Abstract
Breast cancer (BC) is the most common cancer among women, and current available therapies often have high success rates. Nevertheless, BC might acquire drug resistance and sometimes relapse. Current knowledge about the most aggressive forms of BC points to the role of specific cells with stem properties located within BC, the so-called “BC stem cells” (BCSCs). The role of BCSCs in cancer formation, growth, invasiveness, therapy resistance and tumor recurrence is becoming increasingly clear. The growth and metastatic properties of BCSCs are regulated by different pathways, which are only partially known. Sex steroid receptors (SSRs), which are involved in BC etiology and progression, promote BCSC proliferation, dedifferentiation and migration. However, in the literature, there is incomplete information about their roles. Particularly, there are contrasting conclusions about the expression and role of the classical BC hormonal biomarkers, such as estrogen receptor alpha (ERα), together with scant, albeit promising information concerning ER beta (ERβ) and androgen receptor (AR) properties that control different transduction pathways in BCSCs. In this review, we will discuss the role that SRs expressed in BCSCs play to BC progression and recurrence and how these findings have opened new therapeutic possibilities.
Keywords: Breast cancer, Steroids, Sex steroid receptors, Cancer stem cells, Therapeutic implications
Core tip: Many studies have reported the presence of cancer stem cells (CSCs) in breast cancer (BC), highlighting the correlation between CSCs and BC aggressiveness. Sex steroids and steroid receptors play a pivotal role in BCSCs. By controlling different pathways, BCSCs are able to influence both BC recurrence and drug resistance. Therefore, better knowledge of BCSC features and behavior would be useful to employ these cells as BC prognostic factors, and develop new promising therapies targeting these cells and improving BC recurrence.
INTRODUCTION
Breast cancer (BC) is the most common cancer in women worldwide and the second most common cancer overall[1]. Although it is considered to be a postmenopausal disease, genetic predisposition, aging, gender, age of menarca, null parity, late age menopause and familial history of BC still represent the leading risk factors for BC[2].
Transformation of breast stem/progenitor cells is involved in breast carcino-genesis[3] and many studies have reported the presence of cancer stem cells (CSCs) in malignant BC[4-6]. CSCs might positively affect tumor survival, metastatic spreading and therapy escape[7]. Specifically, secretion of interleukins 6 and 8 (IL-6 and IL-8) by tumor associated fibroblasts, mesenchymal stem cells and macrophages promote CSCs self-renewal in BC, further pointing to the role of tumor micro-environment in cancer progression[7]. Estradiol also influences the breast cancer stem cells (BCSCs) population in a paracrine manner, as well as other factors, including metalloproteases (MMPs), insulin growth factor (IGF), platelet growth factor (PDGF) released by cancer-surrounding cells, which might affect proliferation, invasiveness and metastatic spreading of BC cells[8,9].
The presence and frequency of CSCs are, however, related to BC type, and many findings have shown a strong correlation between CSCs and BC aggressiveness. Meta-analyses from twelve published studies have shown that BCSCs are significantly associated with high histological grade, human epidermal receptor-2 (Her-2) positivity, estrogen receptor (ER) and progesterone receptor (PR) negativity, as well as the absence of any correlation with tumor size or nodal status[10]. Moreover, BCSCs are resistant to classical therapies. By enriching for the BCSC population, anti-cancer treatments often fail. Chemo- or radio-resistance of BCSCs has been attributed to different factors. As it occurs in SCs, they persistently remain in a quiescent state (G0 phase), while the cancer cells quickly replicate. Therefore, the standard therapies, which only act on rapidly dividing cells, are ineffective against BCSCs[11]. Again, BCSCs have enhanced expression of ATP-binding cassette (ABC) transporters and aldehyde dehydrogenase (ALDH), both capable of reducing the drug concentration inside cells[12]. Lastly, BCSCs exhibit an altered response to DNA damage, which protects them from apoptosis[11]. All of these properties make them resistant to the currently available antineoplastic therapies.
The role of sex steroids (estrogens, progestins and androgens) as well as SSRs in BC is largely recognized[13]. It is also currently accepted that sex steroids sustain the stem cell population in normal and malignant breast. An increase in the stem cell population might lead to cancer susceptibility in normal breast, while an increase in BCSCs influences both drug resistance and tumor recurrence[14,15]. Taken together, findings collected thus far suggest that CSCs represent a very promising prognostic factor in BC, although additional studies are required to confirm their importance in clinical practice.
In this review, we will present the recent findings on the role of sex steroid receptors (SSRs) in BCSCs. The therapeutic implication of these studies will also be discussed, since BCSC-targeted therapies seem very promising in the clinical management of BC patients.
BCSCs
Mammary gland morphology continuously changes throughout life. At birth, human mammary gland epithelium is made up of a network of ducts. During puberty, mammary ducts form side branches, while also forming numerous lobulo-acinar structures containing the milk-secreting alveolar cells during pregnancy and lactation. By activating massive apoptosis and tissue remodeling, the mammary gland then reduces its dimensions at the end of lactation[16]. To do this, a group of cells with high proliferative potential and differentiation ability have to be localized within the mammary tissue. Despite different studies demonstrating the presence of SCs in mammary tissue, these cells have not yet been identified and isolated to date[3]. Mammary (Ma)SCs are undifferentiated and their cell division can be symmetric, resulting in the production of two self-renewing or asymmetric cells. As such, a variety of pluripotent differentiated cells, including luminal and basal SCs as well as pluripotent progenitors, might differentiate into ductal, alveolar and myoepithelial cells. Consistent with the CSCs theory, both MaSCs and progenitor cells can give rise to BCSCs during these cell divisions, thereby fostering carcinogenesis[17]. A different theory claims that BCSCs are derived from de-differentiated cancer cells induced by changes in the tumor microenvironment, or chemotherapy or other targeted therapies. Through genetic or epigenetic modifications, transformed cells might acquire a stem-like phenotype[17-20].
BCSCs are more resistant than MaSCs, and are characterized by the expression of specific cell surface markers, such as high levels of cluster of differentiation 44 (CD44) and low levels of cluster of differentiation 24 (CD24). Particularly, high expression of CD44 maintains BCSC multipotency, while low levels of CD24 maintain cell stemness[21]. More recently, additional markers have been identified, including ALDH1, which oxidizes retinol to retinoic acid, thereby playing a role in the first step of BCSC differentiation. Elevated expression of ALDH1 identifies BCSCs and correlates with poor prognosis in receptor-negative BCs[22,23]. Again, other cell surface markers, such as the cluster of differentiation 133 (CD133), 49f (CD49f), and 90 (CD90) have been identified as CSC markers and are associated with drug resistance, poor prognosis, and reduced BC survival[24].
These findings, summarized in Table 1, have made it possible to design and synthesize specific antibodies to target these BCSC markers and create more efficacious therapies for aggressive BCs. To make this landscape more intricate, a plethora of pathways activated in MaSCs are deregulated in BCSCs. These include the Notch, Wnt, Hedgehog and Hippo pathways that, in addition to cross-reacting with each other, intersect with the main signaling pathways (PI3-K/Akt; MEK-dependent pathway) in BCSCs. As such, their successful targeting is very ambitious, since inhibition of one circuit frequently induces up-regulation and/or hyper-activation of the other pathways[24]. Unfortunately, less is known about the classical and non-classical pathways commonly activated by SSRs in BC cells. In the subsequent sections of this review, we will discuss the scant data in the literature that integrates and improves our knowledge about this topic.
Table 1.
Breast cancer stem cell biomarkers
| Biomarkers | Expression | Role | Ref. |
| CD44 | Positive/high | Maintenance of breast cancer stem cell multipotency, cell proliferation and cell migration | Schabath et al[21], 2006 |
| CD24 | Negative/low | Cell migration and metastases | Jaggupilli et al[82], 2012 |
| ALDH1 | Positive/high | Stemness, cell migration, invasion, and tumor metastases | Ma et al[28], 2017 |
| CD133 | Positive | Cellular differentiation | Sin et al[83], 2017 |
| CD49f | Positive | Tumor initiation and metastases | Sin et al[83], 2017 |
| CD90 | Positive | Drug-resistance and poor prognosis | Schabath et al[21], 2006 |
CD44: Cluster of differentiation 44; CD24: Cluster of differentiation 24; ALDH1: Aldehyde dehydrogenase 1; CD133: Cluster of differentiation 133; CD49f: Cluster of differentiation 49f; CD90: Cluster of differentiation 90.
ER in BCSCs
Two isoforms of ER, ERα and ERβ, are expressed in BCs[25-28], with ERα representing the most important hormonal biomarker in this cancer. ERα is expressed in almost 75% of BCs, and its presence positively correlates with response to endocrine therapy[29]. In some studies, ERβ has also been associated with improved survival in tamoxifen-treated patients[30,31]. The two ER subtypes are encoded by genes on different chromosomes, and differentially activate common estrogen response elements (ERE) in gene reporter assays[32,33]. In target cells, both ER isoforms act through transcriptional and non-transcriptional mechanisms, thereby controlling cell cycle progression, invasiveness and metastatic phenotypes[34-36]. Recently, a new 36KDa truncated variant of ERα (ERα36) has been identified, which is expressed in both ERα-positive and negative BC cells. ERα36 lacks both of the transactivation ER domains, localizes to plasma membrane as well as cytoplasm, and responds to estrogens and anti-estrogens. It also regulates BC cell proliferation and contributes to BC aggressiveness[37].
The expression and role of each ER isoform in BCSCs, however, still remains under debate. The majority of studies points to the absence of ERα in BCSCs[38]. It has been consistently reported that CD44+/CD24-/ALDH+ CSCs lack ER or express it at very low levels[15,39]. Although considered ERα-negative, the number of both BCSCs and MaSCs can be increased by stimulation with estradiol[38], likely because other receptors (for instance, G-protein coupled receptor 30, ERα36 or ERβ) might mediate estrogen action in these cells. These findings will be extensively discussed below.
Additional studies also argue that BCSCs do not harbor ERα, and that the receptor rather arises from the original BC. As a result, ERα would be expressed in BCSCs derived from ERα-positive BCs, while it would be absent in BCSCs derived from ERα-negative BCs[40]. As it occurs in prostate CSCs[41,42], these quite divergent findings may be due to experimental differences, such as ER assays, cell culture conditions and BC cell populations.
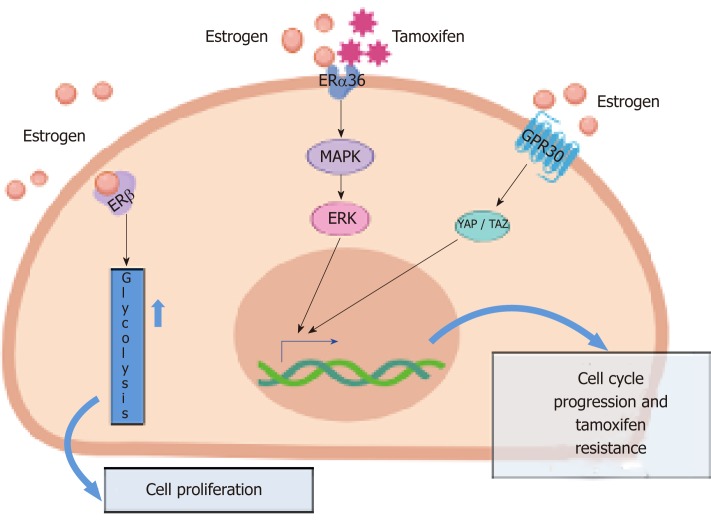
It is, however, currently accepted that estrogens act on BCSCs via non-genomic signaling, by activating GPR30, a seven domain trans-membrane receptor expressed in both ER-positive and ER-negative breast cancers[43]. It has been reported that this influences the Hippo pathway via tafazzin (TAZ) activation. In BCSCs, TAZ activation is responsible for BC metastatic features[44]. Again, elevated levels of TAZ combined with its increased activation can be detected in poorly differentiated BCs, where it confers self-renewal capacity to non-CSCs[45]. Other reports indicate that estrogens act by activating ERα or its variant, ERα36. In ERα-positive, MCF-7-derived tumor spheres collected on day 21 (tertiary tumor spheres), when they possess high levels of stemness markers and self-renewal ability, estrogen stimulation increases the levels of PI-9, a granzyme B inhibitor. Such an effect impairs immune surveillance, and increases both the number and size of tumor spheres[46]. ERα36, which lacks transcriptional activity and exclusively acts through non-genomic action, could mediate these responses since estrogen treatment of tertiary tumor spheres increases ERα36 levels and decreases the level of the full-length ERα[46]. In spite of ERα36 being predominantly a plasma membrane-based receptor and lacks both the AF-1 and AF-2 transactivation domains of ERα66 (ERαwt), it also acts as a negative regulator of genomic estrogen signaling mediated by both ERα wt and the ERβ[47]. A small amount of ERα36 is located in nuclei where it competes with the two receptors for DNA binding sites (ERE, [47]).
Again, upon estrogen stimulation, ERα36 rapidly activates the MAPKs/ERK pathway, thus triggering cellular proliferation[47]. The MAPK/ERK pathway is activated not only by estrogens but also by the antiestrogen tamoxifen in a stronger and prolonged way[47]. These findings might explain the pivotal role of ERα36 in anti-estrogen BC resistance.
ERβ and stem cell marker expression have been recently studied in mammospheres derived from fresh primary BC specimens and BC cells. In about 50% of cases, ERβ was upregulated in BCSCs. More importantly, it was co-expressed with CD44 and ALDH1 in the absence of ERα. Again, ERβ was responsible for the growth of mammospheres and the upregulation of glycolysis. Thus, ERβ might actually be considered as a stemness marker in BC cells[28]. This study offers new hints for a better understanding of ERβ function in BC and, in contrast with the concept that BCSCs respond to estradiol via paracrine signaling, it proposes that estrogens directly challenge BCSCs through ERβ activation. At last, identification of ERβ-enriched BCSCs offers new therapeutic possibilities based on the use of ERβ antagonists, combined with classical drugs (antiestrogens or aromatase inhibitors) routinely employed in the clinical management of BC.
Altogether, the data discussed thus far show that ERα and ERβ can both be detected in BCSCs. Depending on the specific context, they can be targeted to limit the proliferative and invasive rate of BCSCs. Although these cells are usually resistant to the classical therapies that target ER, the presented data support the idea that ER acts in an unconventional manner in BCSCs, paving the way for the exploration of new GPR30[48] or ERβ [28] inhibitors or drugs/peptides that specifically inhibit the non-genomic action induced by ERs in BC[25,35]. Some of the principal pathways operating in BCSCs are sketched in Figure 1.
Figure 1.
The main pathways activated by different estrogen receptor isoforms in breast cancer stem cells, responsible for cell proliferation and tamoxifen-resistance. GPER: G-protein coupled receptor; ERα36: estrogen receptor alpha 36; ERβ: estrogen receptor beta; MEK: Mitogen activated protein kinase; ERK: extracellular signal-regulated kinase; YAP: Yes-associated protein; TAZ: Tafazzin.
PR in BCSCs
Progesterone and its receptor play a pivotal role in mammary gland side branching that occurs during puberty, as well as lobular-alveolar development during pregnancy. PR exists in two isoforms, PR-A (PR-A, 94KDa) and PR-B (PR-B, 114KDa). The same gene encodes for the two PR isoforms, but PR-A lacks the first 164 amino acids of the PR-B, and might act as a trans-repressor of PR-B transcriptional activity, although it might even trans-repress the activity of ER, androgen receptor (AR), and glucocorticoid and mineralcorticoid receptors[49]. The two isoforms are co-expressed at similar levels in normal breast cells, but this balance is altered in cancer cells, where one of the two isoforms, PR-A, is commonly overexpressed[50].
By enhancing SC proliferation and increasing the number of progenitor cells, progesterone influences mammary gland growth[50] and induces mammary tumor formation[38]. As it occurs for ER and AR, the ligand-activated PR works in BC cells through genomic and non-genomic mechanisms, thus controlling transcriptional machinery, epigenetic modifications and rapid signaling pathways depending on Src or PI3-K activation[51]. This is, however, a simple picture of progesterone action in target cells. We now appreciate that rapid activation of signaling cascades by ligand-bound PR fuels chromatin remodeling and gene transcription, on the one hand[52]. On the other, the progestin-activated transcriptional machinery might regulate cytoplasmic events, which impinges on signaling activation[53].
In women with pre-existing BC, progestins are responsible for the re-activation of ER-/PR- cancer stem-like cells[54]. Progesterone stimulation of differentiated cancer cells (ER+, PR+, CK5-) increases the number of stem-like colony cells (ER-, PR-, CD44+, CK5+) within the tumor. Ligand activation of PR does not modify the cell number, but rather de-differentiates the more abundant ER+/PR+/CK5- cells into ER-/PR-/CK5+ cells harboring stem-like properties[54]. Specifically, activated PR binds two putative progesterone response elements localized within the CK5 promoter. This transcriptional regulation finally leads to an increase in CK5 expression and is more effective in small, almost undetectable BCs, allowing their recurrence.
PRs are commonly considered as an indicator of the transcriptionally intact ER axis[55]. In BC-derived T47D cells, which express the two PR isoforms under basal conditions, PR-A is the principal driver of CSC expansion, while PR-B regulates anchorage-independent growth. Specifically, expansion and biochemical features of CSCs (ALDH1, CD44+/CD24-, CD49f+/CD24-) are linked to PR-A phosphorylation at the Ser 294 residue. PR-A+ tumor spheres are, hence, small but express an enriched basal-like CSC phenotype (CD49f+/CD24-), which is suggestive of increased malignancy and metastatic potential. On the other hand, PR-B+ tumor spheres are larger than the PR-A+ ones and exhibit a CD49f+/CD24+ luminal phenotype. Cells expressing a PR-A mutant that cannot be phosphorylated at the Ser 294 residue display an impaired CSC phenotype associated with an enhancement of anchorage-independent growth[55].
Taken together, the data presented thus far highlight the role of the progestin/PR axis in sustaining the survival and growth of BCSCs, and emphasize the role of each PR isoform in these processes. A better understanding of the role of each PR isoform in BCSCs might open new perspectives in the therapeutic approach of this cancer type, particularly in its recurrent forms.
AR in BCSCs
AR expression is closely associated with a group of hormone-related diseases, including cancers of the prostate, breast, ovary, pancreas, liver and lung. It is also linked to various diseases that include muscle atrophy, osteoporosis, diabetes and neurodegenerative disorders[56-58].
AR is expressed in both ER-positive and ER-negative BCs[59]. In ER-positive BCs, AR correlates with a more favorable prognosis, while it is commonly considered to control progression and drug resistance in triple negative BCs[2,60]. It is largely accepted that AR activation by androgens regulates important changes in gene transcription or signaling pathway activation (i.e. Src/Ras/MAPKs, PI3K/Akt, filaminA/Rac). These actions control different processes, including proliferation, migration, and invasiveness of normal and cancer cells[25,58,61,62].
The role of androgens and AR in BCSCs is poorly explored, and few data have been published in the literature. By perusing the United States National Library of Medicine (https://www.ncbi.nlm.nih.gov/pubmed/), we found only 43 results matching with our analysis. In a recent paper[63], AR expression has been correlated with “stemness” markers (i.e. CD44, CD24 and ALDH1) in 166 BC patients. A significant correlation between AR and CD24 has been observed in stage I-III invasive BC. Such a phenotype correlates with favorable clinicopathological features, and delineates a subgroup of patients with better disease-free survival[63]. However, AR expression in CSCs might foster BC invasiveness. Forced suspension culture of AR-positive MDA-MB453 with SUM195pt cells induces an increase in the BCSC-like population, and protects cells from anoikis. Such effects depend on AR, as shown by experiments with the anti-androgen enzalutamide[64].
Again, dihydrotestosterone treatment increases the CK5+ population in MCF-7 but not T47D cells. Notably, CK5+ cells are therapy resistant, have increased tumor-initiating potential, and express the SC marker CD44[65]. The finding that androgens exert different actions in the two BC-derived cell types might be related to the different intersection of AR with other SSRs occurring at the transcriptional or non-transcriptional level in breast and prostate cancer-derived cells[41,42,66]. Furthermore, AR maintains the BCSC population in AR-positive TNBCs, since its knockdown or treatment with enzalutamide reduces the number of ALDH1+ cells as well as mammosphere formation[67]. It should be noted that synthetic progestins activate AR[68]. Therefore, progestin-induced BCSC enrichment might be due to AR activation[69]. In addition to reinforcing the concept that SRs substitute each other in mediating important biological effects[25,70,71], such a mechanism might take place in BCs expressing high levels of AR in association with low or undetectable PR levels. Consistent with this hypothesis, it might also be argued that progestins launch a double hit by acting on both AR and PR. Altogether, these considerations account for the clinical correlation between progestin-treated women with increased BC risk, and highlight the complexity of AR’s role in BC pathogenesis. The contribution of the androgens/AR axis in BCSC regulation, however, still remains uncertain.
STEROID RECEPTOR-REGULATED MIRNAs IN BCSCs
In BCSCs, steroid receptors are also able to control miRNA levels. ERα regulates microRNA (miRNA) expression, thereby controlling the ability of BCSCs to affect proliferation, death, adhesion and cell-cell communication[72]. In BCSCs, activated ERα binds a specific ERE flanking the promoter region of miRNA-140, thereby suppressing miRNA-140 transcription and enhancing the expression of SOX2, a stemness marker, which maintains SCs[73].
PR regulates different miRNAs in BC. Among them, miR-29 and mi-R 200 families are involved in BCSC formation. The miR-29 family includes three members, miR-29 a, b and c, which are all down-regulated by progestins in BC. Such down-regulation is linked to an increase in the transcription factor KLF4, as well as CD44 and CK5, with the subsequent de-differentiation of cells[74]. It has also been shown that the progestin-induced increase of GATA3 results in miR-29b down-regulation, and a subsequent increase in the BCSC population[75]. Again, the miR-200 family includes miR-141, which is down-regulated by PR. miR-141 increases the CD44+ and CK5+ cell population, while reducing PR and Stat-5 levels, two important transcription factors implicated in the control of mammary cell fate[76].
There are no studies about miRNA regulation by AR in BCSCs. Few obtained data have shown that AR is responsible for miRNA down-regulation[77]. In ER-/PR-/AR+ cells, AR up-regulates the pro-differentiation of miRNA let7a, which, in turn, inhibits cell proliferation by downregulating c-MYC and K-Ras[78].
Altogether, the findings reported here indicate that ER and PR upregulate miRNA levels involved in CSCs formation and differentiation. As such, they represent excellent targets to impair CSCs formation and, likely, BC recurrence.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
A growing number of studies is trying to clarify the role of BCSCs in BC pathogenesis and progression. Although interest in the study of BCSCs is currently high, it is not yet well known how these cells work within the cancer, and the identity of the engaged pathways.
Based on the stem cell hypothesis, cancer might arise from a cell population with the stem property of self-renewal. Such a property can already be owned by cells or can be acquired. As such, cancers originating from these cells are organized in a hierarchical fashion, in which SCs or stem-like cells drive the malignant process and generate a population of non-renewing cells that regulate the cancer bulk[79].
Less is known about the role of SSRs in SCs. Despite the fact that some reports claim that ERs are not expressed in BCSCs, many studies concerning the expression and role of this receptor have been published, with very conflicting data. The classical isoform of ERα acts, for instance, through a genomic pathway that regulates miRNA expression and SC phenotype, while the ERα variant, ERα 36 or GPR30, may act through non-genomic pathways, thereby contributing to cell dedifferentiation, tumor metastases and therapy resistance. Surprisingly, ERβ is commonly considered a marker of stemness in BCSCs. Its targeting by specific antagonists can be envisaged as mono or combinatorial therapy in the clinical management of BC.
Both PR isoforms seem to play a pivotal role in BCSC expansion and proliferation, and are tightly linked to BC metastatic and malignant properties. In this way, deepened knowledge of the machinery controlled by PR in BCSCs might be a big step forward to predict BC relapse and inhibit the growth of BCs resistant to currently employed therapies. The role of AR remains uncertain, and data about its behavior in BCSCs are very scant. Therefore, it is very difficult to draw any conclusions concerning the role of this receptor in BCSCs.
In conclusion, the data discussed thus far points to PR isoforms and ERβ as the more convincing targets to reduce the BCSCs population within human BC. Therefore, a better and more exhaustive understanding of other SSRs is required in order to develop new treatments of BC and control drug resistance, which is often imputable to BCSCs.
Preclinical and clinical evidence indicates that BCSCs control progression, invasion, metastasis as well as drug and radiation therapy resistance. Therefore, eradication of BC strictly depends on the elimination of BCSCs. New molecules such as GDC0449 or eribulin have entered clinical trials for their anticancer stem cell activity [80,81]. Further preclinical and clinical studies are needed to elucidate the relevance of CSCs signaling in BC recurrence and therapy resistance.
ACKNOWLEDGEMENTS
A special thanks to Mrs. Sarah Lamptey for the English revision of the manuscript.
Footnotes
Conflict-of-interest statement: All authors declare they have no potential conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: March 4, 2019
First decision: April 11, 2019
Article in press: August 20, 2019
Specialty type: Cell and tissue engineering
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Demonacos C S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
Contributor Information
Pia Giovannelli, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples 80138, Italy. pia.giovannelli@unicampania.it.
Marzia Di Donato, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples 80138, Italy.
Giovanni Galasso, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples 80138, Italy.
Erika Di Zazzo, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples 80138, Italy.
Nicola Medici, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples 80138, Italy.
Antonio Bilancio, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples 80138, Italy.
Antimo Migliaccio, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples 80138, Italy.
Gabriella Castoria, Department of Precision Medicine, University of Campania “L. Vanvitelli”, Naples 80138, Italy.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Giovannelli P, Di Donato M, Galasso G, Di Zazzo E, Bilancio A, Migliaccio A. The Androgen Receptor in Breast Cancer. Front Endocrinol (Lausanne) 2018;9:492. doi: 10.3389/fendo.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36 Suppl 1:59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Kakarala M, Wicha MS. Cancer stem cells: implications for cancer treatment and prevention. Cancer J. 2007;13:271–275. doi: 10.1097/PPO.0b013e318156da4e. [DOI] [PubMed] [Google Scholar]
- 7.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer CF, Kronsteiner N, Marton E, Kubista M, Cullen KJ, Hirtenlehner K, Seifert M, Kubista E. MMP-2 and MMP-9 expression in breast cancer-derived human fibroblasts is differentially regulated by stromal-epithelial interactions. Breast Cancer Res Treat. 2002;72:69–77. doi: 10.1023/a:1014918512569. [DOI] [PubMed] [Google Scholar]
- 9.LeBedis C, Chen K, Fallavollita L, Boutros T, Brodt P. Peripheral lymph node stromal cells can promote growth and tumorigenicity of breast carcinoma cells through the release of IGF-I and EGF. Int J Cancer. 2002;100:2–8. doi: 10.1002/ijc.10481. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Jiang Y, Yan T, Di G, Shen Z, Shao Z, Lu J. The prognostic role of cancer stem cells in breast cancer: a meta-analysis of published literatures. Breast Cancer Res Treat. 2010;122:795–801. doi: 10.1007/s10549-010-0999-4. [DOI] [PubMed] [Google Scholar]
- 11.Palomeras S, Ruiz-Martínez S, Puig T. Targeting Breast Cancer Stem Cells to Overcome Treatment Resistance. Molecules. 2018:23. doi: 10.3390/molecules23092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nandy SB, Gangwani L, Nahleh Z, Subramani R, Arumugam A, de la Rosa JM, Lakshmanaswamy R. Recurrence and metastasis of breast cancer is influenced by ovarian hormone's effect on breast cancer stem cells. Future Oncol. 2015;11:983–995. doi: 10.2217/fon.14.301. [DOI] [PubMed] [Google Scholar]
- 13.Castoria G, Migliaccio A, D'Amato L, Di Stasio R, Ciociola A, Lombardi M, Bilancio A, Di Domenico M, de Falco A, Auricchio F. Integrating signals between cAMP and MAPK pathways in breast cancer. Front Biosci. 2008;13:1318–1327. doi: 10.2741/2764. [DOI] [PubMed] [Google Scholar]
- 14.Finlay-Schultz J, Sartorius CA. Steroid hormones, steroid receptors, and breast cancer stem cells. J Mammary Gland Biol Neoplasia. 2015;20:39–50. doi: 10.1007/s10911-015-9340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simões BM, O'Brien CS, Eyre R, Silva A, Yu L, Sarmiento-Castro A, Alférez DG, Spence K, Santiago-Gómez A, Chemi F, Acar A, Gandhi A, Howell A, Brennan K, Rydén L, Catalano S, Andó S, Gee J, Ucar A, Sims AH, Marangoni E, Farnie G, Landberg G, Howell SJ, Clarke RB. Anti-estrogen Resistance in Human Breast Tumors Is Driven by JAG1-NOTCH4-Dependent Cancer Stem Cell Activity. Cell Rep. 2015;12:1968–1977. doi: 10.1016/j.celrep.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudland PS, Barraclough R, Fernig DG, Smith JA. Growth and differentiation of the normal mammary gland and its tumours. Biochem Soc Symp. 1998;63:1–20. [PubMed] [Google Scholar]
- 17.Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, Ji X, Liu W, Huang B, Luo W, Liu B, Lei Y, Du S, Vuppalapati A, Luu HH, Haydon RC, He TC, Ren G. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5:77–106. doi: 10.1016/j.gendis.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagadec C, Vlashi E, Della Donna L, Dekmezian C, Pajonk F. Radiation-induced reprogramming of breast cancer cells. Stem Cells. 2012;30:833–844. doi: 10.1002/stem.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koren S, Reavie L, Couto JP, De Silva D, Stadler MB, Roloff T, Britschgi A, Eichlisberger T, Kohler H, Aina O, Cardiff RD, Bentires-Alj M. PIK3CA(H1047R) induces multipotency and multi-lineage mammary tumours. Nature. 2015;525:114–118. doi: 10.1038/nature14669. [DOI] [PubMed] [Google Scholar]
- 20.Poli V, Fagnocchi L, Fasciani A, Cherubini A, Mazzoleni S, Ferrillo S, Miluzio A, Gaudioso G, Vaira V, Turdo A, Gaggianesi M, Chinnici A, Lipari E, Bicciato S, Bosari S, Todaro M, Zippo A. MYC-driven epigenetic reprogramming favors the onset of tumorigenesis by inducing a stem cell-like state. Nat Commun. 2018;9:1024. doi: 10.1038/s41467-018-03264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schabath H, Runz S, Joumaa S, Altevogt P. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci. 2006;119:314–325. doi: 10.1242/jcs.02741. [DOI] [PubMed] [Google Scholar]
- 22.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charafe-Jauffret E, Ginestier C, Birnbaum D. Cancer stem cells: just sign here! Cell Cycle. 2010;9:229–230. [PubMed] [Google Scholar]
- 24.Crabtree JS, Miele L. Breast Cancer Stem Cells. Biomedicines. 2018:6. doi: 10.3390/biomedicines6030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovannelli P, Di Donato M, Giraldi T, Migliaccio A, Castoria G, Auricchio F. Targeting rapid action of sex steroid receptors in breast and prostate cancers. Front Biosci (Landmark Ed) 2011;16:2224–2232. doi: 10.2741/3849. [DOI] [PubMed] [Google Scholar]
- 26.Bado I, Gugala Z, Fuqua SAW, Zhang XH. Estrogen receptors in breast and bone: from virtue of remodeling to vileness of metastasis. Oncogene. 2017;36:4527–4537. doi: 10.1038/onc.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warner M, Huang B, Gustafsson JA. Estrogen Receptor β as a Pharmaceutical Target. Trends Pharmacol Sci. 2017;38:92–99. doi: 10.1016/j.tips.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Ma R, Karthik GM, Lövrot J, Haglund F, Rosin G, Katchy A, Zhang X, Viberg L, Frisell J, Williams C, Linder S, Fredriksson I, Hartman J. Estrogen Receptor β as a Therapeutic Target in Breast Cancer Stem Cells. J Natl Cancer Inst. 2017;109:1–14. doi: 10.1093/jnci/djw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis PJ, Lin HY, Mousa SA, Luidens MK, Hercbergs AA, Wehling M, Davis FB. Overlapping nongenomic and genomic actions of thyroid hormone and steroids. Steroids. 2011;76:829–833. doi: 10.1016/j.steroids.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727–3734. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 31.Rosin G, de Boniface J, Karthik GM, Frisell J, Bergh J, Hartman J. Oestrogen receptors β1 and βcx have divergent roles in breast cancer survival and lymph node metastasis. Br J Cancer. 2014;111:918–926. doi: 10.1038/bjc.2014.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 33.Zhao C, Dahlman-Wright K, Gustafsson JÅ. Estrogen signaling via estrogen receptor {beta} J Biol Chem. 2010;285:39575–39579. doi: 10.1074/jbc.R110.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2006;238:1–14. doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Castoria G, Migliaccio A, Giovannelli P, Auricchio F. Cell proliferation regulated by estradiol receptor: Therapeutic implications. Steroids. 2010;75:524–527. doi: 10.1016/j.steroids.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Castoria G, Giovannelli P, Lombardi M, De Rosa C, Giraldi T, de Falco A, Barone MV, Abbondanza C, Migliaccio A, Auricchio F. Tyrosine phosphorylation of estradiol receptor by Src regulates its hormone-dependent nuclear export and cell cycle progression in breast cancer cells. Oncogene. 2012;31:4868–4877. doi: 10.1038/onc.2011.642. [DOI] [PubMed] [Google Scholar]
- 37.Lee LM, Cao J, Deng H, Chen P, Gatalica Z, Wang ZY. ER-alpha36, a novel variant of ER-alpha, is expressed in ER-positive and -negative human breast carcinomas. Anticancer Res. 2008;28:479–483. [PMC free article] [PubMed] [Google Scholar]
- 38.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. Control of mammary stem cell function by steroid hormone signaling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 39.Morimoto K, Kim SJ, Tanei T, Shimazu K, Tanji Y, Taguchi T, Tamaki Y, Terada N, Noguchi S. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Sci. 2009;100:1062–1068. doi: 10.1111/j.1349-7006.2009.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu WR, Lin HS, Chen XY, Zhang Y. Estrogen receptor of breast cancer stem cells depending on the original breast cancers. Med Hypotheses. 2011;77:71–73. doi: 10.1016/j.mehy.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 41.Di Zazzo E, Galasso G, Giovannelli P, Di Donato M, Di Santi A, Cernera G, Rossi V, Abbondanza C, Moncharmont B, Sinisi AA, Castoria G, Migliaccio A. Prostate cancer stem cells: the role of androgen and estrogen receptors. Oncotarget. 2016;7:193–208. doi: 10.18632/oncotarget.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Zazzo E, Galasso G, Giovannelli P, Di Donato M, Castoria G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front Oncol. 2018;8:2. doi: 10.3389/fonc.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, Li F, Yu FX, Sun Y, Yuan H, Zhu H, Xiong Y, Lei QY, Guan KL. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015;125:2123–2135. doi: 10.1172/JCI79573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi G, Sperati F, Amabile MI, Pilozzi E, Patrizii M, Biffoni M, Maugeri-Saccà M, Piccolo S, De Maria R. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34:681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 45.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, Daidone MG, Dupont S, Basso G, Bicciato S, Piccolo S. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 46.Lauricella M, Carlisi D, Giuliano M, Calvaruso G, Cernigliaro C, Vento R, D'Anneo A. The analysis of estrogen receptor-α positive breast cancer stem-like cells unveils a high expression of the serpin proteinase inhibitor PI-9: Possible regulatory mechanisms. Int J Oncol. 2016;49:352–360. doi: 10.3892/ijo.2016.3495. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 49.Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res. 1999;54:291–313; discussion 313-4. [PubMed] [Google Scholar]
- 50.Graham JD, Clarke CL. Expression and transcriptional activity of progesterone receptor A and progesterone receptor B in mammalian cells. Breast Cancer Res. 2002;4:187–190. doi: 10.1186/bcr450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giraldi T, Giovannelli P, Di Donato M, Castoria G, Migliaccio A, Auricchio F. Steroid signaling activation and intracellular localization of sex steroid receptors. J Cell Commun Signal. 2010;4:161–172. doi: 10.1007/s12079-010-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vicent GP, Ballaré C, Nacht AS, Clausell J, Subtil-Rodríguez A, Quiles I, Jordan A, Beato M. Convergence on chromatin of non-genomic and genomic pathways of hormone signaling. J Steroid Biochem Mol Biol. 2008;109:344–349. doi: 10.1016/j.jsbmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horwitz KB, Sartorius CA. Progestins in hormone replacement therapies reactivate cancer stem cells in women with preexisting breast cancers: a hypothesis. J Clin Endocrinol Metab. 2008;93:3295–3298. doi: 10.1210/jc.2008-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Truong TH, Dwyer AR, Diep CH, Hu H, Hagen KM, Lange CA. Phosphorylated Progesterone Receptor Isoforms Mediate Opposing Stem Cell and Proliferative Breast Cancer Cell Fates. Endocrinology. 2019;160:430–446. doi: 10.1210/en.2018-00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang C, Yeh S, Lee SO, Chang TM. Androgen receptor (AR) pathophysiological roles in androgen-related diseases in skin, bone/muscle, metabolic syndrome and neuron/immune systems: lessons learned from mice lacking AR in specific cells. Nucl Recept Signal. 2013;11:e001. doi: 10.1621/nrs.11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Donato M, Giovannelli P, Cernera G, Di Santi A, Marino I, Bilancio A, Galasso G, Auricchio F, Migliaccio A, Castoria G. Non-genomic androgen action regulates proliferative/migratory signaling in stromal cells. Front Endocrinol (Lausanne) 2015;5:225. doi: 10.3389/fendo.2014.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castoria G, Auricchio F, Migliaccio A. Extranuclear partners of androgen receptor: at the crossroads of proliferation, migration, and neuritogenesis. FASEB J. 2017;31:1289–1300. doi: 10.1096/fj.201601047R. [DOI] [PubMed] [Google Scholar]
- 59.Vera-Badillo FE, Templeton AJ, de Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, Seruga B, Tannock IF, Ocana A, Amir E. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:djt319. doi: 10.1093/jnci/djt319. [DOI] [PubMed] [Google Scholar]
- 60.Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D'Amato NC, Spoelstra NS, Edgerton SM, Jean A, Guerrero J, Gómez F, Medicherla S, Alfaro IE, McCullagh E, Jedlicka P, Torkko KC, Thor AD, Elias AD, Protter AA, Richer JK. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16:R7. doi: 10.1186/bcr3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29:3651–3658. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 62.Giovannelli P, Di Donato M, Auricchio F, Castoria G, Migliaccio A. Androgens Induce Invasiveness of Triple Negative Breast Cancer Cells Through AR/Src/PI3-K Complex Assembly. Sci Rep. 2019;9:4490. doi: 10.1038/s41598-019-41016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riaz N, Idress R, Habib S, Azam I, Lalani EM. Expression of Androgen Receptor and Cancer Stem Cell Markers (CD44+/CD24- and ALDH1+): Prognostic Implications in Invasive Breast Cancer. Transl Oncol. 2018;11:920–929. doi: 10.1016/j.tranon.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barton VN, Christenson JL, Gordon MA, Greene LI, Rogers TJ, Butterfield K, Babbs B, Spoelstra NS, D'Amato NC, Elias A, Richer JK. Androgen Receptor Supports an Anchorage-Independent, Cancer Stem Cell-like Population in Triple-Negative Breast Cancer. Cancer Res. 2017;77:3455–3466. doi: 10.1158/0008-5472.CAN-16-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goodman CR, Sato T, Peck AR, Girondo MA, Yang N, Liu C, Yanac AF, Kovatich AJ, Hooke JA, Shriver CD, Mitchell EP, Hyslop T, Rui H. Steroid induction of therapy-resistant cytokeratin-5-positive cells in estrogen receptor-positive breast cancer through a BCL6-dependent mechanism. Oncogene. 2016;35:1373–1385. doi: 10.1038/onc.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossi V, Di Zazzo E, Galasso G, De Rosa C, Abbondanza C, Sinisi AA, Altucci L, Migliaccio A, Castoria G. Estrogens Modulate Somatostatin Receptors Expression and Synergize With the Somatostatin Analog Pasireotide in Prostate Cells. Front Pharmacol. 2019;10:28. doi: 10.3389/fphar.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barton VN, D'Amato NC, Gordon MA, Christenson JL, Elias A, Richer JK. Androgen Receptor Biology in Triple Negative Breast Cancer: a Case for Classification as AR+ or Quadruple Negative Disease. Horm Cancer. 2015;6:206–213. doi: 10.1007/s12672-015-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bullock LP, Bardin CW, Sherman MR. Androgenic, antiandrogenic, and synandrogenic actions of progestins: role of steric and allosteric interactions with androgen receptors. Endocrinology. 1978;103:1768–1782. doi: 10.1210/endo-103-5-1768. [DOI] [PubMed] [Google Scholar]
- 69.Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol. 2008;26:1260–1268. doi: 10.1200/JCO.2007.13.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Migliaccio A, Varricchio L, De Falco A, Castoria G, Arra C, Yamaguchi H, Ciociola A, Lombardi M, Di Stasio R, Barbieri A, Baldi A, Barone MV, Appella E, Auricchio F. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene. 2007;26:6619–6629. doi: 10.1038/sj.onc.1210487. [DOI] [PubMed] [Google Scholar]
- 71.Dressing GE, Lange CA. Integrated actions of progesterone receptor and cell cycle machinery regulate breast cancer cell proliferation. Steroids. 2009;74:573–576. doi: 10.1016/j.steroids.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhat-Nakshatri P, Wang G, Collins NR, Thomson MJ, Geistlinger TR, Carroll JS, Brown M, Hammond S, Srour EF, Liu Y, Nakshatri H. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009;37:4850–4861. doi: 10.1093/nar/gkp500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Eades G, Yao Y, Li Q, Zhou Q. Estrogen receptor α signaling regulates breast tumor-initiating cells by down-regulating miR-140 which targets the transcription factor SOX2. J Biol Chem. 2012;287:41514–41522. doi: 10.1074/jbc.M112.404871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cittelly DM, Finlay-Schultz J, Howe EN, Spoelstra NS, Axlund SD, Hendricks P, Jacobsen BM, Sartorius CA, Richer JK. Progestin suppression of miR-29 potentiates dedifferentiation of breast cancer cells via KLF4. Oncogene. 2013;32:2555–2564. doi: 10.1038/onc.2012.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chou J, Lin JH, Brenot A, Kim JW, Provot S, Werb Z. GATA3 suppresses metastasis and modulates the tumor microenvironment by regulating microRNA-29b expression. Nat Cell Biol. 2013;15:201–213. doi: 10.1038/ncb2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finlay-Schultz J, Cittelly DM, Hendricks P, Patel P, Kabos P, Jacobsen BM, Richer JK, Sartorius CA. Progesterone downregulation of miR-141 contributes to expansion of stem-like breast cancer cells through maintenance of progesterone receptor and Stat5a. Oncogene. 2015;34:3676–3687. doi: 10.1038/onc.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi Y, Yang F, Sun Z, Zhang W, Gu J, Guan X. Differential microRNA expression is associated with androgen receptor expression in breast cancer. Mol Med Rep. 2017;15:29–36. doi: 10.3892/mmr.2016.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lyu S, Yu Q, Ying G, Wang S, Wang Y, Zhang J, Niu Y. Androgen receptor decreases CMYC and KRAS expression by upregulating let-7a expression in ER-, PR-, AR+ breast cancer. Int J Oncol. 2014;44:229–237. doi: 10.3892/ijo.2013.2151. [DOI] [PubMed] [Google Scholar]
- 79.Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol. 2010;28:4006–4012. doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kurebayashi J, Kanomata N, Yamashita T, Shimo T, Moriya T. Antitumor and anticancer stem cell activities of eribulin mesylate and antiestrogens in breast cancer cells. Breast Cancer. 2016;23:425–436. doi: 10.1007/s12282-014-0580-9. [DOI] [PubMed] [Google Scholar]
- 81.Riobo-Del Galdo A, Lara Montero Á, Wertheimer EV. Role of Hedgehog Signaling in Breast Cancer: Pathogenesis and Therapeutics. Cells. 2019:8. doi: 10.3390/cells8040375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jaggupilli A, Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol. 2012;2012:708036. doi: 10.1155/2012/708036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sin WC, Lim CL. Breast cancer stem cells-from origins to targeted therapy. Stem Cell Investig. 2017;4:96. doi: 10.21037/sci.2017.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]