Abstract
The use of plant growth promoting bacteria as bioinoculant to alleviate salt stress is a sustainable and eco-friendly approach in agriculture. However, the maintenance of the bacterial population in the soil for longer period is a major concern. In the present study, chitosan-immobilized aggregated Methylobacterium oryzae CBMB20 was used as a bioinoculant to improve tomato plant (Solanum lycopersicum Mill.) growth under salt stress. The chitosan-immobilized aggregated M. oryzae CBMB20 was able to enhance plant dry weight, nutrient uptake (N, P, K and Mg2+), photosynthetic efficiency and decrease electrolyte leakage under salt stress conditions. The oxidative stress exerted by elevated levels of salt stress was also alleviated by the formulated bioinoculant, as it up-regulated the antioxidant enzyme activities and enhanced the accumulation of proline which acts as an osmolyte. The chitosan-immobilized aggregated M. oryzae CBMB20 was able to decrease the excess Na+ influx into the plant cells and subsequently decreasing the Na+/K+ ratio to improve tomato plant growth under salt stress conditions. Therefore, it is proposed that the chitosan-immobilized aggregated M. oryzae CBMB20 could be used as a bioinoculant to promote the plant growth under salt stress conditions.
Keywords: Carrier material, Enzyme activity, Immobilization, Methylobacterium oryzae CBMB20, Salt stress, Tomato
Introduction
Soil salinity has been considerably affecting the plant growth and productivity across the globe (Machado and Serralheiro 2017). The excessive amount of salt in soil results in enhanced uptake of Na+ (Tank and Saraf 2010), which in turn reduces the water potential of plants (Chatterjee et al. 2018). The increase in uptake of Na+ also decreases the uptake of other ions such as Ca2+ and K+ thereby leading to dysfunctional physiology and nutrient imbalance in plants (Tank and Saraf 2010). In addition, salt stress affects all the major plant physiological and metabolic pathways such as photosynthesis, protein synthesis and lipid metabolism (Lee et al. 2008). The production of reactive oxygen species (ROS) such as superoxide ion, hydrogen peroxide and hydroxyl radicals are evident oxidative stress markers resulting due to high salinity stress and these prove to be highly detrimental to the plants (De pinto et al. 2012). Significant elevation of ROS scavenging enzymes such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR) have been reported in plants grown under salt stress (de Azevedo Neto et al. 2006; Chatterjee et al. 2017).
The use of plant growth promoting bacteria (PGPB) as inoculants in agriculture and stress alleviation has been recommended to achieve sustainable and eco-friendly crop production (Jha and Subramanian 2014). Plant growth promotion using PGPB has been suggested to be achieved either by direct or indirect mechanisms (Glick 2012). The direct mechanisms include biological nitrogen fixation, indole-3-acetic acid (IAA) synthesis, lowering of plant ethylene levels, and solubilization of phosphate, zinc, silica, potassium, and iron and on the other hand, indirect mechanisms refer to competition for nutrient, antibiosis, lytic enzyme production and induced systemic resistance (Glick 2012; Joe et al. 2016; Parray et al. 2016; Chatterjee et al. 2017). Methylobacterium oryzae CBMB20, a potential plant growth promoting bacteria isolated from the phyllosphere of rice plant are capable of utilizing various compounds such as methanol, methylamine and can colonize the phyllosphere of plants (Madhaiyan et al. 2007a). Methylobacterium oryzae CBMB20 is a 1-aminocyclopropane-1-carboxylate deaminase (ACCD) producing bacteria which is also capable to produce IAA and extracellular polysaccharide (EPS), accumulate polyhydroxybutyrate and form biofilm (Chanratana et al. 2017). However, the efficacy of PGPB as potential bioinoculants for agricultural crop production depends on their ability to compete for colonization of root and promote plant growth with various amalgamated mechanisms (Bhattacharyya and Jha 2012). The application of PGPB is often erratic under field conditions because of the various stresses exerted on the inoculants (Souza et al. 2015). Researchers have been trying to develop methods to enhance survivability of bioinoculants by altering their physiological states under stress environment (Ambrosini et al. 2016). A wide number of approaches have been recommended to overcome this issue, such as carriers and formulations of inoculants including liquid, organic, inorganic, polymeric, and encapsulated formulations (Bashan et al. 2014). We have demonstrated in our previous study about the preparation of flocculated or aggregated cells to maintain high survival rate of bioinoculant during storage and on the rhizosphere of the plants (Joe et al. 2010). In addition, in this study, it has also been demonstrated that the aggregation of Methylobacterium oryzae CBMB20 cells in high C/N ratio enables their enhanced tolerance to UV radiation, heat, desiccation, different temperature regimes, oxidative stress and starvation (Chanratana et al. 2017). The aggregated CBMB20 cells were able to produce higher amount of extracellular polysaccharides, accumulate poly-β-hydroxybutyrate and proline, higher amount of production of IAA, enhanced ACC deaminase activity and biofilm formation at 0–200 mM salt concentration (Chanratana et al. 2017). The M. oryzae CBMB20 had also been shown to reduce the stress ethylene levels of plants induced by salinity by scavenging the ethylene precursor ACC by the production of ACC deaminase (Madhaiyan et al. 2007b).
Population of bacteria inoculated without proper carrier declines rapidly for most species of PGPB. The unprotected, inoculated bacteria often had to compete with the better—adapted native microflora and withstand predation by soil microfauna in soils with heterogeneity (Bashan et al. 2014). Higher number of viable cells during prolonged storage at room temperature and after inoculation into the soil is the prerequisite for any bioinoculants preparations. A potent carrier material is characterized by its mechanical stability, cost effectiveness, biodegradability, non-toxicity, eco-friendly and availability (Liu et al. 2008). Alginate beads have been reported to protect microbes from biotic and abiotic stresses and improve persistence and physiological activity as well as cell densities (Schoebitz et al. 2013).
Chitosan, a natural, safe, inexpensive, abundant, polycationic, biodegradable polymer produced from chitin, has been evaluated as a potential bioinoculant carrier (Dar et al. 2015). The porous nature of chitosan has been attributed to support the immobilized cell growth and aid physiological activity of microorganisms (Angelim et al. 2013). It has been reported that the chelating nature of chitosan is helpful for nutrient and mineral sequestration and make them available for uptake by plants (Ramírez et al. 2010). In addition, chitosan also induces plant responses to biotic and abiotic stresses and support the growth of beneficial microorganisms (Yen and Mau 2007). In our previous report, we have illustrated higher survival rate of chitosan-immobilized M. oryzae CBMB20 on tomato spermosphere and enhanced the tomato plant growth under greenhouse condition using chitosan-immobilized M. oryzae CBMB20 (Chanratana et al. 2018).
The objective of this study was to evaluate the chitosan-immobilized M. oryzae CBMB20 on tomato plant growth and its potential to alleviate salt stress in relation to productivity, nutrient uptake, antioxidant enzymes, proline content, electrolyte leakage, chlorophyll a (Chl a), chlorophyll b (Chl b), carotenoids, malondialdehyde, hydrogen peroxide, and uptake of ions. We hypothesized that the chitosan formulation of M. oryzae CBMB20 will not only promote the plant growth but also improve the physiological response of plants under to salt stress.
Materials and methods
Methylobacterium oryzae CBMB20 as inoculant
Chitosan-immobilized Methylobacterium oryzae CBMB20 was used as the inoculant in this study (Chanratana et al. 2018). A single colony of M. oryzae CBMB20 was inoculated in ammonium mineral salt (AMS) broth (K2HPO4 0.7 g/L; KH2PO4 0.54 g/L; MgSO4.7H2O 1.0 g/L; CaCl2.2H2O 0.2 g/L; FeSO4.7H2O 4.0 mg/L; ZnSO4.7H2O 100 µg/L; MnCl2.4H2O 30 µg/L; H3BO3 300 µg/L; CoCl2.6H2O 200 µg/L; CuCl2.2H2O 10 µg/L; NiCl2.6H2O 20 µg/L; Na2MoO4.2H2O 60.0 µg/L; NH4Cl 0.5 g/L) supplemented with 0.5% sodium succinate and grown at 30 °C for 72 h in a rotary shaker at 180 rpm. 10 mL of bacterial growth was collected by centrifugation at 10, 000g and washed twice with sterile saline solution (0.85% NaCl). The pellet was suspended in saline solution and the cell concentration was adjusted to ~ 8.0 log10 cfu mL−1 and used for immobilization. 5 mL of saline suspended M. oryzae CBMB20 was mixed in 20 mL of 1.5% (w/v) chitosan solution prepared in 1% (v/v) acetic acid with tween 80 at 0.1% (v/v) concentration. The chitosan–bacterium mixture was incubated with shaking incubator at 150 rpm for 3 h. The mixture was dropped into 1% (w/v) tripolyphosphate (pH 9.0) with 10 mL syringe to obtain approximately 1.50 mm bead size and allowed to harden in the same tripolyphosphate solution for 3 h. Then the beads were collected and washed three times with 0.15 M K2HPO4 (pH 8.0). Finally, the beads were drained and directly stored in 0.1% peptone water at 4 °C until further use.
Plant inoculation and treatment
Tomato seeds (cv: Yeoreum Mujeok Heukchima; Mirae Seed Co.) were surface sterilized by treating the seeds with 70% ethanol for 1 min followed 1% commercial bleach for 10 min and repeated washing with sterile Milli-Q water. Seeds were sown in seedling trays and kept for 15 days in Chungbuk National University (36.63°N and 127.45°E) greenhouse at 25–30 °C temperature with relative humidity of 50–70% and a 15–9 h day/night photoperiod. Seeds were grown using six different treatments as follows: T1—control; T2—aggregated M. oryzae CBMB20 (liquid form); T3—non-aggregated M. oryzae CBMB20 (liquid form); T4—chitosan (blank), T5—chitosan-immobilized aggregated M. oryzae CBMB20 and T6—chitosan-immobilized non-aggregated M. oryzae CBMB20. The control seeds were treated with sterile Milli-Q water. For bacterial inoculation, the seeds (1 g) were imbibed with 10 mL of aggregated and non-aggregated bacterial cells re-suspended in 0.03 M MgSO4 and 10 mL of 1% (w/v) carboxymethyl cellulose. To prepare aggregated cells of M. oryzae CBMB20, a single colony was inoculated in ammonium mineral salt (AMS) broth supplemented with 0.5% sodium succinate and grown at 30 °C for 72 h in a rotary shaker at 180 rpm. Ten mL of bacterial growth was harvested by centrifugation at 1000g and washed twice with sterile saline solution (0.85% NaCl). 10 mL (8.0 log10 cfu mL−1) of bacterial culture was transferred to 100 mL of fresh high C/N medium (K2HPO4 0.7 g/L; KH2PO4 0.54 g/L; MgSO4·7H2O 1.0 g/L; CaCl2·2H2O 0.2 g/L; FeSO4·7H2O 4.0 mg/L; ZnSO4·7H2O 100 µg/L; MnCl2·4H2O 30 µg/L; H3BO3 300 µg/L; CoCl2·6H2O 200 µg/L; CuCl2·2H2O 10 µg/L; NiCl2·6H2O 20 µg/L; Na2MoO4·2H2O 60.0 µg/L; Sodium succinate 5 g/L) incubated in shaking incubator at 180 rpm and maintained at 30 °C for 72 h to obtain aggregated cells. The bacterial cells were inoculated by keeping the level of inoculum equivalent to ~ 8 log CFU g−1 mL−1 for all the treatments. The second round of application of the inoculant were carried out at 15 days after sowing (DAS) when the seedling plugs were transferred to pots containing 2.5 kg of sterilized agricultural field soil acquired from Chungbuk National University, Cheongju, South Korea (pH 6.1; electric conductivity (EC) 0.65 dS m−1; organic matter 1.28 g kg−1 dry soil; cation exchange capacity (CEC) 1.53 cmol (p +); total N content 0.03%; available P2O5 31.34 mg kg−1 dry soil; K 0.3 cmol kg−1 dry soil; Ca2+ 0.21 cmol kg−1 dry soil; Mg 0.44 cmol kg−1 dry soil and Na 0.11 cmol kg−1 dry soil) and allowed to acclimate for 15 days. The pots were maintained in greenhouse at 28 °C/22 °C day/night temperature and relative humidity of 50–70% under natural illumination. The plants were watered with Milli-Q water. On day 30, the treatment plants were watered with salt at different concentrations (0, 50 and 100 mM NaCl). The NaCl solution was added gradually by adding 25 mL of 25 mM NaCl to each pot on alternate days so as to achieve the desired salt concentration on day 8 for 100 mM NaCl. During this period, the plants were supplemented with 25 mL of Hoagland’s nutrient solution on alternate days. The soil water level was maintained below the field capacity at all times for prevention of water leaching. The tomato plants were grown for 30 days after NaCl amendment and then harvested for analysis.
Plant biomass analysis
Plant biomass was recorded after harvesting at 68 days. The plant biomass was expressed in terms of dry weight for individual plants. The plant samples were dried in 80 °C oven for 72 h and the dry weight was recorded.
Photosynthetic pigment analysis
Photosynthetic pigments (Chl a, Chl b and carotenoid) were determined by following the procedures mentioned in Sumanta et al. (2014). Leaf samples (0.5 g) were homogenized in tissue homogenizer with 80% (w/v) acetone. The samples were centrifuged at 6000 × g and the supernatant was used for analysis of pigments. Absorbance of the extracted pigments were measured using a UV—visible spectrophotometer (UV—1601, Shimadzu Corporation, Japan) at 480 nm, 510 nm, 645 nm and 663 nm. The photosynthetic pigment content was expressed as unit’s mg per gram-fresh weight (mg g−1 FW) and calculated using the following equations:
Determination of proline content
The proline content was estimated as per the protocol mentioned in Bates et al. (1973). Leaf samples (0.5 g) were homogenized in 3% sulfosalicylic acid. The homogenate (2 mL) was filtered with Whatman™ filter paper 2 (GE Healthcare Life Sciences) and equal volume of glacial acetic acid (2 mL) and ninhydrin (2 mL) was added to the filtrate. The mixture was kept in 100 °C water bath for 1 h. Later, proline was separated with 4 mL toluene and the absorbance of the chromophore was measured at 520 nm with toluene as blank. The concentration of the free proline in the solution was determined by preparing a range of proline standards. The proline content was expressed as unit’s µg per gram-fresh weight (µg g−1 FW).
Determination of malondialdehyde content
The amount of lipid peroxidation was measured by determining the concentration of malondialdehyde (MDA) in plant cells. The total MDA content was measured using the protocol mentioned in Chatterjee et al. (2018) with little modifications. Leaf samples (0.5 g) were homogenized in liquid nitrogen and the tissue was re-suspended in 5 mL of 0.1% Trichloroacetic acid (TCA) in ice and transferred to a centrifuge tube containing 1 mL of TCA. Centrifugation was done at 6000 × g at 4 °C and the absorbance of the supernatant was recorded at 532 nm and corrected for nonspecific turbidity by subtracting the absorbance measured at 600 nm. The formula used for calculation of MDA content was A600 subtracted from A532 multiplied by the extinction coefficient of 155 mm−1 cm−1 for MDA. The MDA content was expressed as unit’s nmol per gram-fresh weight (nmol g−1 FW).
Determination of hydrogen peroxide content
The hydrogen peroxide (H2O2) content in leaves was measured by the protocol mentioned in Zhou et al. (2006). Leaf samples (0.5 g) were homogenized in liquid nitrogen and re-suspended in 5 mL of 5% TCA in ice. The mixture was centrifuged at 6000 × g. The supernatant was adjusted to pH 8 with 17 M ammonia solution and filtered. The filtrate was divided into two aliquots and catalase (8 µg) was added to one of those that served as a blank and kept at room temperature for 10 min. Colorimetric reagent [10 mg 4-aminoantipyrine, 10 mg phenol, 5 mg peroxidase (150 U mg−1) dissolved in 50 mL of 100 mM acetic acid buffer (pH 5.6)] was added to both the aliquots and absorbance was measured at 390 nm and a range of H2O2 standards were used to determine the total H2O2 content in leaves.
Electrolyte leakage
Electrolyte leakage was measured using a conductivity meter (Orion Star A210, USA) according to a protocol mentioned in Mishra et al. (2011). Leaf samples (1 g) were cut into small pieces with the size of 7.5 mm leaf discs and rinsed with distilled water. The tissues were placed into tubes containing 15 mL of double distilled water for 24 h at 25 °C. The electrical conductivity of the solution was regarded as the amount of electrolyte leakage from the tissues. The percentage of electrolyte leakage was calculated as the ratio of the conductivity after 12 h and after boiling (total ionic conductivity).
Leaf tissue extraction
Leaf tissue (0.5 g) were ground using a mortar and pestle with liquid nitrogen and 0.5 g of powdered sample was added to 10 mL of a solution containing 50 mM of potassium phosphate buffer (pH 7) and 1% (w/v) polyvinylpyrrolidone (pH 7.8) and kept at 4 °C for 10 min. Later, the homogenate was filtered using filter paper followed by centrifugation at 984 × g for 15 min at 4 °C. The supernatant (enzyme extract) was used for estimation of antioxidant enzymes.
Enzyme analysis
Catalase (CAT), superoxide dismutase (SOD) and ascorbate peroxidase (APX) were analyzed as per the protocols mentioned in Chatterjee et al. (2017).
CAT activity was estimated by a hydrogen peroxide assay which is based on the formation of its stable complex with ammonium molybdate. The enzyme extract (0.2 mL) was incubated in 1 mL reaction mixture containing 65 mM hydrogen peroxide in 60 mM sodium phosphate buffer at room temperature for 4 min. The reaction was terminated by addition of 1 mL of 32.4 mM of ammonium molybdate and the absorbance of the resultant yellow mixture was measured at 405 nm.
SOD activity was estimated by mixing the enzyme extract with 50 mM phosphate buffer (pH 7.8), 9.9 mM l-methionine, 57 µM nitro-blue tetrazolium (NBT), 0.025% (w/v) Triton X-100 and 0.0044% (w/v) riboflavin. The SOD activity was measured at 560 nm by monitoring the decrease in the absorbance due to the photochemical reduction of NBT.
APX activity was estimated by the oxidative conversion of ascorbic acid to dehydroascorbate with respect to the decrease in the absorbance. It was calculated by the means of extinction coefficient 2.8 mM−1 cm−1 at 290 nm. The reaction mixture consists of potassium phosphate buffer (50 mM, pH 7.0), ascorbic acid (0.3 mM), H2O2 (0.1 mM), EDTA (0.1 mM) and 50 µL of the enzyme extract.
Glutathione reductase (GR) activity was estimated by the protocol mentioned in de Azevedo Neto et al. (2006) with little modifications. The GR activity was measured as the rate of NADPH oxidation. The enzyme extract (0.1 mL) was mixed with 0.1 M phosphate buffer (pH 7.0), 1 mM EDTA, 10 mM glutathione, 1 mM NaN3, 1 unit of glutathione reductase, 1.5 mM NADPH and incubated at 37 °C for 10 min. H2O2 was added to each sample to a final concentration of 1 mM. The rate of oxidation of NADPH was measured as GR activity at 340 nm. The antioxidant enzyme activities were expressed as their specific enzyme activities in terms of units per minute–milligram protein (min−1 mg−1 protein).
Total protein estimation
Total protein content was estimated by the protocol mentioned in Bradford (1976). Different concentrations (10–100 μg mL−1) of bovine serum albumin (BSA) were used as standards and the absorbance was recorded at 595 nm.
Plant nutrient accumulation
Harvested tomato plants were oven dried at 80 °C for 72 h and the samples were blended into powder and analyzed according to a protocol mentioned in Lee et al. (2016). For other nutrients such as Ca2+, Mg2+ and K+, dried plant samples (0.5 g) were digested with the pre-digestion solution containing perchloric acid, sulfuric acid, and distilled water (9:1:2) and incubated at room temperature for 24 h. Later the incubated solution was filtered (No. 6, Advantec Tokyo) and the volume was made up to 100 mL. 10 mL solution was analyzed using the inductively coupled plasma optical emission spectroscopy (ICP-OES, Optima 5300DV, Perkin Elmer, USA). Plant total nitrogen content was estimated as per the standard protocol given by Bradstreet (1954). The nutrient accumulation was expressed in terms of total dry weight of individual plants (mg plant−1).
Statistical analysis
All data were normalized and subjected to analysis of variance (ANOVA). Significant differences among means were tested with Duncan’s Multiple Range Test (DMRT) at P < 0.05 using SAS Version 9.1.3 service pack 4 (designed by SAS Institute Inc., Cary, North Carolina, USA) for all data in the experiments.
Results
The chitosan formulation containing immobilized aggregated M. oryzae CBMB20 were tested on tomato plant growth under salt stress. The different formulation treatments were done to the seeds on the day of sowing (day 0). During transplantation on day 15, the plugs containing the plants and the support system were transferred to larger pots.
Plant dry weight
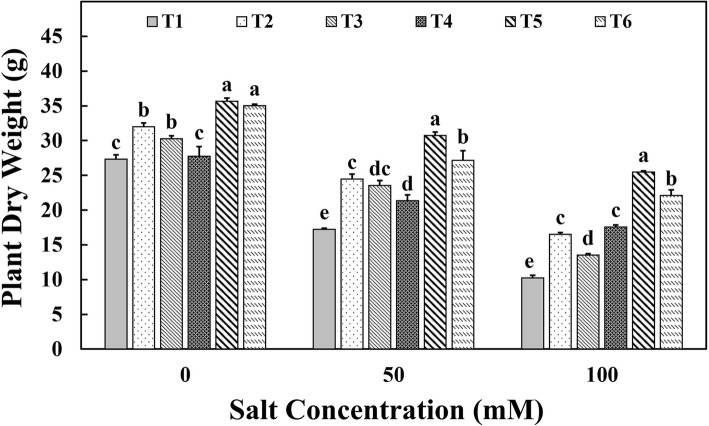
The total biomass of the plants determined in terms of total dry weight showed a drastic reduction in plants grown under salinity stress conditions irrespective of the treatments (Fig. 1). The plants treated with chitosan beads (T4) had shown an increase of 28% and 67% of plant dry weight compared to the non-inoculated control plants at 50 mM and 100 mM of salt stress conditions, respectively. On the other hand, the chitosan-immobilized aggregated M. oryzae CBMB20 (T5) registered significantly higher dry weight of plants grown under 50 mM and 100 mM salinity stress conditions compared to other treatments. The chitosan-immobilized aggregated M. oryzae CBMB20 had shown 78.02% increase in plant dry weight compared to the non-inoculated control plants under 50 mM salt stress conditions. Similarly, the increase was 1.5 fold compared to the control plants under 100 mM salinity stress conditions. Among the liquid formulations used, plants inoculated with aggregated cells biomass resulted in 61.07% increase in total dry weight compared to the un-inoculated control plants, whereas the non-aggregated cells enhanced the dry weight of the plants by 32.07% under 100 mM salt concentration.
Fig. 1.
Effect of treatments on the dry weight of tomato plants under salinity stress conditions. T1—control; T2—aggregated M. oryzae CBMB20 (liquid form); T3—non-aggregated M. oryzae CBMB20 (liquid form); T4—chitosan (blank), T5—chitosan-immobilized aggregated M. oryzae CBMB20 and T6—chitosan-immobilized non-aggregated M. oryzae CBMB20
Photosynthetic pigment analysis
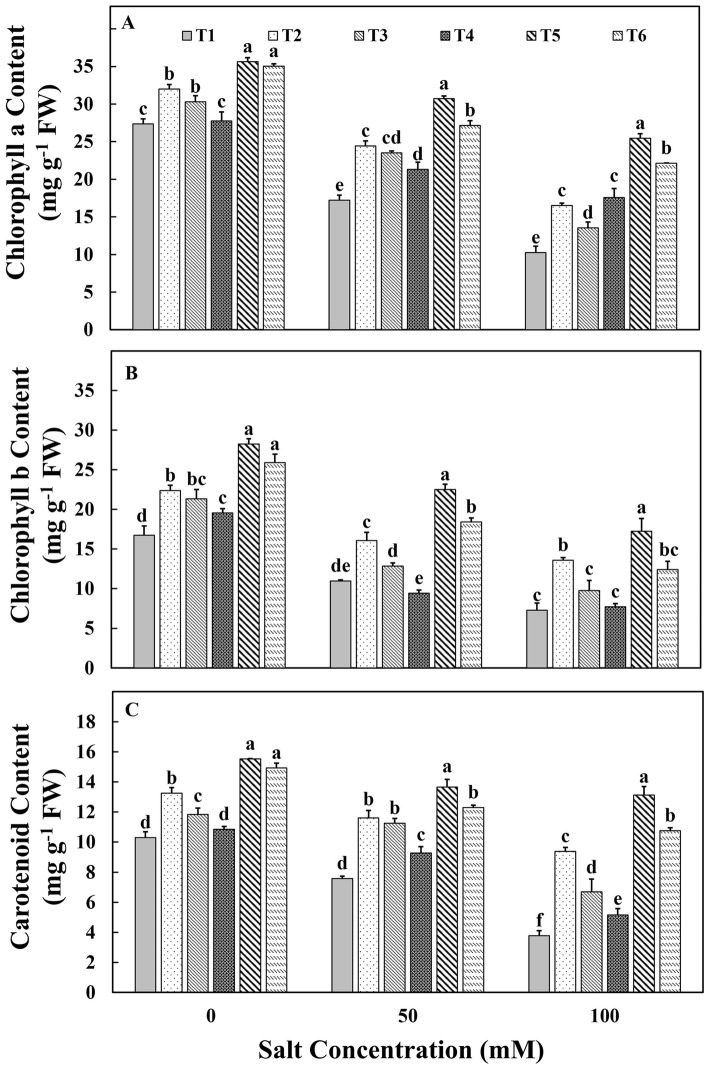
The photosynthetic efficiency was measured in terms of Chl a Chl b and carotenoid concentration. The results showed that a gradual decline with an increase in salt concentration (Fig. 2). However, the chlorophyll and carotenoid content of plants inoculated with chitosan-immobilized aggregated M. oryzae CBMB20 (T5) and chitosan-immobilized non-aggregated M. oryzae CBMB20 (T6) were significantly higher than plants inoculated with liquid formulation and control plants under normal conditions. However, the T5 treatment had shown significantly higher pigment contents compared to the other treatments under 50 mM and 150 mM salt stress conditions.
Fig. 2.
Effect of treatments on a Chlorophyll a b Chlorophyll b and c Carotenoid content of tomato plants under salinity stress conditions. T1—control; T2—aggregated M. oryzae CBMB20 (liquid form); T3—non-aggregated M. oryzae CBMB20 (liquid form); T4—chitosan (blank), T5—chitosan-immobilized aggregated M. oryzae CBMB20 and T6-chitosan-immobilized non-aggregated M. oryzae CBMB20
Proline accumulation and electrolyte leakage
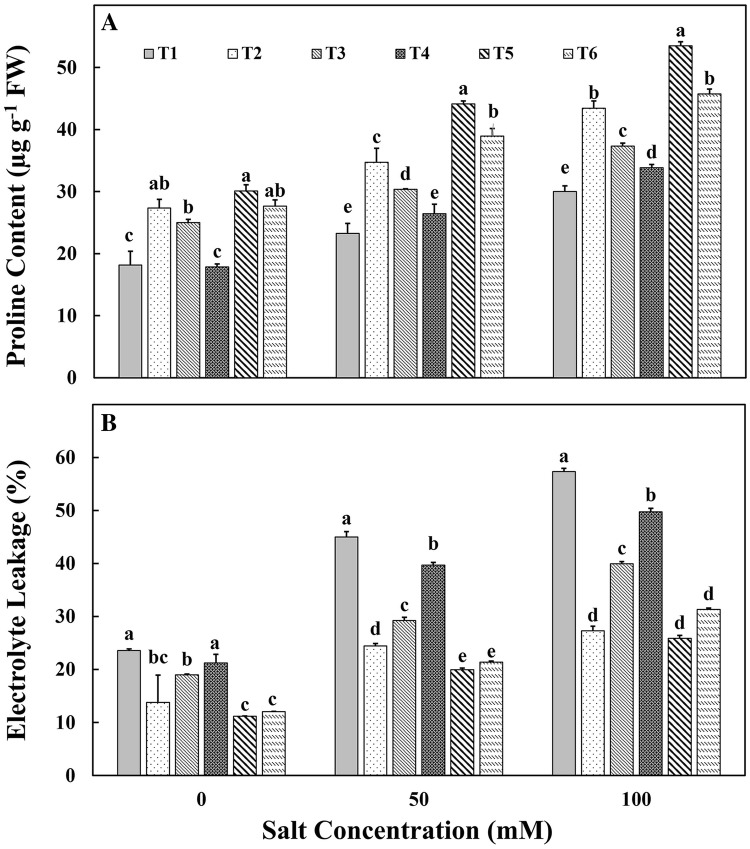
The proline content of tomato leaves were measured and elevated level of proline was observed upon NaCl amendment irrespective of any inoculation. However, T5 and T6 treatments registered significantly higher proline content (89.8% and 78.21%) compared to control plants stressed with 50 mM and 100 mM salt (Fig. 3a). The damage on leaves was measured in terms of electrolyte leakage. The electrolyte leakage had increased among all treatments under salinity stress conditions. The treatments T5 and T6 had significantly lower amount of damage in terms of electrolyte leakage under 50 mM and 100 mM NaCl amendment (Fig. 3b). In addition, T5 showed 20% decrease in damage compared to the control plants in 100 mM NaCl amendment plants. The damage measured in terms of electrolyte leakage showed that the inoculation of both liquid and chitosan-immobilized bacteria significantly improved the salt tolerance of the tomato plants (Fig. 3b). The tomato plants stressed with 100 mM salt produced more electrolyte leakage than 50 mM and plants receiving only Milli-Q water. Among the treatments, the maximum leakage values were recorded in control plants (T1) followed by chitosan alone (T4), non-aggregated cells (T3), chitosan-immobilized non-aggregated cells (T6), aggregated cells (T2) and chitosan-immobilized aggregated cells (T5) inoculated plants when stressed with 100 mM salt.
Fig. 3.
Effect of inoculation of various treatments on a proline content and b percentage of electrolyte leakage of tomato leaves under salt stress conditions. T1—control; T2—aggregated M. oryzae CBMB20 (liquid form); T3—non-aggregated M. oryzae CBMB20 (liquid form); T4—chitosan (blank), T5—chitosan-immobilized aggregated M. oryzae CBMB20 and T6—chitosan-immobilized non-aggregated M. oryzae CBMB20
MDA and H2O2 content
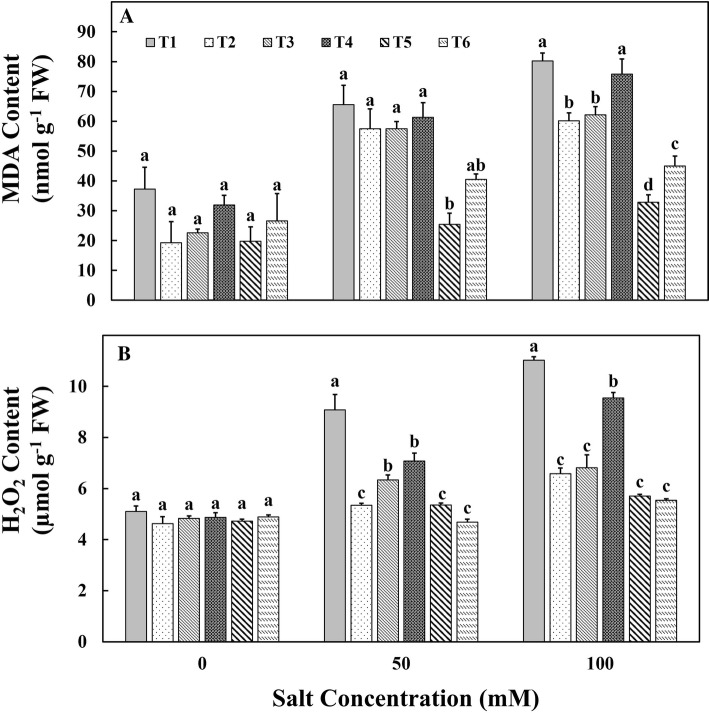
Both MDA and H2O2 contents serve as an indicator to determine the redox status of the plant. The MDA content of tomato leaves increased with an increase in NaCl concentration in all the treatments. However, the inoculation of both liquid and chitosan-immobilized bacterial cells registered lesser MDA content showing their impact against salt stress. However, the T5 treatment registered significantly lower MDA content than other treatments (Fig. 4a). The MDA content in T5 was 32 nmol g−1 of fresh weight (FW) compared to 80 nmol g−1 of FW for control plants. The amount of oxidative stress was determined in terms of H2O2 content. While the H2O2 content increased in all the plants under salt stress, it was higher in the control plants with salt stress followed by T4, T3, T2, T5, T6 (Fig. 4b). The significantly lower H2O2 content of 5.7 µmol g−1 FW was observed in T5 treatment.
Fig. 4.
Effect of treatments on a malondialdehyde content and b hydrogen peroxide content of tomato plants under salt stress conditions. T1—control; T2—aggregated M. oryzae CBMB20 (liquid form); T3—non-aggregated M. oryzae CBMB20 (liquid form); T4—chitosan (blank), T5—chitosan-immobilized aggregated M. oryzae CBMB20 and T6—chitosan-immobilized non-aggregated M. oryzae CBMB20
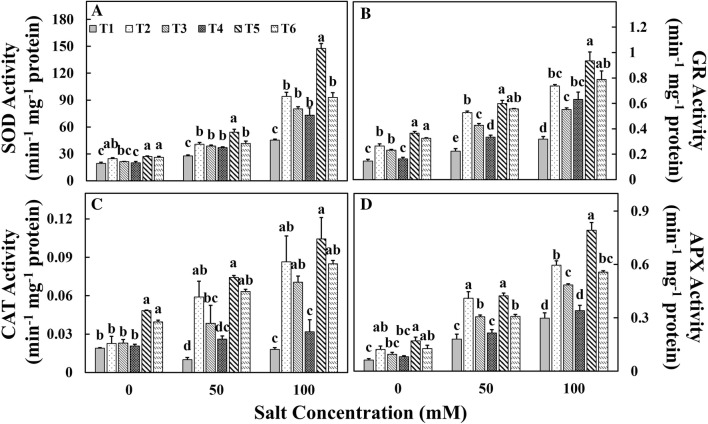
Antioxidant enzymes
The oxidative stress ameliorating response was determined by estimating the activity of various enzymes such as SOD, CAT, GR and APX in plants. The activity of these enzymes increased across the salt stress conditions, irrespective of the treatment applied on plants (Fig. 5). The plants treated with chitosan beads had shown an increase of 56% increase in SOD activity at 100 mM of salt stress conditions. Whereas, a onefold increase in GR activity was observed when plants were treated with chitosan beads at 100 mM of salt stress conditions. On the other hand, the chitosan-immobilized aggregated M. oryzae CBMB20 formulation recorded the maximum activity among other treatments. The increase in SOD activity was double the amount compared to the control plants under 50 mM salt stress condition (Fig. 5a). Similarly, the increase of 2.2 fold was recorded when chitosan-immobilized aggregated M. oryzae CBMB20 inoculated plants were subjected to 100 mM of salt stress condition. Similarly, glutathione reductase activity was maximum when plants were inoculated with chitosan-immobilized aggregated M. oryzae CBMB20 (Fig. 5b). The maximum catalase activity of 0.074 min−1 mg−1 protein and 0.104 min−1 mg−1 protein was recorded for chitosan-immobilized aggregated M. oryzae CBMB20 inoculated plants at 50 mM and 100 mM of salt stress condition (Fig. 5c). Ascorbate peroxidase activity was maximum when plants were inoculated with chitosan-immobilized aggregated M. oryzae CBMB20 (Fig. 5d).
Fig. 5.
Antioxidant enzyme activities a superoxide dismutase, b glutathione reductase, c catalase, d ascorbate peroxidase of tomato plants after application of treatments under salt stress conditions. T1—control; T2—aggregated M. oryzae CBMB20 (liquid form); T3—non-aggregated M. oryzae CBMB20 (liquid form); T4—chitosan (blank), T5—chitosan-immobilized aggregated M. oryzae CBMB20 and T6—chitosan-immobilized non-aggregated M. oryzae CBMB20
Nutrient accumulation
The uptake of nitrogen, phosphorus, potassium and other micronutrients like Ca2+, Mg2+, and Na+ were found significantly increased in the tomato plants inoculated with chitosan-immobilized aggregated and non-aggregated M. oryzae CBMB20 under salt stress conditions (Table 1). At the same time, increase in nutrient uptake was observed up on microbial treatment compared to control at 0 mM NaCl treated plants (Table 1). Increased Na+ uptake was observed in all the plants stressed with NaCl. In general, plants under increasing NaCl concentration in the soil showed that the uptake of Na+ was inversely proportional to the uptake of K+. However, inoculation with aggregated M. oryzae CBMB20 decreased the Na+/K+ ratio (Table 1). Chitosan-immobilized aggregated M. oryzae CBMB20 significantly improved nutrient accumulation compared to all other treatments (Table 1). The nitrogen uptake was improved by 70.2% and 142.57% by T5 compared to control plants under 50 mM and 100 mM of salt stress condition, respectively. Similarly, the phosphorus uptake was improved by 180.29% and 211. 6%; calcium uptake was improved by 145.65% and 429.3%; magnesium uptake was improved by 203.9% and 317.16% by T5 treatment compared to the control plants under 50 mM and 100 mM of salt stress conditions, respectively.
Table 1.
Effect of chitosan-immobilized M. oryzae CBM20 on the nutrient uptake of tomato under salt stress
| Salt concentration | Treatments | Total N | P− | Ca2+ | Mg2+ | K+ | Na+ | Na+/K+ |
|---|---|---|---|---|---|---|---|---|
| mg plant−1 | ||||||||
| 0 mM | T1 | 462.5 ± 13.1e | 116.4 ± 0.8d | 1801.7 ± 33.4d | 403.4 ± 2.1d | 768.5 ± 15.2c | 192.6 ± 3.3b | 0.25 |
| T2 | 674.8 ± 5.9b | 152.1 ± 10.7c | 2543.0 ± 92.9b | 596.2 ± 9.7b | 962.7 ± 11.0b | 193.6 ± 4.8b | 0.20 | |
| T3 | 595.2 ± 6.8c | 150.3 ± 4.7c | 2253.3 ± 167.0c | 536.0 ± 17.9c | 938.6 ± 24.5b | 175.4 ± 4.8c | 0.19 | |
| T4 | 524.2 ± 6.4d | 156.4 ± 5.8c | 2068.5 ± 18.7 cd | 410.3 ± 4.8d | 815.2 ± 5.5c | 212.6 ± 2.7a | 0.26 | |
| T5 | 783.7 ± 2.8a | 258.5 ± 7.1a | 3171.8 ± 147.9a | 696.6 ± 44.3a | 1164.7 ± 52.7a | 184.9 ± 8.4bc | 0.16 | |
| T6 | 697.3 ± 5.6b | 189.7 ± 12.6b | 2751.5 ± 53.9b | 682.1 ± 14.7a | 1115.5 ± 21.0a | 227.1 ± 5.0a | 0.20 | |
| 50 mM | T1 | 453.7 ± 9.8f | 61.9 ± 6.6d | 907.3 ± 104.5d | 174.3 ± 13.0e | 337.9 ± 9.8e | 582.9 ± 48.9a | 1.73 |
| T2 | 600.3 ± 1.8c | 108.5 ± 1.1c | 1826.1 ± 38.6b | 413.8 ± 14.6b | 578.7 ± 1.8c | 525.9 ± 2.8ab | 0.91 | |
| T3 | 540.0 ± 6.4d | 97.7 ± 5.3c | 1570.2 ± 74.5c | 348.1 ± 14.0c | 534.5 ± 6.5c | 563.2 ± 18.0ab | 1.05 | |
| T4 | 491.3 ± 5.5e | 77.2 ± 5.4d | 1371.7 ± 46.7c | 285.5 ± 13.4d | 441.7 ± 5.5d | 582.7 ± 25.5a | 1.32 | |
| T5 | 771.1 ± 5.7a | 173.5 ± 6.3a | 2228.8 ± 73.4a | 529.7 ± 21.0a | 844.9 ± 5.7a | 485.1 ± 21.7b | 0.57 | |
| T6 | 653.6 ± 2.9b | 127.5 ± 4.2b | 1935.3 ± 26.8b | 425.8 ± 3.2b | 682.1 ± 2.9b | 530.7 ± 8.9ab | 0.78 | |
| 100 mM | T1 | 256.7 ± 4.1e | 31.9 ± 1.6e | 334.1 ± 33.9f | 97.3 ± 7.4e | 161.7 ± 4.1f | 762.7 ± 41.8a | 4.72 |
| T2 | 367.9 ± 4.6c | 58.2 ± 2.4c | 1124.4 ± 18.4c | 229.5 ± 1.5c | 364.9 ± 4.6c | 662.3 ± 19.2bc | 1.82 | |
| T3 | 269.8 ± 1.4e | 49.6 ± 3.7d | 839.6 ± 25.5e | 165.4 ± 2.9d | 260.7 ± 1.4e | 701.4 ± 13.1ab | 2.69 | |
| T4 | 329.7 ± 6.3d | 34.6 ± 1.4e | 1019.1 ± 49.9d | 171.1 ± 3.6d | 293.6 ± 6.3d | 725.5 ± 20.9ab | 2.47 | |
| T5 | 622.7 ± 8.8a | 99.4 ± 3.8a | 1768.3 ± 7.9a | 405.9 ± 5.1a | 657.4 ± 8.8a | 573.9 ± 5.4d | 0.87 | |
| T6 | 509.9 ± 0.4b | 74.3 ± 2.2b | 1444.2 ± 6.8b | 306.1 ± 3.9b | 550.1 ± 0.4bs | 603.3 ± 5.7 cd | 1.10 | |
T1—control, T2—aggregated M. oryzae CBMB20 (liquid form), T3—non-aggregated M. oryzae CBMB20 (liquid form), T4—chitosan (blank), T5—chitosan-immobilized aggregated M. oryzae CBMB20 and T6—chitosan-immobilized non-aggregated M. oryzae CBMB20. Each value represents mean ± S.E (standard error) of three replications, letters show differences between treatments according to DMRT test (P ≤ 0.05)
Discussion
Plant growth and productivity in saline soils are severally affected by salt stress induced ion toxicity and oxidative damage (Ilangumaran and Smith 2017). Plant growth promoting bacteria (PGPB) or chemical substances are widely used for mitigating various biotic and abiotic stress in plants (Ilangumaran and Smith 2017). PGPB protect plants against the inhibitory effects of ethylene-producing stresses that include drought, flooding, temperature extremes, high salt, metal and organic contaminants, insect and nematode predation, and both fungal and bacterial phytopathogens (Joe et al. 2016). ACCD produced by PGPB stimulates plant growth by decreasing the ethylene levels thereby relieving some of the growth inhibition that increased ethylene causes (Glick 2012). In addition, IAA produced by bacteria enhances plant lateral root and root hair formation thereby enhancing wide range of environmental stress tolerance (Ramadoss et al. 2013; Duca et al. 2014; Singh and Jha 2016). In this study, we have inoculated both liquid and chitosan-immobilized aggregated and non-aggregated M. oryzae CBMB20 to tomato plants and demonstrated the effectiveness of chitosan-immobilized aggregated cells over non-aggregated cells in inducing salt tolerance and tomato plant under salt stress. M. oryzae CBMB20 is known for higher ACCD activity (600 pmol α-ketobutyrate mg−1 protein h−1) and IAA production (3.7 µg mL−1) under salt stress conditions (Chanratana et al. 2017) and enhanced plant growth promotion, when applied in the form of chitosan and alginate immobilized M. oryzae CBMB20 formulations (Chanratana et al. 2018).
Salt stress is linked to oxidative stress with respect to overproduction of ROS (OH−, O2−, H2O2), detrimental for plant’s survival (Patel and Saraf 2013). In this study, chitosan-immobilized aggregated M. oryzae CBMB20 induced defense mechanism against ROS by significantly promoting higher antioxidants in tomato plants resulting in higher plant biomass. Malondialdehyde (MDA) content is directly correlated with the oxidative damage of the cell membrane in plant (Arbona et al. 2008). In addition, the plants treated with chitosan beads (T4 treatment) had shown higher plant dry weight and antioxidant activity compared to the non-inoculated control plants at 50 mM and 100 mM of salt stress conditions. Abdel-Mawgoud et al. (2010) had reported that the enhancement of strawberry plant growth after chitosan solution treatment under field conditions. A similar study had also shown the increase in germination percentage, plant growth and antioxidant enzymes after treating safflower and sunflower plants with chitosan under salt stress conditions (Jabeen and Ahmad 2013). In the present study, tomato plants under salt stress conditions showed lower levels of MDA content when inoculated with aggregated M. oryzae CBMB20 entrapped in chitosan. Lower MDA concentrations have been reported to represent less membrane damage or increased salt tolerance of the plants (Jha and Subramanian 2014). Accumulation of ROS results in activation of SOD activity, which leads to synthesis of H2O2, a toxic signal molecule for oxidative stress (Miller et al. 2010). Proline is an osmolyte which accumulates in plant during salt stress condition to protect the cells from osmotic stress. Osmolytes contributes in preventing cell membrane disruption and increase membrane stability under salt stress condition. It is reported that proline enhances plant salt/osmotic stress tolerance, by not only adjusting the osmotic pressure but also involving in stabilizing many proteins, enzymes and cell membrane (Ramadoss et al. 2013). Enhanced proline concentration was observed in tomato leaves inoculated with PGPB (Mohamed and Gomaa 2012). In the present study, inoculation with chitosan-immobilized aggregated M. oryzae CBMB20 triggered a significant accumulation of proline in tomato under increasing salt concentration compared to the un-inoculated control. This could be due to upregulation of proline biosynthesis pathway to keep proline in high levels, which helps in maintaining cell water status, protects membranes and proteins from stress. In other causes increased accumulation of Ca2+ ions also increase plant proline content, which leads to higher water potential gradient thereby enhance water uptake under salt stress condition (Misra and Gupta 2006).
Antioxidant enzymes play a major role in reducing reactive oxygen species content in plants which produced under stress condition. Previously, many reports showed the importance of antioxidant enzymes in enhancing plant growth under salt stress condition (Patel and Saraf 2013; Jha and Subramanian 2014). Accumulation of H2O2 triggers the CAT (catalase), APX (ascorbate peroxidase) and POD (peroxidase) activities to reduce its concentration by converting into oxygen and water (Heidari and Golpayegani 2012). This may be the energy efficient mechanisms of removing hydrogen peroxide (Patel and Saraf 2013). In agreement with earlier reports (Heidari and Golpayegani 2012), the present study showed that the inoculation with chitosan-immobilized aggregated M. oryzae CBMB20 increased the production of antioxidant enzymes such as SOD, CAT, APX and GR.
Leaf chlorophyll concentration is an important indicator of salt tolerance and responses to increasing salinity (Percival 2005). Inoculation of PGPB has been reported to increase the chlorophyll content in different plants including tomato plants compared to control. It was proven that inoculation of ACCD containing bacteria significantly suppress ethylene synthesis so that the chlorophyll decay was decreased and stop leaf senescence due to increased ethylene levels (Arshad et al. 2008). Numerous others have also reported increased chlorophyll content in plants inoculated with PGPB (Mohamed and Gomaa 2012; Patel and Saraf 2013).The increase in chlorophyll content and reduced membrane damage by bacterial inoculation can also lead to increase the concentration of ascorbic acid in plant cells (Nadeem et al. 2007).
In salt stress condition, nutrient imbalance occurs in plants due to the higher accumulation of Na+ and Cl− in plants (Mohamed and Gomaa 2012). Earlier studies showed that inoculation of bacteria enhances the uptake of N, P, K+, Ca2+ and Mg2+ and maintain nutrient balance in plant cells (Singh and Jha 2016). In line with this, the present study results exhibited inoculation of chitosan-immobilized aggregated M. oryzae CBMB20 increased the accumulation of N, P, K+, Ca2+ and Mg2+, and on the other hand the accumulation of Na+ in plant cell was reduced compared to control plants.
Conclusions
The present study demonstrated that the inoculation of chitosan-immobilized aggregated M. oryzae CBMB20 increased the resistance of tomato plants under elevated salt concentrations. The immobilized bacterium induced the production of proline and antioxidant enzymes, which resulted in decrease in oxidative stress on tomato plants measured in terms of H2O2 and MDA content. On the other hand, the chitosan-immobilized M. oryzae CBMB20 inoculation reduced electrolyte leakage and enhanced biomass and nutrient uptake of the plants under salt stress. The bacterium applied as liquid formulation with aggregated cells also increased all the parameters compared to control. In addition, the plants treated with chitosan beads had resulted in increase in plant dry weight and antioxidant enzyme activities especially the activities of SOD and GR enzymes. The use of chitosan to immobilize bacteria explains the synergistic effect of both chitosan and the bacterium for promoting the plant growth under salt stressed conditions. Based on the results obtained in this study it could be concluded that the chitosan formulation of M. oryzae CBMB20 can be used as inoculant for obtaining better salt tolerance without any requirement for genetic manipulation of the target plants. Therefore, the chitosan-immobilized PGPB may be used as an effective formulation which can be scaled up for enhancing plant growth under salt stress condition.
Acknowledgements
This work was supported by the Basic Science Research Program, National Research Foundation of Korea (NRF), Ministry of Education, Science and Technology [2015R1A2A1A05001885], Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that we have no competing interests.
Footnotes
Mak Chanratana and Manoharan Melvin Joe authors have contributed equally to this work.
References
- Abdel-Mawgoud AMR, Tantawy AS, El-Nemr MA, Sassine YN. Growth and yield responses of strawberry plants to chitosan application. Eur J Sci Res. 2010;39(1):170–177. [Google Scholar]
- Ambrosini A, de Souza R, Passaglia LM. Ecological role of bacterial inoculants and their potential impact on soil microbial diversity. Plant Soil. 2016;400:193–207. doi: 10.1007/s11104-015-2727-7. [DOI] [Google Scholar]
- Angelim AL, Costa SP, Farias CS, Freitas FL, Melo MVM. An innovative bioremediation strategy using a bacterial consortium entrapped in Chitosan beads. J Enviorn Manag. 2013;127:10–17. doi: 10.1016/j.jenvman.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Arbona V, Hossain Z, Lopez-Climent MF, Perez-Clement RM, Gomez Cadenas A. Antioxidant enzymatic activity is linked to water-logging stress tolerance in citrus. Physiol Plant. 2008;132:452–466. doi: 10.1111/j.1399-3054.2007.01029.x. [DOI] [PubMed] [Google Scholar]
- Arshad M, Shaharoona B, Mahmood T. Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.) Pedosphere. 2008;18:611–620. doi: 10.1016/S1002-0160(08)60055-7. [DOI] [Google Scholar]
- Bashan Y, de Bashan LE, Prabhu SR, Hernandez JP. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013) Plant Soil. 2014;378:1–33. doi: 10.1007/s11104-013-1956-x. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradstreet RB. Kjeldahl method for organic nitrogen. Anal Chem. 1954;26:185–187. doi: 10.1021/ac60085a028. [DOI] [Google Scholar]
- Chanratana M, Han GH, Choudhury AR, Sundaram S, Halim MA, Krishnamoorthy R, Kang Y, Sa T. Assessment of Methylobacterium oryzae CBMB20 aggregates for salt tolerance and plant growth promoting characteristics for bioinoculant development. AMB Express. 2017;7:208. doi: 10.1186/s13568-017-0518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanratana M, Han GH, Joe MM, Choudhury AR, Sundaram S, Halim MA, Sa T. Evaluation of chitosan and alginate immobilized Methylobacterium oryzae CBMB20 on tomato plant growth. Arch Agro Soil Sci. 2018;2018:1–14. doi: 10.1080/03650340.2018.1440390. [DOI] [Google Scholar]
- Chatterjee P, Samaddar S, Anandham R, Kang Y, Kim K, Selvakumar G, Sa T. Beneficial soil bacterium Pseudomonas frederiksbergensis OS261 augments salt tolerance and promotes red pepper plant growth. Front Plant Sci. 2017 doi: 10.3389/fpls.2017.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P, Samaddar S, Niinemets Ü, Sa T. Brevibacterium linens RS16 confers salt tolerance to Oryza sativa genotypes by regulating antioxidant defense and H+ ATPase activity. Microbial Res. 2018;215:89–101. doi: 10.1016/j.micres.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Dar TA, Uddin M, Masroor AK, Ali A, Mir SR, Varshney L. Effect of Co-60 gamma irradiated Chitosan and phosphorus fertilizer on growth, yield and trigonelline content of Trigonella foenum-graecum L. J Radiat Res Appl Sci. 2015;8:446–458. doi: 10.1016/j.jrras.2015.03.008. [DOI] [Google Scholar]
- de Azevedo Neto AD, Prisco JT, Enéas-Filho J, de Abreu CEB, Gomes-Filho E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot. 2006;56:87–94. doi: 10.1016/j.envexpbot.2005.01.008. [DOI] [Google Scholar]
- De Pinto MC, Locato V, Gara DL. Redox regulation in plant programmed cell death. Plant Cell Environ. 2012;35:234–244. doi: 10.1111/j.1365-3040.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- Duca D, Lorv J, Patten CL, Rose D, Glick BR. Indole-3-acetic acif in plant-microbe interactions. Antonie Van Leeuwenhoek. 2014;106:85–125. doi: 10.1007/s10482-013-0095-y. [DOI] [PubMed] [Google Scholar]
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari M, Golpayegani A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.) J Saudi Soc Agric Sci. 2012;11:57–61. doi: 10.1016/j.jssas.2011.09.001. [DOI] [Google Scholar]
- Ilangumaran G, Smith DL. Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci. 2017 doi: 10.3389/fpls.2017.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabeen N, Ahmad R. The activity of antioxidant enzymes in response to salt stress in safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) seedlings raised from seed treated with chitosan. J Sci Food Agric. 2013;93(7):1699–1705. doi: 10.1002/jsfa.5953. [DOI] [PubMed] [Google Scholar]
- Jha Y, Subramanian RB. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol Mol Biol Plants. 2014;20:201–207. doi: 10.1007/s12298-014-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe M, Karthikeyan MB, Sekar C, Deiveekasundaram M. Optimization of biofloc production in Azospirillum brasilense (MTCC-125) and evaluation of its adherence with the roots of certain crops. Indian J Microbiol. 2010;50:21–25. doi: 10.1007/s12088-010-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe MM, Devaraj S, Benson A, Sa T. Isolation of phosphate solubilizing endophytic bacteria from Phyllanthus amarus Schum and Thonn: evaluation of plant growth promotion and antioxidant activity under salt stress. J Appl Res Med Aromat Plants. 2016;3:71–77. doi: 10.1016/j.jarmap.2016.02.003. [DOI] [Google Scholar]
- Lee G, Carrow RN, Duncan RR, Eiteman MA, Rieger MW. Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ Exp Bot. 2008;63:19–27. doi: 10.1016/j.envexpbot.2007.10.009. [DOI] [Google Scholar]
- Lee YW, Krishnamoorthy M, Selvakumar G, Kum KY, Sa T. Alleviation of salt stress in maize plant by co-inoculation of arbuscular mycorrhizal fungi and Methylobacterium oryzae CBMB20. J Korean Soc Appl Biol Chem. 2016;58:533–540. doi: 10.1007/s13765-015-0072-4. [DOI] [Google Scholar]
- Liu CH, Wu JY, Chang JS. Diffusion characteristics and controlled the release of bacterial Fertilizer from modified calcium alginate capsules. Bioresour Technol. 2008;99:1904–1910. doi: 10.1016/j.biortech.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Machado RMA, Serralheiro RP. Soil Salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae. 2017;3:30. doi: 10.3390/horticulturae3020030. [DOI] [Google Scholar]
- Madhaiyan M, Kim BY, Poonguzhali S, Kwon SW, Song MH, Ryu JH, Sa TM. Methylobacterium oryzae sp. nov., an aerobic, pink-pigmented, facultatively methylotrophic, 1-aminocyclopropane-1-carboxylate deaminase-producing bacterium isolated from rice. Int J Syst Evol Microbiol. 2007;57(2):326–331. doi: 10.1099/ijs.0.64603-0. [DOI] [PubMed] [Google Scholar]
- Madhaiyan M, Poonguzhali S, Sa T. Characterization of 1-aminocyclopropane-1-carboxylate (ACC) deaminase containing Methylobacterium oryzae and interactions with auxins and ACC regulation of ethylene in canola (Brassica campestris) Planta. 2007;226(4):867–876. doi: 10.1007/s00425-007-0532-0. [DOI] [PubMed] [Google Scholar]
- Miller GAD, Suzuki N, Ciftci-Yilmaz SULTAN, Mittler RON. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Mishra PK, Bisth SC, Ruwari P, Selvakumar G, Joshi GK, BisthJK Bhatt JC, Gupta HS. Alleviation of cold stress in inoculated wheat (Triticum aestivum L.) seedlings with psychrotolerant Pseudomonas from NW Himalayas. Arch Microbiol. 2011;193:497–513. doi: 10.1007/s00203-011-0693-x. [DOI] [PubMed] [Google Scholar]
- Misra N, Gupta AK. Interactive effects of sodium and calcium on proline metabolism in salt tolerant green gram cultivar. Am J Plant Physiol. 2006;1:1–12. doi: 10.3923/ajpp.2006.1.12. [DOI] [Google Scholar]
- Mohamed HI, Gomaa EZ. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescens on growth and pigment composition of radish plants (Raphanus sativus) under NaCl stress. Photosynthetica. 2012;50:263–272. doi: 10.1007/s11099-012-0032-8. [DOI] [Google Scholar]
- Nadeem SM, Zahir ZA, Naveed M, Arshad M. Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can J Microbiol. 2007;53:1141–1149. doi: 10.1139/W07-081. [DOI] [PubMed] [Google Scholar]
- Parray JA, Jan S, Kamili AN, Qadri RA, Egamberdieva D, Ahmad P. Current perspectives on plant growth-promoting rhizobacteria. J Plant Growth Regul. 2016;35:877. doi: 10.1007/s00344-016-9583-4. [DOI] [Google Scholar]
- Patel D, Saraf M. Influence of soil ameliorants and microflora on induction of antioxidant enzymes and growth promotion of Jatropha curcas L. under saline condition. Euro J Soil Bio. 2013;55:47–54. doi: 10.1016/j.ejsobi.2012.12.004. [DOI] [Google Scholar]
- Percival GC. The use of chlorophyll fluorescence to identify chemical and environmental stress in leaf tissue of three oak (Quercus) species. J Arboric. 2005;31:215. [Google Scholar]
- Ramadoss D, Lakkineni VK, Bose P, Ali S, Annapurna K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springer Plus. 2013;2:6. doi: 10.1186/2193-1801-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez MÁ, Rodriguez AT, Alfonso L, Peniche C. Chitin and its derivatives as biopolymers with potential agricultural applications. Biotecnol Apl. 2010;27:270–276. [Google Scholar]
- Schoebitz M, López MD, Roldán A. Bioencapsulation of microbial inoculants for better soil–plant fertilization. A review. Agron Sustain Dev. 2013;33:751–765. doi: 10.1007/s13593-013-0142-0. [DOI] [Google Scholar]
- Singh RP, Jha PN. Mitigation of salt stress in the wheat plant (Triticum aestivum) by ACC deaminase bacterium Enterobacter sp. SBP-6 isolated from Sorgum bicolor. Acta Physiol Plant. 2016;38:110. doi: 10.1007/s11738-016-2123-9. [DOI] [Google Scholar]
- Souza RD, Amborosini A, Passaglia MP. Plant growth—promoting bacteria as inoculants in agricultural soils. Genet Mol Biol. 2015;38:401–409. doi: 10.1590/S1415-475738420150053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanta N, Haque CI, Nishika J, Suprakash R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res J Chem Sci. 2014;4:63–69. [Google Scholar]
- Tank N, Saraf M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J Plant Interact. 2010;5(1):51–58. doi: 10.1080/17429140903125848. [DOI] [Google Scholar]
- Yen MT, Mau JL. Selected physical properties of chitin prepared from shiitake stipes. Food Sci Technol. 2007;40:558–563. doi: 10.1016/j.lwt.2005.10.008. [DOI] [Google Scholar]
- Zhou B, Wang J, Guo Z, Tan H, Zhu X. A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul. 2006;49:113–118. doi: 10.1007/s10725-006-9000-2. [DOI] [Google Scholar]