Abstract
The prognostic nutritional index (PNI) has been applied in acute myocardial infarction (AMI) recently.However, the application of PNI in AMI needs verification. This was a prospective cohort study. Patients diagnosed with AMI were enrolled. PNI was calculated as (serum albumin (SA in g/L)) + (5 × total lymphocyte count (TLC) × 109/L). Modified PNI (mPNI) was analyzed by logistic regression analysis to reset the proportion of SA and TLC. The primary outcome was all-cause death. A total of 598 patients were enrolled; 73 patients died during follow-up. The coefficient of SA and TLC in the mPNI formula was approximately 2:1. The area under the receiver operating characteristic curve of SA, TLC, PNI, mPNI and GRACE in predicting death for patients with AMI was 0.718, 0.540, 0.636, 0.721 and 0.825, respectively. Net reclassification improvement (NRI) between PNI and mPNI was 0.230 (p < 0.001). Integrated discrimination improvement (IDI) was 0.042 (p = 0.001). Decision curve analysis revealed that mPNI had better prognostic value for patients with AMI than PNI; however, it was not superior to SA. Thus, PNI may not a reliable prognostic predictor of AMI; after resetting the formula, the value of PNI in predicting prognosis of AMI is almost entirely due to SA.
Subject terms: Prognostic markers, Myocardial infarction, Risk factors
Introduction
Ischemic cardiovascular disease is the most common cause of death, and its frequency is increasing worldwide. The annual incidence of hospital admission for acute myocardial infarction (AMI) varies between 90-312/100,000 per year in Europe1. Through the advent of modern antithrombotic therapy2, secondary prevention, and percutaneous coronary intervention (PCI)3,4, there has been a decrease in acute and long-term mortality due to cardiac causes; however, the one-year mortality due to ST segment elevation myocardial infarction (STEMI) is approximately 10%5. Thus, early risk stratification management has been utilized, and many risk predictive indicators have been applied to predict the short- and long-term prognosis of patients with AMI6,7.
The prognostic nutritional index (PNI) was first proposed by Buzby et al. to assess the prognosis of patients undergo gastrointestinal surgery in 19808. Computer-based stepwise linear regression was conducted using serum albumin (SA), serum transferrin, triceps skinfold, and delayed hypersensitivity to reflect the baseline nutritional status. In 1984, Onodera simplified the linear predictive model using immune-nutritional indicators according to the following equation: PNI = SA (g/L) + (5 × total lymphocyte count [TLC] × 109/L). This study showed that the model could be used safely when PNI was over 459, but some reports showed that the optimal cut-off value of PNI is variable10,11. PNI was used to prognosticate various malignancies12–16, pulmonary embolism17, and other diseases. PNI proved to be an effective indicator for assessing nutritional and immunological conditions of patients with cancer and was shown to influence patient prognosis through the local immune response16.
PNI was mostly used to predict prognosis of cancer patients and has been applied in AMI in some recent studies18,19. Myocardial infarction is closely related to SA levels20,21 and to inflammation processes22. However, low SA levels in myocardial infarction may be due to its role as an antioxidant, anti-inflammatory, and antiplatelet aggregation agent, and not primarily due to its nutritional aspect23–25. The pathophysiological mechanisms of SA and TLC in cancer and AMI may not be the same. SA and TLC may have different roles when predicting prognosis for patients with cancer versus AMI, which means that the Onodera PNI calculation used for patients with cancer may not necessarily be applicable to patients with AMI. PNI may be valuable in evaluating the prognosis of AMI patients, however, the application of PNI in assessing prognosis of AMI patients has not been verified and neither compared with traditional risk score such as Global Registry of Acute Coronary Events (GRACE) in these studies18,19. The aims of this study were 1) to investigate whether Onodera’s PNI calculation is applicable to patients with AMI and 2) to adjust the PNI formula and determine if it improves the prognosticating value of PNI for AMI, and compared with traditional GRACE risk score.
Results
Baseline characteristics
This study enrolled 626 patients with AMI; 28 were excluded based on the exclusion criteria. Thus, 598 patients were analyzed (95.5%). The mean age was 64 ± 13 years, and 456 (76.4%) were male patients. On prospective follow-up, 73 patients died at a median of 14.8 (9.3–17.8) months. Baseline characteristics are shown in Table 1. The mean value of continuous PNI was 47.8 ± 6.0, and 182 patients (30.4%) had a PNI score of 1. Dead patients were older, had a higher rate PNI of 1, had higher heart rates, Killip class, D-Dimer, creatinine, interleukin 6, C-reaction protein, NT-proBNP, troponin and GRACE score, and had lower body mass index, SA levels, and left ventricular ejection fraction (LVEF) than those survived.
Table 1.
Demographic variables and baseline clinical characteristics.
| Variable | Survival(n = 525) | Death(n = 73) | p Value |
|---|---|---|---|
| Demographics | |||
| Age, years | 63.4 ± 13.1 | 73.0 ± 10.3 | <0.001 |
| BMI, kg/m2 | 24.3 ± 5.7 | 22.2 ± 3.8 | 0.002 |
| Males, n (%) | 405(77.1) | 51(69.9) | 0.162 |
| Smoking, n (%) | 290(55.2) | 38(52.1) | 0.670 |
| SBP, mmHg | 125.3 ± 23.5 | 122.9 ± 26.2 | 0.430 |
| DBP, mmHg | 77.8 ± 15.8 | 76.2 ± 18.1 | 0.433 |
| HR, /min | 79.0 ± 17.5 | 90.6 ± 22.4 | <0.001 |
| Killip class ≥2, n(%) | 189(36) | 56(76.7) | <0.001 |
| Laboratory findings | |||
| WBC, ×109/L | 10.1 ± 3.5 | 10.8 ± 4.6 | 0.19 |
| TLC, ×109/L | 1.3(1.0–1.7) | 1.2(0.8–1.8) | 0.247 |
| Platelet, ×1012/L | 174.5 ± 74.0 | 164.2 ± 61.9 | 0.258 |
| D-dimer, mg/L | 0.4(0.2–0.9) | 1.1(0.5–2.8) | <0.001 |
| Creatinine, μmol/L | 77(65–91) | 93(70–132) | <0.001 |
| TG, mmol/L | 1.5(1.0–2.4) | 1.1(0.8–1.6) | <0.001 |
| TC, mmol/L | 4.6 ± 1.1 | 4.2 ± 1.4 | 0.026 |
| HDL, mmol/L | 1.2 ± 0.3 | 1.2 ± 0.4 | 0.257 |
| LDL, mmol/L | 2.8 ± 0.9 | 2.6 ± 1.2 | 0.051 |
| Serum albumin, g/L | 41.0 ± 4.0 | 37.7 ± 5.3 | <0.001 |
| Proteinuria, n(%) | 138(26.3) | 19(26.0) | 0.348 |
| IL-6,pg/ml | 9.3(5.6–21.1) | 17.0(7.9–55.2) | 0.003 |
| CRP, mg/L | 4.6(2.5–12.3) | 8.5(4.2–25.7) | 0.001 |
| NT-proBNP, pg/ml | 593(151–2042) | 2225(532–6425) | <0.001 |
| cTnT, ng/L | 402(68–1468) | 716(141–2543) | 0.031 |
| LVEF, % | 55.5 ± 22.0 | 43.4 ± 12.4 | <0.001 |
| Gensini score | 67.6 ± 44.5 | 71.2 ± 44.4 | 0.609 |
| GRACE score | 155.3 ± 36.6 | 208.1 ± 41.5 | <0.001 |
| PNI = 1, n(%) | 142(27.0) | 40(54.8) | 0.001 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; WBC, white blood cell; TLC, total lymphocyte count; TG, triglyceride; TC, cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; IL-6, interleukin 6; CRP, C-reactive protein; NT-BNP, N-terminal pro-brain natriuretic peptide; cTnT, troponin; LVEF, left ventricular ejection fraction; GRACE, Global Registry of Acute Coronary Events; PNI, prognostic nutritional index.
mPNI formula
mPNI was calculated using logistic regression to reset the coefficient of SA and TLC for all-cause death. The equation used was as follows: Logit (P) = (5.142–0.184 × SA + 0.104 × TLC); the coefficient of SA and TLC in the mPNI formula was approximately 2:1. The conditional probability of mPNI in predicting all-cause death can also be calculated.
mPNI and long-term mortality
The conditional probability of mPNI was divided according to the average into low- and high-risk groups. The high-risk group showed significantly higher mortality than the low-risk group (19.1% vs. 5.4%, p < 0.001). Consistently, cumulative survival was significantly lower in the high-risk group than the low-risk group during follow-up (53.7% vs. 84.6%, p < 0.001) (Fig. 1).
Figure 1.
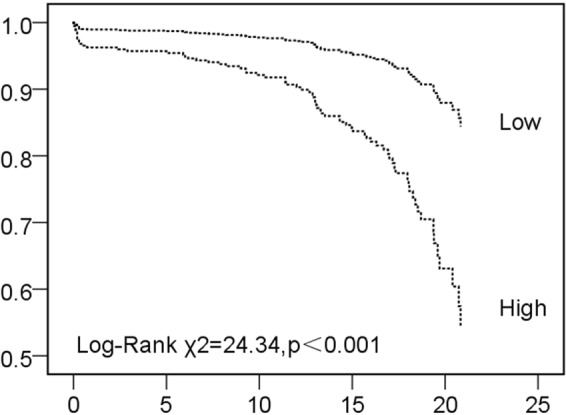
Kaplan-Meier survival analysis of high risk and low risk of mPNI. mPNI, modified prognostic nutritional index.
Univariate logistic regression indicated that PNI, mPNI, BMI, serum albumin, triglyceride, cholesterol, low-density lipoprotein, LVEF and GRACE were associated with all-cause death of AMI patients during follow-up (Table 2). After adjusting for potential confounders in the multivariate logistic regression analysis, mPNI was still independently associated with all-cause death (mPNI high risk vs. low risk, OR = 2.595, 95% CI, 1.084–6.212, p = 0.032), as well as LVEF and GRACE. However, PNI was not an independent factor (PNI 1 vs. 0, OR = 0.535, 95% CI, 0.241–1.188, p = 0.124).
Table 2.
Univariate and multivariate logistic regression analysis of all-cause death.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| BMI | 0.832 | 0.769–0.899 | <0.001 | 0.929 | 0.834–1.034 | 0.177 |
| Serum albumin | 0.854 | 0.812–0.898 | <0.001 | 1.019 | 0.946–1.099 | 0.618 |
| TG | 0.994 | 0.990–0.998 | <0.001 | 0.996 | 0.990–1.001 | 0.142 |
| TC | 0.994 | 0.988–0.999 | 0.037 | 1.013 | 0.985–1.042 | 0.355 |
| LDL | 0.763 | 0.583–0.998 | 0.048 | 0.596 | 0.184–1.927 | 0.387 |
| LVEF | 0.909 | 0.882–0.936 | <0.001 | 0.933 | 0.904–0.964 | <0.001 |
| GRACE | 1.036 | 1.028–1.044 | <0.001 | 1.026 | 1.016–1.036 | <0.001 |
| PNI (1 vs. 0) | 3.208 | 1.941–5.302 | <0.001 | 0.535 | 0.241–1.188 | 0.124 |
| mPNI (high vs. low) | 4.052 | 2.265–7.249 | <0.001 | 2.595 | 1.084–6.212 | 0.032 |
BMI, body mass index; TG, triglyceride; TC, cholesterol; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; GRACE, Global Registry of Acute Coronary Events; PNI, prognostic nutritional index; mPNI, modified prognostic nutritional index.
Calibration and discrimination abilities of PNI and mPNI
After reset the formulation of SA and TLC, goodness of fit test showed mPNI was non-statistical significance using the Hosmer-Lemeshow test (p = 0.950), which means mPNI had a good goodness of fit. Calibration histogram also graphically showed the observed frequency is almost the same as the predicted frequency in each event risk groups (Fig. 2).
Figure 2.
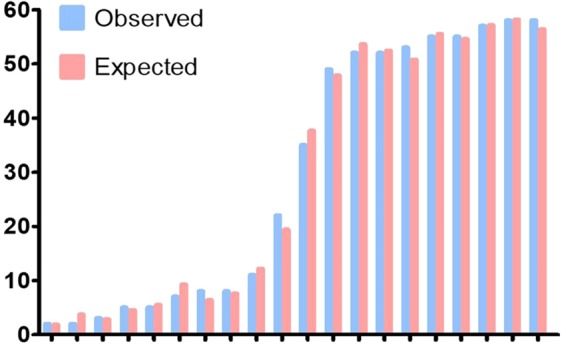
Calibration histogram of mPNI of predicting death based on logistic regression. mPNI, modified prognostic nutritional index.
The AUCs of SA and TLC in predicting death were 0.718 (95% CI, 0.680–0.754, p < 0.001) and 0.540 (95% CI, 0.499–0.581, p = 0.272), respectively. The AUC of mPNI in predicting death was significantly higher than that of PNI (0.721 vs. 0.638, p = 0.004), but less than that of GRACE score (0.825, 95% CI, 0.792–0.855, p < 0.001, Fig. 3), and the difference was significant (p = 0.002).
Figure 3.
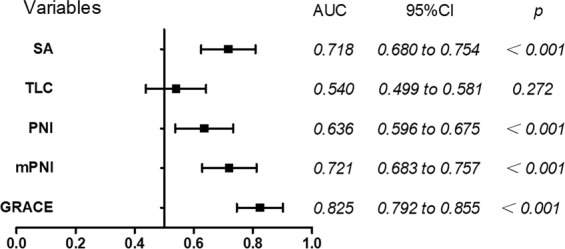
AUC of SA, TLC, PNI, mPNI and GRACE of predicting all-cause death. AUC, area under the receiver-operating characteristic curve; SA, serum albumin; TLC, total lymphocyte count; PNI, prognostic nutritional index; mPNI, modified prognostic nutritional index; GRACE, Global Registry of Acute Coronary Events.
The risk discrimination ability of PNI and mPNI was compared by net reclassification improvement (NRI), the mortality of AMI patients in our study was 12.2%, thus we used 8% and 25% as the arbitrary thresholds to define low, intermediate and high risk. The category NRI of PNI and mPNI was 0.229 (95% CI, 0.093–0.366, p < 0.001), and IDI was 0.042 (95% CI, 0.017–0.068, p = 0.001). Seven patients in the death group were reclassified to the moderate or high-risk group and 70 patients in the survival group were reclassified to the low or moderate risk group. mPNI showed good prognostic performance and significant net reclassification than PNI (Table 3). The NRI between SA and mPNI was no statistical significance, NRI less than 0.001 (95% CI, −0.062–0.063, p = 0.978,) and IDI was 0.009 (95% CI, −0.002–0.020, p = 0.106).
Table 3.
Reclassification across pre-defined risk thresholds.
| Death | mPNI | |||
|---|---|---|---|---|
| PNI | <0.08 | 0.08–0.25 | ≥0.25 | total |
| <0.08 | 5 | 4 | 0 | 9 |
| 0.08–0.25 | 7 | 35 | 11 | 53 |
| ≥0.25 | 0 | 1 | 10 | 11 |
| total | 12 | 40 | 21 | 73 |
| NRI+ = 0.096 | ||||
| Survival | ||||
| <0.08 | 107 | 39 | 0 | 145 |
| 0.08–0.25 | 124 | 221 | 18 | 363 |
| ≥0.25 | 0 | 3 | 13 | 16 |
| total | 231 | 263 | 31 | 525 |
| NRI− = 0.133 | ||||
PNI, prognostic nutritional index; mPNI, modified prognostic nutritional index.
Since the AUCs of SA and mPNI were close to each other, DCA was performed to compare the prognostic value of both. DCA showed that mPNI had better prognostic value for patients with AMI than PNI at any threshold probability; however, it was not superior to SA. TLC did not show any net benefit in the current study (Fig. 4). The DCA graphically showed that mPNI had a higher predictive prognosis value of AMI patients than PNI, but almost same as SA.
Figure 4.
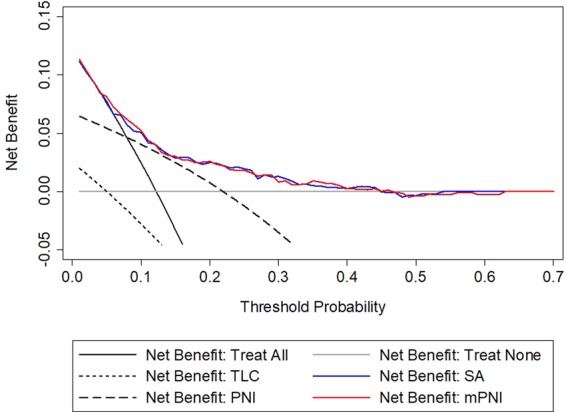
Decision curve analysis for SA, TLC, PNI and mPNI. SA, serum albumin; TLC, total lymphocyte count; PNI, prognostic nutritional index; mPNI, modified prognostic nutritional index.
Discussion
In the present study, we investigated the prognostic value of PNI in patients with AMI and reset the PNI formula to see if this would improve its prognostic value in patients with AMI. Results showed that PNI may not be a reliable prognostic predictor of AMI. After modifying the PNI calculation, mPNI did not show better predictability than SA and was inferior to the traditional GRACE score. The value of PNI in predicting prognosis in patients with AMI comes almost entirely from SA. To the best of our knowledge, the present study is the first to reset the calculation and verify the prognostic value of PNI in patients with AMI.
PNI was proposed to evaluate surgery risk and prognosis of patients with gastrointestinal malignancy9. SA, synthesized in the liver, is the most abundant protein in circulation and is a good indicator reflecting the nutritional status of patients with cancer. Malnutrition is associated with increased morbidity and mortality in patients with cancer26,27. On the other hand, lymphocytes play an important role in eradicating the formation and progression of tumors28, and they can also eliminate cancer cells and inhibit cancer cell proliferation, invasion, and migration29,30. Thus, PNI is a significant prognostic factor in patients with cancer.
In recent studies, PNI was associated with prognosis in patients with STEMI18,19. However, these studies did not separately analyze the prognostic value of SA and TLC, neither did not reset the formula used for AMI, and neither compared with GRACE score. Hypoalbuminemia is an independent predictor of in-hospital and long-term adverse outcomes of AMI. SA is a negative indicator of inflammation, which means that its concentration decreases in the presence of inflammation31. It is also an abundant and important circulating antioxidant using ligand binding and free radical-trapping activities during AMI23,32. Lastly, it is a significant inhibitor of platelet activation and aggregation, which play an important role in the development of thrombosis in AMI33. Thus, the role of SA in AMI may due to its inflammation, antioxidant activity, and antiplatelet aggregation24,34. Lymphocyte count is an index of immunoreaction; a low lymphocyte count may be associated with a pre-existing immunosuppression process, which indicates an inadequate immunological reaction in cardiovascular diseases35. AMI patients with lymphocytopenia are more likely to develop endothelial dysfunction, platelet activation, and thrombogenesis36,37. Since the pathophysiology of cancer and AMI is not the same, we hypothesized that SA and TLC have different roles when predicting prognosis in patients with cancer versus AMI. The coefficient of SA and TLC in the mPNI formula was approximately 2:1 in our study, not 1:5 in Onodera PNI calculation, agreed to our hypothesis.
The present study showed that the AUC of SA in predicting death was higher than that of TLC and PNI, meaning that SA plays a vital role in the pathophysiologic mechanism of AMI. PNI showed weak prognostic value. After adjusting the formula of PNI, we found that mPNI had higher prognostic value than PNI but was not significantly superior to SA; this indicates that TLC may have a limited role in predicting the outcomes of patients with AMI. There was no significant difference in TLC between the survival group and death group in this study. TLC is an immune-inflammatory biomarker, and patients with AMI are more likely to develop lymphocytopenia due to increased inflammatory-related lymphocytes apoptosis. Decreasing lymphocyte count, and the smaller proportion of TLC in mPNI may partly explain that TLC does not play an important role in the current study. Besides, lymphocytopenia acts as a marker for ongoing nonspecific atherosclerotic inflammatory processes. AMI is an acute inflammatory, thrombotic disease and closed related to blood lipid, thus many studies emphasized that the early recognition and management is important to improve prognosis of patients with AMI2,38,39.
GRACE score is a traditional easy and cheap tool used for prognosis of AMI patients, our study indicated that the AUC of mPNI for predicting mortality of AMI patients was inferior to GRACE score. GRACE score has been recognized for its high efficiency and simplicity, but still need eight variables. The mPNI was not originally used for prognostic assessment of AMI, and only included two indicators, thus the results were acceptable.
The present study has several limitations. First, it is a single-center study and included a small sample size. Results in the present study need to be validated further in larger populations. Second, only the calculation of PNI was verified, and other inflammatory biomarkers to rebuild PNI were not analyzed. Third, only the admission PNI and mPNI were evaluated; it is unclear if subsequent changes in the values can provide additional prognostic value.
Our study showed that PNI may not be a reliable prognostic predictor of AMI. After adjusting the formula, mPNI had higher discrimination and calibration abilities than PNI, but was comparable to SA and inferior to GRACE score. The value of PNI in predicting prognosis in patients with AMI comes almost entirely from SA. TLC plays a small role in calculating PNI. This result may prompt us to investigate other biomarkers to rebuild PNI when applied to predict the prognosis of patients with AMI.
Methods
Study design
This was a single-center, prospective cohort study. The study adhered to the tenets of the Declaration of Helsinki; the Human Ethical Committee of West China Hospital of Sichuan University approved the study protocol. We obtained informed consent from all participants involved in the study.
Study population
Patients diagnosed with AMI at the Emergency Department of West China Hospital of Sichuan University between October 2016 and September 2017 were enrolled in the current study. The diagnostic criteria for AMI was based on the fourth definition of myocardial infarction40: a rise and/or fall of troponin values with at least one value above the 99th percentile upper reference limit (URL) together with clinical evidence of acute myocardial ischemia (i.e., ischemic symptoms, electrocardiogram new ischemic changes or development of pathological Q waves, imaging evidence, angiography, or autopsy identification). Exclusion criteria included usage of antithrombotic drugs within 24 hours, lack of laboratory data, and patients with cancer, active or chronic infections diseases, end-stage renal disease, or liver failure.
Data collection
Baseline and clinical data such as vital signs, medical history, past history, and laboratory data were obtained using standard case report forms on admission. An electrocardiogram was promptly obtained upon admission using an electrocardiograph (iMAC1200, Wuhan Zoncare Bio-Medical, Hubei, China). Current smokers were defined as those who had smoked at least 100 cigarettes during their lifetime and were still smoking within the previous 1 month41. Hypertension was diagnosed when the systolic or diastolic blood pressure was ≥140/90 mmHg using a mercury-column sphygmomanometer after 10 minutes of rest or when taking antihypertensive drugs. Diabetes mellitus was defined as a fasting glucose level of ≥7 mmol/L, hemoglobin A1C level of ≥6.5%, random venous blood glucose level of ≥11.1 mmol/L, or use of antidiabetic drugs. Venipuncture was completed at room temperature, and blood samples were filled in standard tubes and centrifuged rapidly. Hematology analytes, including hemoglobin, platelet count (PLT), white blood count (WBC), and TLC were analyzed using the automated hematology analysis system Beckman Coulter LH750 (Beckman Coulter Inc., Brea, CA, USA). SA, alanine aminotransferase (AST), aspartate aminotransferase (ALT), urea nitrogen, and creatinine levels were analyzed using the Architect c16000 analyzer (Abbott Diagnostics).
PNI calculation
PNI was calculated according to the patients’ baseline clinical characteristics using the formula: SA (g/L) + (5 × TLC × 109/L). Scores of ≥45 or <45 were assigned a PNI of 0 or 1, respectively.
Endpoint and follow-up
Prospective clinical follow-up after discharge was accomplished by telephone or questionnaire forms; in-hospital data were reconfirmed using hospital records. Only patients who completed the follow-up were included in this study. The primary outcome was all-cause death.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median with interquartile range. Continuous variables were tested for normality distribution using the Kolmogorov-Smirnov test; normally distributed variables were evaluated using the independent sample t-test, and non-normally distributed variables were tested using the Mann-Whitney U-test. Categorical variables are presented as frequencies and percentages and were analyzed using the chi-square test or Fisher’s exact test. Variables showing p < 0.05 in univariate logistic regression analysis were selected in the multivariate model.
Modified prognostic nutritional index (mPNI) was calculated by logistic regression analysis to reset the coefficient of SA and TLC with all-cause death. The area under the curve (AUC) was analyzed to compare the prognostic value of AMI. A calibration histogram combined with the Hosmer-Lemeshow test was applied to evaluate the calibration of mPNI. Reclassification analysis is widely recommended for assessing the discrimination of risk prediction42, so net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were applied to analyze the degree by which mPNI improved predictive ability as compared to PNI or SA. Vickers et al.43 suggested the use of decision curve analysis (DCA) to evaluate the diagnostic and prognostic models and to compare the prediction value of these models. Two tailed p-values of <0.05 were considered statistically significant. All analyses were performed using SPSS for Windows version 21.0 (SPSS Inc., Chicago, IL, USA) and Stata version 14.0 (StataCorp LP, College Station, TX).
Acknowledgements
This work was supported financially by grants from the Science Foundation of Science and Technology Department of Sichuan (No. 2017SZ0190, 2018RZ0139, 19MZGC0097, 2018JY0577 and 2019YFSY0030), 1•3•5 Project for Disciplines of Excellence-Clinical Research Incubation Project, Sichuan University West China Hospital (No. 2018HXFH001, 2018HXFH027).
Author Contributions
Y.C. and H.L. conceived of the study design, analyzed and interpreted the data, and drafted the manuscript. D.L., L.L., Y.J. and L.Z. contributed to collecting the data and performing the statistical analysis. F.L., X.Z., H.Q. and N.H. contributed substantially to interpreting the data and critically revised the manuscript for important intellectual content. Z.Z., R.Z., Y.C. and Z.W. participated in the design of the study, acquired the data, and helped to revise the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yisong Cheng and Hong Li contributed equally.
Contributor Information
Yu Cao, Email: yuyuer@126.com.
Zhi Wan, Email: 303680215@qq.com.
References
- 1.Widimsky P, et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. European heart journal. 2010;31(8):943–957. doi: 10.1093/eurheartj/ehp492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Covic A, et al. Practical issues in clinical scenarios involving CKD patients requiring antithrombotic therapy in light of the 2017 ESC guideline recommendations. BMC medicine. 2018;16(1):158. doi: 10.1186/s12916-018-1145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puymirat E, et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. Jama. 2012;308(10):998–1006. doi: 10.1001/2012.jama.11348. [DOI] [PubMed] [Google Scholar]
- 4.Gale CP, et al. Trends in hospital treatments, including revascularisation, following acute myocardial infarction, 2003–2010: a multilevel and relative survival analysis for the National Institute for Cardiovascular Outcomes Research (NICOR) Heart (British Cardiac Society) 2014;100(7):582–589. doi: 10.1136/heartjnl-2013-304517. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen F, et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. Journal of the American College of Cardiology. 2014;64(20):2101–2108. doi: 10.1016/j.jacc.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Wlazel RN. (2007)Soluble urokinase plasminogen activator receptor in one-year prediction of major adverse cardiac events in patients after first myocardial infarction treated with primary percutaneous coronary intervention. Archives of medical science, AMS. 2019;15(1):72–77. doi: 10.5114/aoms.2016.63596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burlacu A, et al. Clinical SYNTAX Score - a good predictor for renal artery stenosis in acute myocardial infarction patients: analysis from the REN-ACS trial. Archives of medical science: AMS. 2017;13(4):837–844. doi: 10.5114/aoms.2016.60374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. American journal of surgery. 1980;139(1):160–167. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 9.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai zasshi. 1984;85(9):1001–1005. [PubMed] [Google Scholar]
- 10.Kurumisawa S, Kawahito K. Risk analysis using the prognostic nutritional index in hemodialysis-dependent patients undergoing cardiac surgery. Journal of artificial organs: the official journal of the Japanese Society for Artificial Organs. 2018;21(4):443–449. doi: 10.1007/s10047-018-1056-z. [DOI] [PubMed] [Google Scholar]
- 11.Nozoe T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surgery today. 2012;42(6):532–535. doi: 10.1007/s00595-011-0061-0. [DOI] [PubMed] [Google Scholar]
- 12.Tokunaga R, et al. Prognostic Nutritional Index Predicts Severe Complications, Recurrence, and Poor Prognosis in Patients With Colorectal Cancer Undergoing Primary Tumor Resection. Diseases of the colon and rectum. 2015;58(11):1048–1057. doi: 10.1097/DCR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin WJ, Jr., Torres J. The value of the prognostic nutritional index in the management of patients with advanced carcinoma of the head and neck. Head & neck surgery. 1984;6(5):932–937. doi: 10.1002/hed.2890060507. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, et al. Prognostic value of pretreatment prognostic nutritional index in non-small cell lung cancer. A systematic review and meta-analysis. The International journal of biological markers. 2018;33(4):372–378. doi: 10.1177/1724600818799876. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Jiang S, Yang X, Li X, Wang N. The Significant Value of Preoperative Prognostic Nutritional Index for Survival in Pancreatic Cancers: A Meta-analysis. Pancreas. 2018;47(7):793–799. doi: 10.1097/MPA.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 16.Okadome K, et al. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Annals of surgery (2018). [DOI] [PubMed]
- 17.Hayiroglu MI, et al. A Novel Independent Survival Predictor in Pulmonary Embolism: Prognostic Nutritional Index. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2018;24(4):633–639. doi: 10.1177/1076029617703482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keskin M, et al. A novel and useful predictive indicator of prognosis in ST-segment elevation myocardial infarction, the prognostic nutritional index. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2017;27(5):438–446. doi: 10.1016/j.numecd.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Chen QJ, et al. Prognostic nutritional index predicts clinical outcome in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Scientific reports. 2017;7(1):3285. doi: 10.1038/s41598-017-03364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang LJ, et al. Serum albumin levels might be an adverse predictor of long term mortality in patients with acute myocardial infarction. International journal of cardiology. 2016;223:647–648. doi: 10.1016/j.ijcard.2016.08.251. [DOI] [PubMed] [Google Scholar]
- 21.Zeng R, et al. Hypoalbuminemia predicts clinical outcome in patients with type B acute aortic dissection after endovascular therapy. The American journal of emergency medicine. 2016;34(8):1369–1372. doi: 10.1016/j.ajem.2016.03.067. [DOI] [PubMed] [Google Scholar]
- 22.Jia Y, et al. Inflammation-based Glasgow Prognostic Score in patients with acute ST-segment elevation myocardial infarction: A prospective cohort study. Medicine. 2018;97(50):e13615. doi: 10.1097/MD.0000000000013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazini A, et al. Investigation of ischemia modified albumin, oxidant and antioxidant markers in acute myocardial infarction. Postepy w kardiologii interwencyjnej = Advances in interventional cardiology. 2015;11(4):298–303. doi: 10.5114/pwki.2015.55600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arques S. Human serum albumin in cardiovascular diseases. European journal of internal medicine. 2018;52:8–12. doi: 10.1016/j.ejim.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Dhindsa S, Ghanim H, Dandona P. Nonesterified fatty acids, albumin, and platelet aggregation. Diabetes. 2015;64(3):703–705. doi: 10.2337/db14-1481. [DOI] [PubMed] [Google Scholar]
- 26.Chida M. (2016)Worsened long-term outcomes and postoperative complications in octogenarians with lung cancer following mediastinal lymph-node dissection. Interactive cardiovascular and thoracic surgery. 2009;8(1):89–92. doi: 10.1510/icvts.2008.193383. [DOI] [PubMed] [Google Scholar]
- 27.Paccagnella A, Morassutti I, Rosti G. Nutritional intervention for improving treatment tolerance in cancer patients. Current opinion in oncology. 2011;23(4):322–330. doi: 10.1097/CCO.0b013e3283479c66. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 31.Wiedermann Christian J. Anti-inflammatory activity of albumin. Critical Care Medicine. 2007;35(3):981–982. doi: 10.1097/01.CCM.0000257234.87784.91. [DOI] [PubMed] [Google Scholar]
- 32.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS letters. 2008;582(13):1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 33.Chien SC, Chen CY, Lin CF, Yeh HI. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomarker research. 2017;5:31. doi: 10.1186/s40364-017-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lalor PF, Hepburn EA. Introduction to Lymphocyte Trafficking in Disease. Methods in molecular biology (Clifton, NJ) 2017;1591:169–176. doi: 10.1007/978-1-4939-6931-9_12. [DOI] [PubMed] [Google Scholar]
- 35.Widmer A. (2007)Mechanical complications after myocardial infarction reliably predicted using C-reactive protein levels and lymphocytopenia. Cardiology. 2003;99(1):25–31. doi: 10.1159/000068448. [DOI] [PubMed] [Google Scholar]
- 36.Kurtul A, Acikgoz SK. Usefulness of Mean Platelet Volume-to-Lymphocyte Ratio for Predicting Angiographic No-Reflow and Short-Term Prognosis After Primary Percutaneous Coronary Intervention in Patients With ST-Segment Elevation Myocardial Infarction. The American journal of cardiology. 2017;120(4):534–541. doi: 10.1016/j.amjcard.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Guo TM. (2007)Prognostic Value of Neutrophil to Lymphocyte Ratio for In-hospital Mortality in Elderly Patients with Acute Myocardial Infarction. Current medical science. 2018;38(2):354–359. doi: 10.1007/s11596-018-1887-0. [DOI] [PubMed] [Google Scholar]
- 38.Dyrbus K, et al. Characteristics of lipid profile and effectiveness of management of dyslipidaemia in patients with acute coronary syndromes - Data from the TERCET registry with 19,287 patients. Pharmacological research. 2019;139:460–466. doi: 10.1016/j.phrs.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Burlacu A, et al. The quest for equilibrium: exploring the thin red line between bleeding and ischaemic risks in the management of acute coronary syndromes in chronic kidney disease patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association. European Renal Association. 2017;32(12):1967–1976. doi: 10.1093/ndt/gfx041. [DOI] [PubMed] [Google Scholar]
- 40.Thygesen K, et al. Fourth Universal Definition of Myocardial Infarction (2018) Circulation. 2018;138(20):e618–e651.. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 41.Neuberger JS, Lai SM. Cigarette Consumption and Cigarette Smoking Prevalence Among Adults in Kansas. Preventing chronic disease. 2015;12:E93. doi: 10.5888/pcd12.150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pencina MJ, D’Agostino RB, Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in medicine. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vickers, A. J., Van Calster, B. & Steyerberg, E. W. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ (Clinical research ed), 352:i6. (2016). [DOI] [PMC free article] [PubMed]


