Abstract
Background:
Many studies have demonstrated associations between surgical resections at academic centers and improved outcomes, particularly for complex operations. However, few have examined this relationship in intrahepatic cholangiocarcinoma (ICC). The hypothesis of this study is that facility type is associated with improved postoperative outcomes and survival in patients with ICC who undergo resection.
Methods:
Patients with stage I-III ICC who underwent hepatectomy were identified using the NCDB (2004-2014). Facilities were categorized as academic or community centers per Commission on Cancer designations. High-volume hospitals were those that performed ≥11 hepatectomies/year. Multilevel logistic mixed-effects models and parametric survival-time models were used to determine predictors of outcomes and overall survival (OS), respectively.
Results:
A total of 2,256 patients were identified, of whom 423 (18.8%) patients were treated at community centers and 1,833 (81.3%) at academic centers. Nearly all high-volume centers were academic facilities (98.5% academic vs. 1.5% community), while low-volume centers were mixed (65.5% academic vs. 34.5% community) (p<0.001). Undergoing surgery at an academic center was an independent predictor of decreased positive margins (OR 0.71, 95% CI 0.51-0.98, p=0.04), lower 90-day mortality (OR 0.62, 95% CI 0.39-0.97, p=0.03), and improved OS (HR 0.78, 95% CI 0.63-0.96, p=0.02). Facility hepatectomy volume was not independently associated with any short-term or long-term outcomes.
Conclusions:
Treatment at an academic center is associated with fewer positive resection margins, decreased 90-day mortality, and improved OS in patients who undergo ICC resection. Facility surgical volume was not significantly associated with any postoperative outcomes after adjusting for patient and disease characteristics.
Keywords: Intrahepatic cholangiocarcinoma, academic center, facility type, volume, survival
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is a rare malignancy, with an estimated 3,000 cases diagnosed each year in the United States (US), although its incidence has been increasing steadily.1 Operative resection offers the only chance for cure, and often requires complex hepatobiliary surgical and multidisciplinary intervention.2 However, even following surgical resection, up to two-thirds of patients develop disease recurrence and 5-year overall survival (OS) rates range from 15 to 40 percent.1,3,4
Many studies have demonstrated associations between treatment at academic, highvolume centers with improved outcomes, particularly for complex operations and rare malignancies.5 This has led to mandated centralization of complex procedures in several European countries,6 and ‘passive’ centralization and calls for restricting complex operations to high-volume ‘centers of excellence’ in the US.6 However, there has been increasing discussion that hospital volume itself may not be the most accurate quality indicator, as it may be a surrogate for other factors that have stronger impact on outcomes.7 Due to the rarity of ICC, few studies have evaluated relationships between facility type, volume, and outcomes following resection of ICC.6,8
We performed a retrospective analysis of patients who underwent hepatectomy for nonmetastatic ICC, using the National Cancer Database (NCDB). The primary objective of the study was to compare postoperative outcomes and OS based on facility type (community vs. academic centers). Secondary objectives included (1) determining if outcomes were more strongly correlated with facility type or facility surgical volume, and (2) identifying predictors of undergoing surgery at an academic center.
METHODS
Study Population
We conducted a retrospective analysis of patients who underwent hepatectomy for clinical stage I-III ICC diagnosed between 2004-2014 in the NCDB. The NCDB is a nationwide, facility-based dataset that captures 70% of all newly diagnosed malignancies in the US. It is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society.9 This study was exempt from Institutional Review Board review due to the de-identified nature of the database.
Variable Definitions
The NCDB includes basic demographic characteristics, including age, sex, race, and Charlson/Deyo Comorbidity Score (CDCC).10 CoC facility types are defined based on cancer program structure, services provided, and number of cancer cases accessioned per year. These include community cancer programs (accessions 100-500 newly diagnosed cancer cases each year; training resident physicians is optional), comprehensive community cancer programs (accessions >500 newly diagnosed cancer cases each year; training resident physicians is optional), academic/research programs (accessions >500 newly diagnosed cancer cases each year; trains resident physicians in at least four program areas, including internal medicine and general surgery), and integrated network cancer programs (multiple facilities providing integrated cancer care, at least one facility is a hospital; training resident physicians is optional; no minimum caseload requirement). We excluded integrated network cancer programs due to the heterogeneous types of facilities with that designation, consistent with prior work.11,12 For our analysis, we compared community centers (community cancer programs and comprehensive community cancer programs) to academic programs, which is also consistent with prior work.13
Clinical stage was designated based on American Joint Commission on Cancer (AJCC) 7th edition. Tumor size was defined as the largest dimension of the diameter of the primary tumor in centimeters. Hepatectomy included any procedure ranging from partial hepatectomy or segmental resection to major lobectomy or extended hepatectomy. Margin status (negative or positive) was obtained from final surgical pathologic analysis. Number of lymph nodes examined and node status (negative, positive, or nodes not examined) were also obtained from surgical pathology. Examining ≥6 nodes was considered necessary for accurate oncologic staging, per AJCC 8th edition guidelines.14 Adjuvant chemotherapy was defined as receipt of chemotherapy after primary site surgery, as part of the first course of treatment. OS was defined as months from diagnosis to death.
Facility Volume Calculations
Facility hepatectomy volume was calculated using de-identified facility identification codes assigned by the NCDB. Facility codes are assigned regardless of cancer site and therefore may be used to identify the same facilities across cancer sites. The NCDB participant user files for the ‘liver’ and ‘intrahepatic bile duct’ cancer sites were used to calculate each facility’s annual hepatectomy volume between 2004-2014. Liver transplantations were excluded from volume calculations. Only facilities that submitted at least one case to the NCDB every year of the study were included, to ensure a consistent population of hospitals and to ensure that hospital volume did not appear falsely low due to lack of membership in the CoC in certain years, per recommendations from the practical guide to the NCDB.15 Patients were only included in the cohort if they underwent hepatectomy for ICC at the reporting facility.
We found that the median number of hepatectomies per year was 11 and therefore defined low-volume facilities as those that performed <11 hepatectomies per year and high-volume facilities as those that performed ≥11 hepatectomies per year.
Statistical Analysis
Variables were summarized as median with interquartile range (IQR) or count with percentage. Categorical variables were compared with the Pearson’s chi-squared test, and continuous variables were compared with the 2-sample t-test. Kaplan-Meier curves were used to analyze OS. Multilevel logistic mixed-effects models were used for adjusted analyses of categorical outcomes, assigning fixed-effects to patient-level predictors and random-effects to individual hospitals to account for intra-class correlation for patients nested within the same facility. Similarly, multilevel mixed-effects parametric survival-time models were used for the survival analyses. Because facility type and facility volume were highly collinear, separate logistic models and parametric survival-time models were run with either facility type or volume, along with potential confounders. Results of the logistic regressions and parametric survival-time models were reported as odds ratios (OR) and hazard ratios (HR), respectively, with corresponding 95% confidence intervals (CI) and p-values.
All statistical analyses were performed using Stata software, version SE 14.0 (StataCorp, College Station, TX, USA). All tests were 2-sided and statistical significance was accepted at the p<0.05 level.
RESULTS
We identified 2,256 patients diagnosed with stage I-III ICC between 2004-2014, who underwent hepatectomy at 308 different facilities, of whom 423 (18.8%) patients underwent resection at a community center and 1,833 (81.3%) underwent resection at an academic center. Half (50.0%) of facilities were academic centers. Nearly all high-volume centers were academic facilities (98.5% academic vs. 1.5% community), while low-volume centers were mixed (65.5% academic vs. 34.5% community) (p<0.001). The median (IQR) number of hepatectomies per year was 1.9 (0.9-3.5) at community centers and 14.1 (6.4-22.8) at academic centers (p<0.001). Median (IQR) follow-up time was 25.9 (12.6-44.3) months.
In terms of type of hepatectomy, of the 804 patients who underwent partial hepatectomy or segmental resection, 20.1% were treated at community centers and 79.9% were treated at academic centers. Of the 742 patients who underwent hepatic lobectomy, 18.1% were treated at community centers and 81.9% were treated at academic centers. Of the 318 patients who underwent extended hepatic lobectomy, 19.5% were treated at community centers and 80.5% were treated at academic centers. Of the 392 patients who underwent hepatectomy not otherwise specified, 16.6% were treated at community centers and 83.4% were treated at academic centers.
Predictors of Treatment at an Academic Center
Univariate analysis demonstrated baseline differences between patients who underwent operations at community or academic centers (Table 1). Patients who were treated at academic centers were more likely to be <65 years old, with fewer comorbidities and private insurance, and tended to travel >40 miles between their home zip code and the hospital (all p<0.001). There were no differences in clinical stage, tumor size, or tumor grade based on facility type (all p>0.05).
Table 1.
Predictors of undergoing intrahepatic cholangiocarcinoma resection at an academic (versus community) facility, based on univariate and multivariable analysis.
| Characteristic | Community Center (n=423) | Academic Center (n=1,833) | Unadjusted P-value | Odds Ratio (95% CI) | Adjusted P-value |
|---|---|---|---|---|---|
| Age ≥65 years old | 253 (59.8%) | 859 (46.9%) | <0.001 | 0.78 (0.56-1.09) | 0.14 |
| Male sex | 180 (42.6%) | 866 (47.2%) | 0.08 | 1.21 (0.94-1.55) | 0.14 |
| Race | 0.12 | ||||
| White | 360 (86.1%) | 1,468 (82.0%) | Reference | ||
| Black | 16 (3.8%) | 121 (6.8%) | 2.34 (1.28-4.29) | 0.01 | |
| Hispanic | 22 (5.3%) | 108 (6.0%) | 1.58 (0.92-2.71) | 0.10 | |
| Asian | 20 (4.8%) | 93 (5.2%) | 1.28 (0.74-2.20) | 0.37 | |
| Charlson/Deyo score | <0.001 | ||||
| CDCC 0 | 247 (58.4%) | 1,269 (69.2%) | Reference | ||
| CDCC 1 | 108 (25.5%) | 404 (22.0%) | 0.74 (0.55-0.99) | 0.04 | |
| CDCC ≥2 | 68 (16.1%) | 160 (8.7%) | 0.45 (0.31-0.65) | <0.001 | |
| Insurance status | <0.001 | ||||
| Private | 147 (35.2%) | 776 (44.0%) | Reference | ||
| Medicaid | 10 (2.4%) | 88 (5.0%) | 1.87 (0.86-4.09) | 0.12 | |
| Medicare | 247 (59.1%) | 835 (47.4%) | 0.81 (0.58-1.15) | 0.24 | |
| None or other government | 14 (3.4%) | 64 (3.6%) | 0.82 (0.41-1.67) | 0.59 | |
| Distance between patient zip code and hospital | <0.001 | ||||
| <10 miles | 166 (39.2%) | 392 (21.4%) | Reference | ||
| 10-40 miles | 154 (36.4%) | 640 (34.9%) | 1.97 (1.47-2.65) | <0.001 | |
| >40 miles | 103 (24.4%) | 801 (43.7%) | 3.77 (2.73-5.21) | <0.001 | |
| Clinical stage | 0.37 | ||||
| Stage 1 | 157 (37.2%) | 609 (33.3%) | Reference | ||
| Stage 2 | 81 (19.2%) | 344 (18.8%) | 0.94 (0.67-1.33) | 0.74 | |
| Stage 3 | 40 (9.5%) | 178 (9.7%) | 1.02 (0.64-1.62) | 0.93 | |
| Unknown | 144 (34.1%) | 700 (38.2%) | 1.34 (1.00-1.81) | 0.051 | |
| Tumor size | 0.13 | ||||
| <3 cm | 67 (17.1%) | 360 (20.9%) | Reference | ||
| 3-5 cm | 129 (32.9%) | 498 (28.9%) | 0.84 (0.59-1.22) | 0.36 | |
| >5 cm | 196 (50.0%) | 866 (50.2%) | 0.86 (0.61-1.21) | 0.38 | |
| Tumor grade | 0.44 | ||||
| Well differentiated | 54 (14.6%) | 198 (12.5%) | Reference | ||
| Moderately differentiated | 208 (56.4%) | 894 (56.2%) | 1.32 (0.91-1.93) | 0.14 | |
| Poorly differentiated or undifferentiated | 107 (29.0%) | 499 (31.4%) | 1.25 (0.83-1.89) | 0.28 | |
CI, confidence interval; CDCC, Charlson/Deyo Comorbidity Score
Multivariable analysis was then performed to identify independent predictors of undergoing treatment at an academic facility (Table 1). Higher comorbidity scores were predictive of receiving care at a community facility (p<0.001), while traveling >40 miles to the treating hospital predicted treatment at an academic center (p<0.001).
Clinical and Oncologic Outcomes, Based on Facility Type and Facility Volume
Univariate analysis demonstrated differences in clinical and oncologic outcomes associated with facility type (Table 2). Patients who underwent resection at academic centers were less likely to have positive surgical margins (21.4% vs. 26.1%, p=0.04), more likely to have ≥6 lymph nodes examined (12.6% vs. 8.0%, p=0.01), and had decreased rates of 30-day mortality (4.0% vs. 7.5%, p=0.01) and 90-day mortality (8.0% vs. 12.8%, p=0.004).
Table 2.
Odds of oncologic and clinical outcomes in patients with intrahepatic cholangiocarcinoma who underwent resection at academic centers (vs community centers) and high-volume centers (vs low-volume centers), based on multivariable analysis*.
| FACILITY TYPE | FACILITY VOLUME | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Community Center | Academic Center | Unadjusted P-value | OR (95% CI) | Adjusted P-value | Low-Volume Center | High-Volume Center | Unadjusted P-value | OR (95% CI) | Adjusted P-value |
| Positive surgical margins | 105 (26.1%) | 370 (21.4%) | 0.04 | 0.71 (0.51-0.98) | 0.04 | 238 (23.0%) | 216 (21.9%) | 0.57 | 0.87 (0.64-1.19) | 0.39 |
| Lymph nodes examined ≥6 | 33 (8.0%) | 230 (12.6%) | 0.01 | 1.28 (0.75-2.16) | 0.37 | 113 (10.6%) | 140 (13.4%) | 0.050 | 1.17 (0.72-1.91) | 0.53 |
| Length of stay ≥7 days | 234 (55.3%) | 1,056 (57.6%) | 0.39 | 1.12 (0.82-1.55) | 0.47 | 607 (55.9%) | 608 (57.6%) | 0.43 | 1.06 (0.76-1.47) | 0.73 |
| Readmission within 30 days | 30 (7.1%) | 154 (8.5%) | 0.36 | 0.82 (0.44-1.52) | 0.53 | 76 (7.1%) | 99 (9.4%) | 0.052 | 1.39 (0.73-2.65) | 0.32 |
| Adjuvant chemotherapy administered | 112 (27.8%) | 530 (30.6%) | 0.28 | 1.26 (0.91-1.74) | 0.17 | 315 (29.9%) | 325 (31.1%) | 0.57 | 1.14 (0.84-1.55) | 0.39 |
| 30-day mortality | 26 (7.5%) | 62 (4.0%) | 0.01 | 0.60 (0.34-1.07) | 0.09 | 52 (5.9%) | 33 (3.6%) | 0.02 | 0.68 (0.40-1.16) | 0.16 |
| 90-day mortality | 44 (12.8%) | 123 (8.0%) | 0.004 | 0.62 (0.39-0.97) | 0.03 | 92 (10.5%) | 70 (7.7%) | 0.04 | 0.68 (0.46-1.01) | 0.06 |
| 1 year overall survival^ | 71.5% | 81.1% | 0.01 | 0.78~ (0.63-0.96) | 0.02^ | 76.8% | 81.7% | 0.32 | 0.95~ (0.79-1.14) | 0.59^ |
Multilevel logistic mixed-effects model adjusted for Commission on Cancer (CoC) facility type or facility volume, age, sex, race, Charlson score, clinical stage, tumor size, tumor grade, insurance type, and distance from patient’s zip code to hospital zip code.
Multilevel mixed-effects parametric survival-time model adjusted for CoC facility type or facility volume, age, sex, race, Charlson score, clinical stage, tumor size, tumor grade, insurance type, distance from patient’s zip code to hospital zip code, margin status, and receipt of adjuvant chemotherapy.
Hazard ratio
OR, odds ratio; CI, confidence interval
On multilevel mixed-effects multivariable analysis (Table 2), undergoing surgery at an academic facility remained significantly associated with fewer positive resection margins (OR 0.71, 95% CI 0.51-0.98, p=0.04) and lower 90-day mortality (OR 0.62, 95% CI 0.39-0.97, p=0.03). On subset analysis of only patients with negative margins, academic facility type remained significantly correlated with decreased 90-day mortality (OR 0.57, 95% CI 0.33-0.997, p=0.049).
In contrast, while univariate analysis revealed an association between undergoing surgery at a high-volume center and increased likelihood of examining ≥6 lymph nodes (p=0.050), decreased 30-day mortality (p=0.02), and decreased 90-day mortality (p=0.04), none of those associations remained significant on multilevel mixed-effects multivariable analysis (Table 2). In fact, on multivariable analysis, hospital hepatectomy volume was not an independent predictor of any postoperative outcome.
Analysis of Overall Survival
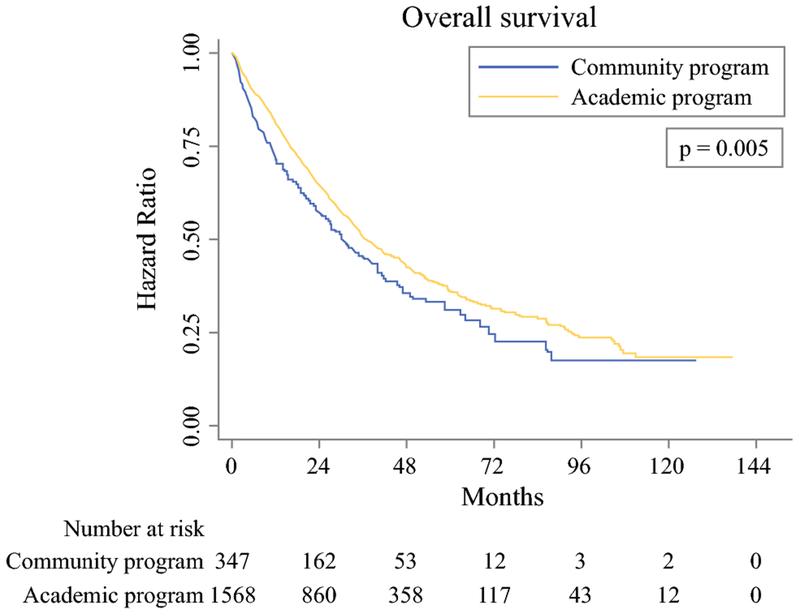
Kaplan-Meier curves demonstrated improved OS in patients who underwent resection at academic facilities compared to patients treated at community centers (p=0.005) (Figure 1). Rates of 1-year OS were 71.5% in patients at community hospitals compared to 81.1% in patients at academic centers (p<0.05), while rates of 5-year OS were 31.2% and 36.3% in patients at community centers and academic hospitals, respectively (p>0.05). When excluding patients who died within 90 days of surgery, 1-year OS remained significantly improved in patients treated at academic centers (88.0% vs 82.0%, p<0.05), while 5-year OS again did not significantly differ based on facility type (39.5% vs 35.7%, p>0.05).
Figure 1.
Kaplan-Meier curves depicting overall survival in patients with resected intrahepatic cholangiocarcinoma, based on facility type.
Multilevel mixed-effects parametric survival-time analysis demonstrated that academic facility type was independently associated with decreased risk of mortality (HR 0.78, 95% CI 0.63-0.96, p=0.02) compared to community facility type (Table 3). Independent predictors of worse OS included age ≥65 years (p=0.002), male sex (p<0.001), advanced clinical stage (p<0.001), tumor size >5 cm (p=0.01), poorly differentiated tumor grade (p<0.001), and positive surgical margins (p<0.001). Receipt of adjuvant chemotherapy was an independent predictor of decreased mortality (p=0.02). On subset analysis of only patients with negative margins, academic facility type continued to be significantly associated with lower mortality (HR 0.71, 95% CI 0.55-0.91, p=0.01). In a separate subset analysis of only patients who survived >90 days after surgery, academic facility type again was associated with statistically improved mortality (HR 0.79, 95% CI 0.63-1.00, p=0.05).
Table 3.
Multilevel mixed-effects parametric survival-time model of predictors of mortality in patients with resected intrahepatic cholangiocarcinoma.
| Variable | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Academic center | 0.78 (0.63-0.96) | 0.02 |
| Age ≥65 years old | 1.38 (1.12-1.70) | 0.002 |
| Male sex | 1.33 (1.15-1.54) | <0.001 |
| Race | ||
| White | Reference | |
| Black | 0.90 (0.64-1.26) | 0.54 |
| Hispanic | 0.89 (0.63-1.24) | 0.49 |
| Asian | 0.81 (0.57-1.14) | 0.22 |
| Insurance status | ||
| Private | Reference | |
| Medicaid | 1.10 (0.74-1.63) | 0.65 |
| Medicare | 0.95 (0.77-1.18) | 0.65 |
| None or other government | 1.00 (0.63-1.57) | 0.99 |
| Distance between patient zip code and hospital (miles) | ||
| <10 miles | Reference | |
| 10-40 miles | 1.09 (0.89-1.33) | 0.43 |
| >40 miles | 1.08 (0.88-1.33) | 0.46 |
| Charlson/Deyo score | ||
| CDCC 0 | Reference | |
| CDCC 1 | 1.11 (0.93-1.32) | 0.23 |
| CDCC ≥2 | 1.25 (0.99-1.58) | 0.06 |
| Clinical stage | ||
| Stage 1 | Reference | |
| Stage 2 | 1.40 (1.13-1.74) | 0.002 |
| Stage 3 | 1.72 (1.33-2.22) | <0.001 |
| Unknown | 1.34 (1.12-1.61) | 0.002 |
| Tumor size | ||
| <3 cm | Reference | |
| 3-5 cm | 0.94 (0.75-1.17) | 0.58 |
| >5 cm | 1.32 (1.07-1.61) | 0.01 |
| Tumor grade | ||
| Well differentiated | Reference | |
| Moderately differentiated | 1.38 (1.08-1.76) | 0.01 |
| Poorly differentiated or undifferentiated | 1.61 (1.24-2.08) | <0.001 |
| Positive surgical margins | 2.03 (1.70-2.41) | <0.001 |
| Received adjuvant chemotherapy | 0.82 (0.69-0.96) | 0.02 |
CI, confidence interval; CDCC, Charlson/Deyo Comorbidity Score
In contrast, hepatectomy volume was not independently associated with OS (p=0.59).
Temporal and Regional Patterns in Utilization of Academic and Community Centers
Over the course of the study period, our data demonstrate a slow but steady decline in the percentage of hepatectomies for ICC being performed at academic centers, from 87.4% in 2004 to 77.7% in 2014. During that same time-period, the percentage of ICC resections at community centers increased from 12.6% in 2004 to 22.3% in 2014.
For the subset of patients treated at community centers, outcomes appeared improved when comparing those diagnosed in 2012-2014 to those diagnosed in 2004-2007, although none reached statistical significance. The rates of positive margins decreased from 34.8% to 22.4% (p=0.15), adequate lymph node harvest increased from 4.3% to 8.7% (p=0.60), 30-day mortality decreased from 12.2% to 7.0% (p=0.40), and 90-day mortality decreased from 16.3% to 12.0% (p=0.73).
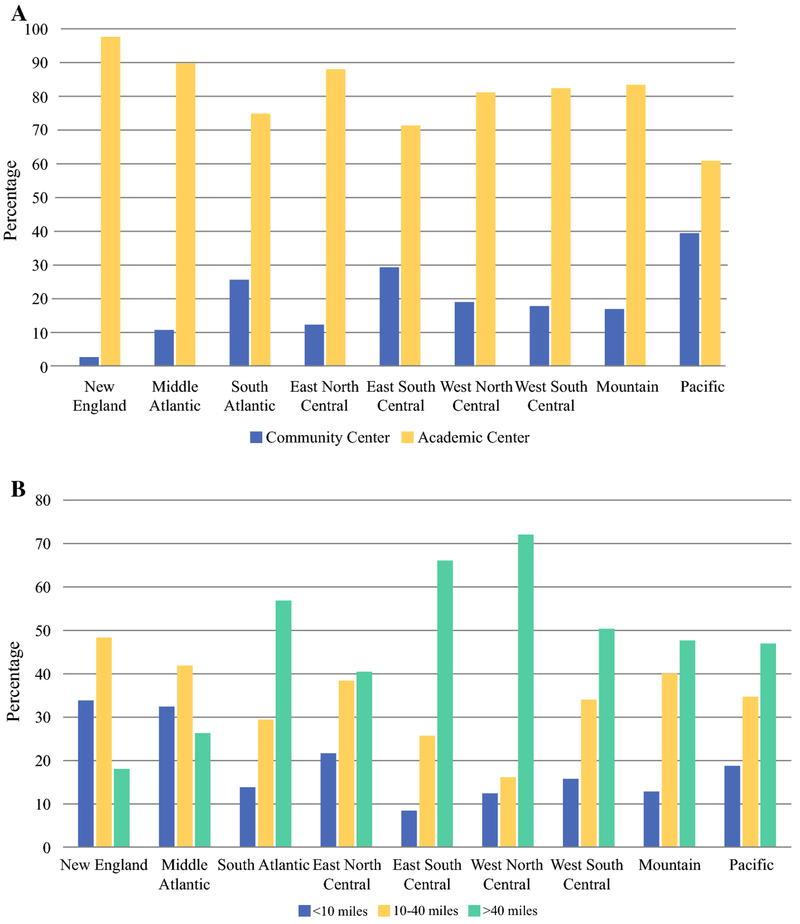
Interestingly, there were vast regional variations in the percentage of ICC resections being performed at community vs. academic centers (Figure 2a). In the New England region, 97.5% of hepatectomies for ICC were performed at academic centers, while in the Pacific region, only 60.7% were performed at academic centers (p<0.001). Looking only at patients treated at academic centers, there were dramatic differences in distances that patients traveled to reach the hospitals, based on US region (Figure 2b). The median (IQR) distance traveled for treatment at an academic center ranged from 16.0 (7.1-32.8) miles in New England to 98.6 (33.0-250.4) miles in the West North Central region (p<0.001).
Figure 2.
United States regional variations in (a) distribution of hepatectomies for intrahepatic cholangiocarcinoma between academic and community centers, and (b) distances patients traveled to academic centers for intrahepatic cholangiocarcinoma resection.
DISCUSSION
In this analysis of 2,256 patients who underwent hepatectomy for ICC at 308 facilities, we found that treatment at an academic center was associated with fewer positive resection margins, lower 90-day mortality, and improved OS compared to treatment at a community center, even after adjusting for multiple patient factors and disease characteristics. Facility type was significantly correlated with facility annual hepatectomy volume, with academic centers performing an average of 14.1 hepatectomies/year and community centers averaging 1.9 hepatectomies/year. However, there were no significant associations between facility volume and postoperative outcomes after adjusting for other factors. Taken together, these data suggest that facility type is a more important predictor of mortality and long-term survival than facility surgical volume.
The reasons why academic facility type may be associated with improved postoperative outcomes are multifactorial. ICC is a rare disease that requires carefully coordinated, multidisciplinary care to optimize survival.2 Favorable margin status may be related to increased surgeon experience and specialization at tertiary care centers, while decreased 90-day mortality may be related to facility infrastructure and the ability to rescue patients from complications (readily-open intensive care units, experienced nursing staff, resident presence 24 hours/day, and availability of consulting services) at academic centers accustomed to high-risk complex surgical procedures.8,11,16–18
Academic facility type was not only associated with improved short-term outcomes, but also OS. An explanation for this finding may be the lower rate of positive resection margins in patients who underwent hepatectomy at academic centers, given the recognized importance of complete tumor excision for long-term survival.19 Academic centers also may have better longitudinal cancer care, with experienced multidisciplinary teams, involvement in clinical trials, and close patient follow up.12 In addition, patients treated at academic centers tend to be younger, with fewer comorbidities and private insurance.11,20 Although we attempted to adjust for these characteristics, it is possible that favorable outcomes associated with academic hospitals remained confounded by their patients’ better overall health and socioeconomic status than patients at community centers.
While 1-year OS was significantly improved in patients treated at academic facilities, there was no significant difference in 5-year OS on unadjusted analysis. This was likely due to lack of power at the 5-year time-point (data was only available for 28 community center patients and 212 academic center patients). Using the more accurate methodology of time-to-event analysis, the data demonstrate a significant improvement in OS for patients treated at academic centers (unadjusted log rank p=0.005; adjusted p=0.02). Other potential reasons for why there was no significant difference in 5-year OS could be that some patients were managed at different types of facilities for their longer-term oncologic care, as the NCDB only captures the ‘first stage’ of treatment. Another reason could be that the benefit of academic facility type was most impactful at the time of diagnosis and operative intervention.
Interestingly, we found a stronger association of outcomes with facility type than facility hepatectomy volume. While numerous studies have demonstrated a clear association between high volume and improved outcomes, there is recent consensus that volume is likely a ‘proxy measure’ for more influential drivers of postoperative outcomes.7,21 Our data would suggest that academic facility type is perhaps one of those influential drivers, as the infrastructure and specialist care provided at academic hospitals are likely to be associated with improved outcomes as well as increased surgical volume. Other studies have demonstrated similar findings; Hyder and colleagues found that even among high-volume hospitals, teaching hospitals were associated with improved outcomes after complex hepatopancreaticobiliary operations compared to non-teaching hospitals.22 It is important to note, however, that this concept is not uniformly validated. Dimick and colleagues demonstrated that the association between facility type and outcomes disappeared after adjustment for surgical volume, thereby concluding that lower mortality rates at teaching hospitals may still be explained by higher procedural volume.23
Finally, it is important to appreciate that there are significant differences in density of academic centers across US regions, which influences patients’ decisions to undergo treatment at academic or community centers and may affect how far patients are willing to travel to receive care at academic institutions. The distance required to travel to academic centers may exacerbate disparities in access to care, as prior work has demonstrated that older patients, racial minorities, and patients with Medicaid insurance are less likely to travel for care than their counterparts.24
Limitations of this study include those inherent to retrospective analyses, such as selection bias and potential unmeasured confounders that we were unable to control for. The NCDB does not include outcomes such as postoperative morbidity or disease recurrence, and variables related to patients’ underlying liver function suffered from significant missing data and therefore were not included in our analysis. Furthermore, although we compared academic centers to community centers, all hospitals that contributed to this dataset are members of the CoC, which may limit generalizability to non-CoC facilities.
CONCLUSIONS
In this analysis of 2,256 patients who underwent ICC resection at 308 facilities, we found that academic facility type was an independent predictor of fewer positive resection margins, lower 90-day mortality, and improved OS. Facility type was likely a superior quality metric compared with facility hepatectomy volume, which was not significantly associated with any postoperative outcomes. While these data may appear to support centralization of complex surgical procedures such as ICC resection at academic, rather than simply high-volume, centers, access to care may be limited by marked variations in regional density of academic centers.
SYNOPSIS.
In patients who undergo intrahepatic cholangiocarcinoma resection, academic facility type is associated with fewer positive resection margins, decreased 90-day mortality, and improved overall survival. Facility surgical volume was not associated with postoperative outcomes after adjusting for patient and disease characteristics.
ACKNOWLEDGMENTS
GCL was supported by the National Institutes of Health T32 Research Training in Alimentary Tract Surgery grant DK007754-13.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Presented at the American College of Surgeons Clinical Congress, Boston, MA, October 21-25, 2018.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Chun YS, Javle M. Systemic and Adjuvant Therapies for Intrahepatic Cholangiocarcinoma. Cancer Control 2017;24:1073274817729241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268–1289. [DOI] [PubMed] [Google Scholar]
- 4.Spolverato G, Vitale A, Cucchetti A, et al. Can hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma? Cancer 2015;121:3998–4006. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 6.Idrees JJ, Merath K, Gani F, et al. Trends in centralization of surgical care and compliance with National Cancer Center Network guidelines for resected cholangiocarcinoma. HPB (Oxford) 2018. [DOI] [PubMed] [Google Scholar]
- 7.Finlayson SR. The volume-outcome debate revisited. Am Surg 2006;72:1038–1042; discussion 1061-1039, 1133-1048. [PubMed] [Google Scholar]
- 8.Csikesz NG, Simons JP, Tseng JF, Shah SA. Surgical specialization and operative mortality in hepato-pancreatico-biliary (HPB) surgery. J Gastrointest Surg 2008;12:1534–1539. [DOI] [PubMed] [Google Scholar]
- 9.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722–1728. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 11.Chu QD, Zhou M, Peddi P, Medeiros KL, Zibari GB, Shokouh-Amiri H, Wu XC. Influence of facility type on survival outcomes after pancreatectomy for pancreatic adenocarcinoma. HPB (Oxford) 2017;19:1046–1057. [DOI] [PubMed] [Google Scholar]
- 12.Chapman BC, Paniccia A, Hosokawa PW, et al. Impact of Facility Type and Surgical Volume on 10-Year Survival in Patients Undergoing Hepatic Resection for Hepatocellular Carcinoma. J Am Coll Surg 2017;224:362–372. [DOI] [PubMed] [Google Scholar]
- 13.Berger NG, Silva JP, Mogal H, et al. Overall survival after resection of retroperitoneal sarcoma at academic cancer centers versus community cancer centers: An analysis of the National Cancer Data Base. Surgery 2018;163:318–323. [DOI] [PubMed] [Google Scholar]
- 14.Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol 2018;7:52. [DOI] [PubMed] [Google Scholar]
- 15.Merkow RP, Rademaker AW, Bilimoria KY. Practical Guide to Surgical Data Sets: National Cancer Database (NCDB). JAMA Surg 2018;153:850–851. [DOI] [PubMed] [Google Scholar]
- 16.Ghaferi AA, Osborne NH, Birkmeyer JD, Dimick JB. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J Am Coll Surg 2010;211:325–330. [DOI] [PubMed] [Google Scholar]
- 17.Raval MV, Wang X, Cohen ME, et al. The influence of resident involvement on surgical outcomes. J Am Coll Surg 2011;212:889–898. [DOI] [PubMed] [Google Scholar]
- 18.Ejaz A, Spolverato G, Kim Y, et al. The impact of resident involvement on surgical outcomes among patients undergoing hepatic and pancreatic resections. Surgery 2015;158:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Squires MH, Cloyd JM, Dillhoff M, Schmidt C, Pawlik TM. Challenges of surgical management of intrahepatic cholangiocarcinoma. Expert Rev Gastroenterol Hepatol 2018;12:671–681. [DOI] [PubMed] [Google Scholar]
- 20.Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, Ko CY. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA 2006;296:1973–1980. [DOI] [PubMed] [Google Scholar]
- 21.Mesman R, Faber MJ, Westert GP, Berden H. Dutch surgeons’ views on the volumeoutcome mechanism in surgery: A qualitative interview study. Int J Qual Health Care 2017;29:797–802. [DOI] [PubMed] [Google Scholar]
- 22.Hyder O, Sachs T, Ejaz A, Spolverato G, Pawlik TM. Impact of hospital teaching status on length of stay and mortality among patients undergoing complex hepatopancreaticobiliary surgery in the USA. J Gastrointest Surg 2013;17:2114–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimick JB, Cowan JA Jr., Colletti LM, Upchurch GR Jr. Hospital teaching status and outcomes of complex surgical procedures in the United States. Arch Surg 2004;139:137–141. [DOI] [PubMed] [Google Scholar]
- 24.Fong ZV, Loehrer AP, Fernandez-Del Castillo C, et al. Potential impact of a volume pledge on spatial access: A population-level analysis of patients undergoing pancreatectomy. Surgery 2017;162:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]