Abstract
Objectives:
Immune checkpoint inhibitors (ICIs) have modest activity in ovarian cancer (OC), yet little is known about their effects on subsequent treatment. Preclinical studies suggest immunotherapy may enhance response to chemotherapy. We sought to evaluate the impact of ICIs on subsequent therapies and survival in recurrent OC.
Methods:
A retrospective review was conducted to identify women with recurrent OC who received ICI from 01/2013–5/2017 and ≥1 subsequent treatment. Treatment duration after ICI was calculated using time-to-event analysis. Kaplan-Meier survival analysis and Cox proportional hazards models were used to calculate overall survival (OS) from first treatment after ICI and to assess survival differences by clinical benefit from ICI, defined by long (≥24 weeks) versus short (<24 weeks) ICI treatment duration.
Results:
Of 79 evaluable women identified, 66 (84%) had platinum-resistant OC. Median age at diagnosis was 57 years. Median time from diagnosis to ICI was 39.7 months, with median of 4 prior treatments (range, 1–12). Median number of treatments after ICI was 2 (range, 1–8). Median duration of first-line treatment after ICI was 3.7 months (95% CI, 2.9–6.0) and declined with each subsequent line. The most common therapies after ICI were taxanes, platinum-based regimens, and pegylated liposomal doxorubicin. Bevacizumab was used in 47 women (59%). Median OS after ICI was 18.3 months (95% CI, 11.8–22.7) and did not differ between long versus short ICI.
Conclusions:
In this heavily pretreated population of patients with recurrent OC, therapies after ICI resulted in promising survival, suggesting that ICI may improve efficacy of subsequent chemotherapy.
Keywords: immunotherapy, checkpoint inhibition, ovarian cancer, platinum resistance, survival
Introduction
Recurrent and platinum-resistant ovarian cancers are therapeutically challenging, with poor overall survival (OS) ranging from 12–14 months.1 Therapies are limited, and response rates to approved chemotherapy agents range from 10–15%.1,2
Immune checkpoint inhibitors (ICIs) block inhibitory receptors on the surface of T cells or their corresponding ligands, preventing exhaustion and promoting activation of T cells to enhance tumor detection and destruction.3 Although immunotherapy is a promising treatment for recurrent ovarian cancer,4 single-agent checkpoint inhibitors have only produced modest results in recurrent ovarian cancer, with response rates ranging from 10–15%.5–8
One of the most promising aspects of immunotherapy is the ability to produce durable responses.9 Pharmacokinetic studies of these antibodies have shown their half-lives range from 3–4 weeks;10 however, the duration of ICI-activated T cell response is unknown and is often maintained after ICI discontinuation. More recent studies have identified a subset of T cells called tissue-resident memory T cells that may play a role in the success and durability of ICIs.11,12
Numerous studies have shown no differences in progression-free survival (PFS) with ICIs compared to traditional chemotherapy but have shown significant improvements in overall survival (OS).13 This suggests the effects of ICIs may endure and influence subsequent therapies, thereby prolonging life, despite the lack of an apparent effect on the initial response rates. Furthermore, ICI and chemotherapy combination regimens have resulted in improved survival in several cancer types, including non-small cell lung cancer (NSCLC),14,15 small cell lung cancer (SCLC),16 and triple-negative breast cancer (TNBC).17 These findings suggest that ICIs may have a positive impact on either concurrent or subsequent chemotherapy.
An increasing number of patients with ovarian cancer are being treated with ICIs on protocols and under compassionate use programs; many of these women will continue to receive subsequent chemotherapy after ICI treatment, and little is known about ICIs’ effects on these subsequent treatments and survival. A small case series of 2 women treated on protocol with nivolumab, a programmed cell death protein 1 (PD-1) inhibitor, reported good responses to subsequent chemotherapy and promising survival;18 however, larger studies are needed to confirm these effects. Our study sought to investigate if ICI use provides benefit from subsequent therapies and results in prolongation of OS in this patient population.
Methods
Patient Selection
We conducted a retrospective review of women with recurrent ovarian cancer treated with ICI at Memorial Sloan Kettering Cancer Center (MSK) between 01/2013 and 05/2017. Women were considered eligible if they had received treatment with an antibody targeted to PD-1, programmed death-ligand 1 (PD-L1), cytotoxic T-Lymphocyte associated protein 4 (CTLA-4), lymphocyte-activation gene 3 (LAG3), or any combination involving one of these agents. We then identified patients who went on to receive at least one subsequent therapy.
Data Collection
Patient characteristics were abstracted and verified by 2 independent reviewers from 3/2018–10/2018. Patient age, stage, and histology were defined at pathological diagnosis using International Federation of Gynecology and Obstetrics (FIGO) staging.19 Initial treatment (neoadjuvant chemotherapy followed by interval debulking or primary debulking followed by adjuvant chemotherapy) and surgical outcomes (optimal vs. suboptimal debulking) were abstracted from the clinical record. CA-125 level, body mass index (BMI), and platinum status were defined at the initiation of ICI treatment. Platinum resistance was defined as disease progression per imaging within 6 months of last platinum (carboplatin or cisplatin) treatment. Platinum-free interval (PFI) was calculated as the time from date of last platinum treatment prior to ICI use to the date of disease progression per imaging/biopsy.
Duration of time from pathological diagnosis to start of ICI therapy was calculated in months. Duration of time on an ICI was defined as the time from the date of the first dose of an ICI to the date of study end of treatment, as all patients completed ICI treatment and were taken off protocol due to progression or intolerability. The reason for discontinuation of ICI treatment, progression or toxicity, was collected. Patients were then stratified into 2 groups based on duration of ICI therapy: short (ICI time <24 weeks) or long (ICI time ≥24 weeks), as defined by prior studies.20,21
Number of treatments, defined as systemic therapy with chemotherapy or hormonal therapy, prior to and after ICI treatment were collected. Total treatment lines were calculated as the sum of prior and subsequent treatments plus 1 to account for the ICI regimen. In those with missing information about prior lines (n=8), total lines were not calculated. Treatment regimens after ICI use were abstracted for each subsequent line of treatment and categorized into the following regimens for each line: 1) platinum based, 2) pegylated liposomal doxorubicin (PLD), 3) gemcitabine, 4) taxanes, 5) topotecan/irinotecan, 6) hormone-based therapies 7) poly (ADP-ribose) polymerase (PARP) inhibitors, 8) pemetrexed, or 9) other, which included clinical trials. The use of bevacizumab, as monotherapy or in combination with chemotherapy, was noted. Patients were categorized as using specific treatment if they received the corresponding treatment in at least one line after ICI treatment.
Statistical Analysis
Patient characteristics and clinical variables were reported using summary statistics. The proportion of patients ever receiving specific treatment regimens and bevacizumab after ICI treatment was reported.
As some patients were still being treated on their first line of therapy after ICI treatment, durations of subsequent treatments were calculated as time-to-event variables using event-free survival (EFS). EFS was defined as the time from initiation of treatment to the time of event (initiation of new treatment, radiographic disease progression in those without subsequent treatment, or death in those without subsequent treatment or progression) or last follow-up for those without events.
Due to limited sample size, EFS was calculated for the first through fourth line of subsequent therapies after ICI only. Analyses were performed separately for each line. The median EFS and 95% confidence interval was also tabulated for each type of treatment after ICI treatment and for use of bevacizumab (monotherapy or in combination) in the first line only given limited sample size and patient heterogeneity.
Kaplan-Meier survival analysis was used to estimate EFS for each line separately and OS, for all patients and stratified by short versus long ICI time. OS was defined as the time from initiation of first therapy after ICI treatment to death or last follow-up in those still living. The log-rank test and Cox proportional hazards (CoxPH) model were used to assess differences in survival in univariate setting. A multivariate CoxPH model was built to examine the relationship between OS and ICI group by adjusting for relevant clinical covariates.
Results
Patient Characteristics
We identified 115 women with recurrent ovarian cancer treated with an ICI (110 on protocol and 5 off-study). Of these, 80 went on to receive at least one subsequent therapy. One woman was excluded because she had disease of granulosa cell histology. Of the 35 women who did not go on to receive additional therapies after ICI treatment, 2 remained on ICI therapy at the time of analysis. Of the remaining 33 women, 28 died and 5 were lost to follow-up before receiving another therapy.
Of the 79 women evaluable for analysis, the median age at diagnosis was 57 years (range, 24–72), and the median age at initiation of ICI treatment was 61 years (range, 28–74). Sixty-eight women (86%) had disease of high-grade serous histology; the remaining 11 women had clear cell carcinoma (n=5), endometrioid carcinoma (n=2), low grade serous (n=2), mixed serous and endometrioid (n=1), or adenocarcinoma not otherwise specified (NOS) (n=1). Most women had stage III disease at diagnosis (n=45, 57%). Twenty-one (27%) of women had germline or somatic mutations in BRCA1/2, and 6 patients did not undergo testing. Of patients with available data regarding surgery, 41 (61%) underwent primary surgical debulking and 26 (39%) received neoadjuvant chemotherapy followed by interval debulking surgery. Optimal debulking was achieved in 44 (82%) patients with available data. At the time of ICI treatment, 66 (84%) women had platinum-resistant disease.
Median time from diagnosis to ICI initiation was 39.7 months (range, 10.1–205.9). The median number of treatment lines before ICI treatment was 4 (range, 1–12). Median CA-125 levels at initiation of ICI treatment was 158 (range, 9–7087), and median BMI at ICI initiation was 25.9 kg/m2 (range, 18.5–50.2).
Most women received treatment with a PD-1/PD-L1 inhibitor alone (n=35, 44%) or a combination of a PD1/PD-L1 with another agent (n=39, 49%), most commonly a CTLA-4 inhibitor. Median duration of ICI therapy was 2.8 months (range, 0.6–13.8). Median number of treatments post-ICI therapy was 2 (range, 1–8). Total lines of treatment, including ICI use, ranged from 4–16, with a median of 7 lines (Table 1).
Table 1:
Clinical characteristics of the cohort (N=79)
| Variables | N=79 |
|---|---|
| Age at Diagnosis, Median (Mean), range | 57 (55.9), 24–72 |
|
Histology | |
| High-grade serous | 68 (86%) |
| Other* | 11 (14%) |
|
Stage at diagnosis | |
| I/II | 3 (4%) |
| III | 45 (57%) |
| IV | 31 (39%) |
|
BRCA 1/2 Mutations (germline or somatic) | |
| Wildtype | 52 (66%) |
| BRCA-mutated | 21 (27%) |
| Not tested | 6 (7%) |
|
Optimal debulking (25 missing) | |
| No | 10 (18%) |
| Yes | 44 (82%) |
|
Primary chemo treatment (12 missing) | |
| Neoadjuvant | 26 (39%) |
| Adjuvant | 41 (61%) |
|
Platinum status at ICI initiation | |
| Sensitive | 13 (16%) |
| Resistant/Refractory | 66 (84%) |
|
ICI target | |
| PD-1/PD-L1 | 35 (44%) |
| PD-1/PD-L1+CTLA-4 | 27 (34%) |
| PD-1/PD-L1+Other | 12 (16%) |
| Other | 5 (6%) |
| CA-125, Median(Mean), range | 158 (618.5), 9–7087 |
| BMI, Median(Mean), range | 25.9 (27.2), 18.5–50.2 |
| ICI duration in months, Median (Mean), range | 2.8 (3.9), 0.6–13.8 |
| Long ICI (ICI duration ≥24 weeks) | 16 (20%) |
| Short ICI (ICI duration <24 weeks) | 63 (80%) |
| Months lag from Dx to ICI, Median(Mean), range | 39.7 (45.9), 10.1–205.9 |
|
Treatment Lines | |
| Pre-ICI (8 missing), Median (Mean), range | 4 (4.1), 1–12 |
| Post-ICI Median (Mean), range | 2 (2.9), 1–8 |
| Total (8 missing), Median(Mean), range | 7 (8.1), 4–16 |
ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; CTLA-4, cytotoxic T-Lymphocyte associated protein 4; dx, diagnosis
Other histologies: clear cell carcinoma (n=5), endometrioid carcinoma (n=2), low grade serous (n=2), mixed serous and endometrioid (n=1), or adenocarcinoma not otherwise specified (NOS) (n=1)
Long versus Short ICI Treatment
Of the 79 women studied, 16 (20%) derived clinical benefit from ICI therapy, with a long duration of ICI use (ICI ≥24 weeks); the other 63 (80%) did not derive clinical benefit, with a short duration of ICI use (<24 weeks). Five women (6%) discontinued ICI therapy due to toxicity; the remaining 74 discontinued due to disease progression. Those with clinical benefit from ICI therapy (long, ≥24 weeks) had a significantly longer time from diagnosis to ICI initiation compared to those with a shorter duration of ICI therapy (ICI<24 weeks), with median times of 54.4 and 32.5 months, respectively (p=0.023). There were no differences in any of the other clinical characteristics examined, including stage at diagnosis, histology, and type of ICI (p>0.05) (Supplemental Table 1).
Treatments after ICI Therapy (Any Line Thereafter)
After ICI treatment, 42 women (53%) received treatment with a taxane, 37 (47%) received platinum-based treatments, 24 (30%) received PLD, 18 (23%) received gemcitabine, 17 (22%) received topotecan/irinotecan, and 23 (29%) received an “other” treatment. Less common treatments included pemetrexed (n=15, 19%), PARP inhibitors (n=10, 13%), and hormonal therapies (n=6, 8%). Forty-seven women (59%) received bevacizumab at some point after ICI treatment (Table 2).
Table 2:
Regimens after ICI therapy and bevacizumab use (N=79)
| Treatment regimens after ICI therapy | n (%) |
|---|---|
| Platinum-based | 37 (47%) |
| Pegylated liposomal doxorubicin (PLD) | 24 (30%) |
| Gemcitabine | 18 (23%) |
| Taxanes | 42 (53%) |
| Topotecan/Irinotecan | 17 (22%) |
| Hormones | 6 (8%) |
| PARP | 10 (13%) |
| Pemetrexed | 15 (19%) |
| Other | 23 (29%) |
|
Bevacizumab after ICI therapy | |
| Yes | 47 (59%) |
| No | 32 (41%) |
ICI, immune checkpoint inhibitor; PARP, poly (ADP-ribose) polymerase
EFS after ICI Treatment
For the first line of treatment after ICI therapy, median EFS (EFS1) was 3.7 months (95% CI: 2.9–6.0 months). For those with a long compared to short duration of ICI use, median EFS1 was 5.7 months (95% CI: 3.5–7.6 months) and 3.7 months (95% CI; 2.8–5.5), respectively (p=0.225).
For subsequent lines of therapy, median EFS was shorter. (EFS2, 3.6 months [95% CI: 2.5–4.7 months]; EFS3, 3.0 months [95% CI: 2–4.6 months]; and EFS4, 3.1 months [95% CI: 2.5–5.3 months]) (Figure 1 and Supplemental Table 2). Subsequent lines were not reported due to limited sample sizes.
Figure 1: Duration of Treatment after ICI, EFS1–4.
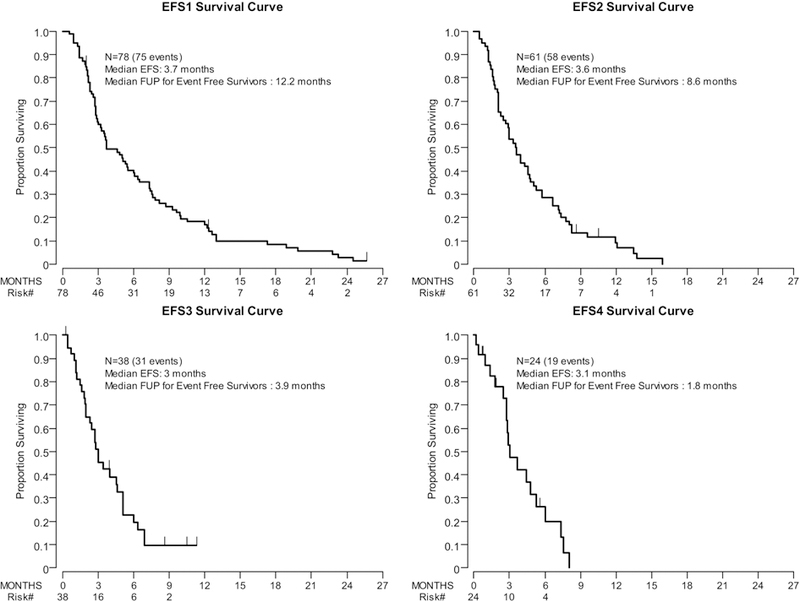
Kaplan-Meier EFS survival curves for the first through fourth subsequent lines of treatment after ICI therapy. EFS was defined as the time from initiation of treatment to the time of event (initiation of new treatment, radiographic disease progression in those without subsequent treatment, or death in those without subsequent treatment or progression) or last follow-up for those without events.
EFS1: Durations by Regimen and Bevacizumab
The most common therapies in the first-line after ICI treatment included PLD (n=16), taxanes (n=15), platinum-based regimens (n=13), gemcitabine (n=8), and topotecan/irinotecan (n=7). Among these regimens, platinum-based therapies had the longest treatment duration (median EFS1, 7.3 months; 95% CI: 3.3–9.9). Two of 13 women received platinum monotherapy; the remaining 11 women received platinum doublets, 3 of whom also received bevacizumab. PLD was associated with a median EFS1 of 4.5 months (95% CI: 2.8–7.6), taxanes with a median EFS1 of 3.7 months (95% CI: 2.0–5.5), gemcitabine with a median EFS1 of 5.1 months (95% CI: 0.6–10.5), and topotecan/irinotecan with a median EFS1 of 2.3 months (95% CI: 0.9–12.4). Use of bevacizumab, either as monotherapy or in combination with chemotherapy, resulted in a median EFS1 of 6.7 months (95% CI: 4.8–9.9). In those who did not receive bevacizumab, the median EFS1 was 3.2 months (95% CI: 2.8–4.6; p=0.059) (Figure 2 and Supplemental Table 3).
Figure 2: Duration of first treatment after ICI by regimen (In those with events only, n=75).
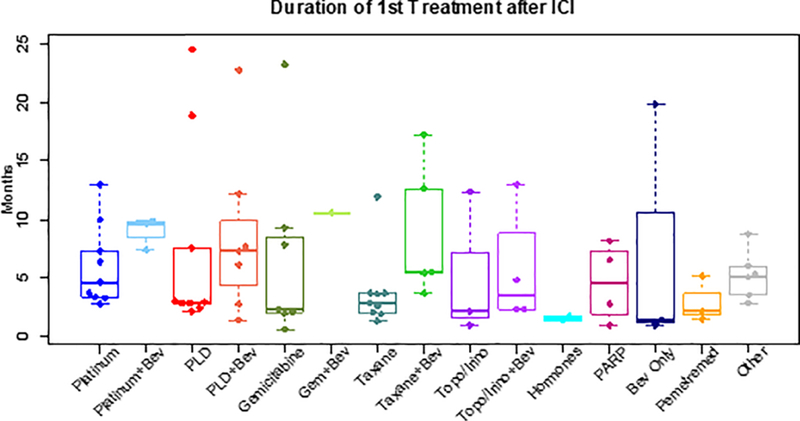
Box and whisker plots depicting durations of treatment in months for the first treatment line after ICI by treatment regimen. Durations of treatments after ICI were calculated as time-to-event variables using EFS. The 3 women remaining on first-line therapy after ICI treatment were censored for this analysis, and only the 75 women with events who completed first-line therapy were included.
Survival Analysis
Among the 78 women included in the post-ICI survival analysis (1 woman was excluded due to a missing start date of first treatment after ICI therapy), there were 50 observed deaths. Median follow-up for the survivors was 18.3 months (range, 1.9–37.0 months). Median post-ICI OS for the cohort was 18.3 months (95% CI: 11.8–22.7 months). The 1-year OS rate was 59.9% (95% CI: 47.8–70%) (Figure 3 and Supplement Table 4).
Figure 3: Overall Survival (Entire Cohort).
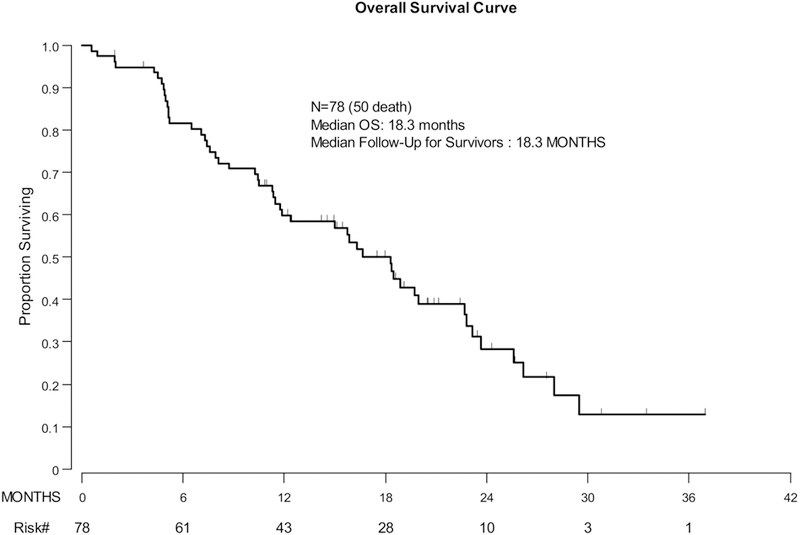
Kaplan-Meier curve depicting overall survival from first treatment after ICI therapy for the entire cohort.
On univariate analysis, increasing lines of treatment prior to ICI use was associated with worse OS (HR, 1.21; 95% CI: 1.06–1.37; p=0.003). Platinum-sensitive disease at initiation of ICI therapy was associated with borderline better OS than platinum-resistant disease (HR, 0.47; 95% CI: 0.22–1.04; p=0.06). Age at ICI therapy, histology (high-grade serous vs. not), and stage at diagnosis (IV vs. other) were not significantly associated with OS (Supplement Table 4).
In patients who derived benefit from ICI therapy (long ICI), median OS was 28 months (95% CI: 10.4–28 months) and the 1-year OS rate was 67% (95% CI: 37.9–84.7%). In those who did not benefit (short ICI), median OS was 16.3 months (95% CI: 11.4–19.7 months) and the 1-year OS rate was 58.2% (95% CI: 44.7–69.5%). On univariate analysis, long ICI was not significantly associated with OS (HR, 0.58; 95% CI: 0.25–1.36; p=0.203) (Supplemental Table 4).
On multivariate analysis, long ICI was not significantly associated with improved OS after adjustment for platinum status at ICI initiation and treatment lines prior to ICI therapy (HR, 0.55; 95% CI: 0.23–1.33; p=0.19) (Table 3).
Table 3:
Multivariate overall survival analysis: long vs. short ICI therapy
| Variables | HR | 95% CI LB |
95% CI UB |
p |
|---|---|---|---|---|
| ICI therapy: | ||||
| Long (≥24wk) vs. short <24wk) ICI duration | 0.55 | 0.23 | 1.33 | 0.19 |
| Platinum status at ICI initiation: | ||||
| Sensitive vs. resistant/refractory | 0.59 | 0.26 | 1.36 | 0.22 |
| Prior Chemo Lines: (continuous variable) | 1.19 | 1.04 | 1.36 | 0.012 |
ICI, immune checkpoint inhibitor
Discussion
We have characterized a cohort of women with heavily pretreated recurrent ovarian cancer, most with platinum-resistant disease, who received subsequent treatments after ICI therapy. Many of these women were re-challenged with platinum as well as other chemotherapy agents, with or without bevacizumab, with suggestion of prolonged clinical benefit from these subsequent lines of therapy. The median OS of 18.3 months from initiation of first treatment after ICI is promising compared to the inferior outcomes of other recent studies of similar patients with fewer prior treatment lines.
The phase 3 AURELIA study randomized women with platinum-resistant epithelial ovarian cancer with 1–2 prior lines of therapy to physician’s choice of chemotherapy (paclitaxel, PLD, or topotecan) with bevacizumab or placebo.22 The median PFS was 3.4 months in the chemotherapy-only arm and was significantly improved to 6.7 months with the addition of bevacizumab. The median OS was 13.3 months in the chemotherapy-only group and 16.6 months in the combination group. The median OS in our cohort of patients with a median of 5 lines of prior therapy compares favorably (18.3 months), especially considering only 59% of our patients received bevacizumab after ICI treatment. In the 65 evaluable patients with platinum-resistant disease at the time of ICI, the median OS from initiation of first treatment after ICI was 16.3 months (95% CI 10.5–20). This is still favorable when compared to AURELIA, particularly given our heavily pre-treated population. In addition, among our platinum-sensitive patients, many progressed very quickly after platinum-based therapy with median platinum-free interval (PFI) of 7.6 months (range 6.2–24.5).
Of note, a large proportion of the women in our study (47%) were re-challenged with platinum-based therapy despite the high prevalence of platinum-resistant disease in our cohort. The median EFS1 for the 13 women who received platinum-based therapies in the first line after ICI treatment was promising at 7.3 months as compared to median EFS1 of 3.7 months in those receiving other therapies, HR 0.77 (95% CI 0.41–1.44, p= 0.41). Higher PFI at time of ICI was associated with improved EFS1, HR 0.91 (95% CI 0.84–0.98, p= 0.014). This is hypothesis generating and suggests that ICIs may help overcome platinum resistance in certain populations. This is also comparable to a recent phase 1/2 study of combination pembrolizumab and low-dose carboplatin in heavily-pretreated, platinum resistant patients with a mPFS of 4.6 months.23
Other frequently used post-ICI regimens included taxanes, PLD, gemcitabine, and topotecan. These agents resulted in similar durations of therapy after ICI treatment to those of the AURELIA study. Interestingly, the median EFS1 for gemcitabine (5.1 months) was higher than what has been reported in similar platinum-resistant populations24 and should be investigated further. Furthermore, 47 of 79 women received bevacizumab at some point after ICI treatment, and as in AURELIA, the addition of bevacizumab prolonged duration on therapy after ICI treatment. There is some evidence that anti-VEGF agents such as bevacizumab may enhance checkpoint inhibition by augmenting T cell infiltration into tumors,25 and their use after ICI appears to be promising in our cohort. Combination regimens using VEGF and checkpoint inhibition are currently being investigated in a number of gynecological cancers.26
Although we did not observe a statistically significant difference in survival with subsequent treatments after ICI therapy when stratified by clinical benefit (long vs. short ICI) on univariate or multivariate analyses, the median OS for the long ICI group was almost a year longer (28 vs. 16.3 months). This comparison is limited in power due to the few women (n=16) with long ICI duration. Of note, the long ICI group had a significantly longer time from diagnosis to ICI treatment compared to the short ICI group (median 54.4 vs. 33.1 months, respectively; p=0.032), with no differences in median lines of therapy (4 for both groups, p=0.396). This argues against the hypothesis that neoepitopes from multiple prior lines of treatment are beneficial for subsequent receipt of immunotherapy. This observation is supported by the KEYNOTE-100 study, which recently reported interim results on 376 women with recurrent ovarian cancer treated with the PD-1 antibody pembrolizumab. Although the overall response rate was 8%, it was not significantly different between those with 1–3 prior treatment lines (RR, 7.4%) and those with 4–6 prior lines (RR, 9.9%).27 This finding is hypothesis generating and may suggest that the disease biology of patients in the long ICI group is favorable and more likely to respond to both chemotherapy and immunotherapy. Understanding the genetic and immunologic parameters underlying this biology is key to the development of predictive biomarkers, and these studies are ongoing.
Stage at diagnosis was not associated with survival after ICI treatment in our study, as this was a highly pretreated and mostly platinum-resistant group. Duration of ICI did not vary by histology (high-grade serous vs. non-high-grade serous), and of the 4 patients with “other histologies” and long duration of ICI, 2 had clear cell carcinoma, 1 had low grade serous carcinoma, and 1 had endometrioid carcinoma. Of these 4 patients, only 1 of the clear cell patients had a deleterious BRCA1/2 mutation. Although histology was also not significantly associated with survival, the non–high-grade serous group had a lower median OS after ICI treatment (18.3 vs. 11.4 months), suggesting potentially different responses to treatments after ICI therapy based on histology, which should be further investigated. Age was not associated with survival after ICI therapy, possibly because our patients were relatively young (median age, 57 years at diagnosis) in this mostly clinical trial population.
Our study limitations include a heterogeneous population with vastly different numbers of prior treatments, several different ICI treatments, and a limited sample size, particularly for analyses comparing long vs. short ICI duration and different treatment regimens after ICI and their effects on survival. Our promising survival may reflect a selected population that was eligible for clinical trials and was healthy enough to receive at least one more line of therapy after ICI treatment. Nevertheless, to our knowledge, this is the largest study to date reporting on post-ICI therapies in ovarian cancer. Our findings suggest that even in the absence of apparent clinical benefit from ICI, these drugs may positively impact the response to subsequent treatments and OS. These results provide a reason for collection of post-ICI therapy data prospectively within the context of ongoing trials and re-enforce the rationale for evaluation of ICIs in combination with chemotherapy in patients with ovarian cancer.
Conclusions
Women with recurrent OC who receive subsequent treatment after ICI therapy represent a heterogeneous group, although most are platinum resistant. Subsequent treatments vary, and many patients are re-challenged with platinum with most receiving bevacizumab. OS after ICI treatment is promising in this heavily pretreated population, suggesting future studies should explore ICI-chemotherapy combinations.
Supplementary Material
Research Highlights.
Median OS from 1st treatment after immunotherapy was 18.3 months in heavily pre-treated ovarian cancer patients
OS after immunotherapy was favorable in all, including those who did not derive clinical benefit from immunotherapy
Many patients were re-challenged with platinum-based regimens after immunotherapy with promising durations of therapy
Bevacizumab was used in 59% of women after immunotherapy and increased duration of therapy
Acknowledgments
Funding: Memorial Sloan Kettering Cancer Center is supported in part by the NIH/NCI Core grant P30 CA008748
Outside the submitted work, Dr. Zamarin reports grants from Merck, personal fees from Merck, personal fees from Synlogic Therapeutics, personal fees from Psioxus Therapeutics, personal fees from Biomed Valley Discoveries, personal fees from Tizona Therapeutics, personal fees from ACM Biolabs, and personal fees from Tesaro; in addition, Dr. Zamarin has a patent for Newcastle Disease Virus and uses thereof, with royalties paid to Merck, and a patent for Chimeric Newcastle Disease Virus and uses thereof, with royalties paid to Merck. Dr. Iasonos reports personal fees from Mylan. Dr. Friedman reports grants from Conquer Cancer Foundation/Merck YIA, personal fees from AstraZeneca, non-financial support from Genentech, research salary support from Bristol Myers Squibb, and personal fees from GLG Consulting. Dr. Konner reports personal fees from Clovis, personal fees from AstraZeneca, and personal fees from Immunogen. Dr. O’Cearbhaill reports personal fees from Clovis and personal fees from Tesaro. Dr. Aghajanian reports personal fees from Tesaro, personal fees from Immunogen, grants and personal fees from Clovis, personal fees from Mateon Therapeutics, personal fees from Cerulean Pharma, grants from Genentech, grants from AbbVie, and grants from Astra Zeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest related to the submitted work.
References
- 1.Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nature reviews Clinical oncology 2013;10(4):211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naumann RW, Coleman RL. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs 2011;71(11):1397–1412. [DOI] [PubMed] [Google Scholar]
- 3.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39(1):1–10. [DOI] [PubMed] [Google Scholar]
- 4.Zamarin D, Jazaeri AA. Leveraging immunotherapy for the treatment of gynecologic cancers in the era of precision medicine. Gynecologic oncology 2016;141(1):86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Disis ML, Patel MR, Pant S, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: Safety and clinical activity. Journal of Clinical Oncology 2016;34(15_suppl):5533–5533. [Google Scholar]
- 6.Hamanishi J, Mandai M, Ikeda T, et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33(34):4015–4022. [DOI] [PubMed] [Google Scholar]
- 7.Hamanishi J, Mandai M, Konishi I. Immune checkpoint inhibition in ovarian cancer. Int Immunol 2016;28(7):339–348. [DOI] [PubMed] [Google Scholar]
- 8.Matulonis U, Shapira-Frommer R, Santin A, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Interim results from the phase 2 KEYNOTE-100 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36(suppl; abstr 5511). [DOI] [PubMed] [Google Scholar]
- 9.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. The New England journal of medicine 2017;377(14):1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheng J, Srivastava S, Sanghavi K, et al. Clinical Pharmacology Considerations for the Development of Immune Checkpoint Inhibitors. Journal of clinical pharmacology 2017;57 Suppl 10:S26–s42. [DOI] [PubMed] [Google Scholar]
- 11.Blanc C, Hans S, Tran T, et al. Targeting Resident Memory T Cells for Cancer Immunotherapy. Frontiers in immunology 2018;9:1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mami-Chouaib F, Blanc C, Corgnac S, et al. Resident memory T cells, critical components in tumor immunology. Journal for immunotherapy of cancer 2018;6(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. The New England journal of medicine 2017;376(11):1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. The New England journal of medicine 2018;378(22):2078–2092. [DOI] [PubMed] [Google Scholar]
- 15.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. New England Journal of Medicine 2018;379(21):2040–2051. [DOI] [PubMed] [Google Scholar]
- 16.Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. New England Journal of Medicine 2018;379(23):2220–2229. [DOI] [PubMed] [Google Scholar]
- 17.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. The New England journal of medicine 2018;379(22):2108–2121. [DOI] [PubMed] [Google Scholar]
- 18.Inayama Y, Hamanishi J, Matsumura N, et al. Antitumor Effect of Nivolumab on Subsequent Chemotherapy for Platinum-Resistant Ovarian Cancer. The oncologist 2018;23(11):1382–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prat J Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014;124(1):1–5. [DOI] [PubMed] [Google Scholar]
- 20.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine 2012;366(26):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukuya T, Mori K, Amann JM, et al. Relationship between Overall Survival and Response or Progression-Free Survival in Advanced Non-Small Cell Lung Cancer Patients Treated with Anti-PD-1/PD-L1 Antibodies. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2016;11(11):1927–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32(13):1302–1308. [DOI] [PubMed] [Google Scholar]
- 23.Liao JB, Gwin WR, Urban R, et al. Pembrolizumab with low dose carboplatin for recurrent platinum resistant ovarian, fallopian tube, and primary peritoneal cancer-interim results 2019;37(15_suppl):5519–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshino K, Hiramatsu K, Enomoto T, et al. Salvage chemotherapy using gemcitabine for taxane/platinum-resistant recurrent ovarian cancer: a single institutional experience. Anticancer research 2012;32(9):4029–4033. [PubMed] [Google Scholar]
- 25.Wallin JJ, Bendell JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun 2016;7:12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YL, Zamarin D. Combination Immune Checkpoint Blockade Strategies to Maximize Immune Response in Gynecological Cancers. Current oncology reports 2018;20(12):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matulonis U, Shapira-Frommer R, Santin A, et al. Antitumor Activity and Safety of Pembrolizumab in Patients with Advanced Recurrent Ovarian Cancer: Interim Results from the Phase 2 KEYNOTE-100 Study SGO; 2019; Honolulu, Hawaii. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


