Abstract
BACKGROUND:
Rodents and humans show an attenuation of fear extinction during adolescence, which coincides with the onset of several psychiatric disorders. Although the ethological relevance and the underlying mechanism are largely unknown, the suppression of fear extinction during adolescence is associated with a diminished plasticity in the glutamatergic neurons of the infralimbic-medial prefrontal cortex (IL-mPFC), a brain region critical for fear extinction. Given the putative effect of synaptic inhibition on glutamatergic neuron activity, we studied whether GABAergic neurons in the IL-mPFC are involved in the suppression of fear extinction during adolescence.
METHODS:
We assessed membrane and synaptic properties in parvalbumin (PVIN)-and somatostatin-positive interneurons (SSTIN) in male pre-adolescent, adolescent and adult mice. The effect of fear conditioning and extinction on PVIN-pyramidal neuron and SSTIN-pyramidal neuron synapses in male pre-adolescent, adolescent and adult mice was evaluated using an optogenetic approach.
RESULTS:
The development of the membrane excitability of PVINs is delayed and reaches maturity only by adulthood, while the SSTIN membrane properties are developed early and remain stable during development from pre-adolescence to adulthood. Although the synaptic inhibition mediated by PVINs undergoes a protracted development, it does not exhibit a fear behavior-specific plasticity. However, the synaptic inhibition mediated by SSTINs undergoes an adolescence-specific enhancement, and this increased inhibition is suppressed by fear learning but not restored by extinction training. This altered plasticity during adolescence overlapped with a reduction in calcium-permeable glutamate receptors in SSTINs.
CONCLUSIONS:
The adolescence-specific plasticity in the SSTINs might play a role in fear extinction suppression during adolescence.
Keywords: Adolescence, Infralimbic medial prefrontal cortex, GABA, Fear extinction, Synaptic plasticity, Parvalbumin-positive neurons, Somatostatin-positive neurons
INTRODUCTION
A prior knowledge of potential threats allows an organism to detect future dangers and take the appropriate actions for its safety. However, a suppression of threat memory in the absence of danger is equally important to permitting other survival functions. An impairment of such coping mechanisms to attenuate threat memory might result in maladaptive behaviors. A diminished ability to extinguish fear memory has been implicated in anxiety- and trauma-related disorders (1–3). Consistently, behavioral therapy for anxiety- and trauma-related disorders relies on extinction learning. Given the high incidence of psychiatric disorders during adolescence (4, 5), it is important to understand whether fear regulation is affected during the transition into and out of adolescence. Past studies on fear extinction in rodents and humans have made a remarkable observation, i.e., a lack of a prototypical fear extinction during adolescence (6, 7). The diminished fear extinction during adolescence might be advantageous to exert a constraint on risky behaviors during the transition from dependence upon a caregiver to being an independent adult. However, an interaction of this reduced fear extinction with potential environmental and genetic risk factors could exacerbate anxiety and trauma-like behaviors in adolescents.
A significant number of adults who are diagnosed with anxiety and fear-related disorders exhibit symptoms during their childhood and adolescent years (8, 9). Understanding the mechanism involved in the onset of anxiety- and fear-related disorders might be critical for the prevention and management of these disorders across the lifespan. It is possible that the brain regions involved in the regulation of fear behavior exhibit a development-dependent plasticity, which might be responsible for the suppression of fear extinction during adolescence (6, 7, 10). Consistently, we observed a lack of fear extinction-dependent synaptic and intrinsic plasticity in the layer 5 pyramidal neurons of the adolescent infralimbic-medial prefrontal cortex (IL-mPFC), a brain region critical for fear extinction (6, 11, 12). However, the mechanism underlying the lack of IL-mPFC plasticity during adolescence is unclear. Since synaptic inhibition plays important roles in sculpting cortical circuitry, pyramidal neuron activity and the generation of cortical rhythms (13, 14), GABAergic neuron development could play a role in the attenuation of IL-mPFC plasticity and fear extinction during adolescence. Consistent with the notion that the phylogenetically newer brain areas undergo a protracted development (15), the medial prefrontal cortex (mPFC) is believed to undergo a prolonged rearrangement (16, 17). Earlier studies have demonstrated a protracted synapse development in the GABAergic neurons of the mPFC (18–21). However, an in-depth understanding of synapse development in IL-mPFC GABAergic neurons from pre-adolescence to adulthood has been lacking. Therefore, we undertook a systematic analysis of the membrane and synaptic properties of the IL-mPFC parvalbumin (PVIN)- and somatostatin (SSTIN)-positive interneurons, which primarily innervate the somatic/proximal dendritic areas and the distal dendrites, respectively, of the pyramidal neurons, in pre-adolescent, adolescent and adult mice (22).
Methods
Animals
The following mouse lines were purchased from Jackson Laboratory and subsequently bred in the Skirball division of New York University Medical School animal facility: B6 PVcre (017320, C57BL6J), sttm2.1(cre)Zjh/J (013044, C57BL6/129S4SvJae/C57BL6J), Ai32(RCL-ChR2(H134R)/EYFP) (024109, C57BL6J), B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (007914, C57BL6J), and GAD67-EGFP (G42, 007677, BALBc/C57BL6J) mice. PV-ChR2 mice were generated from homozygous B6 PVcre and homozygous Ai32(RCL-ChR2(H134R)/EYFP) mice. SST-ChR2 mice were generated from homozygous sttm2.1(cre)Zjh/J and homozygous Ai32(RCL-ChR2(H134R)/EYFP) mice. SST-tdTomato mice were generated from homozygous sttm2.1(cre)Zjh/J and homozygous B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J mice. We have used the same line for specific tests across three developmental stages. Naïve mice were used for data presented in Figures 2–6. Mice were maintained on a 12:12 light-dark cycle at 23 °C with access to food and water ad libitum. All experiments were carried out in male mice. The Institutional Animal Care and Use Committee of the New York University School of Medicine approved all the procedures.
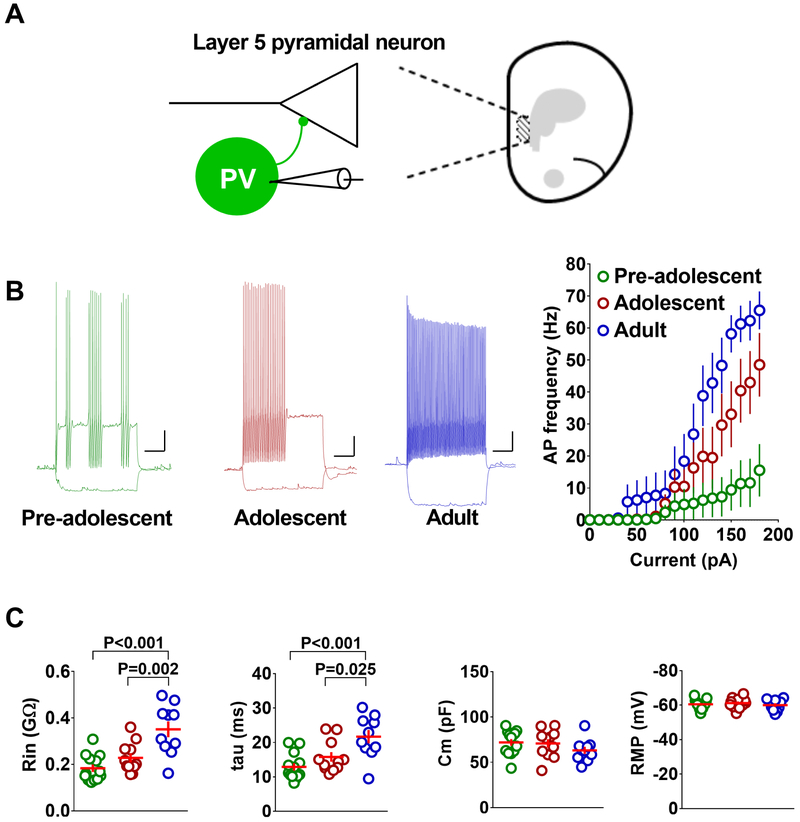
Figure 2.
Protracted development of membrane excitability in the IL-mPFC PV-INs. A) Schematic presentation of whole cell recording in PVINs. B) Mean action potential frequency in response to current injection in PVINs from pre-adolescent (13 neurons/5 mice), adolescent (11 neurons/4 mice), and adult mice (9 neurons/4 mice) (F (2, 30) = 5.7, p = 0.008; pre-adolescent vs adolescent: p =0.12; adolescent vs adult: p = 0.243; pre-adolescent vs adult: p = 0.006). Left panels show example traces of voltage responses to hyperpolarizing (−50 pA) and depolarizing (+180 pA) current steps in PV-INs. Scale: 250 ms/10 mV. C) Passive membrane properties, input resistance (Rin) (F (2, 31) = 13.76, p < 0.001), membrane time constant (tau) (F (2, 31) = 9.334, p = 0.0007), membrane capacitance (Cm) and resting membrane potential (RMP), in the PVINs of pre-adolescent (13 neurons/5 mice), adolescent (11 neurons/4 mice), and adult mice (9 neurons/4 mice). Horizontal line in each group represents mean and vertical line represents standard error of the mean.
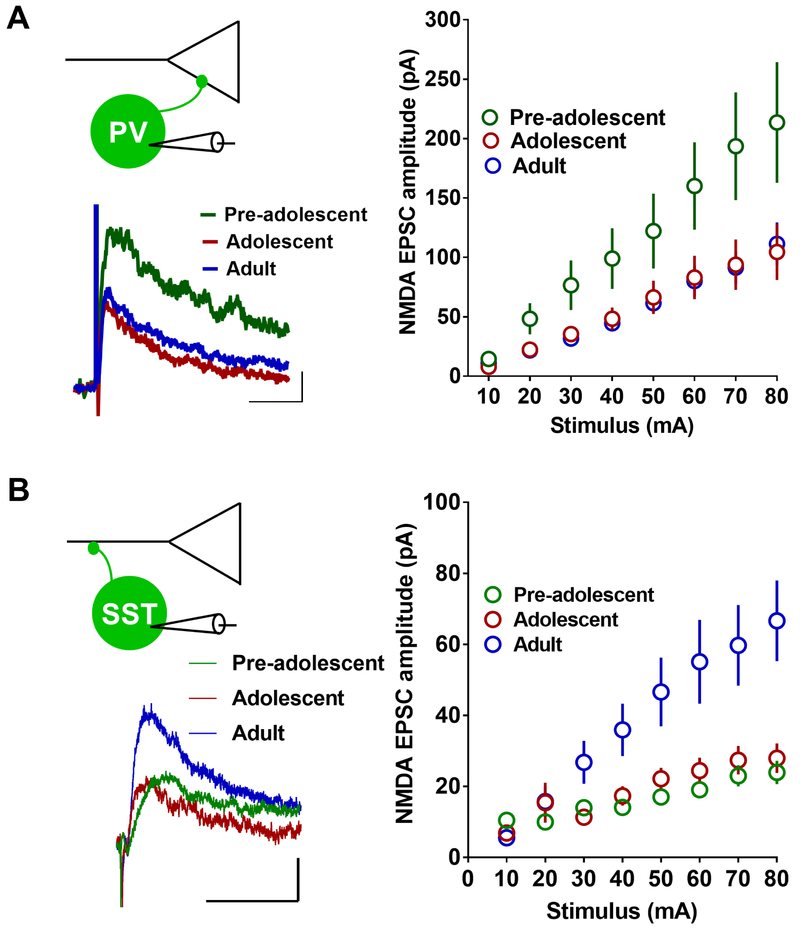
Figure 6.
Development-dependent changes in NMDA receptor transmission in PVINs and SSTINs in the IL-mPFC. A) NMDA receptor-mediated currents in the PVINs of pre-adolescent (13 neurons/3 mice), adolescent (10 neurons/3 mice), and adult mice (14 neurons/3 mice) (F (2, 32) = 3.8, p = 0.033; pre-adolescent vs adolescent: p = 0.075; pre-adolescent vs adult: p = 0.043; adolescent vs adult: p = 0.99). Left panel show example traces. Scale: 50 ms/20 pA. B) NMDA receptor-mediated currents in the SSTINs of pre-adolescent (10 neurons/5 mice), adolescent (10 neurons/5 mice), and adult mice (13 neurons/6 mice) (F (2, 30) = 5.8, p = 0.007; pre-adolescent vs adolescent: p = 1; pre-adolescent vs adult: p = 0.013; adolescent vs adult: p = 0.033). Left panel show example traces. Scale: 50 ms/20 pA.
Behavior
Mice were categorized as pre-adolescent (P24), adolescent (P29) and adult (>P60) as described before (11, 23). Fear conditioning (FC) or tone alone (TA) exposure was performed on P22 for pre-adolescent, on P27 for adolescent or ≥ P60 for adult mice. For conditioning, a mouse was placed in the conditioning chamber (context A) within a soundproof box (Coulbourn Instruments). After a 2 min. exploration, mice received two habituation tones (5 kHz, 30 dB, 30 sec. duration) followed by three tones (30 sec. duration) that co-terminated with foot shocks (2 sec. duration, 0.5 mA, 30 sec. interval). Mice were returned to the home cages 30 sec. after the final tone-shock pairing. The tone alone group received the same tone presentations as the fear conditioned group but without foot shocks. Fear extinction (FE) training was performed 24 hr. after conditioning and consisted of 30 tone presentations (30 sec. duration) at 30 sec interval (context B). For all groups, fear memory was tested 48 hr. after the initial conditioning by exposing the animal to three tones (30 sec. duration) at 30 sec. interval (context B). Freezing was measured using FreezeFrame software (Coulbourn Instruments). Mice were anesthetized for electrophysiology studies 1 hr. after fear memory test on day 3.
Electrophysiology
Mice were anesthetized by an intraperitoneal injection of pentobarbital (120 mg/kg). A transcardial perfusion with ice-cold and oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl (118), glucose (10), KCl (2.5), NaH2PO4 (1), CaCl2 (1) and MgSO4 (1.5) (325 mOsm, pH 7.4), was performed for approximately 45 sec. Immediately following the perfusion, brain was isolated and 300 μm brain slices were prepared on a vibratome (Campden Instruments). In order to allow for recovery, slices were maintained for at least 1 hr. at room temperature. Following recovery, one slice was transferred to the recording chamber and superfused with the aforementioned ACSF containing 2.5 mM CaCl2 at 32 °C at 2–3 ml/min. The IL-mPFC was located us ing a 4x objective. The layer 5 pyramidal neurons were visualized using a 40x water immersion objective and video-enhanced differential interference contrast microscopy and confirmed by their morphology and accommodating action potential firing characteristics. EGFP/EYFP-positive PVINs and EYFP/tdTomato-positive SSTINs were identified under fluorescence microscopy. Recording pipettes of 3–5 M resistance were filled with internal solution containing (in mM): K-gluconate (130), KCl (10), MgCl2 (5), MgATP (5), NaGTP (0.2), EGTA (0.5), HEPES (5), pH adjusted to 7.4 with KOH. Electrophysiological recordings were performed with a Multiclamp 700B amplifier connected to a Digidata 1550A (Molecular Devices, CA, USA). Signals were sampled at 20–100 kHz and filtered at 2 kHz. Neuronal excitability was measured in current clamp mode by injecting currents from −50 to 200 pA (10 pA increments). Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded at −60 mV in the presence of bicuculline (20 μM). In experiments involving electrical stimulation, a concentric bipolar stimulating electrode was placed in layer 2/3. NMDA receptor currents were measured at +40 mV in the presence of bicuculline (20 μM) and DNQX (10 μM) using an electrode solution containing (in mM): CsCl (130), HEPES (10), EGTA (0.5), MgATP (5), Sucrose (10), QX314 (5), pH adjusted to 7.4 with CsOH. The same CsCl internal solution containing 10 mM spermine was used for rectification index experiments. Currents were evoked at different holding potentials from −60 to +60 mV (20 mV increments) in the presence of bicuculline (20 μM) and APV (50 μM), and rectification index was calculated as the ratio of the slopes of the linear current/voltage relationship at positive (0–60 mV) and negative (−60–0 mV) holding potentials (24). For light stimulation of GABAergic terminals in PV-ChR2 and SST-ChR2 mice, blue light (470 nm) was emitted from a Lumen 1600-LED (Prior) at increasing durations (0.05 – 0.5 ms, 0.1 Hz). Electrophysiological recordings were rejected when series resistance or holding current changed by 10% or more.
Data analysis
Membrane properties and evoked currents were analyzed using Clampfit 10.5. Passive membrane properties were measured from the membrane voltage response to hyperpolarizing current injections of -50 to -10 pA. Input resistance (Rin) was calculated as the slope of the current-voltage relationship from -50 to 0 pA and membrane time constant tau was calculated by fitting the initial change in membrane voltage in response to a −50 pA step to a single exponential function. Membrane capacitance (Cm) was calculated from tau and Rin. Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, Inc) or IBM SPSS statistics software. One-way ANOVA with Tukey’s post hoc test or Kruskal-Wallis with Dunn’s post hoc test were used to compare passive membrane properties, spontaneous neurotransmission and fear extinction memory. Two-way ANOVA with Tukey’s post hoc test was used to analyze neuronal excitability.
RESULTS
Suppression of fear extinction in adolescent mice
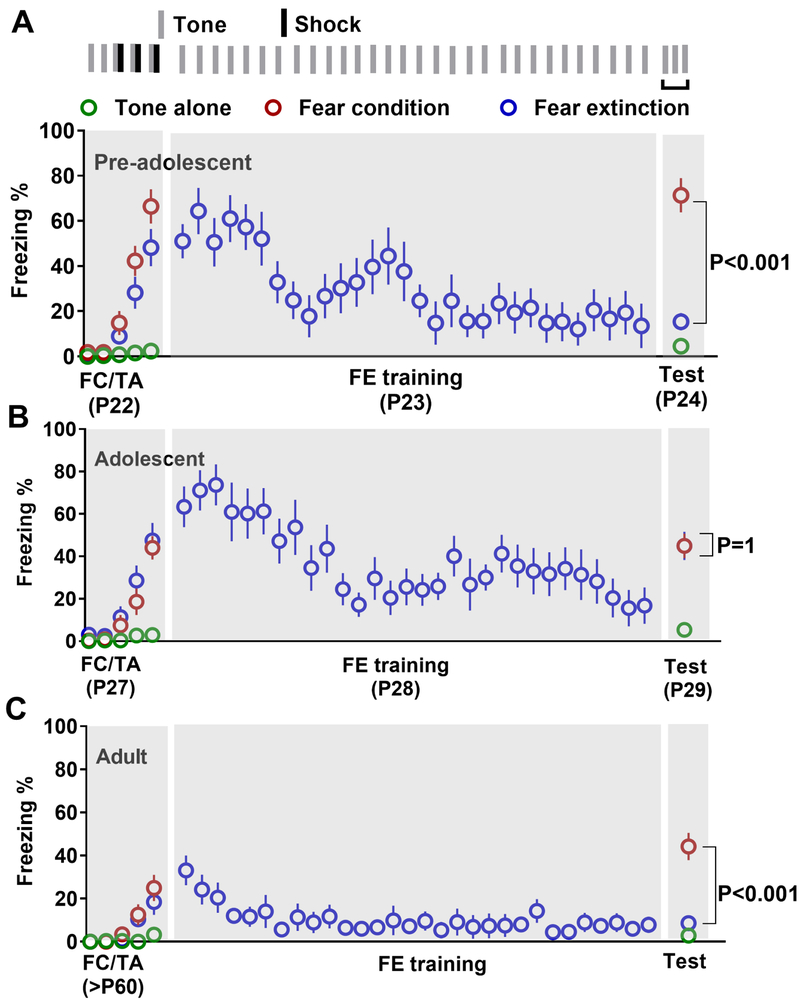
In order to compare fear extinction in pre-adolescent, adolescent and adult mice, we have pooled together behavioral data from PV-ChR2 (Figure S1) and SST-ChR2 mice (Figure S2). There were three treatment groups; tone alone (TA), fear conditioning (FC) and fear extinction groups. In pre-adolescent mice, fear conditioning resulted in a robust freezing behavior compared to the TA group (Figure 1A). Furthermore, fear extinction caused a significant reduction in freezing compared to the FC group (Figure 1A). Similar to the pre-adolescent group, adolescent mice exhibited a significant increase in freezing following fear conditioning (Figure 1B). However, fear extinction training did not result in the reduction of freezing 24 hrs. later in adolescent mice (Figure 1B). Although the magnitude of fear conditioning was lower in adult mice compared to the younger mice, they exhibited a strong fear memory (Figure 1C). Furthermore, fear extinction resulted in a significant decrease in freezing (Figure 1C). These behavioral data further confirm the suppression of fear extinction during adolescence (6, 7).
Figure 1.
Suppression of fear extinction during adolescence. Average freezing in tone alone (TA), fear conditioning (FC) and fear extinction (FE) groups [A. pre-adolescent: tone alone (10 mice), fear conditioning (10 mice), and fear extinction (10 mice), comparison of freezing on day 3: (F (2, 27) = 50.6, p < 0.001; TA vs FC: p < 0.001; TA vs FE: p = 0.4; FC vs FE: p < 0.001)], [B. adolescent: tone alone (11 mice), fear conditioning (10 mice), and fear extinction (10 mice), comparison of freezing on day 3: (F (2, 28) = 17.2, p < 0.001; TA vs FC: p < 0.001; TA vs FE: p < 0.001; FC vs FE: p = 1)], and [C. adult: tone alone (10 mice), fear conditioning (9 mice), and fear extinction (9 mice), comparison of freezing on day 3: (F (2, 25) = 26.7, p < 0.001; TA vs FC: p < 0.001; TA vs FE: p = 1; FC vs FE: p < 0.001)] on days 1 (tone alone or fear conditioning, 2 (extinction training) and 3 (memory test). A part of this behavioral data appeared in an earlier publication (11). We also observed a development-dependent decrease in fear acquisition (F (2, 55) = 12.9, p < 0.001; pre-adolescent vs adolescent: p = 0.25; pre-adolescent vs adult: p < 0.001; adolescent vs adult: p = 0.005) and fear memory (F (2, 26) = 4.7, p = 0.014; pre-adolescent vs adolescent: p = 0.017; pre-adolescent vs adult: p < 0.016; adolescent vs adult: p = 0.93) in pre-adolescent, adolescent and adult mice.
Protracted development of intrinsic excitability in IL-mPFC PVINs
Since membrane excitability plays an important role in neuronal output, an adolescence-specific modulation of the membrane properties of IL-mPFC PVINs could influence fear extinction. To determine whether the IL-mPFC PVINs show an adolescence-specific change in membrane excitability, we compared the number of action potentials in response to current injection in EYFP-positive PVINs in the IL-mPFC of pre-adolescent, adolescent and adult PV-ChR2 mice. We observed a development-dependent increase in the number of action potentials from pre-adolescence to adulthood (Figure 2B). The development-dependent increase in membrane excitability was accompanied by a progressive increase in input resistance and membrane time constant (tau) without any significant change in membrane capacitance or resting membrane potential (Figure 2C). These results strongly suggest that PVINs in the IL-mPFC undergo a protracted development and reach maturity only by adulthood.
Early maturation of intrinsic excitability in IL-mPFC SSTINs
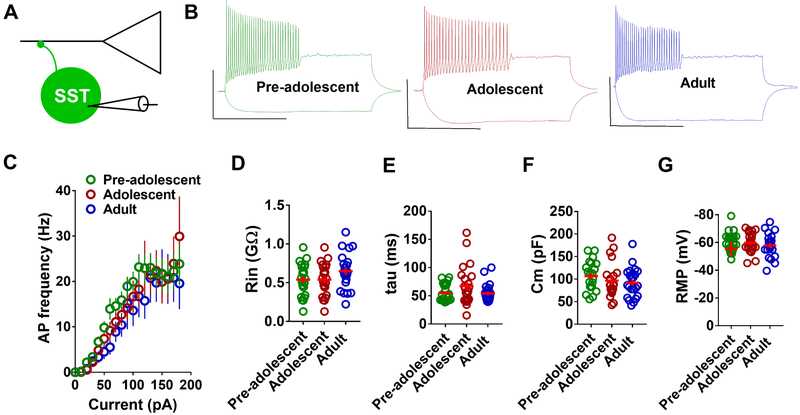
Given the protracted development of PVIN membrane properties, we examined whether the active or passive membrane properties of tdTomato-positive SSTINs in the IL-mPFC change during development. A comparison of the number of evoked action potentials in response to current injection in pre-adolescent, adolescent and adult mice did not reveal a statistically significant effect (Figure 3B,C). Consistent with the lack of change in membrane excitability, we did not observe any change in input resistance, membrane time constant, membrane capacitance or resting membrane potential in the SSTINs of the pre-adolescent, adolescent and adult groups (Figure 3D–G). Therefore, unlike the PVINs, the SSTINs in the IL-mPFC undergo an early maturation and exhibit stable membrane properties during development from pre-adolescence to adulthood.
Figure 3.
Early maturation of membrane excitability in the IL-mPFC SSTINs. A) Schematic presentation of whole cell recording in SSTINs. B) Example traces of voltage responses to hyperpolarizing (−50 pA) and depolarizing (+180 pA) current steps in SSTINs. Scale: 500 ms/50 mV. C) Mean action potential frequency in response to current injection in SSTINs from pre-adolescent (21 neurons/7 mice), adolescent (21 neurons/7 mice), and adult mice (21 neurons/7 mice) (F (2, 60) = 0.944, p = 0.395). D-G) Passive membrane properties, Rin, tau, Cm, and RMP, in the IL-mPFC SSTINs of pre-adolescent (21 neurons/7 mice), adolescent (21 neurons/7 mice), and adult mice (21 neurons/7 mice). Horizontal line in each group represents mean and vertical line represents standard error of the mean.
Comparison of spontaneous glutamatergic transmission in the IL-mPFC PVINs and SSTINs of pre-adolescent, adolescent and adult mice
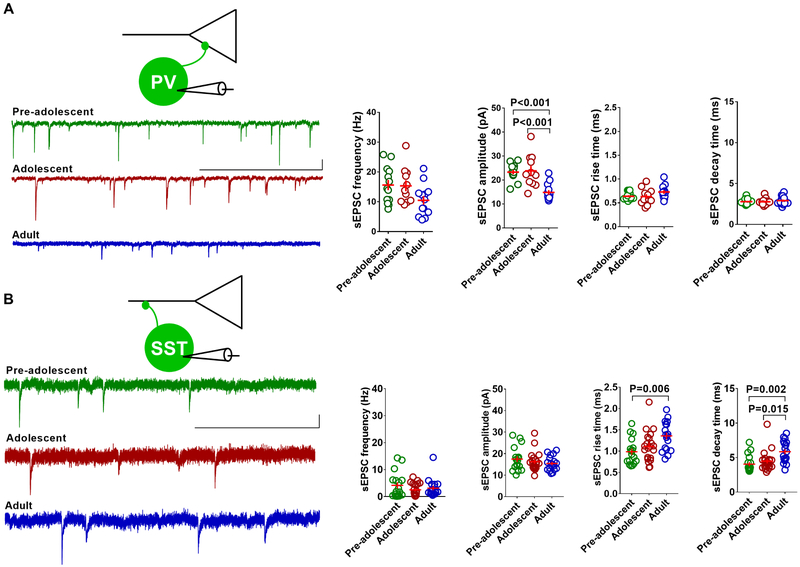
In addition to intrinsic membrane excitability, the excitatory synaptic drive could play an important role in the GABAergic output of PVINs and SSTINs. To test whether the glutamatergic input to these GABAergic neurons exhibits any adolescence-specific changes, we measured the frequency, amplitude, rise time and decay time of spontaneous excitatory post-synaptic currents (sEPSCs) in the EYFP-positive PVINs and tdTomato-positive SSTINs of pre-adolescent, adolescent and adult mice. Although the adult group exhibited a decrease in sEPSC frequency and amplitude in PVINs compared to the pre-adolescent and adolescent groups, only the change in amplitude reached statistical significance (Figure 4A). Both the rise time and decay time of sEPSCs in PVINs remained unchanged during development from pre-adolescence to adulthood (Figure 4A). Unlike the PVINs, SSTINs did not show any change in sEPSC frequency or amplitude in the pre-adolescent, adolescent or adult groups (Figure 4B). Interestingly, we observed an increase in both the rise time and decay time in adult mice compared to the younger groups (Figure 4B). This modification of sEPSC kinetics might be due to a change in AMPA receptor subunit composition during development.
Figure 4.
Development-dependent modulation of spontaneous glutamatergic transmission on to PVINs and SSTINs in the IL-mPFC. A) Mean sEPSC frequency, amplitude (F (2, 34) = 14.6, p < 0.001, pre-adolescent vs adolescent: p = 1; pre-adolescent vs adult: p < 0.001; adolescent vs adult: p < 0.001), rise time, and decay time in the IL-mPFC PVINs of pre-adolescent (13 neurons/4 mice), adolescent (11 neurons/4 mice) and adult mice (13 neurons/4 mice). Left panels show example traces. Scale: 400 ms/10 pA. B) Mean sEPSC frequency, amplitude, rise time (F (2, 53) = 5.6, p = 0.006, pre-adolescent vs adolescent: p = 0.77; pre-adolescent vs adult: p = 0.006; adolescent vs adult: p = 0.079), and decay time (F (2, 53) = 7.5, p = 0.001, pre-adolescent vs adolescent: p = 1; pre-adolescent vs adult: p = 0.002; adolescent vs adult: p = 0.015) in the IL-mPFC SSTINs of pre-adolescent (17 neurons/8 mice), adolescent (21 neurons/8 mice) and adult mice (18 neurons/6 mice). Left panels show example traces. Scale: 400 ms/10 pA. Horizontal line in each group represents mean and vertical line represents standard error of the mean.
Synaptic calcium permeable AMPA receptors in the IL-mPFC PVINs and SSTINs of pre-adolescent, adolescent and adult mice
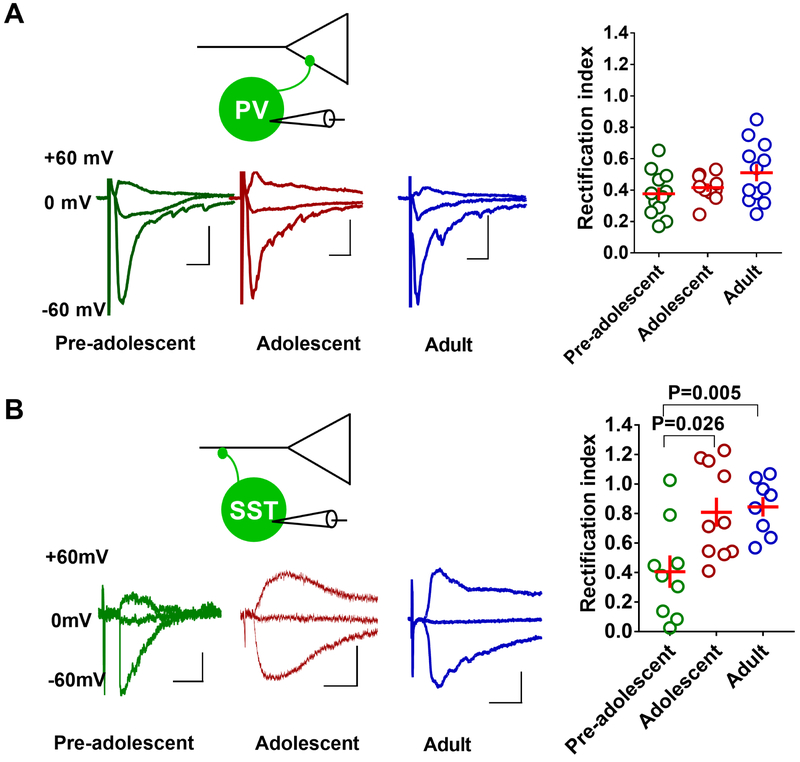
Changes in receptor subunit composition could modify the kinetics of AMPA receptor currents and glutamatergic transmission (25). Therefore, we examined whether the AMPA receptor subunit composition, particularly the presence of GluA2 subunit lacking synaptic calcium permeable AMPA receptors (CPARs), a key mediator of plasticity in GABAergic neurons (26), changes during development from pre-adolescence to adulthood. Based on the inward rectification property of CPARs, we compared the inward rectification of electrically-evoked AMPA currents in the EGFP-positive PVINs of pre-adolescent, adolescent and adult G42 mice (24, 27). A comparison of rectification index in the IL-mPFC PVINs of the pre-adolescent, adolescent and adult groups did not show a statistically significant difference (Figure 5A). However, a similar analysis in the tdTomato-positive SSTINs showed a higher rectification index in the adolescent and adult groups compared to the pre-adolescent group, indicating a development-dependent decrease in synaptic CPARs (Figure 5B). These results suggest a development-dependent switch in the subunit composition of AMPA receptors, leading to an increase in synaptic calcium impermeable AMPA receptors in SSTINs, while PVINs exhibited a stable presence of synaptic CPARs during development from pre-adolescence to adulthood.
Figure 5.
Development-dependent changes in synaptic CPARs in PVINs and SSTINs in the IL-mPFC. A) Rectification index in the PVINs of pre-adolescent (12 neurons/3 mice), adolescent (10 neurons/3 mice), and adult mice (12 neurons/3 mice) (F (2, 31) = 2.577, p = 0.0922). Left panel show example traces. Scale: 10 ms/20 pA. B) Rectification index in the SSTINs of pre-adolescent (9 neurons/5 mice), adolescent (10 neurons/5 mice), and adult mice (10 neurons/5 mice) (F (2, 25) = 6.9, p = 0.004, pre-adolescent vs adolescent: p = 0.026; pre-adolescent vs adult: p = 0.005; adolescent vs adult: p = 1). Left panel show example traces. Scale: 10 ms/20 pA.
NMDA receptor transmission in the IL-mPFC PVINs and SSTINs of pre-adolescent, adolescent and adult mice
Similar to CPARs, NMDA receptors are a major source of calcium signaling at glutamatergic synapses and play an important role in synaptic plasticity. Therefore, we examined whether NMDA receptor transmission is diminished in PVINs or SSTINs during adolescence. A comparison of the amplitude of electrically-evoked NMDA EPSCs in EGFP-positive PVINs of G42 mice revealed a development-dependent decrease in NMDA receptor transmission. The adult group showed a significantly lower NMDA receptor transmission compared to the pre-adolescent mice (Figure 6A). Although the NMDA receptor transmission in the adolescent group was lower than the pre-adolescent group, this effect did not reach statistical significance. A similar examination of NMDA EPSC amplitude in the tdTomato-positive SSTINs of pre-adolescent, adolescent and adult mice showed an increase in NMDA EPSC amplitude in adult mice compared to pre-adolescent and adolescent mice (Figure 6B). Therefore, PVINs and SSTINs in the IL-mPFC exhibit an opposite effect on synaptic NMDA receptors during the transition from pre-adolescence to adulthood.
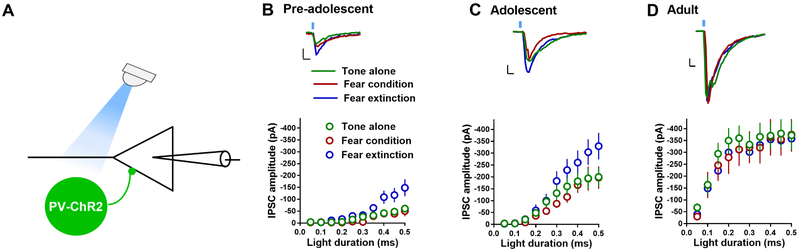
Experience-dependent plasticity at PVIN-pyramidal neuron synapses of pre-adolescent, adolescent and adult IL-mPFC
Given the development-dependent changes in membrane excitability and glutamatergic transmission in PVINs, it is interesting to know whether PVIN-pyramidal neuron GABAergic synapses undergo an experience (fear conditioning and extinction)-and/or development-dependent plasticity. Therefore, we measured the amplitude of light-evoked inhibitory post-synaptic currents (IPSCs) in the IL-mPFC layer 5 pyramidal neurons of TA, FC and FE PV-ChR2 mice. In the pre-adolescent mice, the FE group but not the FC group showed an increase in IPSC amplitude (Figure 7B). Although there was a similar trend in adolescent mice, the FE group did not show a statistically significant increase in IPSC amplitude compared to the TA or FC groups (Figure 7C). A similar analysis in the adult mice showed a lack of effect of fear conditioning and extinction on PVIN-pyramidal neuron GABAergic transmission (Figure 7D). Therefore, a successful extinction in pre-adolescent mice, but not adult mice, was associated with a potentiation of PVIN-pyramidal neuron transmission.
Figure 7.
Experience-dependent modulation of PVIN-mediated inhibition of IL-mPFC layer 5 pyramidal neurons. A) Schematic presentation of light-evoked IPSC recording in the IL-mPFC layer 5 pyramidal neurons of PV-ChR2 mice. B) Mean IPSC amplitude in the IL-mPFC pyramidal neurons of pre-adolescent tone alone (15 neurons/5 mice), fear conditioning (15 neurons/5 mice), and fear extinction groups (17 neurons/5 mice) (F (2, 44) = 4.9, p = 0.011; TA vs FC: p = 0.88; TA vs FE: p = 0.051; FC vs FE: p = 0.015). C) Mean IPSC amplitude in the IL-mPFC pyramidal neurons of adolescent tone alone (17 neurons/5 mice), fear conditioning (19 neurons/5 mice), and fear extinction groups (14 neurons/5 mice) (F (2, 47) = 2.12, p = 0.13; TA vs FC: p = 0.82; TA vs FE: p = 0.33; FC vs FE: p = 0.11). D) Mean IPSC amplitude in the IL-mPFC pyramidal neurons of adult tone alone (12 neurons/4 mice), fear conditioning (12 neurons/4 mice), and fear extinction groups (14 neurons/4 mice) (F (2, 35) = 0.15, p = 0.85; TA vs FC: p = 0.9; TA vs FE: p = 0.86; FC vs FE: p = 0.99). Comparison of IPSC amplitude in pre-adolescent, adolescent and adult TA groups: (F (2, 41) = 26.6, p = 0.007; pre-adolescent vs adolescent: p = 0.07; pre-adolescent vs adult: p < 0.001; adolescent vs adult: p < 0.001). Upper panels show example traces. Scale: 5 ms/50 pA.
In order to determine whether PVIN-pyramidal neuron GABAergic synapses exhibit a development-dependent plasticity, we compared IPSC amplitude in control TA group from pre-adolescent, adolescent and adult mice. We observed an increase in PVIN-pyramidal neuron GABAergic transmission with development (Figure 7B–D), suggesting that PVIN-pyramidal neuron GABAergic transmission undergoes a protracted development and reaches maturity only by adulthood. These results are similar to the protracted development of the membrane properties of PVINs (Figure 2).
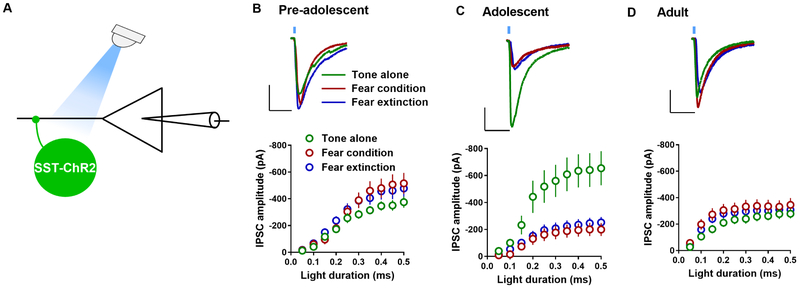
Experience-dependent plasticity at SSTIN-pyramidal neuron synapses of pre-adolescent, adolescent and adult IL-mPFC
Next, we studied the effect of fear conditioning and extinction on light-evoked IPSCs in the IL-mPFC layer 5 pyramidal neurons of pre-adolescent, adolescent and adult SST-ChR2 mice. Neither fear conditioning nor extinction affected IPSC amplitude in pre-adolescent mice (Figure 8B). However, in the adolescent mice, we observed a robust suppression of IPSC amplitude in the FC group compared to the TA group. Furthermore, fear extinction failed to modify fear conditioning-induced suppression of IPSC amplitude (Figure 8C). The effect of fear conditioning and extinction on SSTIN-pyramidal similar to that of pre-adolescent mice, as neither fear conditioning nor extinction affected IPSC amplitude (Figure 8D).
Figure 8.
Experience-dependent modulation of SSTIN-mediated inhibition of IL-mPFC layer 5 pyramidal neurons. A) Schematic presentation of light-evoked IPSC recording in the IL-mPFC layer 5 pyramidal neurons of SST-ChR2 mice. B) Mean IPSC amplitude in the IL-mPFC pyramidal neurons of pre-adolescent tone alone (13 neurons/5 mice), fear conditioning (14 neurons/5 mice), and fear extinction groups (15 neurons/5 mice) (F (2, 39) = 1.14, p = 0.33; TA vs FC: p = 0.57; TA vs FE: p = 0.6; FC vs FE: p = 1). C) Mean IPSC amplitude in the IL-mPFC pyramidal neurons of adolescent tone alone (18 neurons/6 mice), fear conditioning (15 neurons/5 mice), and fear extinction groups (18 neurons/5 mice) (F (2, 48) = 7.8, p = 0.001; TA vs FC: p = 0.003; TA vs FE: p = 0.006; FC vs FE: p = 1). D) Mean IPSC amplitude in the IL-mPFC pyramidal neurons of adult tone alone (15 neurons/6 mice), fear conditioning (15 neurons/5 mice), and fear extinction groups (18 neurons/5 mice) (F (2, 45) = 1.05, p = 0.36; TA vs FC: p = 0.49; TA vs FE: p = 1; FC vs FE: p = 1). Comparison of IPSC amplitude in pre-adolescent, adolescent and adult TA groups: (F (2, 43) = 4.45, p = 0.017; pre-adolescent vs adolescent: p = 0.071; pre-adolescent vs adult: p = 1; adolescent vs adult: p < 0.031). Upper panels show example traces. Scale: 20 ms/200 pA.
To test whether SSTIN-pyramidal neuron GABAergic synapses exhibit a development-dependent plasticity, we compared IPSC amplitude in control TA group from pre-adolescent, adolescent and adult mice. Interestingly, we observed a potentiation of SSTIN-pyramidal neuron GABAergic transmission during adolescence (Figure 8B–D). Therefore, the SSTIN-mediated inhibition is enhanced during adolescence, and these potentiated synapses undergo a depression in response to fear conditioning. Furthermore, fear extinction failed to restore fear conditioning-induced suppression of SSTIN-pyramidal neuron synapses in adolescent mice.
Discussion
Our current study reports the changes in the membrane properties, glutamatergic input and GABAergic output of PVINs and SSTINs in the IL-mPFC during the transition from pre-adolescence to adulthood in mice. While PVINs undergo a protracted development and reach maturity only by adulthood, SSTINs are developed early but exhibit an adolescence-specific GABAergic plasticity. The surge in SSTIN-mediated inhibition of pyramidal neurons during adolescence and an irreversible suppression of this GABAergic transmission after fear conditioning might play a role in diminished fear extinction in adolescents. Although a recent study has demonstrated the effect of SSTINs on glutamatergic transmission (28), future studies will be necessary to understand how SSTIN activity affects plasticity in the IL-mPFC pyramidal neurons, which regulate the amygdala in a top-down fashion to mediate fear extinction (29–34).
The prolonged development of PVINs described in the current study is consistent with the notion that the mPFC undergoes a protracted development (15, 17, 20, 35, 36). Congruent with the earlier suggestion that mPFC development during adolescence is characterized by a slowly increasing inhibitory synaptic transmission and a diminishing glutamatergic transmission (17), we observed a progressive increase in PVIN-pyramidal neuron GABAergic transmission and a simultaneous decrease in glutamatergic transmission in PVINs, particularly those mediated by NMDA receptors, during the transition from pre-adolescence to adulthood. A previous study also reported a similar suppression of NMDA receptor transmission in older mice compared to juvenile mice (19). These results are particularly relevant given the purported role of a NMDA receptor hypofunction in the mPFC fast spiking GABAergic neurons in schizophrenia, a mental disorder with an adolescent onset (17, 37, 38). However, the presence of CPARs, a key mediator of synaptic plasticity in PVINs (26), did not change during development. It is possible that CPARs are sufficient to mediate synaptic calcium signaling in PVINs and therefore, a decrease in NMDA receptors in older mice might not affect plasticity in PVINs. The successful fear extinction was associated with a potentiation of PVIN-pyramidal neuron synapses in pre-adolescent but not adult mice, suggesting that the PVIN-pyramidal neuron synaptic plasticity might play a selective role in fear extinction in pre-adolescents, but not adults. Our earlier study suggested the possibility of distinct fear extinction mechanisms in pre-adolescents and adults, as only adults but not pre-adolescents show fear relapse following extinction (6).
Unlike in the PVINs, the membrane properties of SSTINs did not change during development from pre-adolescence to adulthood. Consistently, a recent study in the anterior cingulate cortex SST-positive neurons showed stable membrane characteristics in pre-adolescent and adolescent stages (18). However, the SSTIN-pyramidal neuron GABAergic transmission underwent a potentiation during adolescence. Consistently, somatostatin expression in the mPFC shows an increase during the 4th postnatal week (36). It is possible that an adolescence-specific neuromodulation is responsible for the increase in SSTIN-pyramidal neuron GABAergic transmission during adolescence. A recent study suggested that dendritic BDNF in the excitatory neurons regulates their SSTIN input (39). These potentiated GABAergic synapses were depotentiated by fear conditioning. Consistent with the lack of fear extinction in adolescent mice, fear extinction training failed to restore this fear conditioning-induced suppression of SSTIN-pyramidal neurons GABAergic transmission. Since SSTIN activity could modulate glutamatergic synapses (28), this adolescence-specific plasticity in the SSTINs could play an important role in the lack of intrinsic and synaptic plasticity in the layer 5 pyramidal neurons (6, 11). Therefore, fear conditioning-induced suppression of SSTIN-pyramidal neuron GABAergic transmission and its irreversible nature might be a key mechanism in the suppression of fear extinction during adolescence. We also observed a switch in synaptic calcium signaling in SSTINs during development. The CPAR-predominant glutamatergic synapses in pre-adolescent mice become calcium impermeable AMPA receptor-predominant glutamatergic synapses in adolescent and adult mice. Consistently, the rise and decay times of sEPSCs in older animals were slower compared to pre-adolescent mice (25). Although this development-dependent decrease in synaptic calcium signaling was compensated by an increase in synaptic NMDA receptors, this effect occurred only by adulthood. Therefore, the glutamatergic synapses in adolescent SSTINs lacked a robust calcium signaling due to low levels of synaptic CPARs and NMDA receptors. Future studies are necessary to understand whether a lack of synaptic calcium signaling is responsible for the aforementioned development-and experience-dependent plasticity in SSTINs. Furthermore, it will be very interesting to examine the mechanism underlying the development-dependent regulation of synaptic CPARs and NMDA receptors. Although we observed a development-dependent decrease in fear conditioning, this effect was not correlated to either fear extinction or experience-dependent plasticity.
We have previously shown that both synaptic and intrinsic plasticity in IL-mPFC pyramidal neurons are involved in fear extinction in pre-adolescents and adults but not adolescents (6, 11). Specifically, fear extinction involves a potentiation of glutamatergic transmission in IL-mPFC layer 5 pyramidal neurons in pre-adolescents and adults but not in adolescents (6). Meanwhile, pre-adolescent and adult mice exhibited a bidirectional modulation of the excitability of IL‐mPFC layer 5 pyramidal neurons following fear conditioning and extinction, i.e., fear conditioning reduced membrane excitability, whereas fear extinction reversed this effect (11). However, fear extinction training failed to reverse fear conditioning-induced suppression of membrane excitability in adolescent mice (11). A medial prefrontal cortex-mediated top-down regulation of the amygdala is believed to be involved in fear extinction (29, 30, 32–34, 40–47). Therefore, the altered SSTIN-pyramidal neuron transmission during adolescence could affect plasticity in the IL-mPFC pyramidal neurons and hence fear extinction. Our current findings shine a light on potential opportunities to enhance IL-mPFC plasticity and hence fear extinction during adolescence. A recent study showed that a pharmacological enhancement of GABAA receptor transmission restores the mPFC gamma oscillation and cognitive flexibility in Dlx5/6+/– mice with impaired GABAergic neuron development (48). Given the delayed development of PVINs, an enhancement of PVIN-mediated GABAergic transmission during adolescence might enhance IL-mPFC plasticity and fear extinction. Also, an enhancement of NMDA receptor transmission might benefit both IL-mPFC plasticity and fear extinction in adolescents, as they show a diminished synaptic calcium signaling in SSTINs (7, 49). Finally, understanding whether and how neuromodulators contribute to the development of the IL-mPFC might benefit efforts to enhance fear extinction during adolescence. Although our study is limited to the examination of two classes of GABAergic neurons, PVINs and SSTINs, and the current study does not demonstrate the causal role of these neurons in the adolescence-specific suppression of fear extinction, we have demonstrated a protracted development of both the membrane and synaptic properties of PVINs and an adolescence-specific plasticity in SSTIN-pyramidal synapses in the IL-mPFC. These findings might facilitate a further understanding of how this differential development of PVIN- and SSTIN-mediated inhibition shapes pyramidal neuron activity, and hence regulates fear extinction during development. There is a higher incidence of affective and anxiety disorders in women compared to men, and this sex difference is believed to emerge after mid-puberty (50–54). The dynamic changes in GABAergic neuron synapses, described in the current study along with the known effects of sex hormones on both pre-and post-synaptic plasticity (55–57), warrant future studies to understand sex differences and the role of sex hormones in the developmental regulation of fear extinction.
Supplementary Material
Figure S1. Suppression of fear extinction during adolescence in PV-ChR2 mice. Average freezing in tone alone (TA), fear conditioning (FC) and fear extinction (FE) groups [A. pre-adolescent: tone alone (5 mice), fear conditioning (5 mice), and fear extinction (5 mice), comparison of freezing on day 3: F (2, 12) = 16.7, p < 0.001], [B. adolescent: tone alone (5 mice), fear conditioning (5 mice), and fear extinction (4 mice), comparison of freezing on day 3: F (2, 11) = 16.1, p < 0.001], and [C. adult: tone alone (4 mice), fear conditioning (4 mice), and fear extinction (4 mice), comparison of freezing on day 3: F (2, 9) = 35.7, p < 0.001] on days 1 (tone alone or fear conditioning), 2 (extinction training) and 3 (memory test).
Figure S2. Suppression of fear extinction during adolescence in SST-ChR2 mice. Average freezing in tone alone (TA), fear conditioning (FC) and fear extinction (FE) groups [A. pre-adolescent: tone alone (5 mice), fear conditioning (5 mice), and fear extinction (5 mice), comparison of freezing on day 3: F (2, 12) = 88.5, p < 0.001], [B. adolescent: tone alone (6 mice), fear conditioning (5 mice), and fear extinction (6 mice), comparison of freezing on day 3: F (2, 14) = 7.4, p = 0.006], and [C. adult: tone alone (6 mice), fear conditioning (5 mice), and fear extinction (5 mice), comparison of freezing on day 3: F (2, 13) = 7.45, p = 0.007] on days 1 (tone alone or fear conditioning), 2 (extinction training) and 3 (memory test).
Key Resource Table
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Chemical Compound or Drug | NaCl | Sigma-Aldrich | S7653 | |
| Chemical Compound or Drug | KCl | Sigma-Aldrich | P9333 | |
| Chemical Compound or Drug | NaH2PO4 | Sigma-Aldrich | S8282 | |
| Chemical Compound or Drug | CaCl2 | Sigma-Aldrich | C4901 | |
| Chemical Compound or Drug | Glucose | Sigma-Aldrich | G7528 | |
| Chemical Compound or Drug | MgSO4 | Sigma-Aldrich | M7506 | |
| Chemical Compound or Drug | K-gluconate | Sigma-Aldrich | G4500 | |
| Chemical Compound or Drug | MgCl2 | Sigma-Aldrich | M8266 | |
| Chemical Compound or Drug | MgATP | Sigma-Aldrich | A9187 | |
| Chemical Compound or Drug | NaGTP | Sigma-Aldrich | G8877 | |
| Chemical Compound or Drug | EGTA | Sigma-Aldrich | E4378 | |
| Chemical Compound or Drug | HEPES | Sigma-Aldrich | H3375 | |
| Chemical Compound or Drug | Bicuculline methiodide | Sigma-Aldrich | 14343 | |
| Chemical Compound or Drug | DNQX | TOCRIS | 2312 | |
| Chemical Compound or Drug | CsCl | Sigma-Aldrich | C4036 | |
| Chemical Compound or Drug | QX314 | Sigma-Aldrich | L5783 | |
| Chemical Compound or Drug | CsOH | Sigma-Aldrich | 232041 | |
| Chemical Compound or Drug | D-APV | TOCRIS | 0105 | |
| Chemical Compound or Drug | spermine | Sigma-Aldrich | S464 | |
| Organism/Strain | Mouse: B6.129P2-Pvalbtm1(cre)Arbr/J | The Jackson Laboratory | RRID:IMSR_JAX:017320 | |
| Organism/Strain | Mouse: STOCK Ssttm2.1(cre)Zjh/J | The Jackson Laboratory | RRID:IMSR_JAX:013044 | |
| Organism/Strain | Mouse: B6.Cg-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J | The Jackson Laboratory | RRID:IMSR_JAX:024109 | |
| Organism/Strain | Mouse: B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | The Jackson Laboratory | RRID:IMSR_JAX:007914 | |
| Organism/Strain | Mouse: CB6-Tg(Gad1-EGFP)G42Zjh/J | The Jackson Laboratory | RRID:IMSR_JAX:007677 |
ACKNOWLEDGEMENTS
This work was supported by NIH (HD076914 to I.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors declare no biomedical financial interests or potential conflicts of interest.
References
- 1.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, et al. (2009): Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 167:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liberman LC, Lipp OV, Spence SH, March S (2006): Evidence for retarded extinction of aversive learning in anxious children. Behav Res Ther. 44:1491–1502. [DOI] [PubMed] [Google Scholar]
- 3.Jovanovic T, Ressler KJ (2010): How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 167:648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 62:593–602. [DOI] [PubMed] [Google Scholar]
- 5.Paus T, Keshavan M, Giedd JN (2008): Why do many psychiatric disorders emerge during adolescence? Nature reviews. 9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. (2012): Altered Fear Learning Across Development in Both Mouse and Human. Proceedings of the National Academy of Sciences of the United States of America. 109:16318–16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCallum J, Kim JH, Richardson R (2010): Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 35:2134–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R (2003): Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Archives of general psychiatry. 60:709–717. [DOI] [PubMed] [Google Scholar]
- 9.Pollack MH, Otto MW, Sabatino S, Majcher D, Worthington JJ, McArdle ET, et al. (1996): Relationship of childhood anxiety to adult panic disorder: correlates and influence on course. Am J Psychiatry. 153:376–381. [DOI] [PubMed] [Google Scholar]
- 10.Gogolla N, Caroni P, Luthi A, Herry C (2009): Perineuronal nets protect fear memories from erasure. Science (New York, NY. 325:1258–1261. [DOI] [PubMed] [Google Scholar]
- 11.Koppensteiner P, Galvin C, Ninan I (2019): Lack of experience-dependent intrinsic plasticity in the adolescent infralimbic medial prefrontal cortex. Synapse (New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do-Monte FH, Manzano-Nieves G, Quinones-Laracuente K, Ramos-Medina L, Quirk GJ (2015): Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci. 35:3607–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacson JS, Scanziani M (2011): How inhibition shapes cortical activity. Neuron. 72:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudy B, Fishell G, Lee S, Hjerling-Leffler J (2011): Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 71:45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson KA, et al. (2016): When Is an Adolescent an Adult? Assessing Cognitive Control in Emotional and Nonemotional Contexts. Psychol Sci. 27:549–562. [DOI] [PubMed] [Google Scholar]
- 17.Insel TR (2010): Rethinking schizophrenia. Nature. 468:187–193. [DOI] [PubMed] [Google Scholar]
- 18.Pan G, Yang JM, Hu XY, Li XM (2016): Postnatal development of the electrophysiological properties of somatostatin interneurons in the anterior cingulate cortex of mice. Sci Rep. 6:28137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HX, Gao WJ (2010): Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol. 588:2823–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JM, Zhang J, Yu YQ, Duan S, Li XM (2012): Postnatal Development of 2 Microcircuits Involving Fast-Spiking Interneurons in the Mouse Prefrontal Cortex. Cereb Cortex. [DOI] [PubMed] [Google Scholar]
- 21.Miyamae T, Chen K, Lewis DA, Gonzalez-Burgos G (2017): Distinct Physiological Maturation of Parvalbumin-Positive Neuron Subtypes in Mouse Prefrontal Cortex. J Neurosci. 37:4883–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wamsley B, Fishell G (2017): Genetic and activity-dependent mechanisms underlying interneuron diversity. Nature reviews. 18:299–309. [DOI] [PubMed] [Google Scholar]
- 23.Spear LP (2000): The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 24:417–463. [DOI] [PubMed] [Google Scholar]
- 24.Adesnik H, Nicoll RA (2007): Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 27:4598–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiagarajan TC, Lindskog M, Tsien RW (2005): Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 47:725–737. [DOI] [PubMed] [Google Scholar]
- 26.Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM (2007): Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science (New York, NY. 315:1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koppensteiner P, Melani R, Ninan I (2017): A Cooperative Mechanism Involving Ca2+-Permeable AMPA Receptors and Retrograde Activation of GABAB Receptors in Interpeduncular Nucleus Plasticity. Cell Rep. 20:1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urban-Ciecko J, Fanselow EE, Barth AL (2015): Neocortical somatostatin neurons reversibly silence excitatory transmission via GABAb receptors. Current biology: CB. 25:722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quirk GJ, Likhtik E, Pelletier JG, Pare D (2003): Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 23:8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milad MR, Quirk GJ (2002): Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 420:70–74. [DOI] [PubMed] [Google Scholar]
- 31.Pare D, Quirk GJ, Ledoux JE (2004): New vistas on amygdala networks in conditioned fear. J Neurophysiol. 92:1–9. [DOI] [PubMed] [Google Scholar]
- 32.Quirk GJ, Garcia R, Gonzalez-Lima F (2006): Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 60:337–343. [DOI] [PubMed] [Google Scholar]
- 33.Amano T, Unal CT, Pare D (2010): Synaptic correlates of fear extinction in the amygdala. Nature neuroscience. 13:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ, et al. (2015): Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 527:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Stradtman GG 3rd, Wang XJ, Gao WJ (2008): A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 105:16791–16796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du X, Serena K, Hwang W, Grech AM, Wu YWC, Schroeder A, et al. (2018): Prefrontal cortical parvalbumin and somatostatin expression and cell density increase during adolescence and are modified by BDNF and sex. Mol Cell Neurosci. 88:177–188. [DOI] [PubMed] [Google Scholar]
- 37.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. (2010): Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nature neuroscience. 13:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen SM, Tsien RW, Goff DC, Halassa MM (2015): The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res. 167:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh H, Piantadosi SC, Rocco BR, Lewis DA, Watkins SC, Sibille E (2019): The Role of Dendritic Brain-Derived Neurotrophic Factor Transcripts on Altered Inhibitory Circuitry in Depression. Biological psychiatry. 85:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santini E, Quirk GJ, Porter JT (2008): Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 28:4028–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan MA, LeDoux JE (1995): Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral neuroscience. 109:681–688. [DOI] [PubMed] [Google Scholar]
- 42.Quirk GJ, Russo GK, Barron JL, Lebron K (2000): The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 20:6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ (2004): Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 24:5704–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004): Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 43:897–905. [DOI] [PubMed] [Google Scholar]
- 45.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS (1999): Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological psychiatry. 45:806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ (2007): Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 53:871–880. [DOI] [PubMed] [Google Scholar]
- 47.Peters J, Kalivas PW, Quirk GJ (2009): Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & memory (Cold Spring Harbor, NY. 16:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho KK, Hoch R, Lee AT, Patel T, Rubenstein JL, Sohal VS (2015): Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/-) mice. Neuron. 85:1332–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgdorf J, Kroes RA, Zhang XL, Gross AL, Schmidt M, Weiss C, et al. (2015): Rapastinel (GLYX-13) has therapeutic potential for the treatment of post-traumatic stress disorder: Characterization of a NMDA receptor-mediated metaplasticity process in the medial prefrontal cortex of rats. Behavioural brain research. 294:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, Yehuda R, et al. (2007): Stress and disease: is being female a predisposing factor? J Neurosci. 27:11851–11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon MB, Herman JP (2009): Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 97:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angold A, Costello EJ, Worthman CM (1998): Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychological medicine. 28:51–61. [DOI] [PubMed] [Google Scholar]
- 53.Zender R, Olshansky E (2009): Women’s mental health: depression and anxiety. The Nursing clinics of North America. 44:355–364. [DOI] [PubMed] [Google Scholar]
- 54.Hankin BL, Abramson LY (1999): Development of gender differences in depression: description and possible explanations. Annals of medicine. 31:372–379. [DOI] [PubMed] [Google Scholar]
- 55.Galvin C, Ninan I (2014): Regulation of the mouse medial prefrontal cortical synapses by endogenous estradiol. Neuropsychopharmacology. 39:2086–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smejkalova T, Woolley CS (2010): Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 30:16137–16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith CC, McMahon LL (2005): Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 25:7780–7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Suppression of fear extinction during adolescence in PV-ChR2 mice. Average freezing in tone alone (TA), fear conditioning (FC) and fear extinction (FE) groups [A. pre-adolescent: tone alone (5 mice), fear conditioning (5 mice), and fear extinction (5 mice), comparison of freezing on day 3: F (2, 12) = 16.7, p < 0.001], [B. adolescent: tone alone (5 mice), fear conditioning (5 mice), and fear extinction (4 mice), comparison of freezing on day 3: F (2, 11) = 16.1, p < 0.001], and [C. adult: tone alone (4 mice), fear conditioning (4 mice), and fear extinction (4 mice), comparison of freezing on day 3: F (2, 9) = 35.7, p < 0.001] on days 1 (tone alone or fear conditioning), 2 (extinction training) and 3 (memory test).
Figure S2. Suppression of fear extinction during adolescence in SST-ChR2 mice. Average freezing in tone alone (TA), fear conditioning (FC) and fear extinction (FE) groups [A. pre-adolescent: tone alone (5 mice), fear conditioning (5 mice), and fear extinction (5 mice), comparison of freezing on day 3: F (2, 12) = 88.5, p < 0.001], [B. adolescent: tone alone (6 mice), fear conditioning (5 mice), and fear extinction (6 mice), comparison of freezing on day 3: F (2, 14) = 7.4, p = 0.006], and [C. adult: tone alone (6 mice), fear conditioning (5 mice), and fear extinction (5 mice), comparison of freezing on day 3: F (2, 13) = 7.45, p = 0.007] on days 1 (tone alone or fear conditioning), 2 (extinction training) and 3 (memory test).