Abstract
Oral treatment with probiotic bacteria has been shown to prevent bone loss in multiple models of osteoporosis. In previous studies we demonstrated that oral administration of Lactobacillus reuteri in healthy male mice increases bone density. The host and bacterial mechanisms of these effects however are not well understood. The objective of this study was to understand the role of lymphocytes in mediating the beneficial effects of L. reuteri on bone health in male mice. We administered L. reuteri in drinking water for 4 weeks to wild type or Rag knockout (lack mature T and B lymphocytes) male mice. While L. reuteri treatment increased bone density in wild type, no significant increases were seen in Rag knockout mice, suggesting that lymphocytes are critical for mediating the beneficial effects of L. reuteri on bone density. To understand the effect of L. reuteri on lymphocytes in the intestinal tissues, we isolated mesenteric lymph node (MLN) from naïve wild type mice. In ex vivo studies using whole mesenteric lymph node (MLN) as well as CD3+ T-cells, we demonstrate that live L. reuteri and its secreted factors have concentration-dependent effects on the expression of cytokines, including anti-inflammatory cytokine IL-10. Fractionation studies identified that the active component of L. reuteri is likely water soluble and small in size (<3 kDa) and its effects on lymphocytes are negatively regulated by a RIP2 inhibitor, suggesting a role for NOD signaling. Finally, we show that T-cells from MLNs treated with L. reuteri supernatants, secrete factors that enhance osterix (transcription factor involved in osteoblast differentiation) expression in MC3T3-E1 osteoblasts. Together, these data suggest that L. reuteri secreted factors regulate T-lymphocytes which play an important role in mediating the beneficial effects of L. reuteri on bone density.
Subject terms: Bacterial host response, Bone
Introduction
Osteoporosis is a growing medical and socioeconomic issue world-wide. Patients with osteoporosis exhibit systemic low bone mineral density and deteriorated bone microarchitecture and therefore are at an increased risk of fracture1,2. Both women (1 in 2) and men (1 in 4) older than 50 years will experience an osteoporotic fracture in their lifetime, with treatment costs in the US alone expected to rise over $25 billion annually by 2025. While numerous therapies are available for reducing bone loss, they are associated with side-effects or high costs3,4. Therefore, some patients choose to either not start the course of treatment or do not see it through to conclusion, increasing their risk of having an osteoporotic fracture and further complications5. For these reasons novel osteoporosis therapeutics that are low-cost and that have fewer side-effects are desired.
In recent years the gut-bone axis has gained significant attention. In this regard, intestinal microbiota dysbiosis is associated with the pathogenesis of diseases including inflammatory bowel disease (IBD), diabetes, obesity and rheumatoid arthritis all of which are associated with bone loss and the development of secondary osteoporosis6–10. In contrast, probiotic bacteria supplementation has been demonstrated to be beneficial to bone health11–13. Our lab has previously shown that administration of Lactobacillus reuteri (L. reuteri) 6475 for 4 weeks to male but not female mice increased femoral trabecular bone density14. The beneficial bone effect of L. reuteri was observed in female mice only under a mild inflammatory state or if they were ovariectomized, suggesting a role for inflammation and/or sex hormones in modulating probiotic activity11,15. Probiotic bacteria have also been tested for their potential therapeutic effects in a number of diseases associated with adverse bone loss16,17. Our lab has demonstrated that L. reuteri 6475 prevents bone loss associated with type 1 diabetes, low estrogen, as well as dysbiosis-induced bone loss in mice11,18,19. Still, the exact mechanism by which L. reuteri 6475 in the intestinal tract exerts a systemic effect to promote bone health remains to be fully elucidated.
The current paradigm for interaction between bacteria and the immune system in the intestine involves the uptake of bacteria by microfold cells (M cells) in the follicle-associated epithelium of Peyer’s patches, transfer of bacteria via channels in goblet cells, or paracellular or transcytotic transport across the epithelium20. These bacteria are then taken up by antigen presenting cells (APCs) in the Peyer’s patches, lamina propria or mesenteric lymph nodes (MLNs) which then activate T-cells20. Given that L. reuteri is administered orally, we wanted to examine whether the immune system, specifically the lymphocytes are involved in the beneficial effects of this probiotic bacteria on bone health. Using Rag knockout mice, deficient in mature T- and B- lymphocytes, we demonstrate that the benefits of L. reuteri on bone health require lymphocytes. In ex vivo studies, we demonstrate that live L. reuteri and its secreted components stimulate T-lymphocytes to further secrete factors that can benefit osteoblasts. Together, our studies provide potential host as well as bacterial mechanisms by which L. reuteri enhances bone density in male mice.
Materials and Methods
Ethical approval
Animal protocols were approved by the Michigan State University Institutional Animal Care and Use Committee and conformed to NIH guidelines.
Animals and experimental design
For all experiments, mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed at Michigan State University animal facility (specific pathogen free). Mice were maintained in a light-dark cycle (12:12-h) at 23 °C (5 animals per cage) for the duration of the studies. Shipped mice were allowed to acclimate for at least one week before experiments were conducted.
For in vivo experiments, male mice (12 weeks of age) wild-type (C57BL/6) and Rag knockout (Rag1tm1M°m, C57BL/6 background) were divided into four cohorts randomly as follows: WT (+/− LR) and KO (+/− LR). Both strains of mice were housed in the same room and on the same rack to ensure adaptation to identical housing environment and to prevent cage effect. L. reuteri treated mice received 3.3 × 108 cfu/ml of L. reuteri in drinking water for four weeks. Water bottles were changed every other day, and fresh L. reuteri added. Control mice received just water. Mice had access of food (Teklad 7914 chow, Madison, WI) and water ad libitum. At the experimental endpoint mice were sacrificed by overdose of isoflurane anesthesia followed by cervical dislocation.
Bacterial culture conditions
L. reuteri ATCC PTA 6475 was cultured as previously reported14,15,19. Breifly, L. reuteri was streaked on deMan, Rogosa, Sharpe media (MRS, Difco) - agar plates and incubated under anaerobic conditions at 37 °C, for a maximum of 1 week. For live bacteria, multiple colonies were selected and anaerobically cultured in 10 ml of MRS broth (16–18 h @ 37 °C). Bacteria were washed with sterile PBS (2 × ) by centrifugation (10 minutes @ 4000 RCF) to remove all traces of MRS broth. Following the final wash, bacteria were re-suspended in 10 ml RPMI (no serum or antibiotics). Bacterial concentration was calculated using the OD600 value. For heat killed bacteria, 1 ml of bacterial suspension was heated to 70 °C for 50 minutes. Bacteria viability was confirmed by culturing anaerobically overnight at 37 °C on MRS plates.
For in vivo experiments L. reuteri was cultured as above with a few modifications to produce a higher concentration of bacteria. After sub-culturing into fresh MRS broth (10 ml) for 16–18 hours, the overnight culture was further incubated in additional fresh MRS broth (800 ml) until log phase (OD600 = 0.4). L. reuteri was pelleted by centrifugation (4000 RCF for 10 minutes) and washed 3x with PBS. The final pellet was re-suspended in sterile PBS (60 ml) and one milliliter (ml) aliquots were made and stored at −80 °C until further use. Colony-forming units per milliliter (cfu/ml) were calculated the day before treatment by plating 10 µl of the aliquots onto MRS agar plates overnight at 37 °C. Mice were administered L. reuteri (3.3 × 108 cfu/ml) reuteri in the drinking water. Drinking water was refilled with fresh water and/or probiotic 3 × /week.
Generation of bacterial conditioned media
Bacterial conditioned media was generated as described before18. Briefly, after culture (as described above), bacteria was resuspended in RPMI and incubated for 3 hours (@ 37 C) anaerobically on a rocker. The culture was then subjected to centrifugation (4000 RCF for 10 min) to pellet the bacteria. The conditioned RPMI media (supernatant) was collected, pH determined and sterile filtered (0.22 µm). Control media was generated by adjusting the pH of RPMI media to that of the bacteria CM using lactic acid (Sigma, St Louis, MO, USA). Control media and CM were aliquoted and stored at −80 °C until further use.
Generation of <3 kDa Fraction of L. reuteri Conditioned Media
L. reuteri conditioned media or RPMI control media was added to an Amicon Ultra-15 3 K centrifugal filter unit (EMD Millipore, Billerica, MA, USA). The filter unit was then centrifuged for 30–60 min (4000 RCF). Flow through containing the <3 kDa fraction was collected, sterile filtered (using 0.22 µm filter), aliquoted and then stored at −80 °C until further use.
Solid-Phase extraction (SPE) fractionation of conditioned media
Fractionation of L. reuteri conditioned media or RPMI control media based on solubility was performed using Oasis PRiME HLB extraction columns (6 cc, 500 mg from Waters, Milford, MA, USA). Briefly, 5.0 mL of CM or RPMI was added to the column and flow through was collected (load). Components retained in the column were collected by washing with 5.0 mL dH20 (wash) followed by elution with 5.0 mL of 90% acetonitrile/10% dH20 v/v (elute). Solvents were removed until dryness from each of the load, wash, and elute fractions by evaporation under vacuum at room temperature using a SpeedVac. Samples were re-dissolved in a comparable volume of RPMI, aliquoted and then stored at −80 °C until further use.
Carboxyfluorescein succinimidyl ester (cfse) staining of bacteria and analysis of translocation to the mesenteric lymph nodes
Live L. reuteri, generated as described, was stained with CellTrace CFSE cell proliferation kit (ThermoFisher Scientific, USA) according to manufacturer’s protocol (ThermoFisher Scientific, USA). Male mice (C57BL/6, 14 weeks old), were gavaged with CFSE-stained L. reuteri (300 µl of 1 × 109 cfu/ml). At various time points after gavage, animals were sacrificed and mesenteric lymph nodes isolated21, homogenized and presence of CFSE+ bacteria analyzed by flow cytometry.
Mesenteric lymph node stimulation
Mesenteric lymph nodes (MLNs) were isolated, homogenized and plated at 1 × 105 cells per well in RPMI media (96-well plate). For CD3+ MLN cultures, CD3+ cells were isolated using magnetic cell sorting (Miltenyi Biotec, San Diego, CA, USA) and plated at 1 × 105 cells per well. Cells were then cultured in the presence or absence of live or heat killed (HK) L. reuteri 6475, at 1, 10 and 100 MOI (multiplicity of infection) for 4 days (37 °C 5% CO2). Cells were then collected and subjected to flow cytometric analysis for the respective cytokines.
Cell isolation from spleen
Spleens were homogenized and re-suspended in RPMI media as described before22. For isolation of naïve CD4+ T cells, magnetic Naïve CD4+ T Cell Isolation Kit (Miltenyi Biotec) was used. Isolated cells were cultured (@1 × 105) under non-polarising conditions, treated with either conditioned media (whole or fractionated) or live or heat killed (HK) L. reuteri 6475 at the indicated MOI for 4 days. Cells were then subjected to flow cytometric analysis. In some experiments cells were also stimulated with CD3/CD28 antibodies (CD3: 10 µg/ml; 145-2C11; CD28 (5 µg/ml; 37.51, BD Biosciences) for 4 days and then subjected to flow cytometry.
Flow cytometry analysis
For analysis, cells were pelleted and supernatant removed. Flow cytometry staining was performed as previously reported15,23,24. Briefly, cells were incubated with Fc block (BD Pharmingen, CA, USA) for 15 min before being stained with anti-mouse CD3-APC AlexaFluor 780 (500A2, eBioscience) and anti-mouse CD4-eF450 (RM 4–5, eBioscience) for 30 minutes at 4 °C. Cells were washed 3X in assay buffer (PBS, 0.5% bovine serum albumin (BSA), 5 mM EDTA) followed by permeabilization using cytofix/cytoperm (BD Pharmingen)23. Intracellular staining for cytokines was performed with anti-mouse IL-10 FITC (JES5-16E3, eBioscience), anti-mouse IL-17A PE (TC11-18H10, BD Bioscience), anti-mouse IFNγ APC (XMG1.2, eBioscience) and anti-mouse LAP (TGFβ) PerCP-eF710 (TW7-16B4, eBioscience). Data were acquired on a BD LSRII (Becton Dickinson, Franklin Lakes, NJ) and analyzed with FlowJo (Version 10; FlowJo, LLC, Ashland OR)15.
In vitro cell culture system
Preosteoblast MC3T3-E1 cells (CRL-2593; ATCC, Manassas, VA) were cultured as described previously25. Briefly, cells were cultured in complete alpha minimal essential media (α-MEM) containing 10% fetal bovine serum (FBS) (Invitrogen and Atlanta Biologicals, Atlanta, GA) and 1% Penicillin-Streptomycin (Life Technologies). Passages between 18 and 24 were used for the experiments. For gene expression analysis, cells were plated in 96-well plates (@ 20,000 cells/well) (Corning Incorporated, Corning NY). Following 24 hours of plating in complete α-MEM, cells were treated as indicated for 6 hours. RNA extraction was completed as described below.
For assessment of intracellular ATP, MC3T3-E1 cells were plated @10,000 cells/well in 96-well plates (white-walled from Corning Incorporated, Corning NY). Twenty four hours after plating, cells were treated as indicated for 6 hours. Intracellular ATP levels were assessed using the ApoSensor Cell Viability Assay kit (BioVision, San Francisco, CA) and Luminescence was measured by Synergy/neo2 multi-mode plate reader and calculated with Gen5 software (Bio-Tek). Each experiment was done in duplicates.
RNA extraction
RNA extraction was performed using TriReagent (Molecular Research Center, Cincinnati, OH) and RNA integrity was verified using agarose-gel electrophoresis15,26,27. Complementary DNA was synthesized by reverse transcription using Superscript II Reverse Transcriptase Kit and oligo dT primers (Invitrogen, Carlsbad, CA)28. Complementary DNA was amplified by PCR using iQ SYBR Green supermix (Bio-Rad Laboratories, Hercules, CA). Real time PCR was carried out for 40 cycles (95 °C for 15 seconds, 60 °C for 30 seconds, and 72° C for 30 seconds) using an iCycler thermal cycler and data evaluated using the iCycler software. Reactions without cDNA were used as negative controls. RNA levels of hypoxanthine guanine phosphoribosyl transferase (HPRT) did not change with treatment and therefore HPRT was used as internal control19. The following primers used for real-time PCR: HPRT (Forward, 5′-AAGCCTAAGATGAGCGCAAG-3′, Reverse, 5′-TTACTAGGCAGATGGCCACA), Osterix (Forward 5′-CTGCGGAAAGGAGGCACAAAGAAG-3′, Reverse, 5′-GGGTTAAGGGGAGCAAAGTCAGAT-3′), Bax (Forward 5′ GACAGGGGGCTTTTTGCTA 3′, Reverse, 5′-TGTCCACGTCAGCAATCATC-3′), Bcl-2 (Forward 5′-GACAGAAGATCATGCCGTCC-3′, Reverse, 5′-GGTACCAATGGCACTTCAAG-3′).
Microcomputed tomography (μCT) bone imaging
Microcomputed tomography was performed as previously described15. After euthanasia, femoral bones were collected and fixed in 10% formalin for 24 hours. Bones were then transferred to 70% ethanol and scanned using a GE Explore Locus microcomputed tomography (μCT) system at a voxel resolution of 20 μm obtained from 720 views28. For each run bone from all groups were included. In addition, a calibration phantom was included to standardize gray scale values and maintain consistency. To separate bone from bone marrow a fixed threshold (841) was used. Femur trabecular bone analyses was performed from 1% of the total length proximal to the growth plate, extending 2 mm toward the diaphysis, and excluding the outer cortical bone. Trabecular bone volume fraction (BVF), bone mineral content (BMC), bone mineral density (BMD), thickness (Tb. Th), spacing (Tb. Sp), and number (Tb. N) were computed using GE Healthcare MicroView software. Femoral trabecular isosurface images were taken from a region in the femur where analyses were performed measuring 1.0 mm in length and 1.0 mm in diameter. Cortical measurements were performed in a 2 × 2 × 2 mm cube centered midway down the length of the bone.
DNA extraction from colonic and fecal samples, 16S rRNA gene amplification, and sequencing
DNA was extracted from colonic and fecal samples as previously described19. Briefly, fecal samples were homogenized in a BioSpec Mini-Beadbeater in the presence of buffer ATL (Qiagen). Following homogenization, Proteinase K (Qiagen) was added and samples were incubated for 30 minutes at 55 °C. Samples were homogenized again for another minute and incubated for additional 30 minutes at 55 C. DNA extraction was performed using Qiagen DNeasy Blood and Tissue kits as described previously11,29. Bacterial 16S sequences spanning variable region V4 were amplified by PCR with primers F515/R806 with a dual indexing approach. Sequencing was done using Illumina MiSeq.30. PCR reactions (20 µl) containing DNA template (40 ng), 1X Phusion High-Fidelity Buffer (New England Biolabs), dNTPs (200 μM from Promega or Invitrogen), primers (10 nM), Phusion DNA Polymerase (0.2 units from New England Biolabs), and PCR grade water were performed in an Eppendorf Prothermal cycler. The initial denaturation was performed at 98 °C for 30 s, followed by 30 cycles of 10 s at 98 °C, 20 s at 51 °C, and 1 min at 72 °C. Replicates samples were pooled and purified with Agencourt AMPure XP magnetic beads (Beckman Coulter). Samples were quantified using the QuantIt High Sensitivity DNA assay kit (Invitrogen) and pooled at equimolar ratios. The quality of the DNA samples was evaluated with the Bioanalyzer High Sensitivity DNA Kit (Agilent).
Microbial community analysis
Sequence data were processed using the MiSeq pipeline for mothur using software version 1.38.131 as described previously19,29. Sequences were clustered into operational taxonomic units (OTUs) with 97% similarity using the average-neighbor algorithm in mothur. A total of 871 OTUs were identified across all samples with an average rarefaction depth of 54,791 reads per sample. Diversity analyses (alpha and beta) and visualization of microbiome communities were performed with R, utilizing the phyloseq package32,33. The Bray-Curtis dissimilarity matrix was used to describe differences in microbial community structure.
Statistical analysis
All measurements are presented as the mean ± SEM or as box plots (whiskers indicate minimum to maximum values). Significance was tested using either Student’s t test (2 groups) or ANOVA (more than 2 groups) with Bonferroni’s multiple comparison test for the post-hoc test. Statistical analysis was performed using GraphPad Prism software version 7 (GraphPad, San Diego, CA, USA). Significant outliers (if present and indicated in figure legend) were removed using the ROUT test for outliers. A p-value of < 0.05 was considered significant.
Results
L. reuteri requires lymphocytes to Exert Beneficial Effects on Bone
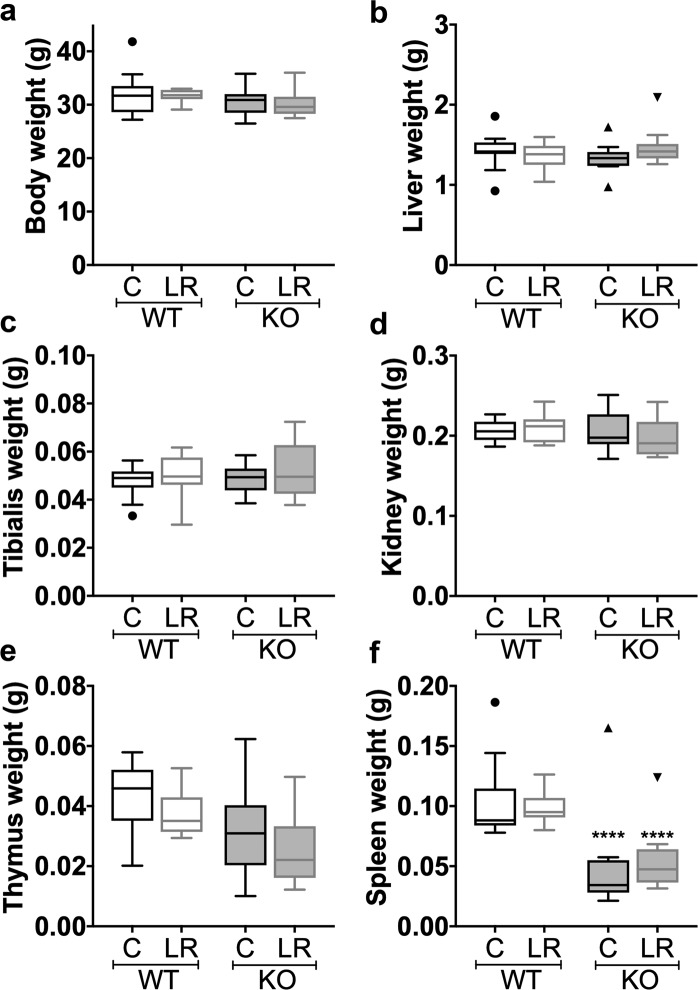
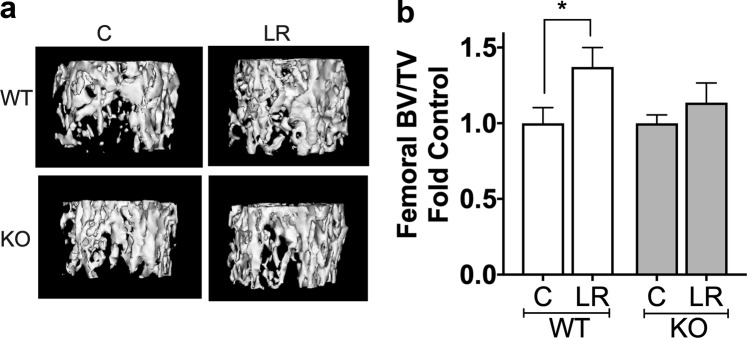
Previous studies have revealed that supplementation with the probiotic L. reuteri ATCC 6475 can have a beneficial effect on bone health11,14,15,19,34 though the exact mechanisms are currently unknown. We and others have shown that L. reuteri has immuno-regulatory properties in vitro and in vivo, suggesting a role for the immune system in modulating the effects of L. reuteri on bone density11,14,35. Although previous studies have shown that L. reuteri can modulate the immune system, the specific role of different immune cells in mediating effects on bone are not well known. To address this, we utilized male mice that are deficient in mature T and B lymphocytes (Rag1 knockout; KO) and wild type (WT) controls. WT and KO mice were administered L. reuteri in their drinking water for 4 weeks. Control mice received water without the probiotic. After 4 weeks, L. reuteri administration had no significant effect on body weight or weights of organs including liver, muscle (tibialis), kidney, thymus and spleen (Fig. 1). As expected, a significant decrease in thymus and spleen weights was observed in the KO group compared to the WT mice (Fig. 1e,f). As we reported earlier14, oral L. reuteri treatment significantly increased trabecular femoral bone density by 37% in the WT mice (Fig. 2, p < 0.05). In contrast, L. reuteri treatment did not enhance trabecular femoral bone density in the KO mice (Fig. 2), suggesting that L. reuteri requires lymphocytes to increase trabecular bone density. Consistent with our findings, bone mineral density (BMD, p < 0.05) and trabecular thickness increased (Tb. Th, p < 0.05), while trabecular spacing decreased (Tb. Sp, p < 0.05) in the L. reuteri treated WT mice (Table 1). Analysis of the cortical regions of the femur (diaphyseal region) showed that marrow area was decreased and outer perimeter increased by L. reuteri in the WT mice but not in the KO. Other parameters did not demonstrate any significant differences between the groups (Table 1).
Figure 1.
General body parameters. 12 weeks old C57BL/6 and Rag KO male mice were supplemented with water (n = 11) or L. reuteri (n = 10) for 4 weeks. General body parameters were measured the day of harvest. (a) body, (b) liver, (c) tibialis, (d) kidney, (e) thymus, and (f) spleen weight in grams (g). L. reuteri treatment had no effect on general body parameters. Data presented as box plots with Whiskers using Tukey method. Statistical analysis performed by One-way ANOVA with Bonferroni’s multiple comparison test. ****p < 0.0001.
Figure 2.
L. reuteri requires lymphocytes to exert beneficial effect on Bone. Twelve week old C57BL/6 and Rag KO male mice were supplemented with water (n = 11) or L. reuteri (n = 10) for 4 weeks. Femoral bone was collected, and trabecular and cortical bone analyzed by µCT. (a) representative micro-computed tomography isosurface images by uCT. (b) bone volume fraction (BV/TV) quantitative data obtained from the distal femur trabecular bone of control and L. reuteri treated mice. Statistical analysis performed one-way ANOVA with Bonferroni’s multiple comparison test. *p < 0.05.
Table 1.
Femoral Bone Parameters.
| Parameter: | Wild Type | Rag knockout | ||
|---|---|---|---|---|
| C (n = 11) | LR (n = 10) | C (n = 11) | LR (n = 10) | |
| Femur trabecular | ||||
| BV/TV | 32.28 ± 3.33 | 44.45 ± 4.16# | 40.53 ± 2.23 | 46.05 ± 5.25 |
| BMD (mg/mL) | 243.5 ± 8.13 | 282.5 ± 12.41# | 265.2 ± 6.27 | 290.3 ± 18.44 |
| BMC (mg) | 0.46 ± 0.02 | 0.52 ± 0.01 | 0.51 ± 0.01 | 0.52 ± 0.02 |
| Tb. Th. (mm) | 0.04 ± 0.002 | 0.06 ± 0.004# | 0.05 ± 0.002 | 0.07 ± 0.006* |
| Tb. N.(1/mm) | 6.94 ± 0.46 | 7.24 ± 0.24 | 7.23 ± 0.23 | 6.47 ± 0.35 |
| Tb. Sp. (mm) | 0.10 ± 0.01 | 0.08 ± 0.008 | 0.08 ± 0.006 | 0.07 ± 0.007 |
| Femur Cortical | ||||
| Cortical area (mm^2) | 0.96 ± 0.05 | 0.93 ± 0.04 | 0.94 ± 0.04 | 0.93 ± 0.04 |
| Marrow area (mm^2) | 0.81 ± 0.04 | 0.68 ± 0.03# | 0.67 ± 0.02 | 0.66 ± 0.04 |
| Mean thickness (mm) Inner | 0.24 ± 0.008 | 0.25 ± 0.006 | 0.25 ± 0.007 | 0.25 ± 0.008 |
| Inner perimeter (mm) | 3.76 ± 0.07 | 3.73 ± 0.04 | 3.62 ± 0.09 | 3.77 ± 0.13 |
| Outer perimeter (mm) | 6.77 ± 0.43 | 7.96 ± 0.26# | 7.24 ± 0.42 | 8.08 ± 0.44 |
| BMD (mg/mL) | 784.3 ± 13.43 | 779.7 ± 7.17 | 784.1 ± 7.14 | 800 ± 11.65 |
| BMC (mg) | 0.01 ± 0.001 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
Effect of L. reuteri on α and β diversity in gut microbiota in male mice
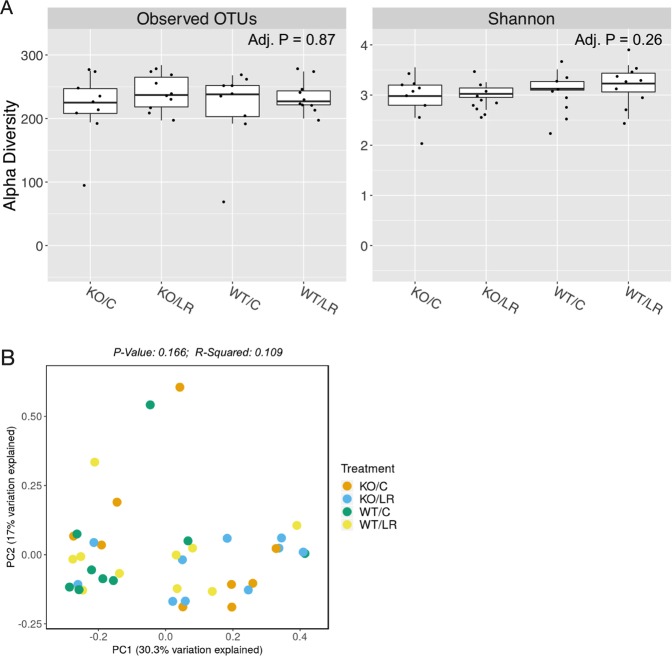
To assess if probiotic administration significantly modulated intestinal microbiota diversity in male mice and whether a difference in microbiota diversity could account for the lack of effect of L. reuteri in the Rag KO mice, we extracted DNA from colonic microbiota and performed 16S rRNA sequencing. Diversity metrics that utilize species richness and evenness (Bray-Curtis) showed no significant separation between the groups (WT±LR and KO±LR) (Fig. 3). Also, no significant differences were found in α diversity both in terms of OTUs and Shannon index (Fig. 3). These data suggest that L. reuteri treatment in either WT or Rag KO mice does not cause broad changes in bacterial communities in male mice. This result does not rule out changes in microbiota function or changes in specific bacterial species that may be impacted by L. reuteri supplementation. Given the absence of mature lymphocytes in Rag KO mice, we reasoned that the lymphocytes likely play an important and direct role in L. reuteri effects on bone.
Figure 3.
L. reuteri effects on gut microbiota. Twelve week old C57BL/6 and Rag KO male mice were supplemented with water or L. reuteri for 4 weeks as indicated in Fig. 2. Fecal samples were collected at the day of harvest and analyzed by 16S rRNA sequencing as described in the methods. (A) Plots of alpha-diversity metrics of observed operational taxonomic units (OTUs) (richness) and Shannon diversity (richness and evenness) of the different treatment groups. (B) Principle coordinate analysis of beta-diversity based on Bray-Curtis (richness and evenness) of the different treatment groups. WT/C = wild-type control, WT/LR = wild-type L. reuteri treated, KO/C = Rag1 KO control, KO/LR = Rag1 KO L. reuteri treated. N = 9–10/group.
Effect of live L. reuteri on cytokine expression in Whole MLN Ex Vivo Cultures
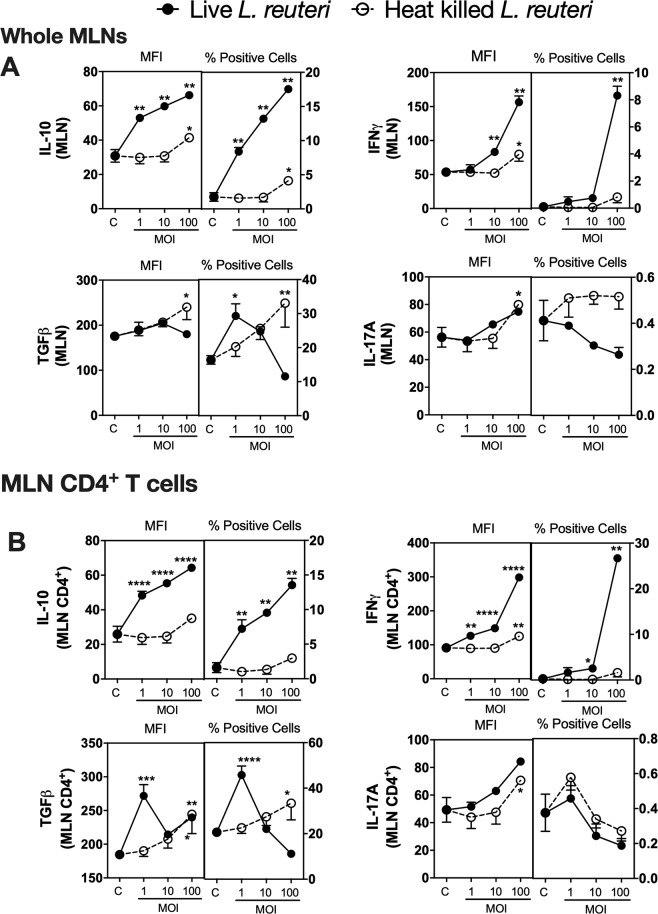
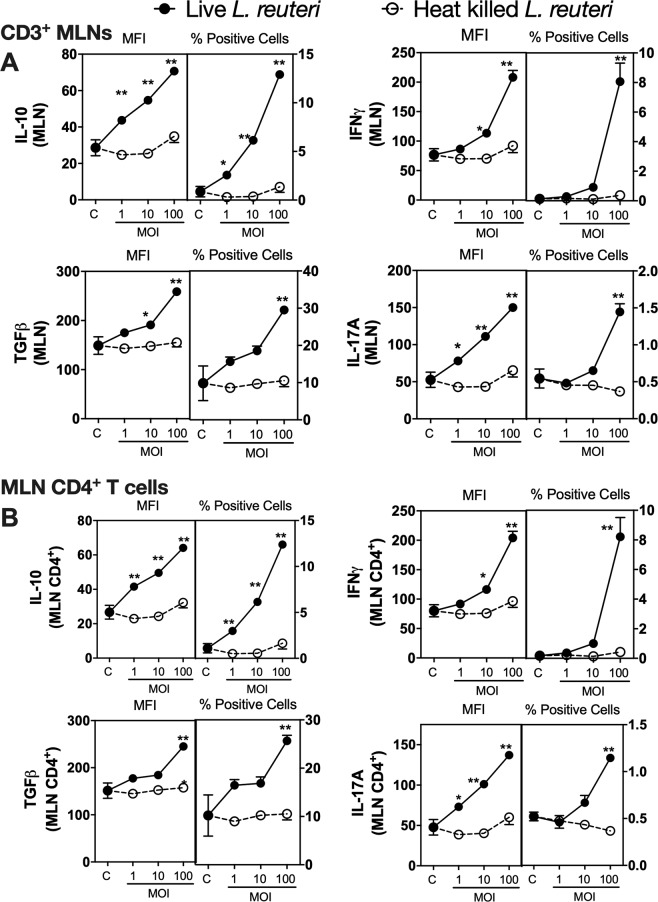
Because our in vivo studies were performed with L. reuteri administered orally, we reasoned that lymphocytes in MLNs would be one of the sites of L. reuteri or its products could interact with lymphocytes. Consistent with our reasoning other studies have shown that probiotics including L. reuteri can translocate to the MLNs following oral administration36. Using a CFSE (5(6)-Carboxyfluorescein N-hydroxysuccinimidyl ester) labelled L. reuteri and flow cytometry analysis, we confirmed that following 6 hours after oral gavage, L. reuteri can be detected in the MLNs (data not shown). To understand the effect of L. reuteri and its secreted products on lymphocytes in MLNs, we treated MLNs ex vivo with L. reuteri for 4 days and assessed expression of cytokines that have been shown to regulate bone health. We focused on IL-10 and TGFβ (can enhance bone health)37–41 as well as IFNγ (negatively influences bone health) and IL-17A (has both negative and positive effects on bone)42–44. These cytokines were assessed using flow cytometry. Treatment of MLN cultures with live L. reuteri significantly increased expression (as indicated by median fluorescent intensity; MFI) of IL-10 and IFNγ in a concentration-dependent manner (p < 0.01) (Fig. 4). In addition, the number of IL-10 + and IFNγ + cells also increased significantly (p < 0.01). In contrast, TGFβ expression showed minimal change with live L. reuteri. However, TGFβ + cell numbers were differentially modulated at different MOI, with an MOI of 1 causing an increase (p < 0.05), and higher MOIs showing no difference. While expression of IL-17A increased only at the highest MOI, IL-17A+ cell numbers were not affected by live L. reuteri (Fig. 4A). Gating within the CD4+ T cell population also revealed similar expression and + cell profiles, except for TGFβ which showed a biphasic response with both MFI for TGFβ as well as TGFβ + cell numbers (Fig. 4B). Compared to live L. reuteri, effect of heat killed L. reuteri was not consistent and in some cases showed a modest response only at high concentrations (p < 0.05) (Fig. 4B). Together, these results demonstrate that live but not heat killed L. reuteri can significantly induce concentration-dependent effects on the MLN immune cell cytokine profiles and the majority of these effects are similar between the whole MLN and the MLN CD4+ T cell population.
Figure 4.
Effect of live and heat killed L. reuteri 6475 on cytokine expression in whole MLN cultures. Mesenteric lymph nodes were isolated from male C57BL/6 mice (12–18 weeks), homogenized and cultured with live or heat killed L. reuteri 6475 at an MOI of 1, 10 or 100 for 4 days. Cells were analyzed using flow cytometry for expression of IL-10, INFγ, IL-17 A and TGFβ. Results for MFI and % positive cells were analysed as (A) the whole MLN or (B) gated on the MLN CD4+ T cells. Statistical analysis performed by 2-way ANOVA with Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to control. n = 5.
Mesenteric lymph nodes contain a multitude of different cells types including antigen presenting cells (APCs). To identify whether the effects of L. reuteri on the CD4+ T cells was indirect via APCs or direct on the T cells, CD3+ cells were isolated from whole MLNs and cultured with either live or heat killed L. reuteri. As with the whole MLN cultures, addition of live L. reuteri to CD3+ MLN cells had a profound effect on cytokine expression (Fig. 5A). Live L. reuteri significantly increased expression of IL-10, IL-17A, TGFβ and IFNγ in a concentration-dependent manner as determined by flow cytometry. Similarly, number of CD3+ cells expressing these respective cytokines was also significantly increased by live L. reuteri treatment. Compared to this, heat killed L. reuteri had no significant effect on expression of any of the cytokines examined. When gated within the CD4+ T-cells, the results were similar to that of CD3+ T cells (Fig. 5A).
Figure 5.
Effect of L. reuteri 6475 on cytokine expression in CD3+ lymphocytes isolated from MLNs. Mesenteric lymph nodes were isolated from male C57BL/6 mice (12–18 weeks), homogenized and CD3+ cells obtained by magnetic separation. CD3+ cells were cultured with live or heat killed L. reuteri 6475 at an MOI of 1, 10 or 100 for 4 days. Cells were analyzed using flow cytometry for expression of IL-10, INFγ, IL-17 A and TGFβ. Results for MFI and % positive cells were analysed as (A) CD3 + cells or (B) gated on the MLN CD4+ T cells. Statistical analysis performed by 2-way ANOVA with Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01 compared to relevant control. n = 5.
Having revealed that L. reuteri directly modulates MLN CD4+ T cell cytokine expression, we next sought to determine the effects of live L. reuteri on naïve CD4+ T cells. CD4+ T cells were isolated from spleens by magnetic isolation and treated with either live or heat killed L. reuteri under non-polarising culture conditions. Comparable to the responses of isolated CD3+ MLN cultures, CD4+ splenic T cells exhibited a concentration-dependent response to live L. reuteri for both IL-10 and IL-17A expression (p < 0.01) (Fig. 6). IFNγ expression increased significantly only at an MOI of 100 (p < 0.01) while no effect was observed for TGFβ. Heat killed L. reuteri increased IL-10 (p < 0.05) and IL-17A (p < 0.01) expression modestly, only at an MOI of 100 (Fig. 6). Together, these data suggest that live L. reuteri is able to stimulate T-cells directly and can modulate cytokine expression in cells that have potential modulatory effects on bone health.
Figure 6.
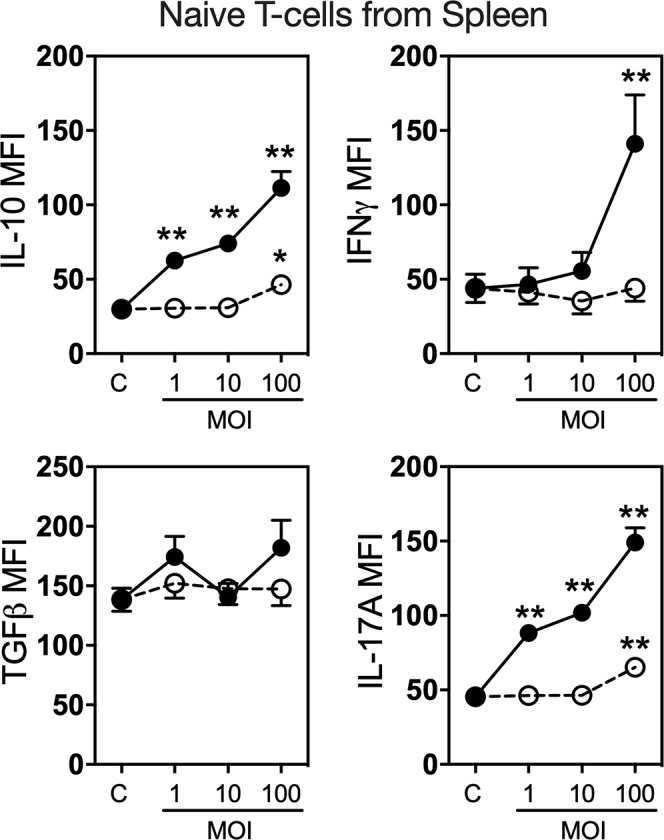
Effect of L. reuteri on naïve splenic CD4+ T Cells cytokine expression. Spleens were isolated from male C57BL/6 mice (12–18 weeks) and naïve CD4+ T cells obtained by magnetic separation. Cells were cultured under non-polarising conditions with (a) live or heat killed L. reuteri 6475 at an MOI of 1, 10 or 100 for 4 days and cytokine expression (IL-10, INFγ, IL17A and TGFβ) analyzed by flow cytometry. Statistical analysis performed by 2-way ANOVA with Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01 compared to control. n = 10.
Effect of L. reuteri secreted factors on T-lymphocyte cytokine expression
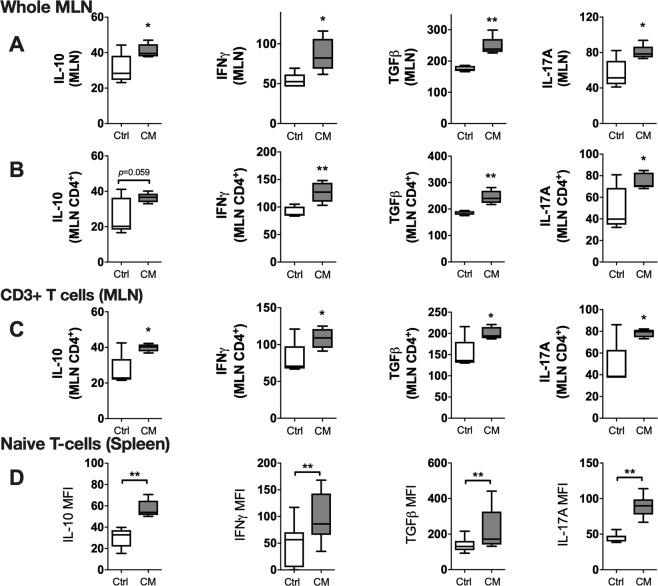
To identify whether the effects of live L. reuteri required direct cell-cell contact or whether the effects are induced by secreted factor(s), whole MLN cultures were treated ex vivo with L. reuteri conditioned media (CM). L. reuteri CM significantly elevated expression of all the cytokines assessed (IL-10, IFNγ, TGFβ and IL-17A) (Fig. 7A). When gated within the CD4+ T-cells, expression of cytokines showed similar pattern (Fig. 7B). Percentage of cytokine positive cells mostly followed a similar pattern to that of the MFI (data not shown). To determine whether L. reuteri secreted factor(s) were responsible for the observed direct effect of L. reuteri on T-lymphocytes, we further treated isolated CD3+ T-cells from MLNs with L. reuteri CM (Fig. 7C). Similar to the effects on whole MLN cultures, L. reuteri CM treatment of isolated T-cells significantly increased CD4+ T cell expression of all four cytokines (Fig. 7C) as well as increased numbers of CD4+ cytokine + cells (data not shown). Similar to MLN cultures, treatment of CD4+ T cells (spleen) with L. reuteri CM also significantly increased the expression of all cytokines assessed (Fig. 7D). Together, these results suggest that secreted component(s) of L. reuteri are able to directly stimulate T-cells and significantly modulate expression of cytokines.
Figure 7.
Effect of L. reuteri conditioned media on cytokine expression in T-cells. Mesenteric lymph nodes and spleens were isolated from male C57BL/6 mice (12–18 weeks), homogenized and cultured with L. reuteri 6475 CM or control media for 4 days. Expression of cytokines (IL-10, IFNγ, TGFβ and IL-17A) was assessed using Flow cytometry. (A) whole MLN cultures, (B) CD3+ T cells, and (C) naïve CD4+ T cells. Statistical analysis performed by unpaired t-test. *p < 0.05, **p < 0.01 compared to control. Whiskers in the box plots represent minimum to maximum values. n = 5.
RIP2 negatively regulates expression of IL-10 and IL-17A in lymphocytes
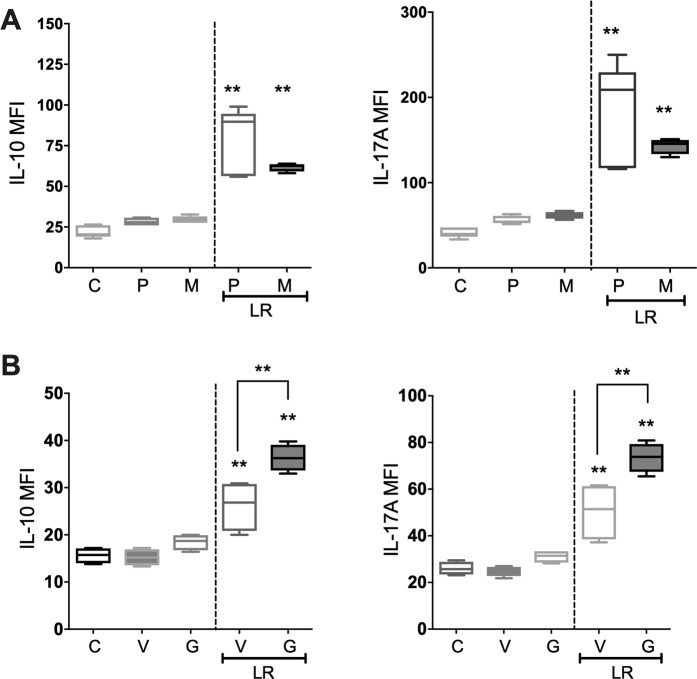
Probiotic bacteria and Lactobacillus bacteria in particular have previously been shown to induce their beneficial effects through toll-like receptor (TLR) signaling, specifically TLR245,46. Furthermore, bacteria are able to induce a signaling cascade through activation of the Nucleotide-binding Oligomerization Domain (NOD) pathway47. To determine whether L. reuteri secreted factors induce their modulatory effects through TLR or NOD signaling we inhibited MyD88 or RIP2 tyrosine kinase in splenic CD4+ T cells prior to L. reuteri stimulation. To determine if L. reuteri was acting through a TLR in a MyD88-dependent manner, we used a MyD88 peptide inhibitor (Fig. 8A). MyD88 inhibitor failed to block the effects of L. reuteri on IL-10 and IL-17A. To further investigate whether L. reuteri signaled via the NOD pathway we utilized Gefitinib, a RIP2 tyrosine kinase inhibitor which inhibits RIP2 tyrosine phosphorylation and NOD2-induced NF-κB activation and cytokine release48. L. reuteri CM significantly increased expression of IL-10, and IL-17A as expected; however, pretreatment of the cells with Gefitinib significantly augmented the expression of IL-10 (p < 0.01), and IL-17A (p < 0.01) suggesting that RIP2 negatively regulates expression of these cytokines in T-cells induced by L. reuteri CM (Fig. 8B).
Figure 8.
Effect of L. reuteri on CD4+ T Cells is negatively regulated by NOD pathway. Spleens were isolated from male C57BL/6 mice (12–18 weeks) and naïve CD4+ T cells obtained by magnetic separation. CD4+ T cells were pre-treated with either (A) MyD88 inhibitor (100 µM) or (B) gefitinib (20 µM) for 24 hours before culture with L. reuteri CM for 4 days and cytokine expression (IL-10 and IL-17A) analyzed by flow cytometry. M = MyD88 inhibitor; P = control peptide; V = DMSO vehicle control; G = Gefitinib; LR = L. reuteri conditioned media. Statistical analysis performed by One-way ANOVA with Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01 compared to control; ^p < 0.05 compared to LR treated P. n = 5.
Fractionation of L. reuteri conditioned media
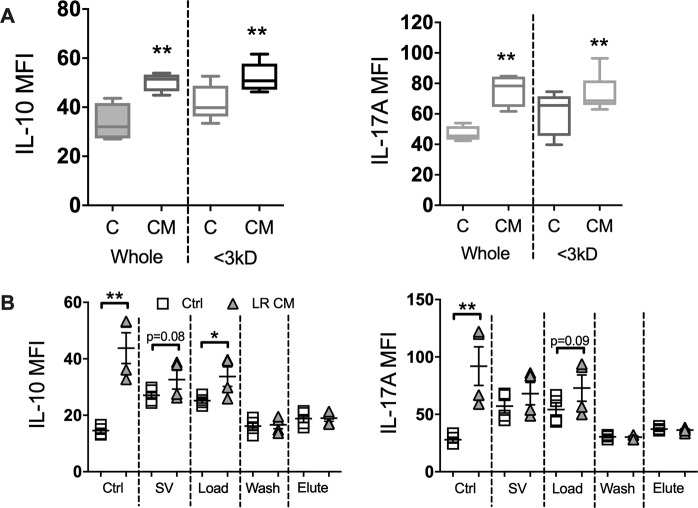
To identify the active components secreted by L. reuteri that influences T-cell cytokine expression, the conditioned media was subjected to a series of fractionations based on size and water solubility. Fractionation based on size revealed that the active component(s) in question is less than 3 kD in size (Fig. 9B). Treatment of splenic CD4+ T cells with the <3 kD fraction of L. reuteri CM significantly increased expression of IL-10 (p < 0.01) and IL-17A (p < 0.01) compared to control. To further define the active component in the L. reuteri CM, whole supernatants were fractionated based on water solubility using a reversed phase solid-phase extraction (SPE) column and the resulting fractions (load, wash and elute) tested for activity (Fig. 9B). Normal RPMI media and L. reuteri CM were also evaporated to dryness through the SpeedVac (SV) to control for any effects of the evaporation process. Analysis of cytokine expression in CD4+ T cells revealed significantly increased expression of IL-10 (p < 0.05) with the L. reuteri CM load fraction (unretained on the SPE column) over the control load fraction. A modest increase in IL-17A was also observed. The wash and elute fractions of the L. reuteri CM failed to induce any cytokine response over the control, consistent with our conclusion that the active molecule in L. reuteri CM is likely water soluble and therefore not retained.
Figure 9.
Effect of L. reuteri conditioned media fractions on naïve CD4+ T Cell cytokine expression. Spleens were isolated from male C57BL/6 mice (12–18 weeks) and naïve CD4+ T cells obtained by magnetic separation. CD4+ T cells were cultured with either a) whole or <3kD CM or b) SPE fractionated CM and expression of cytokines analyzed by flow cytometry. SV = SpeedVac. Statistical analysis performed by unpaired t-test. *p < 0.05, **p < 0.01 compared to comparable control. n = 4–5.
MLN and bone link
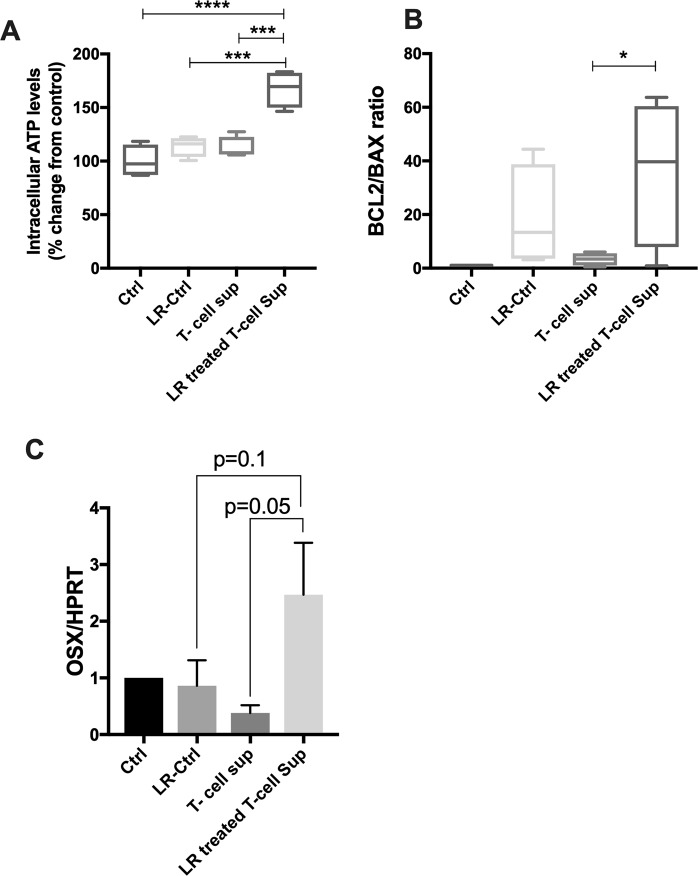
Data thus far suggest that oral L. reuteri administration likely interacts with MLNs and stimulates expression of multiple cytokines that have either osteoblastogenic or anti-osteoblastogenic activities. To determine the net effect of L. reuteri stimulation of T-cells on osteoblasts, we treated MC3T3-E1 pre-osteoblasts with T-cell supernatants (treated with or without L. reuteri stimulation). CD4+ T cells were isolated from MLNs and treated with L. reuteri CM or vehicle for 4 days. The supernatants from the T-cell cultures were then collected and added to osteoblast cultures for 6 hours. To rule out any residual activity of L. reuteri in the T-cell supernatant, the control L. reuteri CM was left in the incubator for 4 days (without any T-cells) similar to the T-cell treatment. Only the supernatants from T-cells treated with L. reuteri stimulated osteoblast ATP levels (Fig. 10A). Importantly, L. reuteri CM, incubated without any cells for 4 days did not have any activity, suggesting that the ATP inducing effect of T-cell supernatants (treated) is due to secreted factors from T-cells. Consistent with this, Bcl2/Bax ratio was also significantly enhanced by L. reuteri treated T-cell supernatant, suggesting that T-cell secretory factor (stimulated by L. reuteri) is likely able to enhance the survival of osteoblasts (Fig. 10B). Similarly, osterix expression (transcription factor important for osteoblast differentiation) was also modestly induced by the L. reuteri treated T-cell supernatant but not by supernatants from other groups (Fig. 10B). These data suggest that L. reuteri is able to stimulate T-cells in MLNs that can indeed beneficially affect osteoblasts. Taken together, these results suggest that L. reuteri-induced bone responses are dependent on lymphocytes and that L. reuteri is able to stimulate T-lymphocytes to increase expression of cytokines and other factors that have potentially osteoblastogenic activity. In addition, our studies reveal that the active fraction that elicits these effects is in the <3 kD fraction in the L. reuteri secreted component.
Figure 10.
Secretory factors from L. reuteri CM treated T cells enhances osteoblast viability and osterix gene expression. Mesenteric lymph nodes were isolated from male C57BL/6 mice (12–18 weeks), homogenized and CD4+ cells obtained by magnetic separation. CD4+ T-cells were cultured with L. reuteri CM for 4 days. Supernatants from these cultures (secreted factors) were collected after 4 days and used to treat osteoblast cells for 6 hours. (A) cell viability was measured by intracellular ATP levels; (B) BCL2/BAX gene expression ratio; (C) osterix gene expression. Ctrl = control, LR-Ctrl = L. reuteri CM with no cells, T-cell sup = T cells with no treatment. Statistical analysis performed one-way ANOVA with Bonferroni’s multiple comparision test. *p < 0.05, ***p < 0.001, ****p < 0.0001. n = 5–6.
Discussion
In recent years growing evidence from murine models has highlighted the benefit of probiotic bacteria in treating adverse bone pathology associated with numerous conditions, including estrogen-deficiency and type 1 diabetes11,13,16,17,34,49. Furthermore, results from a recent randomized, double-blind placebo-controlled clinical trial have demonstrated that L. reuteri 6475 is able to reduce bone loss in older women50, further demonstrating its potential as a novel therapeutic. Although different probiotic bacteria have been shown to have beneficial effects on bone health, the complexity of host and bacterial mechanisms that mediate these effects are not well understood. Our lab has shown that treatment with the probiotic L. reuteri ATCC 6475 can enhance femoral trabecular bone volume fraction, bone mineral density and bone mineral content in intact healthy male mice14. Building upon these previous findings, in this study we have elucidated host as well as the bacterial mechanisms that enhance bone density in healthy male mice.
The intestine is residence to a significant population of immune cells that are in constant interaction with the intestinal microbiota. Immune cells present within the gut-associated lymphoid tissue (GALT), especially the mesenteric lymph nodes, play a critical role in inducing and maintaining tolerance to food proteins and commensal bacteria51; these immune cells can then re-enter the blood stream and circulate throughout the body52. Of the immune cells, T-lymphocytes are key players in maintaining the balance of bone remodeling and can exert an effect on both the bone-forming osteoblasts and the bone-resorbing osteoclasts. T cell-derived cytokines such as IL-10 and IL-17A have been demonstrated to inhibit/stimulate osteoclast differentiation as well as enhance mesenchymal stem cell proliferation and osteoblast differentiation42–44,53. These data suggest that intestinal probiotics could exert systemic bone effects through the modulation of T cell cytokine expression. In this context, we have identified that the presence of lymphocytes is crucial for the beneficial effect of L. reuteri on bone health. Consistent with our studies demonstrating that Rag KO mice do not respond to L. reuteri treatment, Dar et al. suggested that the effect of L. acidophilus in preventing OVX-induced bone loss is dependent on T-reg-Th17 balance54. Similarly, other probiotics such as LGG and the VSL#3 can decrease intestinal and bone marrow inflammation49 that could potentially benefit bone.
L. reuteri can influence the host through several mechanisms including modifying the gut microbiome composition and function as well as secreting factors that directly influence host cells. Even though our recent studies suggest that L. reuteri is able to alter the microbial communities in a model of ABX-induced dysbiosis19, taxonomy data suggest that under healthy conditions, L. reuteri does not modify broad, phylum level, bacterial community composition. This however, does not rule out changes in the proportion of specific bacterial species (especially low abundance species that are not sufficiently detected by 16 s sequencing) during/following L. reuteri treatment. Other studies have shown that healthy microbiota is resistant to significant changes in composition55 and that not all probiotics colonize the mouse and human gut56,57. In the current study, L. reuteri was administered orally at a dose of 3.3 × 108 cfu/ml in the drinking water for 4-weeks. During transit, the bacteria feed on luminal nutrients and produce metabolites/factors which can cross the gut barrier. In fact, we have found significant differences in the serum profiles of mice treated with or without L. reuteri (unpublished data). Therefore, we examined direct effects of L. reuteri on the host (versus indirect effects via microbiota modifications). Consistent with previous findings36 we find that orally administered L. reuteri is able to translocate from the intestinal lumen to the mesenteric lymph nodes (not shown). Importantly, our findings also demonstrate that secreted products of L. reuteri can significantly affect MLN cytokine profile by increasing IL-10 expression and numbers of IL-10+ cells as well as other cytokines including TGFβ, IFNγ and IL-17A. Intriguingly we also find that both L. reuteri and its secreted components have immunomodulatory effects on CD4+ T cell cytokine profile and that these actions of L. reuteri on CD4+ T cells are negatively regulated by NOD signaling. In providing further evidence for a link between T-cell modulation of L. reuteri and effect on osteoblasts, we show that L. reuteri secreted products stimulate T-cells from MLNs to produce various factors (including cytokines) and these soluble factors from T-cells are able to modulate osteoblasts in culture. Although the precise identity of the factor(s) from MLN T-cells that stimulate(s) osteoblast gene expression is currently under investigation, taken together with the in vivo evidence from Rag knockout, our results support the possibility of L. reuteri secreted factors signaling MLN T-cells which subsequently contribute to the beneficial bone effects of L. reuteri. We recognize that our in vitro studies used factors produced by L. reuteri while grown in medium under cell culture conditions. These products may not necessarily be made by L. reuteri in the mouse intestine, which provides a different environment compared to a culture dish. Further studies are needed to identify the specific secreted factors and then test their ability to enhance bone health in vivo.
The most widely acknowledged paradigm of bacteria-immune cell interaction in the intestine involves the sampling of bacteria by dendritic cells and the presentation of these antigens to and subsequent programming of T cells20. Recent studies however, have suggested that bacteria can cross the intestinal barrier independent of APCs. In cirrhotic patients with ascites, increased bacterial DNA has been detected in the serum58 while in a murine model of social stress, DNA from commensal Lactobacilli have been detected in the spleen59. In addition to these disease models of bacterial translocation, a study by Schultz et al.21 demonstrated that a green fluorescent protein (GFP) probiotic E. coli strain Nissle 1917 was able to translocate from the intestinal lumen to the Peyer’s patches and MLNs in a time-dependent manner, with peak levels detected 6 hours post-gavage. Together, these data suggest that orally administered probiotics or their secreted products can potentially interact with cells in MLNs, and exert immunomodulatory effect via regulation of immune cells.
In this study, we examined the direct effect of L. reuteri on cells in the MLNs and find that several cytokines including IL-10 and IL-17 are significantly modulated by probiotic bacteria and its secreted products. This observed immunomodulatory effect of L. reuteri is comparable to that reported with other species of lactobacilli; L. rhamnosus Lcr3560, L. casei, L. fermentum Lb20, L. plantarum (Lb1 and 299 v), L. johnsonii La161 and L. gasseri SBT205562, have all been demonstrated to have concentration-dependent effects on dendritic cells. While the results from the whole MLN cultures suggested that the L. reuteri effects could be driven by APCs, the results from the CD3+ MLN cultures raised the possibility that these probiotic bacteria and secretory products could act directly on the T cells. This direct action on T cells was further confirmed in the CD4+ T cells from spleen where, as with the MLN cultures, L. reuteri had a concentration-dependent effect on cytokine expression. Perhaps the most interesting finding in this study however, is the discovery that soluble factors released by L. reuteri are able to modulate MLN and naïve CD4+ T cell cytokine profile at levels comparable to that observed in the whole live bacteria cultures. The identity of these molecules is currently under active investigation. This finding provides a potential mechanism by which oral administration of L. reuteri could have systemic effects and modulate T cells, which is critical for the beneficial bone effects as demonstrated in the Rag knockout mice.
Dendritic cells, as well as other immune cells, recognize components of bacteria known as pathogen-associated molecular pattern (PAMPs), using specific pattern-recognition receptors (PRRs) which are present on the host cell surface and in the cytosolic compartment47,63. Of the cell surface PRRs, the family of toll-like receptors (TLRs) have been demonstrated to respond to a large variety of PAMPs and activate the innate immune system. Of the cytosolic PRRs, the nucleotide binding and oligomerization domain (NOD)-like receptor family members NOD1 and NOD2 are the most well understood64. Studies have suggested that Lactobacillus species potentially interacts with the immune system and induces a response through TLR-245,62,65, TLR-4 and DC-specific intracellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)66. Furthermore, studies utilizing NOD1 and NOD2 knockout mice have identified that these receptors play a key role in the regulation of bone mass by the intestinal microbiota67. Both TLR and NOD2 expression has been reported in T cells, suggesting that L. reuteri or its components could potentially act directly on the T cells, independent of APCs68–70. To understand the signaling mechanisms of how L. reuteri products activate T-cell cytokine expression, we therefore focused on TLR2 and NOD signaling pathways and our results are contrary to expectation in that the NOD signaling negatively affects L. reuteri-induced cytokine expression. While the physiological implication of this is unclear at this point, further extensive studies however are needed to delineate L. reuteri-stimulated signaling pathways in lymphocytes.
Probiotic bacteria, including lactobacilli, are known to secrete many factors that can potentially modulate the immune system including extracellular proteins71, short chain fatty acids (SCFA)72 and soluble peptides73. To try and identify the active molecules produced by L. reuteri, we fractionated the conditioned media. The active molecule was observed to be likely water soluble and within the <3kD fraction, suggesting either a non-protein molecule or a very small extracellular bacterial peptide. The characteristics of the L. reuteri active component in the present study had similar hallmarks to the active molecule(s) produced by the lactic acid bacteria Bifidobacterium breve and Streptococcus thermophilus74; a non-protein molecule <3kD in size. Interestingly, these authors suggest that the effects observed by B. breve and S. thermophilus may not be caused by the same active metabolite as some discordance was observed between experiments. Furthermore, the authors ruled out the SCFA butyrate and lactic acid as the metabolites responsible; as concentrations were too low and no inhibitory effect of LPS-induced TNFα secretion was observed at the levels present74. Butyrate is not a major component of lactobacilli fermentation; with different species and strains producing either very low levels or none at all75–77. In contrast, acetate is produced at much higher concentrations76. This could be of potential importance in suggesting a possible mechanism by which L. reuteri modulates T cell cytokine profile; treatment of CD4+ T cells upon initiation of differentiation with acetate has been demonstrated to promote IL-10 expression in all T cell polarized conditions (T helper (Th)17 and Th1) and non-polarized T cells, without affecting expression of Foxp378.
In summary we have identified that the beneficial effects of the probiotic bacteria L. reuteri 6475 on bone are dependent on mature lymphocytes and that it is able to directly stimulate T-cells in the mesenteric lymph nodes and spleen. We also demonstrate that L. reuteri secretes active metabolites that are able to directly modulate CD4+ T cell cytokine profile and that this mechanism is negatively regulated by NOD signaling pathway. Furthermore, we have demonstrated that secreted factors from L. reuteri treated T cells can be beneficial to osteoblasts providing an important link between the effect of bacteria on lymphocytes and its beneficial bone effects. These findings highlight the potential mechanism by which L. reuteri is able to exert its beneficial systemic bone effect and highlight potential targets for future therapeutic research.
Acknowledgements
The work presented in this study was funded in part by NIH AT007695 (to LRM, RAB and NP), and DK101050 (to LRM and NP); NDR-A was supported by the William Townsend Porter Pre- doctoral Fellowship from the American Physiological Society. ADJ acknowledges support from the USDA National Institute of Food and Agriculture, Hatch project MICL02474.
Author Contributions
F.L.C., N.D.R.-A., L.R.M., N.P. devised experiments; F.L.C., N.D.R.-A., J.D.S., A.D.J., L.S. performed experiments and analyzed data; L.R.M., N.P., R.A.B. analyzed data; F.L.C., N.D.R.-A., L.R.M. and NP prepared graphs, drafted and edited manuscript; All authors reviewed and edited the manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fraser L. Collins, Naiomy Deliz Rios-Arce, Laura R. McCabe and Narayanan Parameswaran contributed equally.
Contributor Information
Laura R. McCabe, Email: mccabel@msu.edu
Narayanan Parameswaran, Email: narap@msu.edu.
References
- 1.Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat. Rev. Rheumatol. 2010;6:99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 2.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–87. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ing-Lorenzini K, et al. Low-energy femoral fractures associated with the long-term use of bisphosphonates: a case series from a Swiss university hospital. Drug Saf. 2009;32:775–785. doi: 10.2165/00002018-200932090-00002. [DOI] [PubMed] [Google Scholar]
- 4.Shannon J, Shannon J, Modelevsky S, Grippo Aa. Bisphosphonates and osteonecrosis of the jaw. J. Am. Geriatr. Soc. 2011;59:2350–2355. doi: 10.1111/j.1532-5415.2011.03713.x. [DOI] [PubMed] [Google Scholar]
- 5.Khosla S, Shane E. A Crisis in the Treatment of Osteoporosis. J. Bone Miner. Res. 2016;31:1485–7. doi: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- 6.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunne JL, et al. The intestinal microbiome in type 1 diabetes. Clin. Exp. Immunol. 2014;177:30–37. doi: 10.1111/cei.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdam FJ, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity. 2013;21:607–615. doi: 10.1002/oby.20466. [DOI] [PubMed] [Google Scholar]
- 9.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat. Rev. Rheumatol. 2011;7:569–578. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, F. L. et al. Immunology of gut-bone signaling. Advances in Experimental Medicine and Biology1033 (2017). [DOI] [PMC free article] [PubMed]
- 11.Britton Ra, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014;229:1822–30. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J-Y, et al. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Invest. 2016;126:2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohlsson C, et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9:e92368. doi: 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCabe LR, Irwin R, Schaefer L, Britton Ra. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J. Cell. Physiol. 2013;228:1793–8. doi: 10.1002/jcp.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins FL, et al. Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS One. 2016;11:e0153180. doi: 10.1371/journal.pone.0153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins FL, Rios-Arce ND, Schepper JD, Parameswaran N, McCabe LR. The Potential of Probiotics as a Therapy for Osteoporosis. Bugs as Drugs. 2017;5:213–233. doi: 10.1128/microbiolspec.BAD-0015-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins, F. L., Kim, S. M., McCabe, L. R. & Weaver, C. M. Intestinal Microbiota and Bone Health: The Role of Prebiotics, Probiotics, and Diet. in Bone Toxicology (eds Smith, S. Y., Varela, A. & Samadfam, R.) 417–443 (Springer International Publishing, 2017).
- 18.Zhang J, et al. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology. 2015;156:3169–3182. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schepper JD, et al. Probiotic Lactobacillus reuteri Prevents Postantibiotic Bone Loss by Reducing Intestinal Dysbiosis and Preventing Barrier Disruption. J. Bone Miner. Res. 2019 doi: 10.1002/jbmr.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mowat Allan M., Agace William W. Regional specialization within the intestinal immune system. Nature Reviews Immunology. 2014;14(10):667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 21.Schultz M, et al. Green fluorescent protein for detection of the probiotic microorganism Escherichia coli strain Nissle 1917 (EcN) in vivo. J. Microbiol. Methods. 2005;61:389–398. doi: 10.1016/j.mimet.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Sharma D, Malik A, Steury MD, Lucas PC, Parameswaran N. Protective Role of β-arrestin2 in Colitis Through Modulation of T-cell Activation. Inflamm. Bowel Dis. 2015;0:1. doi: 10.1097/MIB.0000000000000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins FL, Rios-Arce ND, McCabe LR, Parameswaran N. Cytokine and hormonal regulation of bone marrow immune cell Wnt10b expression. PLoS One. 2017;12:e0181979. doi: 10.1371/journal.pone.0181979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steury, M. D. et al. G-Protein Coupled Receptor Kinase-2 Deficient Mice are Protected from Dextran Sodium Sulfate-Induced Acute Colitis. Physiol. Genomics physiolgenomics. 00006.2018 (2018). [DOI] [PMC free article] [PubMed]
- 25.Coe LM, Irwin R, Lippner D, McCabe LR. The bone marrow microenvironment contributes to type I diabetes induced osteoblast death. J. Cell. Physiol. 2011;226:477–83. doi: 10.1002/jcp.22357. [DOI] [PubMed] [Google Scholar]
- 26.Motyl KJ, et al. Bone inflammation and altered gene expression with type I diabetes early onset. J. Cell. Physiol. 2009;218:575–83. doi: 10.1002/jcp.21626. [DOI] [PubMed] [Google Scholar]
- 27.Lee T, et al. Β-Arrestin-1 Deficiency Protects Mice From Experimental Colitis. Am. J. Pathol. 2013;182:1114–1123. doi: 10.1016/j.ajpath.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raehtz, S., Bierhalter, H., Schoenherr, D., Parameswaran, N. & McCabe, L. R. Estrogen deficiency exacerbates type 1 diabetes-induced bone TNF-a expression and osteoporosis in female mice. Endocrinology158 (2017). [DOI] [PMC free article] [PubMed]
- 29.Quach D, Collins F, Parameswaran N, McCabe L, Britton RA. Microbiota Reconstitution Does Not Cause Bone Loss in Germ-Free Mice. mSphere. 2018;3:1–14. doi: 10.1128/mSphereDirect.00545-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins J, Auchtung JM, Schaefer L, Eaton KA, Britton RA. Humanized microbiota mice as a model of recurrent Clostridium difficile disease. Microbiome. 2015;3:35. doi: 10.1186/s40168-015-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–20. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Team, R. C. R: a language and environment for statistical computing. R Froundation for Statistical Computing, Vienna, Austria (2017).
- 33.McMurdie PJ, Holmes S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Jing, Motyl Katherine J., Irwin Regina, MacDougald Ormond A., Britton Robert A., McCabe Laura R. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the ProbioticLactobacillus reuteri. Endocrinology. 2015;156(9):3169–3182. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas CM, et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012;7:e31951. doi: 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dicksved Johan, Schreiber Olof, Willing Ben, Petersson Joel, Rang Sara, Phillipson Mia, Holm Lena, Roos Stefan. Lactobacillus reuteri Maintains a Functional Mucosal Barrier during DSS Treatment Despite Mucus Layer Dysfunction. PLoS ONE. 2012;7(9):e46399. doi: 10.1371/journal.pone.0046399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, M., Chen, G. & Li, Y. P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 4 (2016). [DOI] [PMC free article] [PubMed]
- 38.Mori G, D’Amelio P, Faccio R, Brunetti G. The Interplay between the bone and the immune system. Clin. Dev. Immunol. 2013;2013:720504. doi: 10.1155/2013/720504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu LX, et al. Interleukin‐10 selectively inhibits osteoclastogenesis by inhibiting differentiation of osteoclast progenitors into preosteoclast‐like cells in rat bone marrow culture system. J. Cell. Physiol. 1995;165:624–629. doi: 10.1002/jcp.1041650321. [DOI] [PubMed] [Google Scholar]
- 40.Al-Rasheed A, Scheerens H, Srivastava AK, Rennick DM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10: Late onset. J. Periodontal Res. 2004;39:194–198. doi: 10.1111/j.1600-0765.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- 41.Hong MH, Williams H, Jin CH, Pike JW. The Inhibitory Effect of Interleukin-10 on Mouse Osteoclast Formation Involves Novel Tyrosine-Phosphorylated Proteins. J. Bone Miner. Res. 2010;15:911–918. doi: 10.1359/jbmr.2000.15.5.911. [DOI] [PubMed] [Google Scholar]
- 42.Sato K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang H, et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009;16:1332–1343. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 44.Croes M, et al. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone. 2016;84:262–270. doi: 10.1016/j.bone.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Ryu SH, et al. The probiotic Lactobacillus prevents citrobacter rodentium-induced murine colitis in a TLR2-dependent manner. J. Microbiol. Biotechnol. 2016;26:1333–1340. doi: 10.4014/jmb.1602.02004. [DOI] [PubMed] [Google Scholar]
- 46.Moratalla A, et al. Bifidobacterium pseudocatenulatum CECT7765 promotes a TLR2-dependent anti-inflammatory response in intestinal lymphocytes from mice with cirrhosis. Eur. J. Nutr. 2016;55:197–206. doi: 10.1007/s00394-015-0837-x. [DOI] [PubMed] [Google Scholar]
- 47.Moreira, L. O. & Zamboni, D. S. NOD1 and NOD2 signaling in infection and inflammation. Front. Immunol. 3 (2012). [DOI] [PMC free article] [PubMed]
- 48.Tigno-Aranjuez JT, Asara JM, Abbott DW. Inhibition of RIP2’s tyrosine kinase activity limits NOD2-driven cytokine responses. Genes Dev. 2010;24:2666–77. doi: 10.1101/gad.1964410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J-Y, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Invest. 2016;126:2049–63. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilsson A. G., Sundh D., Bäckhed F., Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. Journal of Internal Medicine. 2018;284(3):307–317. doi: 10.1111/joim.12805. [DOI] [PubMed] [Google Scholar]
- 51.Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J. Exp. Med. 2006;203:497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter, M. C., Teijeira, A. & Halin, C. T cell trafficking through lymphatic vessels. Front. Immunol. 7 (2016). [DOI] [PMC free article] [PubMed]
- 53.Zaiss MM, et al. Treg cells suppress osteoclast formation: A new link between the immune system and bone. Arthritis Rheum. 2007;56:4104–4112. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- 54.Dar HY, et al. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Reports. 2018;8:46–56. doi: 10.1016/j.bonr.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lozupone Catherine A., Stombaugh Jesse I., Gordon Jeffrey I., Jansson Janet K., Knight Rob. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zmora Niv, Zilberman-Schapira Gili, Suez Jotham, Mor Uria, Dori-Bachash Mally, Bashiardes Stavros, Kotler Eran, Zur Maya, Regev-Lehavi Dana, Brik Rotem Ben-Zeev, Federici Sara, Cohen Yotam, Linevsky Raquel, Rothschild Daphna, Moor Andreas E., Ben-Moshe Shani, Harmelin Alon, Itzkovitz Shalev, Maharshak Nitsan, Shibolet Oren, Shapiro Hagit, Pevsner-Fischer Meirav, Sharon Itai, Halpern Zamir, Segal Eran, Elinav Eran. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell. 2018;174(6):1388-1405.e21. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 57.Ibnou-Zekri N., Blum S., Schiffrin E. J., von der Weid T. Divergent Patterns of Colonization and Immune Response Elicited from Two Intestinal Lactobacillus Strains That Display Similar Properties In Vitro. Infection and Immunity. 2003;71(1):428–436. doi: 10.1128/IAI.71.1.428-436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santiago A, et al. Alteration of the serum microbiome composition in cirrhotic patients with ascites. Sci. Rep. 2016;6:25001. doi: 10.1038/srep25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lafuse William P., Gearinger Rachel, Fisher Sydney, Nealer Connor, Mackos Amy R., Bailey Michael T. Exposure to a Social Stressor Induces Translocation of Commensal Lactobacilli to the Spleen and Priming of the Innate Immune System. The Journal of Immunology. 2017;198(6):2383–2393. doi: 10.4049/jimmunol.1601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evrard B, et al. Dose-dependent immunomodulation of human dendritic cells by the probiotic lactobacillus rhamnosus lcr35. PLoS One. 2011;6:1–12. doi: 10.1371/journal.pone.0018735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 2002;168:171–8. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 62.Sakai F, et al. Lactobacillus gasseri SBT2055 Induces TGF-β Expression in Dendritic Cells and Activates TLR2 Signal to Produce IgA in the Small Intestine. PLoS One. 2014;9:e105370. doi: 10.1371/journal.pone.0105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janeway CA, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 64.Fukata M, Vamadevan AS, Abreu MT. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin. Immunol. 2009;21:242–253. doi: 10.1016/j.smim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Mohamadzadeh M, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. USA. 2005;102:2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smits HH, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 67.Ohlsson Claes, Nigro Giulia, Boneca Ivo Gomperts, Bäckhed Fredrik, Sansonetti Philippe, Sjögren Klara. Regulation of bone mass by the gut microbiota is dependent on NOD1 and NOD2 signaling. Cellular Immunology. 2017;317:55–58. doi: 10.1016/j.cellimm.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J. Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zanello G, et al. Nod2 activates NF-kB in CD4+ T cells but its expression is dispensable for T cell-induced colitis. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0082623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sánchez B, Urdaci MC, Margolles A. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa-bacteria interactions. Microbiology. 2010;156:3232–3242. doi: 10.1099/mic.0.044057-0. [DOI] [PubMed] [Google Scholar]
- 72.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–88. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lebeer S, Vanderleyden J, De Keersmaecker SCJ. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008;72:728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menard S. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut. 2004;53:821–828. doi: 10.1136/gut.2003.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991;70:443–59. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- 76.Kahouli I, et al. Screening and In-Vitro Analysis of Lactobacillus reuteri Strains for Short Chain Fatty Acids Production, Stability and Therapeutic Potentials in Colorectal Cancer. Bioequivalence Bioavailab. 2015;7:39–50. [Google Scholar]
- 77.LeBlanc JG, et al. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017;16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.