procedures, and subjects gave written informed consent to participate in the study. Confidentiality of study subjects has been maintained through routine precautions: we assigned codes to identify the subjects, kept our records of procedures and scans in locked security cabinets. Only information necessary to conduct the study has been distributed to the study staff; all data stored in computers have been kept in a password-protected file, available only to investigators.
Subjects
Fourteen patients with relapsing remitting MS (RRMS), 14 with secondary progressive MS (SPMS) and 16 age-matched healthy controls (HC), all with high- or mixed-affinity binding genotype for the TSPO gene14, were prospectively enrolled in the study. The study cohort overlaps (27/28 MS patients; 13/16 HC) with a previously published study assessing 11C-PBR28 uptake in the brain15.
General inclusion criteria were: age 18-65 years, education ≥ 8 years, absence of any treatment with benzodiazepines, no general PET and MRI contraindications, absence of major medical and/or psychiatric disorders (other than MS for patients). Inclusion criteria for MS were: clinically definite MS16, absence of clinical relapse within 3 months, no corticosteroids therapy within 1 month of study enrollment, stable disease-modifying treatment or no treatment for at least 6 months.
Clinical evaluation
Within one week from imaging procedures, all patients underwent neurological disability assessment through Expanded Disability Status Scale (EDSS)17. Cognitive evaluation was performed using Symbol Digit Modalities Test (SDMT). SDMT raw scores were converted to Z-scores after correction for age and education18.
Imaging Data Acquisition
Patients and HC underwent 11C-PBR28 MR-PET imaging on a Siemens (Erlangen, Germany) BrainPET system, a PET scanner operating in the bore of a 3T whole-body MRI system equipped with an 8-channel head coil19. All subjects received an intravenous bolus injection of 11C-PBR28, which was produced in house (mean±SD administered dose= 11.4±0.6mCi in patients and 11.06±0.8 mCi in HC, p= 0.1), as previously detailed15. The PET data were acquired in list-mode format during a 90 minutes scan. Multiple gradient echo 3-dimensional (3D) magnetization-prepared rapid acquisition (ME-MPRAGE) images (1 mm isotropic voxels) for attenuation correction20, co-registration of PET maps to 3T data, FreeSurfer anatomical reconstruction for brain cortex and WM segmentation, and conventional 3D fluid-attenuated inversion recovery (FLAIR) images (1 mm isotropic voxels) for brain WM lesions segmentation, were simultaneously acquired to PET images. Patients also underwent 7T MRI on a Siemens scanner using a 32-channel head coil, within ~1 week from the MR-PET session to acquire 0.6×0.6×1.5 mm voxels T1-weighted Turbo-FLASH (TFL) images for cerebellar lesion segmentation. Three MS patients did not undergo 7T imaging due to the presence of implants not approved for 7T.
MRI and PET Data Analysis
Cerebellar lesions were segmented by consensus from two expert raters (VTB and CAT) on 7T TFL images using a semiautomated method (3D Slicer v4.2.0) and defined as hypo-intensities extending at least 3 voxels across 2 consecutive slices21. In 3 MS patients that did not undergo 7T imaging lesion segmentation was obtained on ME-MPRAGE 3T scans. Cerebellar lesions were classified as cortical (CL), leukocortical (LCL), white matter (WML) or deep grey matter (DGML). Lesion volume was computed using FSL version 5.0.7 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL). Masks of the whole cerebellum, cerebellar WM and cortical grey matter (cGM) were segmented on 3T T1-weighted images on ME-MPRAGE 3T images using the automated software Volbrain (http://volbrain.upv.es).
Masks of cerebellar normal appearing white matter (NAWM) and cerebellar normal appearing cortical grey matter (cNAGM) were obtained subtracting cerebellar lesion masks from the WM and cGM masks by FSL. To exclude that uptake in normal-appearing tissue could be influenced by that in perilesional tissue, lesion masks were enlarged by 2 mm using FSL, and SUVR were extracted for NAWM and cNAGM before and after excluding perilesional areas. The subtracted NAWM and cNAGM masks were used in further analyses.
Brain WM lesions were segmented with 3D Slicer on FLAIR images. Brain WM and GM masks were segmented automatically by FreeSurfer on ME-MPRAGE images. Brain NAWM masks were obtained with FSL as for the cerebellum.
In-house software was used to compute voxel-wise SUV (mean radioactivity/injected dose/weight) from the 60-90 minutes post-injection data (sampled at 1.25 mm isotropic voxel size) as previously detailed15. In each subject, the SUV map was normalized by a pseudo-reference region (SUVR) located in the brain NAWM, with mean SUV in MS patients around the mean of SUV in HC. As MS is a diffuse disease lacking an anatomically consistent reference region, the pseudo-reference region was identified by a cluster-based approach15. Using FSL and Freesurfer, we identified clusters (minimum size 9 contiguous voxels) in the WM of HC and NAWM of patients that were within ±0.5 SD of the mean 11C-PBR28 SUV for the global WM in HC. This process was conducted separately for high- and mixed-affinity binders15. No differences were found in the clusters’ SUV in patients versus HC (for high-affinity binders: mean SUV 0.71±0.01 in patients versus 0.70±0.01 in HC, p= 0.3; for mixed-affinity binders: mean SUV 0.54±0.002 in patients versus 0.54±0.002 in HC, p= 0.1 by multilinear regression covarying for age and gender).
Masks of normal appearing whole cerebellum, cerebellar NAWM and cNAGM, brain NAWM and GM, and lesions in both the brain and the cerebellum, were registered to SUVR maps using FNIRT/FSL to extract the mean SUVR.
Lesions were grouped in WM lesions and cGM lesions to search for correlations with NAWM and cNAGM, respectively.
Statistical analysis
Statistical analysis was performed using JMP (https://www.jmp.com).
Based on normality, Mann-Whitney test and Fisher’s exact test were used to compare demographics and clinical characteristics in patients versus HC, and in RRMS versus SPMS. Multilinear regression models were used i) to compare 11C-PBR28 cerebellar uptake in patients versus HC in different regions of interest, ii) to correlate uptake in lesions and normal appearing tissue, iii) to search for associations between cerebellar uptake values and clinical metrics (disease duration, EDSS, SDMT), iv) to assess correlation between SUVR in the cerebellum and in the brain. Gender, binding affinity, age and disease duration were included as covariates of no interest when appropriate. Matched pairs t test compared uptake in lesions and non-lesioned tissue in patients. A p-value <0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics
Demographic and clinical data of study subjects are shown in table 1.
Table 1.
Demographic and clinical characteristics of study subjects.
| Characteristic | HC n = 16 | All MS n = 28 | RRMS n = 14 | SPMS n = 14 |
|---|---|---|---|---|
| Age, mean (SD) | 47 (12) | 48 (10) | 43 (9) | 53 (7) |
| Gender, F/M | 11/6 | 22/6 | 11/3 | 11/3 |
| Affinity, high/mixed | 8/8 | 15/13 | 7/7 | 8/6 |
| Disease duration, median | - | 9 | 2 | 21.5 |
| EDSS, median (range) | - | 3.5 (1.5-7.5) | 2 (1.5-6) | 6.5 (2.5-7.5) |
| SDMT z-score, median | - | 0.03 | 0.77 | −1.4 |
| MS treatment | ||||
| Dimethyl fumarate | - | 7 | 5 | 2 |
| Natalizumab | - | 5 | 2 | 3 |
| Glatiramer acetate | - | 4 | 2 | 2 |
| Rituximab | - | 3 | 1 | 2 |
| Interferon beta 1a | - | 2 | 2 | 0 |
| Fingolimod | - | 1 | 0 | 1 |
| None | - | 6 | 2 | 4 |
Abbreviations: HC= healthy controls; MS= multiple sclerosis; RRMS= relapsing remitting multiple sclerosis; SPMS= secondary progressive multiple sclerosis; EDSS= Expanded Disability Status Scale; SDMT= Symbol Digit Modalities Test.
Patients and HC did not differ in age, gender and binding affinity. No differences were found between the two MS sub-groups for gender and binding affinity. As expected, RRMS were younger (p= 0.005), had shorter disease duration (p< 0.001), and lower EDSS (p< 0.001) than SPMS patients. Cognitive scores were also lower in SPMS relative to RRMS cases (p< 0.001). One patient with RRMS was not able to complete the test because of important visual impairment.
Twenty-two patients (12 RRMS and 10 SPMS) were on disease modifying therapy.
Cerebellar lesions
Cerebellar lesions were found in the majority of MS patients: in 11/14 RRMS and 13/14 SPMS. In RRMS, most lesions were localized in the WM; in SPMS, lesions involved more frequently the cerebellar cortex, either intracortically or leukocortically. The detailed spatial localization of cerebellar lesions segmented at 7T is shown in table 2. Figure 1A–D illustrates a few examples of cerebellar lesions at 7T. White matter lesions in the cerebellum typically involved the peduncles, and the hilar regions of the deep nuclei (figure 1B and D); leukocortical lesions showed a broader extension in the WM and involved the cortex to smaller extent (figure 1A); pure cortical lesions were seemingly confined within the folia (figure 1C).
Table 2.
Cerebellar lesion count and volume of according to their location and disease subtype.
| RRMS | SPMS | |
|---|---|---|
| Cortical lesions | ||
| Count, median (range) | 1 (0-2) | 2 (0-4) |
| Volume, mean±SD (range) | 37±19 mm3 (21-58) | 74±47 mm3 (10-127) |
| Number of patients | 3/14 | 6/14 |
| Leukocortical lesions | ||
| Count, median (range) | 2 (0-3) | 2.5 (0-9) |
| Volume, mean±SD (range) | 108±39 mm3 (70-166) | 187±222 mm3 (17-791) |
| Number of patients | 5/14 | 10/14 |
| Deep grey matter lesions | ||
| Count, median (range) | 1 (0-1) | 1 (0-2) |
| Volume, mean±SD (range) | 77±63 mm3 (43-205) | 198±201 mm3 (17-525) |
| Number of patients | 6/14 | 6/14 |
| White matter lesions | ||
| Count, median (range) | 1 (0-4) | 2 (0-6) |
| Volume, mean±SD (range) | 154±168 mm3 (27-521) | 98±127 mm3 (10-340) |
| Number of patients | 7/14 | 7/14 |
Abbreviations: RRMS= relapsing-remitting multiple sclerosis; SPMS= secondary-progressive multiple sclerosis.
Figure 1. Cerebellar lesions on 7 Tesla Turbo-FLASH images.
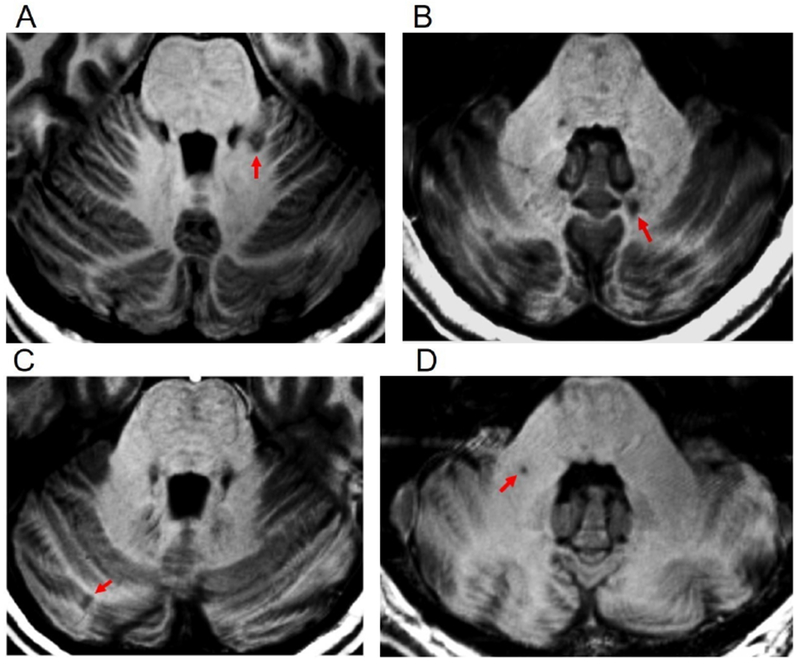
Cerebellar lesions (arrows) in multiple sclerosis patients in different regions of interest. A= leukocortical, B= deep grey matter (left dentate); C= cortical; D= white matter.
No cerebellar lesions were observed in HC.
Tracer uptake in MS compared to healthy controls
The 11C-PBR28 uptake was overall higher in patients relative to HC in all the cerebellar regions examined (figure 2).
Figure 2. Fused MRI and PET images.
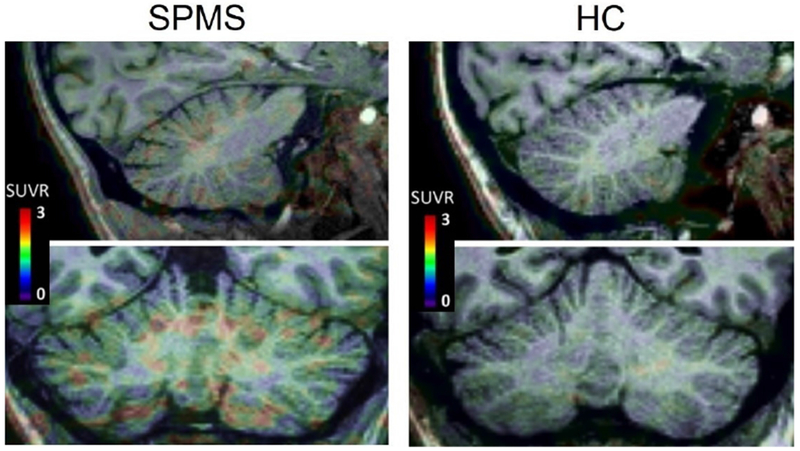
Images from a patient with progressive multiple sclerosis and a healthy subject, both mixed-affinity binders, showing overall higher uptake in the first. Abbreviations: SPMS= secondary progressive multiple sclerosis; HC= healthy control; SUVR= standardized uptake values normalized by a pseudo-reference region.
The highest 11C-PBR28 SUVRs were found in cerebellar lesions (mean SUVR 1.43±0.3), followed by the NAWM and cNAGM in patients (mean SUVR= 1.42±0.3 and 1.38±0.3, respectively) and WM and GM in HC (mean SUVR= 1.23±0.2 and 1.20±0.2, respectively). Figure 3 shows 11C-PBR28 SUVR distribution across cerebellar regions in HC and MS subjects, according to their binding genotype.
Figure 3. Mean 11C-PBR28 standardized uptake values in MS patients and healthy controls.
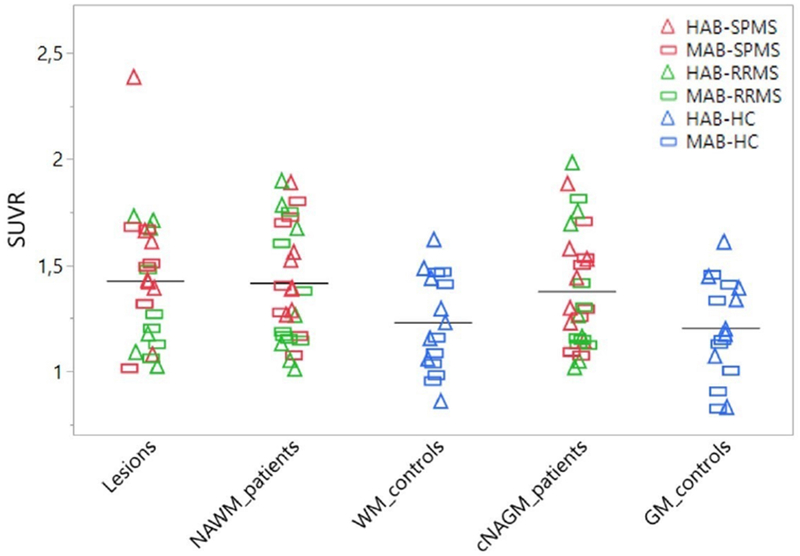
Scatter plot showing individual mean 11C-PBR28 standardized uptake values normalized by a pseudo-reference region (SUVR) in patients and healthy controls. Bars represent mean uptake values. Abbreviations: NAGM/GM= normal appearing grey matter/grey matter; NAWM/WM= normal appearing white matter/white matter; HC= healthy controls; MS= multiple sclerosis; RRMS= relapsing remitting multiple sclerosis; SPMS= secondary progressive multiple sclerosis; HAB= high-affinity binders; MAB= mixed-affinity binders.
When correcting for age, gender and binding affinity, MS patients showed abnormally increased 11C-PBR28 uptake in lesions compared to the HC’s whole cerebellum (p= 0.03), as well as in NAWM (p= 0.03) and cNAGM (p= 0.04) versus WM and cGM in HCs, respectively. As an outlier was present when measuring lesion uptake in patients (figure 3), the analysis was repeated after excluding that datum and the significant difference with HC’s whole cerebellum uptake persisted (p= 0.03).
To assess whether increased TSPO expression in the cerebellar NAWM and cNAGM could be driven by inflammation in perilesional tissue, the analysis was repeated excluding the perilesional area. We found that NAWM and cNAGM SUVR were still higher in patients than in HC (p= 0.03 and p= 0.04 respectively).
Although cerebellar 11C-PBR28 uptake was overall higher in SPMS relative to RRMS patients (NAWM: mean SUVR 1.46±0.2 versus 1.37±0.3; NAGM: mean SUVR 1.4±0.2 versus 1.36±0.3; lesions: mean SUVR 1.51±0.3 versus 1.32±0.3;), these differences were not significant (p= 0.7 for NAWM, p= 0.9 for NAGM, p= 0.4 for lesions by linear regression models after correcting for age, gender and binding affinity).
We did not find differences in 11C-PBR28 uptake in treated versus untreated patients in any region examined.
Finally, we investigated the relationship between tracer uptake in lesions and in corresponding normal appearing tissue. Tracer uptake in cGM lesions correlated with cNAGM SUVR (coefficient 0.56; CI= 0.35,0.78; p<0.0001), though SUVR in lesions were higher than in cNAGM (mean SUVR 1.49±0.4 versus 1.38±0.3, p= 0.02 by matched pairs t test). A modest correlation was seen between WM lesions and NAWM uptake (coefficient= 0.45; CI= 0.05,0.85; p= 0.03), with no differences in the mean values (mean SUVR 1.37±0.3 versus 1.42±0.3, p= 0.4 by t test).
Correlation with brain uptake values
We found positive correlations between tracer uptake in the cerebellum and in the brain. In particular, we tested cerebellar NAWM versus brain NAWM uptake (coefficient= 1.05; CI= 0.83,1.27; p<0.0001), cerebellar cGM versus brain cGM uptake (coefficient= 0.95; CI= 0.77,1.13; p<0.0001), cerebellar lesions uptake versus brain WM lesions uptake (coefficient= 0–87; CI= 0.29,1.44; p=0.005) (figure 4).
Figure 4. Scatter plots showing the correlation between cerebellar and brain SUVR.

Abbreviations: NAWM= normal appearing white matter; SUVR= standardized uptake values normalized by a pseudo-reference region; cGM= cortical grey matter; WM= white matter.
Correlations with clinical metrics
No correlation was found between tracer uptake and disease duration. Both EDSS and SDMT correlated with disease duration (p<0.001 and p= 0.01 respectively). There was a positive correlation between EDSS and SUVR in NAWM (p= 0.02), cNAGM (p= 0.03) and lesions (p= 0.02). We also found a negative correlation between SDMT scores an SUVR in NAWM (p= 0.03), but not in cNAGM or in lesions (see table 3 for more details).
Table 3.
Linear correlations between clinical metrics and tracer uptake.
| disease duration | NAWM suvr | cNAGM suvr | Lesion suvr | |
|---|---|---|---|---|
| EDSS | ||||
| Coefficient | 0.12 | 1.77 | 2.15 | 1.77 |
| CI | 0.06; 0.18 | 0.35; 3.20 | 0.23; 4.06 | 0.35; 3.20 |
| p value | <0.001 | 0.02 | 0.03 | 0.02 |
| SDMT | ||||
| Coefficient | −0.08 | −2.42 | −1.8 | −1.45 |
| CI | −015; −0.02 | −0.64; −0.19 | −4.12; 0.44 | −3.60; 0.7 |
| p value | 0.01 | 0.03 | 0.1 | 0.2 |
Abbreviations: EDSS= expanded disability status scale; SDMT= symbol digit modalities test; NAWM= normal appearing white matter; cNAGM= normal appearing cortical grey matter; SUVR= standardized uptake values normalized by a pseudo-reference region; CI= confidence interval.
No correlations were found between tracer uptake and cerebellar subscores for NAWM (p= 0.2), cNAGM (p= 0.1) and lesions (p= 0.1).
Figure 5 illustrates the correlations between tracer uptake and clinical metrics.
Figure 5. Scatter plots showing the correlation between tracer uptake and clinical metrics in MS patients.
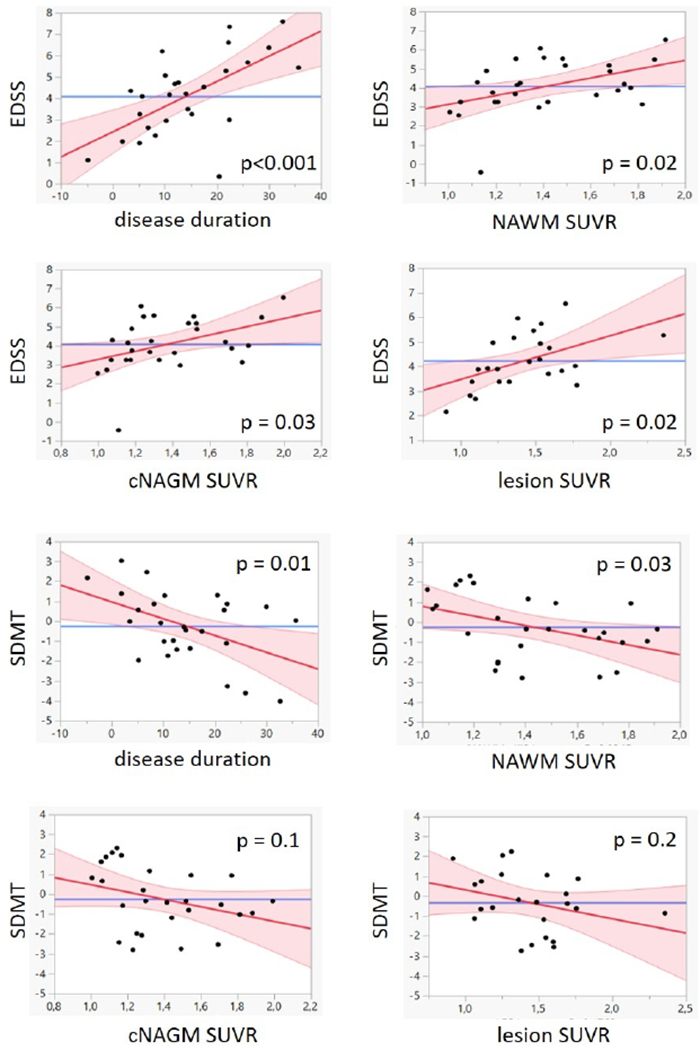
Abbreviations: EDSS= expanded disability status scale; NAWM= normal appearing white matter; SUVR= standardized uptake values normalized by a pseudo-reference region; cNAGM= normal appearing cortical grey matter; SDMT= symbol digit modalities test.
DISCUSSION
In this study, using 11C-PBR28 MR-PET and 7T MRI, we investigated the presence of neuroinflammation in the cerebellum of RRMS and SPMS cases. We found that, relative to HC, patients had abnormally increased TSPO expression, a marker of microglia activation, in both lesioned and normal appearing cerebellar WM and cortex. Neuroinflammation correlated with decreased information processing speed and neurological disability.
Previous PET studies did not report increased TSPO expression in the cerebellum of MS patients6. This could be explained by the fact that previous data were obtained using 11C-PK11195, which shows significantly lower binding specificity than 11C-PBR2822.
Using 7T MRI, we detected cerebellar lesions in the majority of patients. In RRMS, lesions were mainly located in the WM; in SPMS there was a predominance of cortical and leukocortical plaques. Our findings are in line with previous reports on the presence and distribution of cerebellar lesions in the disease23. We segmented lesions on T1-weighted images because previous examinations demonstrated that 7T T1 sequences have higher sensitivity than 3T MRI to cerebellar lesions in MS, especially those located cortically and leukocortically13. Histopathology shows that demyelinated plaques can be widespread in the WM and GM of the cerebellum in MS, since the early phase of the disease. Demyelination seems to mainly involve the cerebellar cortex especially in the progressive phase; in some case WM plaques could be undetectable even in presence of extensive cortical demyelination24.
As we previously reported15, 11C-PBR28 SUVR is a reliable method for characterizing microglial pathology in MS, since it has a strong positive correlation with the radiotracer volume of distribution. We also demonstrated that MS subjects and controls showed similar levels of 11C-PBR28 plasma concentrations, and no differences in the area under the blood curves25.
In our MS cohort, abnormally increased TSPO signal extensively involved the WM, both lesions and normal appearing tissue. Microglia activation can be frequently detected in WM lesions and NAWM7,26 in the brain of MS patients. Within lesioned tissue, inflammation is most severe in active and expanding lesions27.
In NAWM, activated microglia mainly features the progressive disease, while it is less pronounced in the relapsing phase6,7,26. We found similar results for cerebellar NAWM, where tracer uptake was higher in patients than in HC, especially in progressive MS. However, in our analysis cerebellar deep nuclei were not segmented apart from the WM, thus the SUVR in the WM were a combination of WM and deep GM uptake.
Interestingly, as observed previously for the MS brain15, we found that microglia activation also involved the cerebellar cortex, especially lesions and SPMS disease stages, indicating that neuroinflammation is a relevant component of cerebellar cortical pathology in the disease. Ex vivo MS studies have suggested the presence of a link between cortical microglia activation, demyelination and diffuse meningeal inflammation8,24.26. Meningeal inflammation in the brain has been associated to the presence of aggregates of B-cells, organized in ectopic lymphoid follicle-like structures4. These structures seem to accompany a condition of higher cortical inflammation driven by activated microglia28. Although meningeal inflammation was pathologically found also in the cerebellum, evidence of follicle-like structures therein is still lacking8,24 Future studies are needed to clarify the role of meningeal inflammation and microglia activation in cerebellar cortical pathology and its relationship with overlying brain neuroinflammation.
In our study, cerebellar neuroinflammation was widespread throughout all the regions of interest, and not strictly influenced by perilesional uptake. Additionally, 11C-PBR28 uptake in the cerebellum strongly correlated with tracer uptake in the brain, suggesting that neuroinflammation in MS is diffuse to the whole CNS rather than restricted to the supra- or infratentorial compartments.
We previously reported a positive correlation between EDSS and 11C-PBR28 uptake in the brain, especially in the NAWM and thalamus, but also in cortical GM15. This was seen also in the cerebellum, where tracer uptake diffusively correlated with EDSS. As known, cerebellar symptoms contribute to the overall EDSS score. Moreover, cerebellar structural abnormalities seem to correlate with EDSS, even in absence of clinical signs of cerebellar dysfunction29, and MS patients with cerebellar signs are known to have more severe disease with poor prognosis1,2.
We also found that lower SDMT scores correlated with increased TSPO expression in the NAWM, as previously demonstrated for the brain15. Cognitive impairment with information processing speed dysfunction frequently occurs in MS, as the result of damage affecting multiple areas, including the cerebellum3. Patients with motor cerebellar signs specifically showed decreased performance in attention and verbal fluency tasks30, and structural abnormalities in the MS cerebellum have been shown to correlate with SDMT scores and overall cognitive function31,32. Structural alterations in the WM can be responsible for interruption of the pathways connecting cerebellum and supratentorial structures, leading to motor and cognitive impairment. Moreover, diffusion tensor imaging analyses reported diffuse WM microstructural abnormalities in the cerebellum of MS subjects, compared to a more restricted, selective damage in the GM33. There are a few limitations to our study. First, we cannot exclude that the small dimensions of some of the cerebellar lesions detected at 7T might have impacted the accuracy of tracer quantification within lesions, though the effect should be mitigated in normal appearing tissue where perilesional tissue was excluded. However, the finding that 11C-PBR28 uptake in cortical lesions was significantly higher than in cNAGM suggests that partial volume effects in lesion SUVR estimation may not be as relevant. Second, previous investigations have suggested that an endothelial binding component could affect 11C-PBR28 tracer quantification34. Although this has not been definitively proven in MS, future studies will address this component for accurate tracer uptake estimation.
Longitudinal investigations in larger MS cohorts are needed to better clarify the role of cerebellar glial activation in the progression of disease and in the occurrence of neurodegeneration.
Acknowledgments
Funding
This study was supported by National Multiple Sclerosis Society [NMSS; RG 4729A2/1], the US Army, Department of Defense [W81XWH-13-1-0112], NIH [R01 NS078322-01-A1]. Elena Herranz was supported by NMSS fellowship [FG-1507-05459].
Declaration of Conflicting Interests
Carolina Ionete has received research support from Genzyme/Sanofi, Biogen Idec, Genentech and financial compensations for scientific advisory from Genzyme/Sanofi. Jacob A Sloane is a consultant on advisory boards for Biogen, Teva, Genentech, Celgene, Genzyme; has grant funding from Genzyme, Biogen. Caterina Mainero has received research support by Genzyme Sanofi and Serono Merck. Other authors declare no potential conflicts of interest.
References
- 1.Amato MP, Ponziani G. A prospective study on the prognosis of multiple sclerosis. Neurol Sci 2000;21:S831–838. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis. Mult Scler 2003;9:260–274. [DOI] [PubMed] [Google Scholar]
- 3.Weier K, Banwell B, Cerasa A, et al. The role of the cerebellum in multiple sclerosis. Cerebellum 2015;14:364–374. [DOI] [PubMed] [Google Scholar]
- 4.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007;130:1089–1104. [DOI] [PubMed] [Google Scholar]
- 5.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain: a journal of neurology 2011;134:2755–2771. [DOI] [PubMed] [Google Scholar]
- 6.Rissanen E, Tuisku J, Rokka J, et al. In Vivo Detection of Diffuse Inflammation in Secondary Progressive Multiple Sclerosis Using PET Imaging and the Radioligand (1)(1)C-PK11195. J Nucl Med 2014;55:939–944. [DOI] [PubMed] [Google Scholar]
- 7.Giannetti P, Politis M, Su P, et al. Increased PK11195-PET binding in normal-appearing white matter in clinically isolated syndrome. Brain 2015;138:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell OW, Schulz-Trieglaff EK, Carassiti D, et al. Extensive grey matter pathology in the cerebellum in multiple sclerosis is linked to inflammation in the subarachnoid space. Neuropathol Appl Neurobiol 2015;41:798–813. [DOI] [PubMed] [Google Scholar]
- 9.Kreisl WC, Fujita M, Fujimura Y, et al. Comparison of [(11)C]-(R)-PK 11195 and [(11)C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage 2010;49:2924–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park E, Gallezot JD, Delgadillo A, et al. (11)C-PBR28 imaging in multiple sclerosis patients and healthy controls: test-retest reproducibility and focal visualization of active white matter areas. Eur J Nucl Med Mol Imaging 2015;42:1081–1092. [DOI] [PubMed] [Google Scholar]
- 11.Moll NM, Rietsch AM, Thomas S, et al. Multiple sclerosis normal-appearing white matter: pathology-imaging correlations. Ann Neurol 2011;70:764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippi M, Evangelou N, Kangarlu A, et al. Ultra-high-field MR imaging in multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry 2014;85:60–66. [DOI] [PubMed] [Google Scholar]
- 13.Fartaria MJ, O’Brien K, Sorega A, et al. An Ultra-High Field Study of Cerebellar Pathology in Early Relapsing-Remitting Multiple Sclerosis Using MP2RAGE. Invest Radiol 2017;52:265–273. [DOI] [PubMed] [Google Scholar]
- 14.Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 2012;32:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herranz E, Gianni C, Louapre C, et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Annals of neurology 2016;80:776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 18.Parmenter BA, Testa SM, Schretlen DJ, Weinstock-Guttman B, Benedict RH. The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 2010;16:6–16. [DOI] [PubMed] [Google Scholar]
- 19.Kolb A, Wehrl HF, Hofmann M, et al. Technical performance evaluation of a human brain PET/MRI system. European radiology 2012;22:1776–1788. [DOI] [PubMed] [Google Scholar]
- 20.Izquierdo-Garcia D, Hansen AE, Forster S, et al. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2014;55:1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louapre C, Govindarajan ST, Gianni C, et al. Beyond focal cortical lesions in MS: An in vivo quantitative and spatial imaging study at 7T. Neurology 2015;85:1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannestad J, Gallezot JD, Schafbauer T, et al. Endotoxin-induced systemic inflammation activates microglia: [(1)(1)C]PBR28 positron emission tomography in nonhuman primates. Neuroimage 2012; 63:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabrese M, Mattisi I, Rinaldi F, et al. Magnetic resonance evidence of cerebellar cortical pathology in multiple sclerosis. J Neurol Neurosurg Psychiatry 2010;81:401–404. [DOI] [PubMed] [Google Scholar]
- 24.Kutzelnigg A, Faber-Rod JC, Bauer J, et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol 2007;17:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herranz E, Hooker JM, Izquierdo-Garcia D, et al. Reply. Ann Neurol 2017. February;81(2):324–325. [DOI] [PubMed] [Google Scholar]
- 26.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005;128:2705–2712. [DOI] [PubMed] [Google Scholar]
- 27.Oh U, Fujita M, Ikonomidou VN, et al. Translocator protein PET imaging for glial activation in multiple sclerosis. J Neuroimmune Pharmacol 2011;6:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magliozzi R, Howell OW, Reeves C, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 2010;68:477–493. [DOI] [PubMed] [Google Scholar]
- 29.Deppe M, Tabelow K, Kramer J, et al. Evidence for early, non-lesional cerebellar damage in patients with multiple sclerosis: DTI measures correlate with disability, atrophy, and disease duration. Mult Scler 2016;22:73–84. [DOI] [PubMed] [Google Scholar]
- 30.Valentino P, Cerasa A, Chiriaco C, et al. Cognitive deficits in multiple sclerosis patients with cerebellar symptoms. Mult Scler 2009;15:854–859. [DOI] [PubMed] [Google Scholar]
- 31.Weier K, Penner IK, Magon S, et al. Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PLoS One 2014;9:e86916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobyne SM, Ochoa WB, Bireley JD, et al. Cognitive impairment and the regional distribution of cerebellar lesions in multiple sclerosis. Mult Scler 2017:1352458517730132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prosperini L, Sbardella E, Raz E, et al. Multiple sclerosis: white and gray matter damage associated with balance deficit detected at static posturography. Radiology 2013;268:181–189. [DOI] [PubMed] [Google Scholar]
- 34.Rizzo G, Veronese M, Tonietto M, et al. Generalization of endothelial modelling of TSPO PET imaging: Considerations on tracer affinities. J Cereb Blood Flow Metab 2017. January 1:271678X17742004. [DOI] [PMC free article] [PubMed] [Google Scholar]


