Abstract
Atherosclerosis is a cardiovascular disease that affects most people to at least some extent by old age. Many factors contribute to atherogenesis, and although it is extremely common, the mechanisms behind the pathogenesis of the disease remain poorly understood. Endothelial dysfunction is thought to be one of the main causes of atherosclerosis along with numerous other factors, such as oxidative stress and proinflammatory cytokine upregulation. The culmination of the complications that lead to atherogenesis is the formation of a fatty plaque on the intima of the arterial endothelium. In this study, we explore these aspects and others in regard to the treatment potential of dehydrocostus lactone (DHL), which is naturally occurring in certain flora such as the Saussurea lappa costus plant. Having long been used in traditional Chinese medicine, the effects of this plant are only just beginning to be studied by modern science. Among our most noteworthy findings are that DHL exerts an inhibitory effect against the increased expression of VCAM-1 and E-selectin induced by exposure to oxidized low-density lipoprotein (ox-LDL), which has been linked to the development and progression of atherosclerosis. The introduction of DHL also significantly diminished the downstream effects of VCAM-1 and E-selectin, such as the attachment of monocytes to the endothelium and the release of proinflammatory cytokines and chemokines, including TNF-α, MCP-1, and HMGB1. Furthermore, DHL is capable of rescuing the expression of KLF2, an important regulator of VCAM-1 and E-selectin expression. Together, our findings demonstrate the potential of DHL as a prophylactic or therapeutic treatment against ox-LDL-induced atherosclerosis via inhibition of the attachment of monocytes to endothelial cells.
Keywords: Dehydrocostus lactone, endothelial dysfunction, KLF2, atherosclerosis
Introduction
One of the leading health concerns related to the process of aging is the increased risk of the development of atherosclerosis, a cardiovascular disease primarily characterized by the formation of fatty plaque and hardening of the arterial walls. This disease typically begins with few to no symptoms at all, which makes it difficult to diagnose early on. While atherogenesis tends to begin in younger years, it typically does not begin to cause complications until the person afflicted begins to reach old age. The proper function of the endothelium is essential in the natural prevention of atherosclerosis [1]. In its normal state, the endothelium plays a major role in regulating the production of reactive oxygen species (ROS) and the release of nitric oxide (NO). However, in a state of endothelial dysfunction, the production of ROS is upregulated while the expression of NO, a protective factor, is downregulated, thereby leading to sustained oxidative stress [2]. Additionally, endothelial cells produce various transcriptional facotors including Kruppel-like factor 2 (KLF2) that play a part in reducing inflammation and suppressing the adhesion of leukocytes to the endothelium, which is recognized as a major early event in the pathogenesis of atherosclerosis. Endothelial dysfunction results in increased release of proinflammatory cytokines and chemokines, such as tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and high mobility group box 1 (HMGB1) [3].
Oxidized low-density lipoprotein (ox-LDL) is thought to be a result of the reaction between LDL and free radicals, and though thoroughly studied, the exact mechanisms behind the effects of ox-LDL are not fully understood. Thus far, it has been established that ox-LDL plays a significant role in the development of many diseases, including but certainly not limited to atherosclerosis. This is due primarily to the inflammatory properties of ox-LDL. Specifically, in atherogenesis, ox-LDL has been shown to facilitate the adhesion of monocytes to the endothelium, thereby forming the characteristic fatty plaque buildup associated with atherosclerosis [4]. This process leads to constricted blood flow and an increased risk of blood clots, among other negative and dangerous effects. While certain catalysts such as VCAM-1 and E-selectin aid in the development of plaque, others mitigate it, such as KLF2 [5,6]. VCAM-1 is an endothelial ligand which mediates the monocyte attachment process during atherogenesis. Increased gene transcription of adhesion molecules induced by ox-LDL is a common cause of overproduction of VCAM-1. As a selectin cell adhesion molecule, E-selectin contributes to angiogenesis by further promoting monocyte attachment. However, KLF2 has been shown to inhibit the expression of these adhesion molecules in endothelial cells [7]. While expression KLF2 was originally associated with the lungs, it has since been found to regulate the expression of adhesion molecules, proinflammatory cytokines and chemokines induced by ox-LDL, thereby indicating the potential benefit of treatments aimed at targeting KLF2 expression against atherosclerosis [8,9].
Dehydrocostus lactone (DHL) is a sesquiterpene lactone found naturally in the Saussurea lappa plant which has been widely used in traditional Chinese medicine for centuries. Today, this compound is recognized for a myriad of pharmacological effects such as preventing the phosphorylation of Ikβα and inhibiting IKKβ activity. DHL possesses many beneficial effects overall, including anti-inflammatory properties, immunomodulatory capabilities, and anti-tumor action. DHL has been found to limit breast cancer development without significant damage to normal cells in the same area, to reduce the development of prostate cancer, and to trigger apoptosis of DU145 cells [10,11]. DHL is able to suppress the enlargement, activation, and resorption activity of osteoclasts by downregulating the expression of integrin β3, PKC-β, and autophagy-related 5 [12]. Another study demonstrated that DHL protected rat hippocampal slices against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced injuries [13]. Additionally, administration of DHL could alleviate LPS-induced acute lung injury in a rodent model through inhibiting the production of proinflammatory mediators such as iNOS, NO, cytokines including TNF-α, IL-6, IL-1β, and IL-12, and activation of the p38/NF-κB signaling pathway in macrophages [14], thereby suggesting a robust anti-inflammatory capacity of DHL. However, it remains unknown whether DHL possesses a beneficial effect in endothelial cells against ox-LDL-induced inflammation and damage.
Materials and methods
Cell culture and treatment
Primary human aortic endothelial cells (HAECs) were purchased from Lonza (Basel, Switzerland). Human leukemia monocyte cell line THP-1 cells were from the American Type Culture Collection (ATCC) (Manassas, VA). HAECs in low passage numbers (less than 10) were grown in 2% low serum growth medium (EGM2). THP-1 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum. Cells for experimentation were stored in a 5% (v/v) CO2/95% (v/v) nitrogen incubator at a temperature of 37°C. For all experiments, HAECs were exposed to 10 ng/ml in the presence or absence of 15 or 30 µM DHL [15,16] for 24 h, or for 2 h for the ERK5 knockdown experiment.
Real-time polymerase chain reaction (PCR) analysis
Qiazol reagent (Qiagen, USA) was used to extract the total intracellular RNA for spectrophotometric quantification. The isolated RNA was treated with DNase I (4 units) at a temperature of 37°C for 2 h. Then, cDNA was prepared using a high-capacity cDNA synthesis kit purchased from Applied Biosystems (USA) and reverse transcription PCR (RT-PCR) was performed using an RT-PCR kit in accordance with the manufacturer’s instructions [17]. A total of 2 μl cDNA was subjected to polymerase chain reaction (PCR) analysis on an Applied Biosystems 7500 Real-Time PCR System using SYBR Green PCR Master Mix to determine the expression of the target genes. The results are expressed as normalized to GAPDH in each sample and were analyzed using the 2-ΔΔCt method.
The following primers were used in this study: TNF-α: For, 5’-GTCACTCATTGCTGAGCCTCT-3’; Rev, 5’-AGCTTCTTCCCACCCACAAG-3’; MCP-1: For, 5’-ATGCAATCAATGCCCCAGTC-3’; Rev, 5’-TGCAGATTCTTGGGTTGTGG-3’; VCAM-1: For, 5’-CTTAAAATGCCTGGGAAGATGGT-3’; Rev, 5’-GTCAATGAGACGGAGTCACCAAT-3’; E-selectin: For, 5’-GCCTGCAATGTGGTTGAGTG-3’; Rev, 5’-ACGAACCCATTGGCTGGATT-3’; KLF2: For, 5’-GCGCCCCCAGCCTTCGGTCTCT-3’; Rev, 5’-CATGTGCAGCGCCAGGTGAT-3’; GAPDH: For, 5’-GGAGCGAGATCCCTCCAAAAT-3’; Rev, 5’-GGCTGTTGTCATACTTCTCATGG-3’.
Enzyme-linked immunosorbent assay (ELISA)
HAECs were harvested and lysed with cell lysis buffer. The cell culture media was collected, and the expression levels of TNF-α, MCP-1, and HMGB1 were analyzed. The following ELISA kits were used in this study: human TNF-α ELISA kit (#DTA00D, R&D Systems, USA); human MCP-1 ELISA kit (#DCP00, R&D Systems, USA); human HMGB-1 ELISA kit (#OKCD04074, Aviva Systems Biology, USA). Experiments were performed in accordance with the manufacturer’s instructions to determine the protein concentrations.
Western blot analysis
After the necessary treatment, RIPA lysis buffer containing protease inhibitor cocktail was used to prepare cell lysates from HAECs. Samples of extracted proteins (20 µg) were then separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P, Millipore) [18]. Membranes were then washed in blocking buffer containing 5% non-fat milk for 1 h at room temperature (RT) to eliminate non-specific binding sites on the membranes. The membranes were then incubated with primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibody. An enhanced chemiluminescence (ECL) kit was used to visualize the immune-bands.
Attachment of monocytes to endothelial cells
HAECs were cultured with 10 μg/ml ox-LDL in the presence or absence of 15 or 30 µM DHL for 24 h. The dye calcein-AM (2 μM) was used to stain THP-1 monocytes for 30 min at 37°C. Then, 5 × 105 cells/ml stained THP-1 monocytes were added to 1 × 105 confluent HAECs and incubated for 2 h. THP-1 cells that did not adhere to HAECs were washed away. Then, a fluorescence microscope was used to visualize green fluorescence-stained THP-1 cells, and the number of attached cells was quantified.
Dihydroethidium (DHE) staining
After the necessary treatment, dihydroethidium (DHE) staining was used to assess the level of ROS production in HAECs. Briefly, cells were loaded with DHE (10 μM) [19] and incubated at 37°C in darkness for 30 min. After 3 gentle washes, red fluorescence signals were observed under a fluorescence microscope. The software Image J was used to quantify ROS in HAECs. Briefly, regions of interest (ROIs) of fluorescent images were defined, and the average numbers of cells present in the defined ROIs were calculated. The integrated density value (IDV) of red fluorescence in each ROI was assessed. The IDV was divided by the average number of cells and was used to index the average levels of intracellular ROS.
Measurement of reduced GSH
A fluorometric assay was used to assess the intracellular levels of reduced glutathione (GSH) in HAECs. HAECs were stimulated with 10 μg/ml ox-LDL in the presence or absence of 15 or 30 µM DHL for 24 h. The cells were collected and stored in ice-cold 5% meta-phosphoric acid (MPA) until use. Then, the cells were sonicated and centrifuged at 14000 × g for 5 min. The supernatant was incubated with an equal part OPAME (Sigma-Aldrich, USA) in methanol and borate buffer for 15 min at RT. The resulting fluorescent signals were recorded at 350 nm excitation and 420 nm emission.
Statistical analysis
All the experiments were repeated at least for 3 times. Experimental data are expressed as means ± S.E.M. Statistical analysis was performed using SPSS software (Version 20). Multi-group comparisons were analyzed using analysis of variance (ANOVA), followed by Bonferroni post-test comparisons. A P-value of less than 0.05 was considered statistically significant.
Results
Dehydrocostus lactone ameliorates ox-LDL-induced oxidative stress
Recognized as a critical factor in the progression of atherogenesis, oxidative stress is a vital factor of study when testing potential treatments for atherosclerosis. Overproduction of ROS contributes to atherogenesis through several mechanisms, including modification of LDL and promoting leukocyte recruitment [20]. The effects of ROS in the present study are shown in Figure 1A. Briefly, the introduction of ox-LDL increased the intracellular level of ROS by 3.5-fold, which was reduced to approximately 2.3- and 1.6-fold in the presence of 15 and 30 μM DHL, respectively. The naturally occurring antioxidant glutathione (GSH) is known to abolish ROS over-production, making it a valuable treatment target. As shown in Figure 1B, the level of GSH was decreased to approximately 50% upon exposure to ox-LDL alone but was restored to levels of approximately 71% and 89% upon the addition of the two doses of DHL, respectively.
Figure 1.
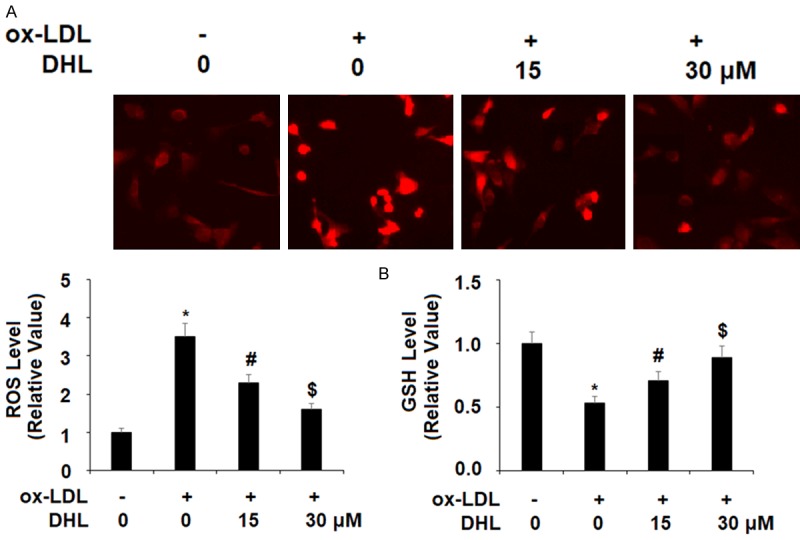
Dehydrocostus lactone attenuated ox-LDL-induced oxidative stress in human aortic endothelial cells (HAECs). Cells were treated with ox-LDL (10 μg/ml) with or without dehydrocostus lactone (DHL) at concentrations of 15 and 30 μM for 24 h. A. Intracellular ROS was determined by staining with dihydroethidium (DHE); B. The levels of intracellular reduced GSH were measured (*, #, $, P < 0.01 vs previous group, n = 6).
Dehydrocostus lactone reduces ox-LDL-induced expression of proinflammatory cytokines
Proinflammatory cytokines are known for their key role in atherogenesis and in the broader context of cardiovascular disease. Both TNF-α and MCP-1 have been found to play a particularly paramount role in these various diseases [21], and for this reason, we selected them as a focus of study. In order to better understand the effect of DHL treatment on the expression of these cytokines induced by ox-LDL, we measured their expression at both the mRNA level using real-time PCR analysis, and at the protein level using ELISA analysis. Our findings demonstrate that upon the introduction of ox-LDL, the mRNA level of TNF-α increased by approximately 6-fold. However, with the addition of DHL (15 and 30 μM), this surge was limited in a dose-dependent manner to only approximately 3- and 2-fold, respectively, as shown in Figure 2. The mRNA level of MCP-1 upon the introduction to ox-LDL alone increased to approximately 8-fold. While upon the addition of the two doses of DHL, this increase was limited to only approximately 3- and 2-fold in the same dose-dependent manner (Figure 2). The protein level of TNF-α in the presence of ox-LDL alone increased by approximately 4.5-fold, but with DHL, this increase was limited to only approximately 2- and 1.5-fold, respectively, as presented in Figure 2. As for the protein level of MCP-1, upon the addition of ox-LDL, the expression level of MCP-1 increased approximately 5.5-fold, while treatment with the two doses of DHL reduced this increase to approximately 3- and 2-fold, respectively, as shown in Figure 2. Another important cytokine is the protein known as high mobility group box-1 (HMGB1). One of the more significant chromatin proteins, it is known to be involved in the process of DNA organization and transcription as well as for its role in inflammation [22]. For this reason, neutralization of HMGB1 secretion is a potentially important aspect of the treatment of atherosclerosis. We found that when cells were treated with ox-LDL alone, the level of HMGB1 secretion increased by 4.2-fold. This increase was limited by the introduction of DHL in a dose-dependent manner, limiting the secretion of HMGB1 to respective levels of 2.5- and 1.6-fold, as shown in Figure 3.
Figure 2.
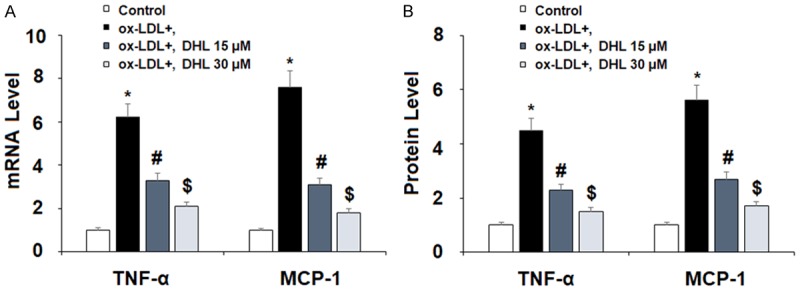
Dehydrocostus lactone attenuated ox-LDL-induced expression and secretion of pro-inflammatory cytokines in human aortic endothelial cells (HAECs). Cells were treated with ox-LDL (10 μg/ml) with or without dehydrocostus lactone (DHL) at concentrations of 15 and 30 μM for 24 h. A. mRNA levels of TNF-α and MCP-1 were measured by real-time PCR analysis; B. Protein levels of TNF-α and MCP-1 were measured by ELISA analysis (*, #, $, P < 0.01 vs previous group, n = 5-6).
Figure 3.
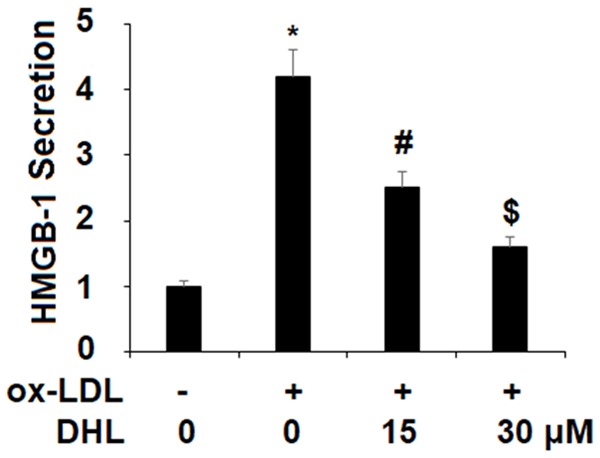
Dehydrocostus lactone prevented ox-LDL-induced secretion of high mobility group box-1 protein (HMGB1) in human aortic endothelial cells (HAECs). Cells were treated with ox-LDL (10 μg/ml) with or without dehydrocostus lactone (DHL) at concentrations of 15 and 30 μM for 24 h. Secretions of HMGB-1 was measured by ELISA (*, #, $, P < 0.01 vs previous group, n = 6).
Dehydrocostus lactone prevents the attachment of monocytes to endothelial cells
Being arguably the single most crucial consequence of atherosclerosis, the buildup of plaque on artery walls is therefore also an important target for treatment during atherogenesis. Two important factor in this process are VACM-1 and E-selectin. In the present study, we measured the expression of these two adhesion molecules as well as the attachment of THP-1 monocytes to the endothelium induced by ox-LDL in the presence or absence of DHL. Again, we studied the mRNA and protein levels via real-time PCR and western blot analysis, respectively, using the same doses of DHL described above. For VCAM-1 mRNA expression, the addition of ox-LDL alone increased the expression level approximately 5.6-fold, while the presence of the two doses of DHL reduced the level in a dose-dependent manner to approximately 2.9- and 1.9-fold, as shown in Figure 4A. For E-selectin, we found that ox-LDL increased the mRNA level approximately 4.9-fold, while the two doses of DHL helped to lower the expression of E-selectin to only approximately 2.6- and 1.6-fold, respectively, as shown in Figure 4A. As for protein levels of VCAM-1, basal levels were nearly undetectable, but the addition of ox-LDL increased the protein expression of VCAM-1 by nearly 100%. Meanwhile, the respective doses of DHL decreased the expression level to only approximately 50% and 35% increases. The protein expression of E-selectin was also nearly undetectable at basal levels but also increased by roughly 100% upon the addition of ox-LDL. However, upon the addition of DHL, we found approximately only 45% and 30% increases, respectively. Next, we determined the effects of DHL treatment on ox-LDL-induced attachment of THP-1 monocytes to endothelial cells. As shown in Figure 5, we found that ox-LDL induced an increase in the number of attached monocytes of approximately 3.9-fold, while this increase was limited to only roughly 2.5- and 1.7-fold by the two doses of DHL, respectively.
Figure 4.
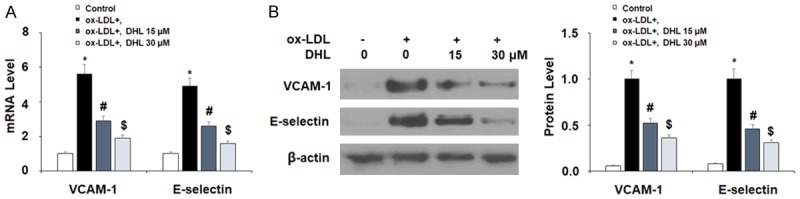
Dehydrocostus lactone reduced ox-LDL-induced expression of VCAM-1 and E-selectin. Cells were treated with ox-LDL (10 μg/ml) with or without dehydrocostus lactone (DHL) at concentrations of 15 and 30 μM for 24 h. A. mRNA levels of VCAM-1 and E-selectin as determined by real-time PCR analysis; B. Protein levels of VCAM-1 and E-selectin as determined by western blot analysis (*, #, $, P < 0.01 vs previous group, n = 5-6).
Figure 5.
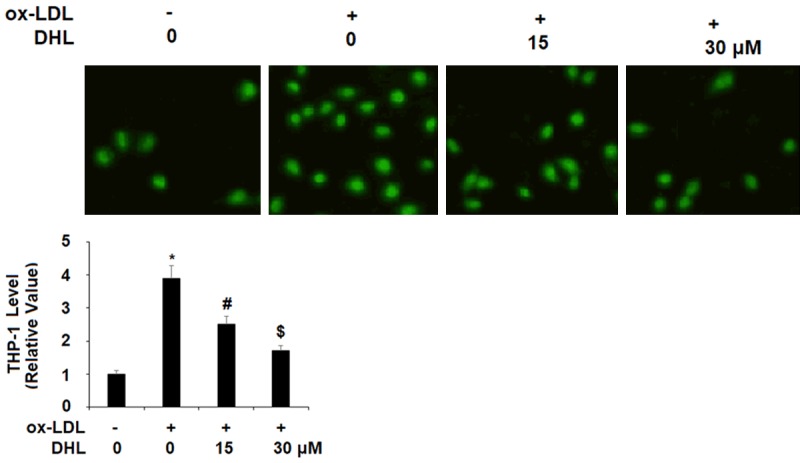
Dehydrocostus lactone inhibited ox-LDL-induced attachment of the monocytes THP-1 cells to human aortic endothelial cells (HAECs). HAECs were treated with ox-LDL (10 μg/ml) with or without dehydrocostus lactone (DHL) at concentrations of 15 and 30 μM for 24 h. Attachment of THP-1 cells to HAECs was measured (*, #, $, P < 0.01 vs previous group, n = 5-6).
Dehydrocostus lactone rescues KLF2 function via ERK5
KLF2 is recognized for its ability to regulate the inflammatory response in atherosclerosis as well as other cardiovascular diseases. Additionally, KLF2 plays an inhibitory role in the activation of cells that promote the buildup of plaque in the arteries and protects the function of the endothelium [23]. For these reasons, the effect of DHL treatment on KLF2 is a subject of significant interest in our research. As shown in Figure 6, we found that KLF2 expression was greatly reduced by ox-LDL at both the mRNA and protein levels, which were reduced to 38% and 43%, respectively. However, KLF2 mRNA expression was rescued by treatment with DHL in a dose-dependent manner to 67% and 91%, respectively. Similarly, the two doses of DHL rescued ox-LDL-induced reduced protein expression of KLF2 to 72% and 93%, respectively. The ERK5 pathway is known to be involved in both cardiovascular support and endothelial cell function via promoting KLF2 expression [24]. As shown in Figure 7A, upon exposure to ox-LDL, the level of phosphorylated ERK5 was reduced by 66%, while the addition of 15 and 30 µM DHL limited this reduction to only 31% and 11%, respectively. In order to examine these findings further, we tested the effects of DHL along with the specific ERK5 inhibitor XMD8-92. The results of western blot analysis in Figure 7B, 7C show that, as previously demonstrated, 30 µM DHL rescued ERK5 expression to near basal levels while inhibition of ERK5 by XMD8-92 abrogated this effect, reducing ERK5 mRNA expression to only 23%. At the protein level, DHL rescued ERK5 expression to 88% while XMD8-92 again abrogated this effect, reducing ERK5 protein expression to only in the presence of 30 µM DHL 32%.
Figure 6.
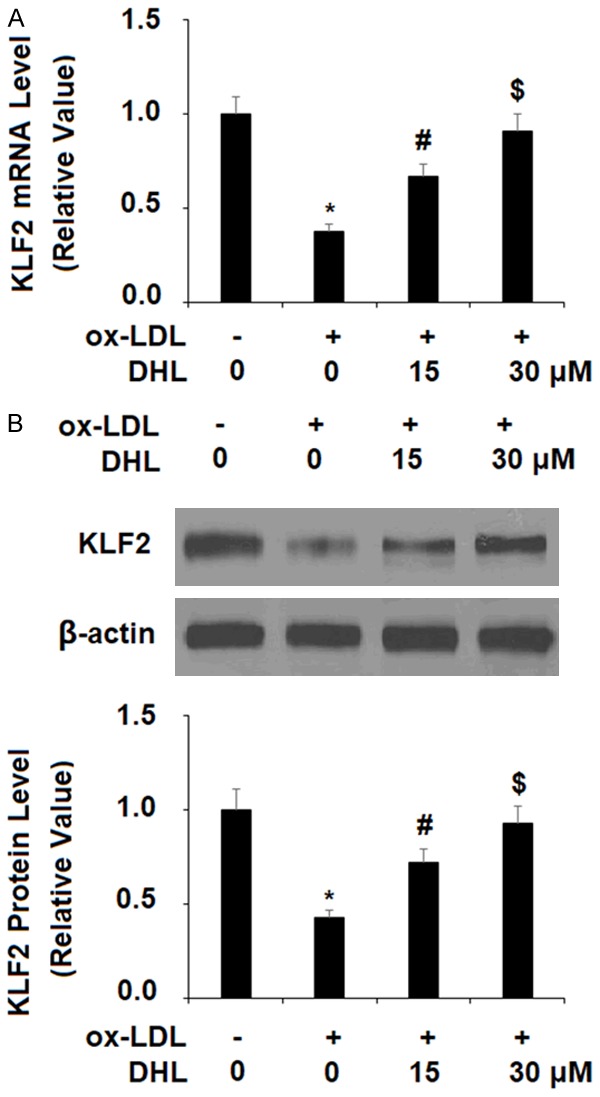
Dehydrocostus lactone resorted ox-LDL-induced reduction of KLF2 in human aortic endothelial cells (HAECs). HAECs were treated with ox-LDL (10 μg/ml) with or without dehydrocostus lactone (DHL) at concentrations of 15 and 30 μM for 24 h. A. mRNA levels of KLF2 as determined by real-time PCR analysis; B. Protein levels of KLF2 as determined by western blot analysis (*, #, $, P < 0.01 vs previous group, n = 5-6).
Figure 7.
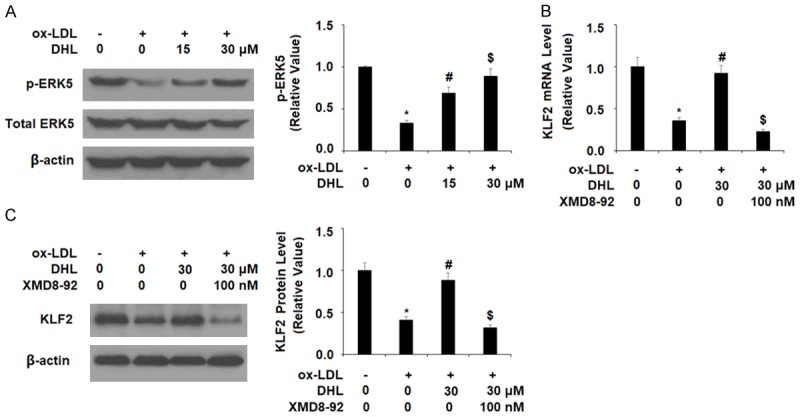
The effects of dehydrocostus lactone in promoting KLF2 expression is mediated by ERK5. A. HAECs were treated with ox-LDL (10 μg/ml) with or without dehydrocostus lactone (DHL) at concentrations of 15 and 30 μM for 2 h. Phosphorylated and total ERK5 was determined by western blot analysis. B, C. Cells were treated with ox-LDL (10 μg/ml) with or without dehydrocostus lactone (DHL) (30 μM) or the specific ERK5 inhibitor XMD8-92 (100 nM). mRNA levels and protein levels of KLF2 were measured (*, #, $, P < 0.01 vs previous group, n = 5-6).
Discussion
Although cardiovascular diseases such as atherosclerosis present a global public health concern, there are few reliable treatments available that can effectively prevent or reverse the development and progression of atherosclerosis. A somewhat recent phenomenon, looking to traditional medicine for answers regarding how to best treat age-old diseases has proven to be a valuable asset to the medical community; if pharmacological benefit is not observed with direct use of a traditional medicinal product, potential treatments are often revealed through further research of its constituents. In the present study, we tested the effects of DHL, a naturally occurring sesquiterpene derived from the Saussurea lappa Costus plant, on various factors commonly associated with the development and progression of atherosclerosis. These include oxidative stress, expression of proinflammatory cytokines and chemokines, attachment of monocytes to endothelial cells, and suppression of the protective transcriptional factor KLF2. ROS are a major signaling catalyst involved in generating downstream atherogenic effects that drive disease development and progression of atherosclerosis [25]. Our results show that while exposure to ox-LDL significantly increased the production of ROS, remarkably, the addition of DHL maintained the level of intracellular ROS near the basal level. Upregulation of ROS can be the result of numerous factors, but ox-LDL is recognized as playing a major role in ROS and superoxide formation through the apoptotic pathway [26]. GSH is an antioxidant healing compound found naturally in many living things and its presence at normal levels aids in healthy endothelial cell function. The antioxidant system mediated by GSH has been shown to prevent atherosclerosis induced by ox-LDL [27]. In the present study, we found that DHL plays a significant role in preserving GSH and its function. While initially reduced to around 50% in ox-LDL-induced atherosclerotic conditions, the higher dose of DHL rescued these levels to nearly 90%. As a key antioxidant, preserving GSH levels is an important strategy for preventing ox-LDL-induced upregulation of ROS and protecting against the development or progression of atherosclerosis. Our results demonstrate a potent antioxidant effect of DHL through its ability to inhibit ox-LDL-induced production of ROS and rescue ox-LDL-induced decreased levels of GSH.
Proinflammatory cytokines are a major driving force behind many of the issues involved in atherosclerosis. The three major cytokines investigated in this study were TNF-α, MCP-1, and HMGB-1. The cytokine TNF-α is particularly noted for its negative effects on cell function, and its association with atherogenesis is well-documented. For example, a 2008 study specifically linked increased expression of TNF-α to higher rates of atherosclerosis [28]. Other more recent studies have taken a deeper look at the involvement of TNF-α and its corresponding pathways in relation to atherosclerosis including a study from 2016 which linked unmitigated upregulation of TNF-α to increased buildup of atherosclerotic plaque [29]. MCP-1 and HMGB1 also have significant potential as treatment targets against atherosclerosis. Previous studies have demonstrated the role of MCP-1 as a proatherogenic cytokine [30,31]. HMGB1 is a chromatin protein and damage-associated molecular pattern (DAMP) that is ubiquitously expressed. HMGB1 is released by cells are injured or activated and has been shown to be upregulated in vascular diseases, including atheromatous plaque and atherosclerotic lesions [32,33]. Interestingly, a diet rich in polyphenols, a natural compound similar to sesquiterpenes, was shown to exert a positive effect in that a polyphenol-rich Mediterranean diet aided in decreasing atherosclerosis-related inflammation [34]. In the present study, the expressions of each of these cytokines in ox-LDL-induced atherogenic conditions were found to be significantly reduced in a dose-dependent manner by the addition of the two doses of DHL. Thus, treatment with the sesquiterpene DHL can exert a significant anti-inflammatory effect in endothelial cells exposed to ox-LDL, thereby demonstrating its potential as a novel antiatherosclerotic agent.
VCAM-1 and E-selectin are essential players in the process of atherosclerosis as they initiate the adhesion of monocytes to the arterial intima. A 2017 study demonstrated the role of VCAM-1 in this process using an atherosclerosis mouse model and showed that the inhibition of VCAM-1 activity directly correlated to a reduction in atherosclerotic lesion formation [35]. In 2018, a study was published in which E-selectin-targeted treatment was applied in a mouse model, which was again found to be effective in limiting the attachment of monocytes to endothelial cells [36]. These results show that the inhibition of VCAM-1 and E-selectin can help to ameliorate one of the main health concerns associated with atherosclerosis. In the present study, we found that DHL successfully reduced the expression of both of these molecules in a dose-dependent manner, thereby demonstrating the potential significance of DHL treatment in combating the formation of atherosclerotic lesions.
KLF2 is a transcription factor that plays a key role in a number of diseases, and its activation is significant in the prevention of atherosclerosis. A 2018 study linked KLF2 expression with protection against atherosclerosis and muscle injury and has been recognized as being highly effective in slowing atherogenesis by upregulating the expression of protective genes and triggering other beneficial processes [37]. The ERK5 pathway is thought to be involved in mediating the activation of KLF2 and its effects. In a previous study investigating the treatment potential of the isoflavone puerarin in regard to atherosclerosis, the antiatherosclerotic effects of puerarin were found to be mediated by the ERK5 pathway, and knockdown of this pathway using specific ERK5 inhibitor greatly decreased downstream KLF2 expression [37]. Concordantly, we found that DHL was capable of rescuing the reduction in KLF2 expression levels induced by exposure to ox-LDL. We further confirmed the link between KLF2 and ERK5, building on a previous nascent understanding of this connection. We used the specific ERK5 inhibitor XMD8-92 to demonstrate that normal ERK5 activation aided in the downstream rescue of KLF2 expression, while this effect was abolished by inhibition of ERK5. These results demonstrate that the effects of DHL on KLF2 expression are dependent on the ERK5 pathway.
In conclusion, we found that DHL has considerable potential as a treatment against atherosclerosis owing to its modulatory effect on various aspects of atherogenesis. Our results suggest that the DHL exerts potent antioxidant effects by suppressing the overproduction of ROS and increasing the production of the antioxidant GSH. DHL also displays a remarkable anti-inflammatory effect by inhibiting the expression of TNF-α, MCP-1, and HMGB1, three key proinflammatory cytokines. Importantly, DHL ameliorates ox-LDL-induced attachment of monocytes to endothelial cells via suppression of VCAM-1 and E-selectin expression. DHL also rescues decreased expression of the key protective factor KLF2, which is mediated through the ERK5 pathway. As a safe and natural sesquiterpene, DHL may provide a new treatment strategy in atherosclerosis as well as other diseases. Further research is required to understand the exact mechanisms behind the effects of DHL observed in this study.
Acknowledgements
Natural Science Foundation of Zhejiang Province (LY16H020010); Medicine Health Science and Technology Plan of Zhejiang Province (2017194804); Grant from Science and Technology Bureau of Wenzhou (Y20160021).
Disclosure of conflict of interest
None.
References
- 1.Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease-a 30th anniversary update. Acta Physiol (Oxf) 2017;219:22–96. doi: 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- 2.Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120:713–35. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 3.Montes VN, Subramanian S, Goodspeed L, Wang SA, Omer M, Bobik A, Teshigawara K, Nishibori M, Chait A. Anti-HMGB1 antibody reduces weight gain in mice fed a high-fat diet. Nutr Diabetes. 2015;5:e161. doi: 10.1038/nutd.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trpkovic A, Resanovic I, Stanimirovic J, Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D, Isenovic ER. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52:70–85. doi: 10.3109/10408363.2014.992063. [DOI] [PubMed] [Google Scholar]
- 5.Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Yoshida T, Wu L, Maiti D, Cebotaru L, Duh EJ. Transcription factor MEF2C suppresses endothelial cell inflammation via regulation of NF-κB and KLF2. J Cell Physiol. 2015;230:1310–1320. doi: 10.1002/jcp.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Sun M, Wang Z, Li X, Zhu Y, Li Y. Omentin-1 ameliorates the attachment of the leukocyte THP-1 cells to HUVECs by targeting the transcriptional factor KLF2. Biochem Biophys Res Commun. 2018;498:152–156. doi: 10.1016/j.bbrc.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Yang X, Zhang Q, Gao Z, Yu C, Zhang L. Down-regulation of MiR-150 alleviates inflammatory injury induced by interleukin 1 via targeting kruppel-like factor 2 in human chondrogenic cells. Cell Physiol Biochem. 2018;47:2579–2588. doi: 10.1159/000491654. [DOI] [PubMed] [Google Scholar]
- 10.Kim HR, Kim JM, Kim MS, Hwang JK, Park YJ, Yang SH, Kim HJ, Ryu DG, Lee DS, Oh H, Kim YC. Saussurea lappa extract suppresses TPA-induced cell invasion via inhibition of NF-κB-dependent MMP-9 expression in MCF-7 breast cancer cells. BMC Complement Altern Med. 2014;14:170. doi: 10.1186/1472-6882-14-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim EJ, Lim SS, Park SY, Shin HK, Kim JS, Park JH. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of saussurea lappa. Food Chem Toxicol. 2008;46:3651–3658. doi: 10.1016/j.fct.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Lee HI, Lee J, Hwang D, Lee GR, Kim N, Kwon M, Lee H, Piao D, Kim HJ, Kim NY, Kim HS, Seo EK, Kang D, Jeong W. Dehydrocostus lactone suppresses osteoclast differentiation by regulating NFATc1 and inhibits osteoclast activation through modulating migration and lysosome function. FASEB J. 2019;33:9685–9694. doi: 10.1096/fj.201900862R. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Q, Chen A, Wang X, Zhang Z, Zhao Y, Huang Y, Ren S, Zhu Y. Protective effects of dehydrocostuslactone on rat hippocampal slice injury induced by oxygen-glucose deprivation/reoxygenation. Int J Mol Med. 2018;42:1190–1198. doi: 10.3892/ijmm.2018.3691. [DOI] [PubMed] [Google Scholar]
- 14.Nie Y, Wang Z, Chai G, Xiong Y, Li B, Zhang H, Xin R, Qian X, Tang Z, Wu J, Zhao P. Dehydrocostus lactone suppresses LPS-induced acute lung injury and macrophage activation through NF-κB signaling pathway mediated by p38 MAPK and Akt. Molecules. 2019;24 doi: 10.3390/molecules24081510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Yu Z, Wang C, Tian X, Huo X, Wang Y, Sun C, Feng L, Ma J, Zhang B, Yang Q, Ma X, Xu Y. Dehydrocostus lactone, a natural sesquiterpene lactone, suppresses the biological characteristics of glioma, through inhibition of the NF-κB/COX-2 signaling pathway by targeting IKKβ. Am J Cancer Res. 2017;7:1270–1284. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu GZ, Zhang M, Kou CZ, Ni YH, Ji CB, Cao XG, Guo XR. Effects of Lyrm1 knockdown on mitochondrial function in 3 T3-L1 murine adipocytes. J Bioenerg Biomembr. 2012;44:225–232. doi: 10.1007/s10863-012-9404-9. [DOI] [PubMed] [Google Scholar]
- 17.Chen YM, Li X, Song GX, Liu M, Fan Y, Wu LJ, Li H, Zhang QJ, Liu YQ, Qian LM. Effect of LYRM1 knockdown on proliferation, apoptosis, differentiation and mitochondrial function in the P19 cell model of cardiac differentiation in vitro. J Bioenerg Biomembr. 2016;48:33–41. doi: 10.1007/s10863-015-9638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanmugam G, Narasimhan M, Tamowski S, Darley-Usmar V, Rajasekaran NS. Constitutive activation of Nrf2 induces a stable reductive state in the mouse myocardium. Redox Biol. 2017;12:937–945. doi: 10.1016/j.redox.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mügge A. The role of reactive oxygen species in atherosclerosis. Z Kardiol. 1998;87:851–864. doi: 10.1007/s003920050241. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad Z, Ng CT, Fong LY, Bakar NA, Hussain NH, Ang KP, Ee GC, Hakim MN. Cryptotanshinone inhibits TNF-α-induced early atherogenic events in vitro. J Physiol Sci. 2016;66:213–220. doi: 10.1007/s12576-015-0410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Souza AW, Westra J, Limburg PC, Bijl M, Kallenberg CG. HMGB1 in vascular diseases: its role in vascular inflammation and atherosclerosis. Autoimmun Rev. 2012;11:909–917. doi: 10.1016/j.autrev.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts OL, Holmes K, Müller J, Cross DA, Cross MJ. ERK5 and the regulation of endothelial cell function. Biochem Soc Trans. 2009;37:1254–1259. doi: 10.1042/BST0371254. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama M. Oxidant stress and atherosclerosis. Curr Opin Pharmacol. 2004;4:110–115. doi: 10.1016/j.coph.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Chan SH, Hung CH, Shih JY, Chu PM, Cheng YH, Tsai YJ, Lin HC, Tsai KL. Baicalein is an available anti-atherosclerotic compound through modulation of nitric oxide-related mechanism under oxLDL exposure. Oncotarget. 2016;7:42881–42891. doi: 10.18632/oncotarget.10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Yao H, Chen Y, Sun L, Li Y, Ma X, Duan S, Li X, Xiang R, Han J, Duan Y. Inhibition of glutathione production induces macrophage CD36 expression and enhances cellular-oxidized low density lipoprotein (oxLDL) uptake. J Biol Chem. 2015;290:21788–21799. doi: 10.1074/jbc.M115.654582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruunsgaard H, Skinhøj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-α) and atherosclerosis. Clin Exp Immunol. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tousoulis D, Oikonomou E, Economou EK, Crea F, Kaski JC. Inflammatory cytokines in atherosclerosis: current therapeutic approaches. Eur Heart J. 2016;37:1723–1732. doi: 10.1093/eurheartj/ehv759. [DOI] [PubMed] [Google Scholar]
- 29.Husain K, Hernandez W, Ansari RA, Ferder L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J Biol Chem. 2015;6:209–217. doi: 10.4331/wjbc.v6.i3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia J, Huang L, Zhang ZH. Correlation of serum cyclophilin a and monocyte chemoattractant protein-1 levels with carotid atherosclerosis in patients with acute cerebral infarction. J Hainan Med Univer. 2017;23:150–153. [Google Scholar]
- 31.Chen Q, Wang ZY, Chen LY, Hu HY. Roles of high mobility group box 1 in cardiovascular calcification. Cell Physiol Biochem. 2017;42:427–440. doi: 10.1159/000477591. [DOI] [PubMed] [Google Scholar]
- 32.Inoue K, Kawahara KI, Biswas KK, Ando K, Mitsudo K, Nobuyoshi M, Maruyama I. HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc Pathol. 2007;16:136–143. doi: 10.1016/j.carpath.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Medina-Remón A, Casas R, Tressserra-Rimbau A, Ros E, Martínez-González MA, Fitó M, Corella D, Salas-Salvadó J, Lamuela-Raventos RM, Estruch R PREDIMED Study Investigators. Polyphenol intake from a mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: a substudy of the PREDIMED trial. Br J Clin Pharmacol. 2017;83:114–128. doi: 10.1111/bcp.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel ME, Idelman G, Konaniah ES, Zucker SD. Bilirubin prevents atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice by inhibiting endothelial VCAM-1 and ICAM-1 signaling. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsoref O, Tyomkin D, Amit U, Landa N, Cohen-Rosenboim O, Kain D, Golan M, Naftali-Shani N, David A, Leor J. E-selectin-targeted copolymer reduces atherosclerotic lesions, adverse cardiac remodeling, and dysfunction. J Control Release. 2018;288:136–147. doi: 10.1016/j.jconrel.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Manoharan P, Radzyukevich TL, Heiny JA, Lingrel JB. Contrasting effects of myeloid specific ablation of statin-responsive KLF2 in atherosclerosis and skeletal muscle injury. FASEB J. 2018;32:568–582. [Google Scholar]
- 37.Deng Y, Lei T, Li H, Mo X, Wang Z, Ou H. ERK5/KLF2 activation is involved in the reducing effects of puerarin on monocyte adhesion to endothelial cells and atherosclerotic lesion in apolipoprotein E-deficient mice. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2590–2599. doi: 10.1016/j.bbadis.2018.04.021. [DOI] [PubMed] [Google Scholar]


