Abstract
In the field of regenerative medicine, generating numerous transplantable functional cells in the laboratory setting on a large scale is a major challenge. However, the in vitro maintenance and expansion of terminally differentiated cells are challenging because of the lack of specific environmental and intercellular signal stimulations, markedly hindering their therapeutic application. Remarkably, the generation of stem/progenitor cells or functional cells with effective proliferative potential is markedly in demand for disease modeling, cell-based transplantation, and drug discovery. Despite the potent genetic manipulation of transcription factors, integration-free chemically defined approaches for the conversion of somatic cell fate have garnered considerable attention in recent years. This review aims to summarize the progress thus far and discuss the advantages, limitations, and challenges of the impact of full chemicals on the stepwise reprogramming of pluripotency, direct lineage conversion, and direct lineage expansion on somatic cells. Owing to the current chemical-mediated induction, reprogrammed pluripotent stem cells with reproducibility difficulties, and direct lineage converted cells with marked functional deficiency, it is imperative to generate the desired cell types directly by chemically inducing their potent proliferation ability through a lineage-committed progenitor state, while upholding the maturation and engraftment capacity posttransplantation in vivo. Together with the comprehensive understanding of the mechanism of chemical drives, as well as the elucidation of specificity and commonalities, the precise manipulation of the expansion for diverse functional cell types could broaden the available cell sources and enhance the cellular function for clinical application in future.
Keywords: Chemical induction, Pluripotent reprogramming, Direct lineage conversion, Direct lineage expansion, Hepatocyte expansion, Cell fate specificity, Transcriptional memory, In vivo induction
Core tip: Chemical-mediated reprogramming is a promising strategy for generating desired cells. However, chemical-mediated pluripotent reprogramming has reproducibility difficulties, and direct lineage conversion shows significant deficiency in cell function maturation. On the other hand, direct lineage expansion from target cells not only bypasses pluripotency-related tumorigenesis but also has superior posttransplantation advantages in engraftment and functional maturation. Recent achievements in chemical expansion of human hepatocytes may help solve the cell source limitation in liver disease treatment.
INTRODUCTION
The barriers to cell fate conversion between somatic cells and pluripotent cells had a breakthrough with the proposition of the induced pluripotent stem cell (iPSC) reprogramming strategy in 2006, when Takahashi et al[1,2] reported a significant discovery that the ectopic expression of four defined transcription factors (TFs; Oct4, Sox2, Klf4a, and c-Myc) could force the cell fate conversion. Remarkably, iPSCs can be converted into multiple functional cells under optimal differentiation strategies, not only contributing to the establishment of patient-specific disease models but also benefiting drug discovery and development[3-5]. Later, combinations of lineage-specific TFs were screened and applied to generate various desired cell types, known as direct lineage conversion, including neurons, cardiomyocytes, hepatocytes, and pancreatic β-cells[6-9]; this strategy bypasses the transition pluripotent stage, rendering the process faster and more effective and, meanwhile, evading the risk of pluripotency-related tumorigenesis, holding great promise for biomedical applications[10,11].
However, to date, TF-based reprogramming approaches face numerous challenges in efficiency, especially in safety, regarding the use of oncogenes and likely genetic integration of exogenous factors. Researchers have made substantial efforts to optimize the reprogramming using nonviral and nonintegrating methods, including synthetic RNAs[12,13], cell membrane–permeable proteins[14], episomal plasmids[15], and chemical compounds[16]. Comparatively, chemicals offer several unique advantages such as structural stability; spatiotemporal flexibility; easy for screening, application, and delivery; amenability to manufacturing and scale-up; and the possibility of fine-tuning their effects by altering their concentrations and combinations[17,18]. To date, several small molecules have been proved to facilitate the iPSCs reprogramming and lineage conversion, either by their substitute role for replacing certain TFs, or synergetic effect for augmenting the efficiency[19-22]. Additionally, the progressive attainment of full chemical-induced human pluripotent and lineage-committed cells merits great promise to resolve the cell source limitation for the therapeutic purpose (Figure 1), as well as offer an alternative option for human disease modeling and drug development.
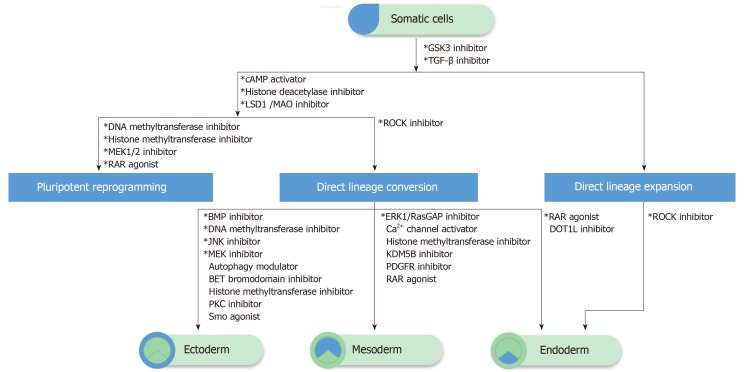
Figure 1.
The schematic of chemical-driven cell fate change and expansion. The transforming growth factor (TGF)-β and GSK3 pathway inhibitors are commonly required in pluripotency reprogramming, direct lineage conversion, and expansion. Additional epigenetic modulators (histone deacetylase inhibitors and/or deoxyribonucleic acid/histone methyltransferase inhibitors) are applied for pluripotency reprogramming, as well as direct lineage conversion, while different lineage commitments require specific signaling modulations. The combination of TGF-β, GSK3, and ROCK pathway inhibitors could induce the direct lineage expansion of endoderm-committed cells such as hepatocytes. The direct expansion of ectoderm and mesoderm-committed cells remains unclear and could not be listed here. *Necessary and/or commonly used compounds.
Perhaps, establishing a stable and efficient chemical induction strategy could fundamentally alter the principal concept of the conventional cell reprogramming strategy. Nevertheless, to date, the specificity of chemical targets and the correlative gene network dynamics remain unclear[17,23]. Thus, elucidation of the individual or synergetic chemical force on the cell pathway signaling and epigenetic pattern modulation would be vital to endorse the induction of desired cell types proficiently. Hence, this review aims to summarize the updated progress and discuss the advantages, limitations, and challenges of the impact of full chemicals on stepwise reprogramming of pluripotency, direct lineage conversion, and direct lineage expansion on somatic cells.
PLURIPOTENT REPROGRAMMING
The first breakthrough for pluripotency reprogramming by full chemical treatment was attained in 2013[24]. By screening, Hou et al[24] first successfully identified the chemical substitutes for Takahashi et al[1,2]’s four TFs, including VPA, CHIR99021, 616452, tranylcypromine, Forskolin, and DZNep (VC6TFZ), which could convert mouse fibroblasts into a partially reprogrammed state with high Oct4 expression; embryonic stem cell-like chemically-iPSs (CiPSCs) could be generated after switching to 2i (CHIR99021 and PD0325901) medium. Additionally, it was revealed that an endogenous pluripotency program could be established by chemically manipulating the cell signaling pathways. Although the routes and mechanisms underlying pluripotency reprogramming remain unclear to date, two primary hypotheses reveal how chemicals drive somatic cells stepwise toward pluripotency.
Stepwise bridges
During the attainment of a pluripotent program, unique extraembryonic endoderm (XEN)–like state was first discovered linking fibroblasts to pluripotency, which markedly expressed XEN genes Sall4, Gata4, Gata6, Sox17, and Sox7[25]. Remarkably, the knockdown of XEN genes during the reprogramming markedly impairs the generation of CiPSCs, whereas their overexpression is adequate to replace essential chemicals (CHIR99021, 616452, and Forskolin) for the Oct4-positive XEN-like colony formation, illustrating that the XEN-like state is a vital intermediate state toward CiPSCs. Of note, despite different cell origins, the similar activation of the XEN-like program was reported during the early stage of reprogramming from neural stem cells (NSCs) and small intestinal epithelial cells (IECs)[26], highlighting that the XEN-like state serves as a vital and exclusive bridge toward pluripotency. Remarkably, the reprogramming kinetics and frequency of XEN-like state were highly distinct between NSCs and IECs. Compared with NSCs, IECs exhibited much rapid and efficient XEN-like colony formation, which could be explained by the fact that the inherent expression of Gata4 and Gata6 in IECs facilitated the conversion of XEN-like program at an early stage.
After 3 years, the same group reported that the 2C (two-cell stage)-like programs were key bridges linking the XEN-like state to pluripotency, and the expression level of the 2C-like program (Zscan4c, Zscan4f, Tcstv1, Tcstv3, Lmx1a, and Sp110) well correlated with the reprogramming potential[27]. The knockdown of 2C genes markedly impaired the reprogramming efficiency of CiPSCs. Additionally, extensive loss of deoxyribonucleic acid (DNA) methylation was detected in this stage, which corroborated the hallmark of global epigenetic reprogramming in early embryogenesis, elucidating further the stepwise establishment of “early embryonic-like programs’’ toward the complete pluripotency network. Notably, when the 2C-like program was enhanced by the optimized treatment of VPA (histone deacetylase inhibitor), the reprogramming efficiency was markedly augmented.
Chromatin accessibility dynamics
BrdU, commonly used in tracing DNA replication, was occasionally reported to facilitate OSKM-induced reprogramming and demonstrated further that it could replace Oct4 in Yamanaka factors–mediated reprogramming and promote full chemical reprogramming[28]. Nevertheless, to date, the precise mechanisms by which BrdU promotes TFs and chemical-mediated reprogramming remain unclear. Based on the benefits of BrdU on closing or opening of chromatin loci, the dynamic chemical reprogramming process was revealed in the view of chromatin accessibility[29]. At stage 1 of chemical induction, the AP1 families–related chromatin loci in fibroblasts were closed gradually, whereas chromatin loci enriched with GATAs, FOXs, KLFs, and SOXs were opened, which highly corroborated the XEN-like intermediate state, as reported previously[25]; however, it markedly differed from TF-mediated reprogramming, which does not need to bypass through this particular state[30], illustrating the unique epigenetic dynamics driven by chemicals. Following the treatment of 2iL at stage 2, GATAs- and FOXs-related loci turned to close gradually, along with the opening for OCT/SOX/KLF families. Notably, BrdU is essential to correctively open and close chromatin loci enriched with the XEN-like and fibroblast program at stage 1. Remarkably, in this study, the overexpression of master XEN genes could not replace BrdU for opening specific loci for the Oct4 expression, which contradicted a previous study[25].
Despite published protocols, currently, the successful induction of CiPSCs is restricted in limited groups, and the core factors and induction efficiency remain debatable[25,27-29,31], which could be partially explained by the nonspecificity of chemicals. Additionally, the targeted signaling pathway and/or epigenetic modulation could change much because of the tiny bias of chemical concentrations and combinations, making it difficult to establish stable intermediate states or correctly open or close the required chromatin loci. Generally, the current low reproducibility raises severe challenges for enhancing the stepwise protocol for generating CiPSCs.
DIRECT LINEAGE CONVERSION
Recently, the advancements of TF-mediated direct lineage conversion from somatic cells to other cell types have garnered considerable attention; this strategy bypasses the acquisition of pluripotent state and serves a promising approach for generating numerous types of functional cells. The impact of chemicals was first reported to facilitate the TF-mediated lineage conversion. Supplemented with specific chemicals, multiple cells from different germ layers have been successfully generated from fibroblasts or other somatic cells with minimal utilization of lineage-specific TFs, including neuronal cells[32-35], cardiac cells[36,37], hepatocytes[22,38], and pancreatic cells[39,40]. Additionally, chemical cocktails could markedly augment the TF-mediated conversion with relatively high efficiency and purity[22,32]. However, from the perspective potential risks of genetic integration, direct lineage conversion by full chemicals merits much anticipation at present.
Ectoderm
The first full chemical cocktail reported to generate neural progenitor cells (NPCs) directly from mouse and human somatic cells was a simple and effective VCR (VPA, CHIR99021, and RepSox) combination[41]. The chemical-induced NPCs (CiNPCs) exhibited similar transcription profiles as brain-derived NPCs and could self-renew and further differentiate into different neural lineage cells both in vitro and in vivo. Later, using alternative chemical cocktails, NSC-like cells were attained by other groups[42,43]. Notably, FGF and Shh signaling pathways recompiled the transcriptional and epigenetic programs from fibroblast to neural lineage by modulating the binding and activation of immediate downstream TFs Elk1/Gli2 to master neural genes[43].
Bypassing the NPC state, direct chemical conversion of mature neurons (CiNs) from mouse and human fibroblasts has been achieved[44-46]. Compared with fibroblasts, astrocytes are extensively considered as a preferable starting cell source for neurogenesis, as well as direct neuron conversion[47,48]. Under chemical-mediated epigenetic silencing of glial genes, as well as the transcriptional activation of crucial neural transcriptional factors (NEUROD1 and NEUROGENIN2), human astroglial cells were reportedly converted into functional neurons efficiently, with the ability to integrate into local circuits in vivo. Additionally, a recent preprint report of in vivo direct conversion garnered considerable attention. A study reported successfully converting resident astrocytes to functional neurons in situ in adult mouse brain[49]. Remarkably, such in situ-generated neurons acquired electrophysiological functions and could functionally interact with resident neurons in the brain.
Mesoderm
Under the two-step chemical treatment based on CRFVPT (CHIR99021, RepSox, Forskolin, VPA, Parnate, and TTNPB), for the first time, mouse fibroblasts were successfully converted to spontaneously beating cardiomyocyte-like cells (CMs) through a Sca-1+ cardiac precursor-like stage[50]. Later, another study reported the generation of mouse chemical-induced CMs (CiCMs) by replacing the core chemical RepSox with another TGF-β inhibitor, A83-01[51]. Notably, CiCMs induced by either approach expressed cardiomyocyte-specific markers and displayed typical cardiac calcium flux and electrophysiological features, resembling primary cardiomyocytes. Remarkably, CiCMs could also be induced directly from resident cardiac fibroblasts in vivo. Despite the relatively low efficiency, the induced cardiomyocytes could markedly enhance cardiac functions in myocardial infarction mice[52].
In particular, during the conversion of human CiCMs, platelet-derived growth factor receptor inhibitors reportedly facilitated the cardiac conversion by suppressing the fibroblast program[53]. Additionally, the synergistic effects of chemicals for epigenetic modulation were determined, enabling cells responsive to extrinsic cardiogenic signals. Furthermore, the chemicals promoted the chromatin accessibility of core cardiogenesis genes loci, enabling effective binding of cardiogenic signal effectors, such as β-catenin and Smad1, and ultimately facilitating the cell fate conversion.
Endoderm
During the chemical induction of mouse CiPSCs, some studies reported a unique XEN-like intermediate state[25,26,29]; these extraembryonic endoderm-like cells shared similar global gene expression patterns and in vivo developmental potential to the embryo-derived XEN cells[25] and displayed high plasticity for directing endoderm and ectoderm lineage cells. Under favorable induction conditions, both hepatocytes and neurons could be generated[54]. Remarkably, when cultured in a lineage-favorable condition, the multipotential intermediate status appeared prone to incline to a specific direction. Combined with the hepatocyte culture medium and activin A, mouse endoderm progenitor cells (EPCs) were induced with the robust expression of endoderm markers Sox17, Foxa2, Gata4/6, and Hnf4a, while lacking ectoderm markers[55]. Additionally, chemically induced EPCs (CiEPCs) displayed marked foregut/liver differentiation potential regarding the markedly elevated expression of Krt8, Krt18, and Krt19. Furthermore, under specific differentiation conditions, both hepatocyte-like cells (HLCs) and pancreatic lineage cells could be generated.
To date, the induction of human EPCs from somatic cells was restricted to the same germ layer. Initiated from gastrointestinal epithelial cells, human CiEPCs were reportedly generated under the support of tissue-specific mesenchymal feeders[56], endowed with bi-potential differentiation capacity toward hepatocytes, pancreatic endocrine cells, and IECs. Nevertheless, despite growing evidence demonstrating that chemicals could facilitate the TF-mediated induction of human endoderm lineage cells from fibroblasts or other distant lineages, such as hepatocytes[22,38] and pancreatic cells[39], there was seldom achievement mediated by a full chemical strategy. Perhaps, gastrointestinal epithelial cells are more amenable for CiEPCs induction than fibroblasts because of their proximity in lineage distance. Regarding the difference in transcriptome and pathway profiles between humans and mice, further chemical cocktail screening is warranted to attain the direct endoderm conversion from other germ layers.
DIRECT LINEAGE EXPANSION IN HEPATOCYTES
Although direct lineage conversion from fibroblasts or other somatic cells has established predominant advantages for regenerative medicine[41,43], the relative deficient functional maturation and engraftment in vivo remain a major obstacle[57]. For decades, human hepatic cell source is in high demand for liver disease treatment because of the shortage of available liver organs[58,59]. The generation of a large number of functional and transplantable hepatic cells merits considerable clinical significance and has garnered substantial attention. In recent years, TF-mediated direct reprogramming of human-induced hepatocytes (hiHeps) has garnered more attention, overwhelming the conventional iPS-derived HLCs, in terms of markedly reduced risk of tumorigenesis. Despite the progressive enhancements in efficiency and purity of hiHeps, the extremely low in vivo repopulation capability, as well as deficient functions regarding metabolism, markedly hampered their transplantation applications[60,61]. Most recently, the successive achievements in the chemical induction of primary hepatocytes highlight the acquisition of highly expandable characteristics (Table 1), which could markedly promote the development of hepatic cell-based liver disease therapies.
Table 1.
Expandable hepatic cells induced from primary hepatocytes to date
| Hepatocyte source(s) | Chemicals | Growth factor(s) | Medium and supplements |
Expansion potential |
Yr | |
| Passage count | Doubling time | |||||
| Mouse and Rat | A83-01, CHIR99021, Y-27632 | EGF | DMEM/F12, HEPES, L-proline, ITS, dexamethasone, nicotinamide, ascorbic acid-2 phosphate, BSA, antibiotic/ antimycotic | Rat: >10; Mouse: >20 | Rat: 14.7 ± 1.1 h | 2017[62] |
| Mouse | A83-01, CHIR99021, Y-27632 | EGF, HGF | DMEM/F12, N2 or ITS, S1P, LPA | >30 | 15-20 h | 2017[63] |
| Human (resected patient liver tissue, non-lesion) | A83-01, CHIR99021 | EGF, HGF | DMEM/F-12 (high glucose), FBS, nicotinamide, dexamethasone, ITS, penicillin/ streptomycin | >10 | 37.9-39.8 h | 2018[64] |
| Human (resected patient liver tissue, non-lesion) | A83-01, CHIR99021, Y-27632 | EGF, HGF | Advanced DMEM/F-12, N2, B27, sodium pyruvate, ascorbic acid, S1P, LPA | >10 | 24.7 ± 1.4 h | 2018[67] |
| Human (normal, cryopreserved) | A83-01, Y-27632 | EGF, FGF10, HGF, Wnt3a | Advanced DMEM/F-12, FBS, N2, B27 (minus vitamin A), N-acetylcysteine, nicotinamide, [Leu15]-gastrin I, penicillin/ streptomycin, | 4 (normoxia); 8 (hypoxia) | 2018[68] | |
| Human (normal, freshly isolated and cryopreserved) | A83-01, CHIR99021, Y-27632 | EGF, FGF7, FGF10, HGF, TGFa | Advanced DMEM/F-12, HEPES, B27 (minus vitamin A), R-spodin1 conditioned medium, N-acetylcysteine, nicotinamide, gastrin, GlutaMAX, penicillin/ streptomycin, | Fetal hepatocytes: >16 | 5-7 d | 2018[70] |
Lineage-committed cells and progenitors
To break the blockage for expansion, a simple and effective combination of three core chemicals YAC (Y-27632, A83-01, and CHIR99021) was first reported in 2017, enabling the conversion of terminally differentiated rat and mouse hepatocytes to highly expandable liver progenitor-like state, with bi-potential differentiation capacity toward mature hepatocytes and biliary epithelial cells[62]. Later, using the same core chemical combination, the induction of progenitor-like state was established further by another group with in vivo repopulation and maturation capability[63].
However, the induction effect of YAC was only restricted to hepatocytes originated from rodents, until the discovery of HGF, which was highlighted to be essential for establishing a human hepatic progenitor-like state through the ERK-1/2 signaling activation[64]. Remarkably, during the induction of human hepatocytes by modified cocktail HAC (HGF, A83-01, and CHIR99021), not only were hepatic progenitor markers markedly elevated but also endoderm and pluripotency markers were detected[64], suggesting the potential acquisition of multilineage differentiation capacity other than the hepatic fate. Additionally, nicotinamide, commonly used for hepatocyte culture[65,66], inhibited the proliferation and even induced apoptosis through the inactivation of SIRT1, offering a clue for long-term culture optimization. Of note, under the three-dimensional differentiation condition, the expanded progenitor-like cells could regain the expression of hepatitis B virus (HBV) receptor sodium taurocholate cotransporting polypeptide, which could markedly support the HBV infection or reactivation modeling[67].
Besides the markedly elevated expression of progenitor-associated markers, the HAC-based induction approach resulted in the suppressed expression of most mature hepatocyte markers[64,67]. However, when one of the core chemicals, CHIR99021, was replaced with Wnt3a, a unique proliferative state was established, which partly retained mature hepatocyte characteristics while exhibiting progenitor-associated identity[68]. Besides the GSK3-mediated Wnt pathway activation, special insight was provided into the crosstalk between Wnt3a and specific cell signaling regarding hepatic progenitor self-renew and proliferation such as the Hippo–YAP pathway[69]. Moreover, it implied that the CHIR99021-mediated pathway modulation could largely erase the initial memory of hepatocyte identity, while the Wnt3a-mediated modulation partially sustained these signatures. Remarkably, when induced cells were transplanted in vivo for over 4 mo, the human albumin secretion and repopulation efficiency could attain a comparable level to primary human hepatocytes, suggesting that these induced cells could serve as the most compatible hepatic cell source that had ever been reported beyond primary hepatocytes.
Organoid expansion
Using a highly similar chemical and cytokine cocktail, both human and mouse primary hepatocytes have been established to form organoids (Hep-Orgs) in a 3D culture system and stably expand for a long term[70]. Hep-Orgs comprise noncycling mature hepatocytes and cycling hepatocyte progenitor cells, exhibiting comparable gene expression profiles and functions to primary hepatocytes. Remarkably, progenitor markers were not markedly elevated in Hep-Orgs, which was considerably different from that in conventional 2D culture[68]. Despite applying a different Wnt agonist, R-spondin, instead of Wnt3a, the major discrepancy could be contributed by the special circumstance in the organoid culture system, which seemingly recapitulated the regeneration microenvironment of the adult liver in vivo. In Hep-Orgs, cell cycle and ribosome synthesis–related gene expression was markedly enhanced, resembling the response of hepatocytes to acute liver damage such as partial hepatectomy.
Limitations
Notably, human hepatocyte–induced proliferative cell sources displayed large scale expandable potential, as well as superior compatibility and functional maturation, for transplantation compared with iPSCs-derived HLCs or hiHeps, exhibiting a remarkably higher repopulation rate and human albumin secretion[61,71]. As the maturation direction of human hepatocyte-induced proliferative cell sources is lineage-committed, they did not necessarily require an extra differentiation process, severing as ready-to-use transplantable sources, and might merit broader prospects for breaking through the obstacle for liver disease treatment. Nevertheless, currently, the expansion efficiency of human hepatocytes is remarkably lower than that of rodent hepatocytes. Compared with rodent hepatocytes, human hepatocytes required approximately a 10 h longer period for cell doubling, whereas the maximum passage counts for stable expansion declined approximately 10 times[63,67]. Regarding the proliferation kinetics, rodent hepatocytes essentially required three to five passages for establishing an accelerated proliferative state, while human hepatocytes exhibited a gradual loss of proliferation potential after three to four passages, implying that the building of a proliferative intermediate state for human hepatocytes might be relatively insufficient or unstable. Although a hypoxic expansion condition possibly inhibited the senescence and prolonged proliferation[68], an understanding of the different transcriptome and pathway network profiles between humans and mice is required to reveal specific signaling for human hepatocyte expansion.
Telomere shortening during prolonged expansion is another limiting factor[70]. The unlimited self-renewal and genomic stability of pluripotent stem cells were facilitated by sufficiently long telomeres[72]. During pluripotent reprogramming, which was either TF- or chemical-mediated, short telomeres of somatic cells were significantly elongated in a process mediated by telomerase activity upregulation or by recombination-based ALT mechanisms[31,73]. Moreover, reprogramming triggers telomere elongation regardless of donor age status[74,75]. Thus, telomere erosion, characterized in aged and senescent cells, can be efficiently rejuvenated after pluripotent reprogramming. However, direct lineage conversion and expansion from somatic cells bypass the pluripotent intermediate status, thereby preserving age-associated features. Compared with the iPSC-derived counterpart, extensive DNA damage, loss of heterochromatin and nuclear organization, and increased SA-β-Gal activity were observed in lineage-converted functional cells[76]. Therefore, even though the conversion is successfully completed, the converted cells seem to inherit aging signatures from parental cells, including short telomeres, and be prone to senescence after passages. During chemical-mediated expansion of primary human hepatocytes, the inherently short telomeres and insufficient telomerase activation may play a key role in eliciting senescence of induced proliferative cells after limited passages[68], which remains a major obstacle for large-scale expansion in vitro. In contrast, we found that when human iPSC-derived HLCs are chemically induced for expansion, they exhibit a superior expansion advantage compared with primary hepatocytes. Induced cells can stably expand for extended passages while sustaining hepatic differentiation potency (unpublished data). The relatively high telomerase activity inherited from iPSCs potentially restores or elongates telomeres, facilitating long-term expansion. The ability to use chemicals to activate telomerase and elongate telomeres in human terminally differentiated cells such as hepatocytes would be of great importance for an extended expansion capability.
Moreover, the advantage of the organoid culture system has been proved to be supportive for the long-term expansion with enhanced functional maturation; however, the current expansion efficiency was much lower than the 2D system, and the culture condition optimization, such as the extracellular matrix, warrants further investigation.
Despite the achievements in hepatocytes, direct expansion of other lineage cells, such as neurons and cardiomyocytes, has been seldom reported. Undoubtedly, the direct conversion from fibroblasts has already proved a feasible option for generating these functional cells. It is expected that when the direct lineage expansion is applied, the in vivo repopulation and functional maturation could be enhanced further.
CHALLENGES
The chemical-mediated direct lineage conversion and expansion, bypassing potential risks using transgenic methods, as well as pluripotency-related tumorigenesis, offer promising options for therapeutic purpose. However, several challenges remain regarding the unrevealed mechanism of chemical effects.
Cell fate specificity
To date, several dozens of chemicals have been identified in various combinations for cell induction[11,16,18,77]. Although the precise mechanism underlying a particular conversion between different cell fates remains unclear, chemical inducers could be classified into two major groups—epigenetic modulators and signaling regulators. Epigenetic modulators, typically HDAC inhibitors and DNA/histone methyltransferase inhibitors, such as VPA and BIX01294, are commonly used for the induction of CiPSCs, CiNSCs, and CiCMs[27,41,53]. Conversely, signaling regulators specify the characteristics of the designated cell identity. Reportedly, ISX9 and AS8351 were specific for the CiNs induction[44,78]. Additionally, SC1 was specific for the CiCMs induction[51,53]. The 2i combination (CHIR99021 and PD0325901) was always required for the late stage of the CiPSCs induction[24,25]. However, RepSox, A83-01, CHIR99021, and Forskolin were extensively used as essential factors for inducing various cell types, including CiPSCs, CiNPCs, CiNs, CiCMs, and CiEPCs[24,43,53,55,78]. The nonspecific feature of a significant portion of the chemicals could be attributed to the fact that same signaling pathways are often shared by the multilineage development such as the Wnt and TGF-β pathways[79]; it may not only extend the induction application for a broad range of cell types but also possibly lead to undesired cell fates. Using the same chemical cocktail for the CiPSCs induction, the generation of an unexpected cardiac fate was uncovered[50]. The possible explanation was that the chemicals might induce a nonspecific multipotential intermediate state, which was highly plastic and unstable; or a heterogeneous mixture of multiple progenitor cell types. Under favorable conditions, the induction direction could incline further to cardiac or pluripotent fate.
To exclude the unanticipated induction fate, it would be challenging to elucidate further the individual and synergistic effect of chemical cocktails regarding cell signaling and epigenetic regulation, as well as the specificity and commonality for distinct lineages (Table 2), and then develop stepwise reliable chemical cocktails that could precisely direct the desired cell types.
Table 2.
The specificity and commonality of chemicals in direct lineage conversion
| Compounds | Function |
Target germ layer and cell types |
Ref. | ||
| Ectoderm: Neural stem/ progenitor cell, neuron | Mesoderm: Cardiomyocyte | Endoderm/ extraembryonic endoderm: Endoderm progenitor cell, XENs | |||
| A83-01 | TGF-βRI (ALK4/5/7) inhibitor | + | + | [42,43,51,53] | |
| AM580 | RAR agonist | + | [25,54] | ||
| AS8351 | KDM5B inhibitor | + | [53] | ||
| Bay K 8644 | Ca2+ channel activator | + | [51] | ||
| BIX01294 | Histone methyltransferase inhibitor | + | + | [42,53] | |
| CHIR99021 | GSK3 inhibitor | + | + | + | [25,41-46,50,51,53-55,78] |
| DMH1 | BMP inhibitor | + | [46] | ||
| EPZ004777 | DOT1L inhibitor | + | [25,54] | ||
| Forskolin | cAMP activator | + | + | + | [25,44-46,50,51,54,55,78] |
| GO6983 | PKC inhibitor | + | [44] | ||
| Hh-Ag1.5 | Smo agonist | + | [43] | ||
| I-BET151 | BET bromodomain inhibitor | + | [78] | ||
| ISX9 | Neurogenic agent | + | [78] | ||
| JNJ10198409 | PDGF-RTK inhibitor | + | [53] | ||
| LDN193189 | BMP type I receptor (ALK2/3) inhibitor | + | [43,45] | ||
| OAC2 | Oct4 activator | + | [53] | ||
| Parnate (Tranylcypromine) | LSD1/MAO inhibitor | + | + | + | [25,43,50,54] |
| PD0325901 | MEK inhibitor | + | [42,45] | ||
| RepSox (616452) | TGF-βRI (ALK5) inhibitor | + | + | + | [25,41,44,46,50,54,55] |
| RG108 | DNA methyltransferase inhibitor | + | [42,43] | ||
| SB431542 | TGF-βRI (ALK4/7) inhibitor | + | [45,78] | ||
| SC1 | ERK1/RasGAP inhibitor | + | [51,53] | ||
| SMER28 | Autophagy modulator | + | [43] | ||
| SP600125 | JNK inhibitor | + | [44,46] | ||
| SU16F | PDGFRβ inhibitor | + | [53] | ||
| TTNPB | RAR agonist | + | + | [50,55] | |
| VPA | Histone deacetylase inhibitor | + | + | + | [25,41,42,44,46,50,54] |
| Y‑27632 | ROCK inhibitor | + | + | + | [44,46,53-55] |
XENs: Extraembryonic endoderm-like cells.
Cell identity memory
Each cell type has its unique transcriptome, which defines its cell identity. Reprogrammed cells more or less inherit the cell identity memory of their ancestor. An analysis of molecular traces during the induction of iPSC-HLCs and hiHeps identified original fibroblast identity efficiently but not completely erased in both cell types[61]. Additionally, iPSC-HLCs exhibited the expression of endoderm progenitor (FOXA2 and GATA6) and hepatoblast (AFP and EPCAM) markers, suggesting that the memory of molecular traces during the differentiation path was also sustained in iPSC-HLCs; however, the hiHeps induction bypasses these cell identities transition, exhibiting no expression of these markers. Overall, the sustained memory during reprogramming and differentiation process could elucidate the fact that both c and hiHeps exhibited distinct characteristics from primary hepatocytes in gene expression and related functions[80-82].
Additionally, chemical-mediated reprogramming was also challenged by the initial cell identity modulation. The investigation of the direct induction of neurons identified a bromodomain inhibitor, IBET151, as a core compound needed to erase the initial fibroblast transcriptional network program for cell identity rewriting[78]; the mechanism could be concerned with the blocking effect that IBET151 disrupted the accessibility of bromodomain proteins to acetylated histones related to fibroblast programs, resulting in transcriptional silencing[83]. Additionally, the bromodomain and extra-terminal domain inhibitors remarkably enhanced iPSC and NPC induction via switching off a large set of initial somatic transcriptional programs[84]. Besides, platelet-derived growth factor receptor (PDGFR) inhibitors, SU16F and JNJ10198409, reportedly accelerated the downregulation of fibroblast genes during the conversion of human cardiomyocytes, markedly enhancing the efficiency[53]. Perhaps, further investigation of chemicals with the erase effect on specific cell types would booster the efficiency of the cell fate conversion.
Conversely, the initial somatic transcriptional memory could also facilitate the transplantation. In a study, when direct lineage expansion was applied in primary hepatocytes, their partly sustained hepatocyte transcriptional memory could facilitate quick engraftment and functional maturation after transplantation in vivo[68]. Of note, based on our study, when cells were chemically induced form iPSC-HLCs, they exhibited relatively low in vivo repopulation capability, which corroborated that before induction (unpublished data). The possible explanation was that the induced cells inherited portions of transcriptional memory of iPSC-HLCs, including the deficiency in repopulation capability. This discrepancy not only highlights the hurdles for transplantation of iPSC-derived functional cells but also emphasizes the elucidation of the cell memory dynamics during induction.
In vivo induction
Conventionally, long-term in vitro expansion is critical to obtain sufficient functional cells for transplantation, whereas the potential risk of functional alteration and genetic mutations from the in vitro microenvironment raises serious problems[57,85]. Theoretically, in vivo reprogramming could produce functional cells followed by inducing resident functional cells with bi-potency or proliferation capability, or directly convert the neighboring cell types proximal in lineage distance and, meanwhile, take advantage of the in situ niche to regenerate the damaged tissue or organ efficiently, which remains a major obstacle for in vitro circumstances.
In recent years, substantial TF-mediated in vivo reprogramming has demonstrated some exciting achievements, including neuroblasts or neurons induced from glial cells[86,87], cardiomyocytes induced from cardiac fibroblasts[88-90], hepatocytes induced from myofibroblasts[91,92], and pancreatic β-like cells induced from exocrine cells[9,93]. Despite great promise for diseases treatment as demonstrated by previous studies, the TF-mediated strategy also poses risks of genome integration, tumorigenesis, as well as manufacturing and delivery problems. Comparatively, chemical-mediated strategy minimized the risk of genetic alteration and is easier for scalable manufacturing, stocking, and delivery[23], eventually preferable for in vivo therapeutic applications. Recently, for the first time, in vivo chemical reprogramming was reported to successfully convert the resident astrocytes into functional neurons in the adult mouse brain, resembling endogenous neurons in both neuron-specific marker expression and electrophysiological properties[49]. Meanwhile, cardiomyocytes were induced from adult cardiac fibroblasts by direct full chemical administration in vivo, and although the reprogramming efficiency is relatively low, the chemical cocktail treatment markedly decreased the scar formation and enhanced cardiac functions in myocardial infarction mice[52]; these encouraging achievements not only provide a general strategy for in vivo reprogramming but also open a novel path to regenerate the diseased organs in situ.
However, the off-target or unspecific-target effects of chemicals pose challenges for the in vivo induction, as the chemical treatment could target undesired cell types in situ or nearby, potentially declining the intrinsic homeostasis of local cell populations. Moreover, exogenous chemicals could also break the homeostasis of local niche and cause unintended tissue damage. Thus, it will be essential to evaluate the side effects in vivo in the long term, and the chemical combinations and concentration should be optimized to adapt physiological homeostasis synergistically. Finally, despite the high permeability advantage of chemicals in tissue, the arrival to unwanted location should be avoided. Advances in the delivery system are warranted to deliver chemicals to designated locations for an expected period precisely. Overall, enhanced targeting and shielding capabilities will be indispensable for attaining effective reprogramming in vivo.
PERSPECTIVES
Generating substantial functional cells with tissue/organ regeneration capability is a major challenge for regenerative medicine. Despite the rapid progress of the conventional strategy regarding the differentiation from pluripotent stem cells[94,95], the direct lineage conversion, bypassing the pluripotent stage, has highlighted a promising alternative strategy in recent years[57]. Additionally, chemical-mediated strategy, targeting the modulation of epigenetic status and signaling pathways without interfering the genome integration, exhibited the unique advantage over transgenic and other approaches for cell reprogramming, especially regarding the expansion potential[23,77]. Remarkably, chemically induced cells, including XEN-like state and lineage-specific stem/progenitors (CiNSCs, CiNPCs, and CiEPCs), exhibited highly expandable characteristics, which could markedly satisfy the predominant requirement for clinical use. Additionally, recently reported direct lineage expansion holds great promise for cell transplantation applications. As these induced cells not only exhibited the robust proliferation capability but also partly sustained the initial cell identity memory, it facilitated direct and rapid revert to mature state after in vitro differentiation or been transplanted in vivo. However, it remains a major obstacle for direct lineage conversion from fibroblasts or other somatic cells, which exhibited a significant deficiency in functional maturation. Although the achievement to date in direct lineage expansion of human functional cells remains highly limited, the success in human hepatocytes would undoubtedly offer a general idea for extending this strategy to other desired cell types. Moreover, the limited expansion capability of induced human hepatocytes also evokes the importance of exploring chemical candidates to activate telomerase and rejuvenate telomeres, which may potentially extend expansion of induced cells in vitro (Table 3).
Table 3.
Advantages and disadvantages of different strategies for functional cell induction
| Strategy | Induction efficiency | Reproducibi-lity/stability | Target specificity | Cellular function | In vivo engraftment | Safety | Scaling up | Cost | Ref. |
| TF-mediated pluripotent reprogramming followed by differentia-tion | Moderate | Highly reproducible/ stable | High | Immature | Low | Genomic integration; tumorigenesis risk | Expandable before differentia-tion | Very high | [11,97-99] |
| TF-mediated direct lineage conversion | Fast and efficient | Reproducible/stable | High | Deficient | Low | Genomic integration | Expandable in progenitors | High | [100-103] |
| Chemical-mediated pluripotent reprogramming followed by differentia-tion | Controversial | Poorly reproducible/ unstable | Low | Not clear | Not clear | Integration-free | Not clear | Low | [24,25,29,31] |
| Chemical-mediated direct lineage conversion | Low | Reproducible/ unstable | Low | Deficient | Low | Integration-free | Expandable in progenitors | Very low | [18,77,104,105] |
| Chemical-mediated direct lineage expansion | Fast and efficient | Reproducible/ unstable | Low | Close to primary | High | Integration free | Expandable in rodents/ Limited in humans | Very low | [62,64,67,68] |
TF: Transcriptional factor; XENs: Extraembryonic endoderm-like cells.
Holding an excellent promise for direct lineage conversion and expansion, spatiotemporal flexibility and nonintegrative characteristics of chemical strategy could also be favorable for the in vivo induction approach, offering superior advantages for therapeutic potential[94]. As safety and efficiency might be the rate-limiting step for in vivo reprogramming[96], assistant technology, such as nano-delivery system, might be required to deliver chemicals to specifically targeted sites in a controlled manner.
Along with the advances of chemical screening, discrete combinations of pathway-specific chemicals have progressively been identified to reprogram somatic cells into many lineages. Nevertheless, improved knowledge of the pathway networks, together with the epigenetic pattern that drives the cell fate conversion and proliferation, is warranted to intelligently enhance the induction efficiency and specificity in vitro and in vivo.
ACKNOWLEDGEMENTS
We thank Dr. Jaejeong Kim of University of Tsukuba Faculty of Medicine for designing the figure image.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: March 4, 2019
First decision: June 5, 2019
Article in press: August 21, 2019
Specialty type: Cell and tissue engineering
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Wu YXJ
Contributor Information
Jian-Yun Ge, Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki 305-8575, Japan.
Yun-Wen Zheng, Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki 305-8575, Japan; Institute of Regenerative Medicine and Affiliated Hospital, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China; Department of Regenerative Medicine, School of Medicine, Yokohama City University, Yokohama 236-0004, Japan. ywzheng@md.tsukuba.ac.jp.
Li-Ping Liu, Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki 305-8575, Japan; Institute of Regenerative Medicine and Affiliated Hospital, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
Hiroko Isoda, Faculty of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki 305-8572, Japan.
Tatsuya Oda, Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki 305-8575, Japan.
References
- 1.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Mungenast AE, Siegert S, Tsai LH. Modeling Alzheimer's disease with human induced pluripotent stem (iPS) cells. Mol Cell Neurosci. 2016;73:13–31. doi: 10.1016/j.mcn.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai S, Miyauchi M, Kurokawa M. Modeling of hematologic malignancies by iPS technology. Exp Hematol. 2015;43:654–660. doi: 10.1016/j.exphem.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Jungverdorben J, Till A, Brüstle O. Induced pluripotent stem cell-based modeling of neurodegenerative diseases: a focus on autophagy. J Mol Med (Berl) 2017;95:705–718. doi: 10.1007/s00109-017-1533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 7.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- 11.Ma T, Xie M, Laurent T, Ding S. Progress in the reprogramming of somatic cells. Circ Res. 2013;112:562–574. doi: 10.1161/CIRCRESAHA.111.249235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, Morrisey EE. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kogut I, McCarthy SM, Pavlova M, Astling DP, Chen X, Jakimenko A, Jones KL, Getahun A, Cambier JC, Pasmooij AMG, Jonkman MF, Roop DR, Bilousova G. High-efficiency RNA-based reprogramming of human primary fibroblasts. Nat Commun. 2018;9:745. doi: 10.1038/s41467-018-03190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Kim KP, Lim KT, Lee SC, Yoon J, Song G, Hwang SI, Schöler HR, Cantz T, Han DW. Generation of integration-free induced hepatocyte-like cells from mouse fibroblasts. Sci Rep. 2015;5:15706. doi: 10.1038/srep15706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma X, Kong L, Zhu S. Reprogramming cell fates by small molecules. Protein Cell. 2017;8:328–348. doi: 10.1007/s13238-016-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Li K, Wei W, Ding S. Chemical approaches to stem cell biology and therapeutics. Cell Stem Cell. 2013;13:270–283. doi: 10.1016/j.stem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie X, Fu Y, Liu J. Chemical reprogramming and transdifferentiation. Curr Opin Genet Dev. 2017;46:104–113. doi: 10.1016/j.gde.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Zhou H, Abujarour R, Zhu S, Young Joo J, Lin T, Hao E, Schöler HR, Hayek A, Ding S. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009;27:2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyssiotis CA, Foreman RK, Staerk J, Garcia M, Mathur D, Markoulaki S, Hanna J, Lairson LL, Charette BD, Bouchez LC, Bollong M, Kunick C, Brinker A, Cho CY, Schultz PG, Jaenisch R. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci U S A. 2009;106:8912–8917. doi: 10.1073/pnas.0903860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Liu J, Yang J, Chen Y, Chen J, Ni S, Song H, Zeng L, Ding K, Pei D. BMPs functionally replace Klf4 and support efficient reprogramming of mouse fibroblasts by Oct4 alone. Cell Res. 2011;21:205–212. doi: 10.1038/cr.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim KT, Lee SC, Gao Y, Kim KP, Song G, An SY, Adachi K, Jang YJ, Kim J, Oh KJ, Kwak TH, Hwang SI, You JS, Ko K, Koo SH, Sharma AD, Kim JH, Hui L, Cantz T, Schöler HR, Han DW. Small Molecules Facilitate Single Factor-Mediated Hepatic Reprogramming. Cell Rep. 2016;15:814–829. doi: 10.1016/j.celrep.2016.03.071. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Xu J, Deng H. Small molecule-induced cellular fate reprogramming: promising road leading to Rome. Curr Opin Genet Dev. 2018;52:29–35. doi: 10.1016/j.gde.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Zhao T, Guan J, Zhang X, Fu Y, Ye J, Zhu J, Meng G, Ge J, Yang S, Cheng L, Du Y, Zhao C, Wang T, Su L, Yang W, Deng H. A XEN-like State Bridges Somatic Cells to Pluripotency during Chemical Reprogramming. Cell. 2015;163:1678–1691. doi: 10.1016/j.cell.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Ye J, Ge J, Zhang X, Cheng L, Zhang Z, He S, Wang Y, Lin H, Yang W, Liu J, Zhao Y, Deng H. Pluripotent stem cells induced from mouse neural stem cells and small intestinal epithelial cells by small molecule compounds. Cell Res. 2016;26:34–45. doi: 10.1038/cr.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao T, Fu Y, Zhu J, Liu Y, Zhang Q, Yi Z, Chen S, Jiao Z, Xu X, Xu J, Duo S, Bai Y, Tang C, Li C, Deng H. Single-Cell RNA-Seq Reveals Dynamic Early Embryonic-like Programs during Chemical Reprogramming. Cell Stem Cell. 2018;23:31–45.e7. doi: 10.1016/j.stem.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Long Y, Wang M, Gu H, Xie X. Bromodeoxyuridine promotes full-chemical induction of mouse pluripotent stem cells. Cell Res. 2015;25:1171–1174. doi: 10.1038/cr.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao S, Yu S, Li D, Ye J, Yang X, Li C, Wang X, Mai Y, Qin Y, Wu J, He J, Zhou C, Liu H, Zhao B, Shu X, Wu C, Chen R, Chan W, Pan G, Chen J, Liu J, Pei D. Chromatin Accessibility Dynamics during Chemical Induction of Pluripotency. Cell Stem Cell. 2018;22:529–542.e5. doi: 10.1016/j.stem.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Li D, Liu J, Yang X, Zhou C, Guo J, Wu C, Qin Y, Guo L, He J, Yu S, Liu H, Wang X, Wu F, Kuang J, Hutchins AP, Chen J, Pei D. Chromatin Accessibility Dynamics during iPSC Reprogramming. Cell Stem Cell. 2017;21:819–833.e6. doi: 10.1016/j.stem.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Fu H, Tian CL, Ye X, Sheng X, Wang H, Liu Y, Liu L. Dynamics of Telomere Rejuvenation during Chemical Induction to Pluripotent Stem Cells. Stem Cell Reports. 2018;11:70–87. doi: 10.1016/j.stemcr.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, Herms S, Wernet P, Kögler G, Müller FJ, Koch P, Brüstle O. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods. 2012;9:575–578. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- 33.Liu ML, Zang T, Zou Y, Chang JC, Gibson JR, Huber KM, Zhang CL. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat Commun. 2013;4:2183. doi: 10.1038/ncomms3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YJ, Lim H, Li Z, Oh Y, Kovlyagina I, Choi IY, Dong X, Lee G. Generation of multipotent induced neural crest by direct reprogramming of human postnatal fibroblasts with a single transcription factor. Cell Stem Cell. 2014;15:497–506. doi: 10.1016/j.stem.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Zhu S, Ambasudhan R, Sun W, Kim HJ, Talantova M, Wang X, Zhang M, Zhang Y, Laurent T, Parker J, Kim HS, Zaremba JD, Saleem S, Sanz-Blasco S, Masliah E, McKercher SR, Cho YS, Lipton SA, Kim J, Ding S. Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res. 2014;24:126–129. doi: 10.1038/cr.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Cao N, Spencer CI, Nie B, Ma T, Xu T, Zhang Y, Wang X, Srivastava D, Ding S. Small molecules enable cardiac reprogramming of mouse fibroblasts with a single factor, Oct4. Cell Rep. 2014;6:951–960. doi: 10.1016/j.celrep.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ifkovits JL, Addis RC, Epstein JA, Gearhart JD. Inhibition of TGFβ signaling increases direct conversion of fibroblasts to induced cardiomyocytes. PLoS One. 2014;9:e89678. doi: 10.1371/journal.pone.0089678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo R, Tang W, Yuan Q, Hui L, Wang X, Xie X. Chemical Cocktails Enable Hepatic Reprogramming of Mouse Fibroblasts with a Single Transcription Factor. Stem Cell Reports. 2017;9:499–512. doi: 10.1016/j.stemcr.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li K, Zhu S, Russ HA, Xu S, Xu T, Zhang Y, Ma T, Hebrok M, Ding S. Small molecules facilitate the reprogramming of mouse fibroblasts into pancreatic lineages. Cell Stem Cell. 2014;14:228–236. doi: 10.1016/j.stem.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu S, Russ HA, Wang X, Zhang M, Ma T, Xu T, Tang S, Hebrok M, Ding S. Human pancreatic beta-like cells converted from fibroblasts. Nat Commun. 2016;7:10080. doi: 10.1038/ncomms10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng L, Hu W, Qiu B, Zhao J, Yu Y, Guan W, Wang M, Yang W, Pei G. Generation of neural progenitor cells by chemical cocktails and hypoxia. Cell Res. 2014;24:665–679. doi: 10.1038/cr.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han YC, Lim Y, Duffieldl MD, Li H, Liu J, Abdul Manaph NP, Yang M, Keating DJ, Zhou XF. Direct Reprogramming of Mouse Fibroblasts to Neural Stem Cells by Small Molecules. Stem Cells Int. 2016;2016:4304916. doi: 10.1155/2016/4304916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, Lin YH, Sun YJ, Zhu S, Zheng J, Liu K, Cao N, Li K, Huang Y, Ding S. Pharmacological Reprogramming of Fibroblasts into Neural Stem Cells by Signaling-Directed Transcriptional Activation. Cell Stem Cell. 2016;18:653–667. doi: 10.1016/j.stem.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu W, Qiu B, Guan W, Wang Q, Wang M, Li W, Gao L, Shen L, Huang Y, Xie G, Zhao H, Jin Y, Tang B, Yu Y, Zhao J, Pei G. Direct Conversion of Normal and Alzheimer's Disease Human Fibroblasts into Neuronal Cells by Small Molecules. Cell Stem Cell. 2015;17:204–212. doi: 10.1016/j.stem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Dai P, Harada Y, Takamatsu T. Highly efficient direct conversion of human fibroblasts to neuronal cells by chemical compounds. J Clin Biochem Nutr. 2015;56:166–170. doi: 10.3164/jcbn.15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wan XY, Xu LY, Li B, Sun QH, Ji QL, Huang DD, Zhao L, Xiao YT. Chemical conversion of human lung fibroblasts into neuronal cells. Int J Mol Med. 2018;41:1463–1468. doi: 10.3892/ijmm.2018.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gotz M, Sirko S, Beckers J, Irmler M. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, in vitro potential, and genome-wide expression analysis. Glia. 2015;63:1452–1468. doi: 10.1002/glia.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magnusson JP, Göritz C, Tatarishvili J, Dias DO, Smith EM, Lindvall O, Kokaia Z, Frisén J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- 49.Ma Y, Xie H, Du X, Wang L, Jin X, Sun S, Han Y, Han Y, Xu J, Huang Z, Chai Z, Deng H. In vivo chemical reprogramming of astrocytes into functional neurons. bioRxiv. 2018:305185. [Google Scholar]
- 50.Fu Y, Huang C, Xu X, Gu H, Ye Y, Jiang C, Qiu Z, Xie X. Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails. Cell Res. 2015;25:1013–1024. doi: 10.1038/cr.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park G, Yoon BS, Kim YS, Choi SC, Moon JH, Kwon S, Hwang J, Yun W, Kim JH, Park CY, Lim DS, Kim YI, Oh CH, You S. Conversion of mouse fibroblasts into cardiomyocyte-like cells using small molecule treatments. Biomaterials. 2015;54:201–212. doi: 10.1016/j.biomaterials.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 52.Huang C, Tu W, Fu Y, Wang J, Xie X. Chemical-induced cardiac reprogramming in vivo. Cell Res. 2018;28:686–689. doi: 10.1038/s41422-018-0036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao N, Huang Y, Zheng J, Spencer CI, Zhang Y, Fu JD, Nie B, Xie M, Zhang M, Wang H, Ma T, Xu T, Shi G, Srivastava D, Ding S. Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science. 2016;352:1216–1220. doi: 10.1126/science.aaf1502. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Liu D, Ma Y, Du X, Jing J, Wang L, Xie B, Sun D, Sun S, Jin X, Zhang X, Zhao T, Guan J, Yi Z, Lai W, Zheng P, Huang Z, Chang Y, Chai Z, Xu J, Deng H. Direct Reprogramming of Fibroblasts via a Chemically Induced XEN-like State. Cell Stem Cell. 2017;21:264–273.e7. doi: 10.1016/j.stem.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 55.Cao S, Yu S, Chen Y, Wang X, Zhou C, Liu Y, Kuang J, Liu H, Li D, Ye J, Qin Y, Chu S, Wu L, Guo L, Li Y, Shu X, Chen J, Liu J, Pei D. Chemical reprogramming of mouse embryonic and adult fibroblast into endoderm lineage. J Biol Chem. 2017;292:19122–19132. doi: 10.1074/jbc.M117.812537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Qin J, Wang S, Zhang W, Duan J, Zhang J, Wang X, Yan F, Chang M, Liu X, Feng B, Liu J, Pei X. Conversion of Human Gastric Epithelial Cells to Multipotent Endodermal Progenitors using Defined Small Molecules. Cell Stem Cell. 2016;19:449–461. doi: 10.1016/j.stem.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015;16:119–134. doi: 10.1016/j.stem.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Bhatia SN, Underhill GH, Zaret KS, Fox IJ. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6:245sr2. doi: 10.1126/scitranslmed.3005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Runge D, Michalopoulos GK, Strom SC, Runge DM. Recent advances in human hepatocyte culture systems. Biochem Biophys Res Commun. 2000;274:1–3. doi: 10.1006/bbrc.2000.2912. [DOI] [PubMed] [Google Scholar]
- 60.Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z, Cen J, Chen X, Liu C, Hu Y, Lai D, Hu Z, Chen L, Zhang Y, Cheng X, Ma X, Pan G, Wang X, Hui L. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14:370–384. doi: 10.1016/j.stem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Gao Y, Zhang X, Zhang L, Cen J, Ni X, Liao X, Yang C, Li Y, Chen X, Zhang Z, Shu Y, Cheng X, Hay DC, Lai D, Pan G, Wei G, Hui L. Distinct Gene Expression and Epigenetic Signatures in Hepatocyte-like Cells Produced by Different Strategies from the Same Donor. Stem Cell Reports. 2017;9:1813–1824. doi: 10.1016/j.stemcr.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katsuda T, Kawamata M, Hagiwara K, Takahashi RU, Yamamoto Y, Camargo FD, Ochiya T. Conversion of Terminally Committed Hepatocytes to Culturable Bipotent Progenitor Cells with Regenerative Capacity. Cell Stem Cell. 2017;20:41–55. doi: 10.1016/j.stem.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Wu H, Zhou X, Fu GB, He ZY, Wu HP, You P, Ashton C, Wang X, Wang HY, Yan HX. Reversible transition between hepatocytes and liver progenitors for in vitro hepatocyte expansion. Cell Res. 2017;27:709–712. doi: 10.1038/cr.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim Y, Kang K, Lee SB, Seo D, Yoon S, Kim SJ, Jang K, Jung YK, Lee KG, Factor VM, Jeong J, Choi D. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J Hepatol. 2019;70:97–107. doi: 10.1016/j.jhep.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Mitaka T, Sattler CA, Sattler GL, Sargent LM, Pitot HC. Multiple cell cycles occur in rat hepatocytes cultured in the presence of nicotinamide and epidermal growth factor. Hepatology. 1991;13:21–30. [PubMed] [Google Scholar]
- 66.Inoue C, Yamamoto H, Nakamura T, Ichihara A, Okamoto H. Nicotinamide prolongs survival of primary cultured hepatocytes without involving loss of hepatocyte-specific functions. J Biol Chem. 1989;264:4747–4750. [PubMed] [Google Scholar]
- 67.Fu GB, Huang WJ, Zeng M, Zhou X, Wu HP, Liu CC, Wu H, Weng J, Zhang HD, Cai YC, Ashton C, Ding M, Tang D, Zhang BH, Gao Y, Yu WF, Zhai B, He ZY, Wang HY, Yan HX. Expansion and differentiation of human hepatocyte-derived liver progenitor-like cells and their use for the study of hepatotropic pathogens. Cell Res. 2019;29:8–22. doi: 10.1038/s41422-018-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang K, Zhang L, Liu W, Ma X, Cen J, Sun Z, Wang C, Feng S, Zhang Z, Yue L, Sun L, Zhu Z, Chen X, Feng A, Wu J, Jiang Z, Li P, Cheng X, Gao D, Peng L, Hui L. In Vitro Expansion of Primary Human Hepatocytes with Efficient Liver Repopulation Capacity. Cell Stem Cell. 2018;23:806–819.e4. doi: 10.1016/j.stem.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 69.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 70.Hu H, Gehart H, Artegiani B, LÖpez-Iglesias C, Dekkers F, Basak O, van Es J, Chuva de Sousa Lopes SM, Begthel H, Korving J, van den Born M, Zou C, Quirk C, Chiriboga L, Rice CM, Ma S, Rios A, Peters PJ, de Jong YP, Clevers H. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell. 2018;175:1591–1606.e19. doi: 10.1016/j.cell.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Ji S, Zhang L, Hui L. Cell fate conversion: direct induction of hepatocyte-like cells from fibroblasts. J Cell Biochem. 2013;114:256–265. doi: 10.1002/jcb.24380. [DOI] [PubMed] [Google Scholar]
- 72.Huang J, Wang F, Okuka M, Liu N, Ji G, Ye X, Zuo B, Li M, Liang P, Ge WW, Tsibris JC, Keefe DL, Liu L. Association of telomere length with authentic pluripotency of ES/iPS cells. Cell Res. 2011;21:779–792. doi: 10.1038/cr.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F, Yin Y, Ye X, Liu K, Zhu H, Wang L, Chiourea M, Okuka M, Ji G, Dan J, Zuo B, Li M, Zhang Q, Liu N, Chen L, Pan X, Gagos S, Keefe DL, Liu L. Molecular insights into the heterogeneity of telomere reprogramming in induced pluripotent stem cells. Cell Res. 2012;22:757–768. doi: 10.1038/cr.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, Lemaitre JM. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–2253. doi: 10.1101/gad.173922.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Groh GI, Buchert PK, Allen WC. A comparison of transfusion requirements after total knee arthroplasty using the Solcotrans autotransfusion system. J Arthroplasty. 1990;5:281–285. doi: 10.1016/s0883-5403(08)80084-8. [DOI] [PubMed] [Google Scholar]
- 76.Tang Y, Liu ML, Zang T, Zhang CL. Direct Reprogramming Rather than iPSC-Based Reprogramming Maintains Aging Hallmarks in Human Motor Neurons. Front Mol Neurosci. 2017;10:359. doi: 10.3389/fnmol.2017.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu A, Cheng L. Chemical transdifferentiation: closer to regenerative medicine. Front Med. 2016;10:152–165. doi: 10.1007/s11684-016-0445-z. [DOI] [PubMed] [Google Scholar]
- 78.Li X, Zuo X, Jing J, Ma Y, Wang J, Liu D, Zhu J, Du X, Xiong L, Du Y, Xu J, Xiao X, Wang J, Chai Z, Zhao Y, Deng H. Small-Molecule-Driven Direct Reprogramming of Mouse Fibroblasts into Functional Neurons. Cell Stem Cell. 2015;17:195–203. doi: 10.1016/j.stem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 80.Camp JG, Sekine K, Gerber T, Loeffler-Wirth H, Binder H, Gac M, Kanton S, Kageyama J, Damm G, Seehofer D, Belicova L, Bickle M, Barsacchi R, Okuda R, Yoshizawa E, Kimura M, Ayabe H, Taniguchi H, Takebe T, Treutlein B. Multilineage communication regulates human liver bud development from pluripotency. Nature. 2017;546:533–538. doi: 10.1038/nature22796. [DOI] [PubMed] [Google Scholar]
- 81.Carpentier A, Nimgaonkar I, Chu V, Xia Y, Hu Z, Liang TJ. Hepatic differentiation of human pluripotent stem cells in miniaturized format suitable for high-throughput screen. Stem Cell Res. 2016;16:640–650. doi: 10.1016/j.scr.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Willenbring H. A simple code for installing hepatocyte function. Cell Stem Cell. 2011;9:89–91. doi: 10.1016/j.stem.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 83.LeRoy G, Rickards B, Flint SJ. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol Cell. 2008;30:51–60. doi: 10.1016/j.molcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shao Z, Yao C, Khodadadi-Jamayran A, Xu W, Townes TM, Crowley MR, Hu K. Reprogramming by De-bookmarking the Somatic Transcriptional Program through Targeting of BET Bromodomains. Cell Rep. 2016;16:3138–3145. doi: 10.1016/j.celrep.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 85.Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, Strom SC. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2010;16:1075–1082. doi: 10.1089/ten.tea.2008.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyamoto K, Akiyama M, Tamura F, Isomi M, Yamakawa H, Sadahiro T, Muraoka N, Kojima H, Haginiwa S, Kurotsu S, Tani H, Wang L, Qian L, Inoue M, Ide Y, Kurokawa J, Yamamoto T, Seki T, Aeba R, Yamagishi H, Fukuda K, Ieda M. Direct In Vivo Reprogramming with Sendai Virus Vectors Improves Cardiac Function after Myocardial Infarction. Cell Stem Cell. 2018;22:91–103.e5. doi: 10.1016/j.stem.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 91.Rezvani M, Español-Suñer R, Malato Y, Dumont L, Grimm AA, Kienle E, Bindman JG, Wiedtke E, Hsu BY, Naqvi SJ, Schwabe RF, Corvera CU, Grimm D, Willenbring H. In Vivo Hepatic Reprogramming of Myofibroblasts with AAV Vectors as a Therapeutic Strategy for Liver Fibrosis. Cell Stem Cell. 2016;18:809–816. doi: 10.1016/j.stem.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, Reetz J, Brandes S, Dai Z, Pützer BM, Araúzo-Bravo MJ, Steinemann D, Luedde T, Schwabe RF, Manns MP, Schöler HR, Schambach A, Cantz T, Ott M, Sharma AD. Direct Reprogramming of Hepatic Myofibroblasts into Hepatocytes In Vivo Attenuates Liver Fibrosis. Cell Stem Cell. 2016;18:797–808. doi: 10.1016/j.stem.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 93.Li W, Nakanishi M, Zumsteg A, Shear M, Wright C, Melton DA, Zhou Q. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. Elife. 2014;3:e01846. doi: 10.7554/eLife.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet. 2014;15:82–92. doi: 10.1038/nrg3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loh KM, Ang LT, Zhang J, Kumar V, Ang J, Auyeong JQ, Lee KL, Choo SH, Lim CY, Nichane M, Tan J, Noghabi MS, Azzola L, Ng ES, Durruthy-Durruthy J, Sebastiano V, Poellinger L, Elefanty AG, Stanley EG, Chen Q, Prabhakar S, Weissman IL, Lim B. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell. 2014;14:237–252. doi: 10.1016/j.stem.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Srivastava D, DeWitt N. In Vivo Cellular Reprogramming: The Next Generation. Cell. 2016;166:1386–1396. doi: 10.1016/j.cell.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Masip M, Veiga A, Izpisúa Belmonte JC, Simón C. Reprogramming with defined factors: from induced pluripotency to induced transdifferentiation. Mol Hum Reprod. 2010;16:856–868. doi: 10.1093/molehr/gaq059. [DOI] [PubMed] [Google Scholar]
- 98.Revilla A, González C, Iriondo A, Fernández B, Prieto C, Marín C, Liste I. Current advances in the generation of human iPS cells: implications in cell-based regenerative medicine. J Tissue Eng Regen Med. 2016;10:893–907. doi: 10.1002/term.2021. [DOI] [PubMed] [Google Scholar]
- 99.Sun N, Longaker MT, Wu JC. Human iPS cell-based therapy: considerations before clinical applications. Cell Cycle. 2010;9:880–885. doi: 10.4161/cc.9.5.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vallier L. Heps with pep: direct reprogramming into human hepatocytes. Cell Stem Cell. 2014;14:267–269. doi: 10.1016/j.stem.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 101.Budniatzky I, Gepstein L. Concise review: reprogramming strategies for cardiovascular regenerative medicine: from induced pluripotent stem cells to direct reprogramming. Stem Cells Transl Med. 2014;3:448–457. doi: 10.5966/sctm.2013-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nizzardo M, Simone C, Falcone M, Riboldi G, Comi GP, Bresolin N, Corti S. Direct reprogramming of adult somatic cells into other lineages: past evidence and future perspectives. Cell Transplant. 2013;22:921–944. doi: 10.3727/096368912X657477. [DOI] [PubMed] [Google Scholar]
- 103.Kim J, Ambasudhan R, Ding S. Direct lineage reprogramming to neural cells. Curr Opin Neurobiol. 2012;22:778–784. doi: 10.1016/j.conb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takeda Y, Harada Y, Yoshikawa T, Dai P. Chemical compound-based direct reprogramming for future clinical applications. Biosci Rep. 2018:38. doi: 10.1042/BSR20171650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ebrahimi B. Chemicals as the Sole Transformers of Cell Fate. Int J Stem Cells. 2016;9:9–20. doi: 10.15283/ijsc.2016.9.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]