Abstract
Background: Mesenchymal stem cells (MSCs) can be efficiently recruited to wound, inflammatory and tumor sites to repair and regenerate tissue. However, its role in colitis and colitis associated colon cancer is still controversial. This study was designed to evaluate the role and mechanisms of inflammatory cytokines-activated-MSCs in mice colitis and colon cancer. Methods: We selected two well-characterized pro-inflammatory cytokines, tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ), to expand the inflammatory microenvironment of MSCs. The severity of colitis and colon cancer was evaluated by measuring colon length, Myeloperoxidase (MPO) activity, Hematoxylin-eosin staining, Western Blot, Immunohistochemistry and Immunofluorescence. These techniques were also performed to analyze the mechanisms of inflammatory cytokines-activated-MSCs in mice colitis and colon cancer. Real-time PCR and Enzyme-linked Immunosorbent Assay (ELISA) were used to measure the secretion of pro-inflammatory factors. Results: We found that the incubation of MSCs with TNF-α and IFN-γ aggravates colitis, where high levels of pro-inflammatory factors, such as interleukin (IL)-17, IL-8, IL-12, IL-1β, transforming growth factor (TGF)-β, TNF-α and IFN-γ, were secreted. Furthermore, this phenomenon was associated with the activation of the nuclear factor-kappa-B (NF-κB)/Signal transducer and activator of transcription three (STAT3) pathway. In addition, our study demonstrated that TNF-α and IFN-γ pretreated MSCs synergistically exacerbated mice colon cancer, which was closely associated with angiogenesis. Conclusions: Taken together, these results indicate that TNF-α and IFN-γ pretreatment effectively inhibited the repair ability of MSCs and accelerated inflammation and tumor progression involving NF-κB/STAT3 pathway and angiogenesis-related factors.
Keywords: Mesenchymal stem cells (MSCs), mice, colitis, colitis-associated colon cancer, TNF-α and IFN-γ
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disorder of the colon, characterized by a wide range of signs and symptoms such as abnormal pain, mucosal ulceration, and hematochezia. UC and Crohn’s disease (CD) is known as the two main forms of inflammatory bowel disease (IBD), that is defined as an autoimmune disease, implicated in an aberrant and persistent inflammation of the bowel [1].
In developed countries, patients with IBD have an increased risk of 10-15% developing colorectal cancer (CRC) [2]. CRC, the third most common cancer in western countries and the second leading cause of cancer death, is one of the most effectively studied topics in recent years [3,4]. Although the percentage of colitis-associated colon cancer (CAC) was approximately 2% among all CRC cases, it drew more attention due to insights into the connection between inflammation and colon cancer [5].
Although the molecular pathogenesis of IBD remains unclear, bacterial translocation across a defective intestinal mucosal barrier is regarded as a key driving mechanism of an imbalanced intestinal immune response and disease progression [6]. Current pharmacotherapeutic approaches for IBD treatment is multimodal, including antibiotics, corticosteroids, thiopurines, folic acid antagonists and biological therapy using anti-inflammatory cytokines. In addition, cellular therapy, especially involving mesenchymal stem cells (MSCs), is a promising strategy to modulate the immune system for IBD treatment [7-10]. Similarly, previous studies reported the role of MSCs in IBD-mediated tumorigenesis that was determined through an AOM/DSS colitis-associated carcinoma model. Chen et al. demonstrated that BMSCs alleviated the severity of CAC in mice by suppressing the expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and by activating STAT3 [11]. Nasuno et al. also demonstrated that BMSCs partially canceled AOM-induced tumor initiation and inhibited the acute apoptotic response to a genotoxic carcinogen (AARGC) in colonic epithelial cells [12]. Furthermore, umbilical cord tissue-derived MSCs could prevent neoplasm and inhibit chronic inflammation mediated by Smad2 signaling in the CAC model [13]. Therefore, it is essential to investigate the molecular mechanisms of MSCs positive effects on the functionality of colitis and in a mouse AOM/DSS colitis-associated carcinoma model.
MSCs possess the characteristics of self-renewal, tissue regeneration, immunosuppressive and potentially immune-modulatory potencies, and are currently under investigation for an IBD alternative therapy via inhibiting the expression of inflammatory factors [7]. Pourgholaminejad et al. showed that MSCs possessed the ability to incorporate into inflammation sites and to maintain the immunomodulatory function in cytokine-based treatment [14]. Among the pro-inflammatory cytokines, TNF-α, IFN-γ, IL-1, IL-4 and IL-6, TNF-α and IFN-γ play crucial roles in the MSCs immune regulation. Meanwhile, our group has found noticeable increases in TNF-α and IFN-γ expression in colitis mice (Supplementary Figure 1B, 1C). Nowadays, many studies reported the combined influence of pro-inflammatory cytokines TNF-α and IFN-γ on MSCs. According to the latest report, the stimulation of MSCs with pro-inflammatory factors, promoted osteoblasts proliferation, migration, differentiation and mineralization through paracrine-related mechanisms. Liu et al. first demonstrated that MSCs pre-stimulation by TNF-α and IFN-γ could provoke C26 colon cancer growth in mice [15-22]. However, little is known about the effect of combining MSCs transplantation with TNF-α and IFN-γ on colitis and CAC in vivo. Therefore, the main aim of the present study was to explore potential effects and mechanisms of TNF-α and IFN-γ pretreated MSCs on DSS-induced colitis and AOM/DSS-induced colitis, and their association with colon cancer, to provide evidence for future studies.
Material and methods
Experimental animals
Male BALB/c and C57/BL6 mice weighing approximately 18-20 g and aged 5 to 6 weeks old were used in the experiments. All mice were maintained under SPF conditions and raised under standard laboratory conditions with a 12 h day/night cycle in Xiamen University Laboratory Animal Center. All animal procedures were approved by the Animal Care and Use Committee of Xiamen University, China (license No: SYXK [Min] 2008-0003, issued on May 6, 2008).
Reagents and cell culture
Dextran Sulfate Sodium (DSS, 36-50 kDa MW, from MP Biomedical, Solon, OH), Antibodies against NF-κB (p65) and IκBa were purchased from Santa Cruz. Anti-pSTAT3 (Y705) was purchased from Cell Signaling Technology. Ki67 and PCNA were from Abcam. MSCs cell line was obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China), and was cultured in α-MEM medium containing 10% FBS, 1% L-glutamine, 100 units/ml penicillin, and 100 mg/L streptomycin at 37°C in a 5% CO2/95% air.
DSS-induced colitis and AOM/DSS-induced colitis-associated colon cancer model
Male BALB/c mice were divided into experimental and control groups. Experimental groups with DSS-induced colitis were treated or not with MSCs (or by TNF-α and IFN-γ treated with for 6 h, 24 h, 48 h). Colitis was induced by orally administered of 3% (w/v) DSS solution daily. The normal control group was treated with double distilled water alone. MSCs group received intravenous injection of mesenchymal stem cell for 3 days after DSS treatment. The body weight, stool consistency, and blood in the feces were examined daily to assess the extent of DSS-induced colitis in mice.
Figure 4A outlines the inducement of this model; briefly, AOM (10 mg/kg body weight, i.p) was administered to mice by a single intraperitoneal (i.p.) injection. Five days later, mice exposed to 3% DSS (wt/vol) in drinking water for 5 consecutive days followed by 12 days of regular drinking water. Then, this cycle was repeated twice by 2% DSS due to the mass mortality of 3% DSS. At the same time, as shown in Figure 4A, MSCs were injected by tail vein for 1×106 cells each mouse. Body weight was assessed according to Figure 4B throughout the course of the experiment.
Figure 4.

TNF-α and IFN-γ synergistically induce the MSCs exacerbating the mice colon cancer. A. Schematic diagram of experimental methods for AOM/DSS-induced colitis-associated colon cancer model with or without MSCs/MSCs treated with TNF-α and IFN-γ for 6 h, 24 h and 48 h. B. Significant body weight loss was observed in AOM/DSS model group and TNF-α and IFN-γ/48 h group. C. Gross view of mice colon tissue and HE staining revealed that cytokines treatment groups up-regulated the incidence, number and size of the tumor and presented more irregular tubular structures, multiple lumens and reduced stroma. (Data were presented with mean ± SD of three independent experiments, *P<0.05).
Analysis of colon injury
Paraffin-embedded tissue sections of Swiss-rolled whole colon (rolled from the distal end to the proximal end) were stained with hematoxylin and eosin for light microscopic examination to assess colon injury and inflammation. Samples from the entire colon were examined by a pathologist blinded to treatment conditions.
Blood samples and mice tissues
After MSCs injection, mice were scarified and 1-mL orbital blood sample was collected. The abdomen was opened along the median line. Then the colon was rapidly excised, gently washed with ice-cold PBS, placed on ice, and opened longitudinally. The colon was incised, and the fecal contents were washed out gently within PBS. The blood samples were injected into dry test tubes and separated by centrifugation, and the serum was stored at -80°C until use. And then the colon was embedded into the dry tubes and stored at -80°C until use.
Immunofluorescence staining
The colon tissues were fixed in 10% formaldehyde and embedded in paraffin. Tissue sections were permeabilized and blocked in PBS containing 0.3% Triton X-100 (Sigma-Aldrich, Milwaukee, WI) and 10% goat serum, followed by staining with primary antibodies for p65 (1:300) overnight at 4°C and secondary antibody (1:200). p65 proteins were then detected and immunolocalized using Mounting Medium containing DAPI. All the sections observed using fluorescence microscopy. Cultured cells immunofluorescence staining was performed as previously described [14]. Image-pro plus software was used to quantify the staining density. Based on the principles of equidistance, we randomly chose five views of distal end of the colonoscope in each section using 20× image, quantified the averaged IOD of each section. Every group has prepared 10 sections. The averaged IOD of each group was compared by Student’s t-test.
Immunohistochemical staining
The colon tissues were fixed in 10% formalin and embedded in paraffin. Tissue sections were cut, dewaxed, and incubated in 0.01 M natrium citricum for antigen retrieval. The slides were rinsed in phosphate-buffered saline and incubated overnight at 4°C with diluted anti-Ki67, CD34, p-Stat3 or anti-PCNA antibodies. Following steps were performed using the immunostaining kit (Maixin BIO, Fuzhou, China) according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay
Weighing the same weight of colon organization, fixed with pre-cooling PBS (1:9), homogeneous mixed, and centrifugated (10000 rpm/min, 10 min). The supernatant was reserved. The MSCs were treated with TNF-α and IFN-γ for 24 hours in the DSS treated mice. After treatment, the supernatants of tissues were collected and centrifugalized (1000 rpm/min, 5 min). The production of IL-1β, IL-17, TNF-α, IFN-γ and IL-10 in colon tissues supernatants and blood serum were determined in duplicate using ELISA kit (R&D System Europe Ltd., UK) as described by the manufacturer.
Western blotting analysis
Samples were collected by lysing cells in RIPA lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.1% SDS, 1% TritonX-100, 1% sodium deoxycholate, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Each sample was size-fractionated using SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidene difluoride (PVDF) transfer membranes (DuPont, Boston, MA, U.S.A.). Blots were incubated for 1 h at room temperature in 5% skim milk for blocking, and proteins were detected with primary antibodies overnight at 4°C, and then blotted with peroxidase-conjugated conjugated secondary antibodies for 1 hour at room temperature. The immunoblots were visualized using ECL (GE Healthcare, Bucks, UK).
Real-time PCR
Cells were collected to extract the total cellular mRNA using TRIzol reagent (Invitrogen). cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (Promega) and 2 μg of total RNA and oligo (dT) 18 primers. Two-microliter aliquots of cDNA were used for PCR amplification. Real-time PCR was performed in triplicate using the SYBR Prime Script RT-PCR Kit (Takara, Dalian, China). Thermocycler conditions included an initial hold at 50°C for 2 min and then 95°C for 10 min; this was followed by a two-step PCR program of 95°C for 15 s and 60°C for 60 s repeated for 45 cycles on a LightCycler®96 system (Roche, Shanghai, China), on which data were collected and quantitatively analyzed.
In vivo imaging of homing ability to tumors
We use Cell Tracer CM-Dill (Invitrogen Life Technologies, CA, USA) to trace MSCs in vivo. CM-Dil working solution was prepared as the manufacturer’s instructions. Briefly, 1 mg CM-Dil/mL stock solution in culture-grade DMSO, 8 μM solutions were made in 500 μL PBS, vortexed, and then combined with 2×106 hUC-MSCs in 500 μL PBS, to give 106 cells/mL in 4 μM CM-Dil labeling solution. CM-Dil cell suspensions were incubated for 30 min at 37°C and then for 15 min at 4°C. After labeling, cells were washed three times with PBS and resuspended in fresh medium. 24 hours after staining, cells were injected into the mice by tail vein, and then optical bioluminescence imaging was conducted to periodically trace the cells using a maestro in vivo imaging system (CRI, MA, USA).
Statistical analysis
All data are presented as the means ± S.D. for at least three separate determinations for each group. The differences between the groups were examined for statistical significance using the Student’s t-test with SPSS software. Differences were considered significant when the *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Results
MSCs pretreatment with TNF-α and IFN-γ aggravates mice colitis
Accumulating evidence showed that migration and homing of MSCs to the injured tissue contribute to the development of inflammation and wound healing, through autocrine or paracrine cytokines interacting with damaged tissues. To investigate this MSCs-mediated function, the DSS-induced experimental model of ulcerative colitis was constructed. It is characterized by a severe inflammation, such as massive mucosal damage, loss of goblet cells, crypt reduction and accumulation of infiltrating neutrophils in the lamina propria (Supplementary Figure 1A). Both TNF-α and IFN-γ were expressed abundantly during the active phase of UC when the DSS-induced colitis model was successfully established (Supplementary Figure 1B and 1C). The expression of the embryonic stem cell markers SOX2 and Nanog [23] indicated that MSCs preferentially migrated toward inflammation sites rather than normal sites (Supplementary Figure 2A). We found that MSCs pre-treated with TNF-α and IFN-γ for 72 h could accumulate in the caecum (Supplementary Figure 2B). In a previous study, it was reported that the ability of MSCs to preferentially migrate toward the sites of tissue damage and inflammation, was related to surface chemokine receptors or inflammation cytokines, such as CXC chemokine receptors 1 and 2 (CXCR1 and CXCR2), CXCR4 and, monocyte chemoattractant protein 1 (MCP-1) [24-26]. We detected CXCR4 and MCP-1 mRNA expression levels in each group, as they are involved in MSCs mobilization (Supplementary Figure 2C and 2D). Consequently, MSCs play an important role by homing to the injured sites.
To compare with the DSS and MSCs treated group, our study confirmed clear decreases in body weight loss and colon length in the TNF-α and IFN-γ treated group (Figure 1A and 1B). Myeloperoxidase (MPO) activity is a representative of neutrophil infiltration and is considered to be an important indicator of inflammation degree. Therefore, we investigated the MPO activity (Figure 1C) and found that its level was clearly increased following the addition of TNF-α and IFN-γ to MSCs. Similarly, cytokines treated group tended to exacerbate the inflammation as determined by HE staining, that showed a loss of structure of the mucosa and lamina propria, a loss of goblet cells, advanced damage to the crypt and increased neutrophils infiltration, when compared to the other groups (Figure 1D).
Figure 1.
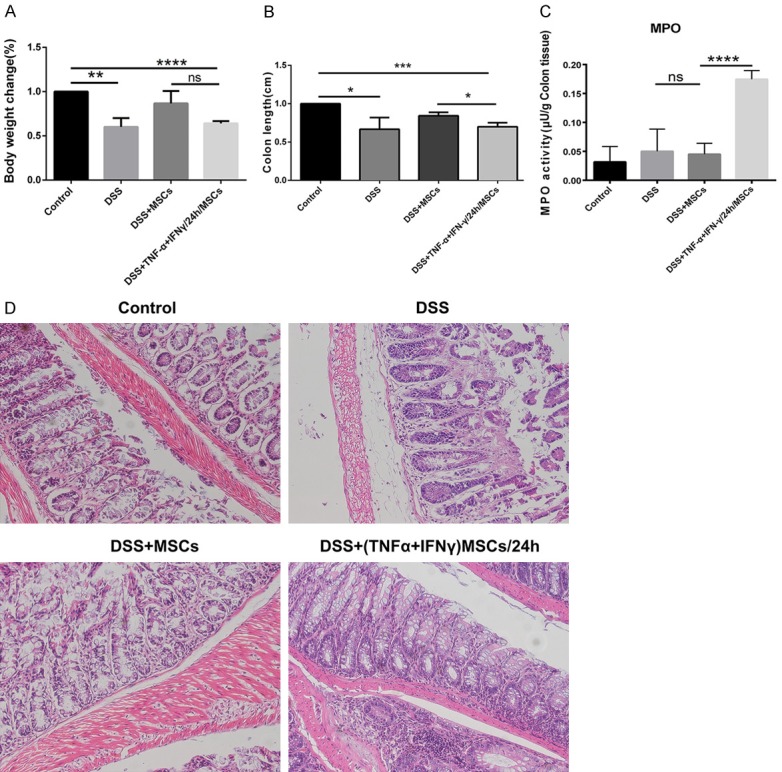
MSCs pretreated by TNF-α and IFN-γ aggravated the mice colitis. A and B. In comparison to the DSS and MSCs treated group, the group treatment with TNF-α and IFN-γ show a more obvious decrease in the loss of body weight and colon length. C. Intervention of TNF-α and IFN-γ to the MSCs induced the elevation of MPO activity in mice colon. D. The HE staining of each group demonstrated more serious destruction epithelial architecture and inflammatory cellular infiltration in TNF-α and IFN-γ treatment group. (Data were presented with mean ± SD of three independent experiments, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Hence, the MSCs group had a remarkable remission that was reflected by the change in body weight, colon length, reduction of MPO level and a noticeable tissue repair in mice with colitis. Yet when the MSCs were injected into mice tail vein, following treatment with TNF-α and IFN-γ, the repair function was profoundly inhibited.
MSCs treated with TNF-α and IFN-γ aggravate mice colitis via the NF-κB/STAT3 signaling pathway
MSCs, which are modulated by cytokines present in the inflammation microenvironment, play an important role in the repair of colitis in mice. However, the underlying mechanisms involved are not well known. According to a previous study, IFN-γ and TNF-α synergistically impair MSCs via the NF-κB-mediated activation of Mothers against decapentaplegic homolog 7 (SMAD7) in ovariectomized (OVX) mice [19]. In this study, we investigated the combined effect of IFN-γ and TNF-α on MSCs by in vitro pretreatments of MSCs with IFN-γ (50 ng/mL) and TNF-α (10 ng/mL) for 6 hours, 24 hours and 72 hours These treatments resulted in the activation of the NF-κB pathway leading to P65 up-regulation and IκBα downregulation (Figure 2A, Supplementary Figure 3). Meanwhile, we observed by immunofluorescence Staining, an accumulation of P65 in the nucleus, 24 h following treatment (Figure 2B). Simultaneously, the expression of p65 and p-STAT3 were both higher in the group treated with TNF-α and IFN-γ for 24 h in vivo (Figure 2C and 2D). The results above infer that the administration of TNF-α and IFN-γ activated the NF-κB/STAT3 pathway of MSCs in mice colitis.
Figure 2.

MSCs treated with TNF-α and IFN-γ affects the mice colitis via NF-κB/stat3 signaling pathway. A. Pre-treated the MSCs with TNF-α (10 ng/mL) and IFN-γ (50 ng/mL) for 6 h, 24 h, 72 h, then detecting the expression of p65 and IκBα. B. Pre-treated the MSCs with TNF-α (10 ng/mL) and IFN-γ (50 ng/mL) for 6 h, 24 h, 72 h, then the expression and location of p65 were detected by immunofluorescence. C. The immunofluorescence results illustrated the enhanced p65 expression when MSCs were pretreated with TNF-α and IFN-γ from colon tissues. D. The higher expression of p-stat3 was detected in the group treated with TNF-α and IFN-γ in colon tissues by immunohistochemistry. (Images were representative of three independent experiments).
Effect of MSCs pretreatment with TNF-α and IFN-γ on the immune microenvironment of mice colitis
Previous studies demonstrated that IBDs have a close connection with a dysregulated dialogue between intestinal microbiota and components of both the innate and adaptive immune systems. Cytokines, including TNF-α, IFN-γ, IL-1β, IL-6 and IL-10, play essential roles in mediating the crosstalk between activated immune and non-immune cells, such as epithelial and mesenchymal cells [27]. For example, TNF-α, which is one of the earliest and most important mediators, released in the process of inflammation, activates neutrophils and lymphocytes that are involved in the increase of vascular permeability and the regulation of metabolism activity of other tissues. This activation also promotes other cytokines production and release, and plays a crucial role in the initiation and amplification of inflammatory reactions involved in tissue damage and repair [28].
Meanwhile, our research indicated that the autocrine and paracrine activity of MSCs was associated with wound healing and inflammatory response. We analyzed protein expression and mRNA levels of inflammatory factors in colon tissue, serum, and plasma by Elisa and RT-PCR. The level of inflammatory cytokines in the serum of each group has been determined when MSCs were stimulated with TNF-α and IFN-γ, especially correlative increases in expression of pro-inflammatory factors, such as IL-17, TNF-α, IL-1β, IFN-γ and the anti-inflammatory IL-10 (Figure 3A-E). Similar expression pattern could also be seen in colon tissue of each group (Figure 3F-J) for TNF-α and IL-1β mRNA levels in plasma (Figure 3K and 3L). Therefore, these results showed that MSCs modulate wound healing through regulating the release of inflammatory factors, and the expression and secretion of inflammatory cytokines, that are associated with the NF-κB pathway in MSCs.
Figure 3.
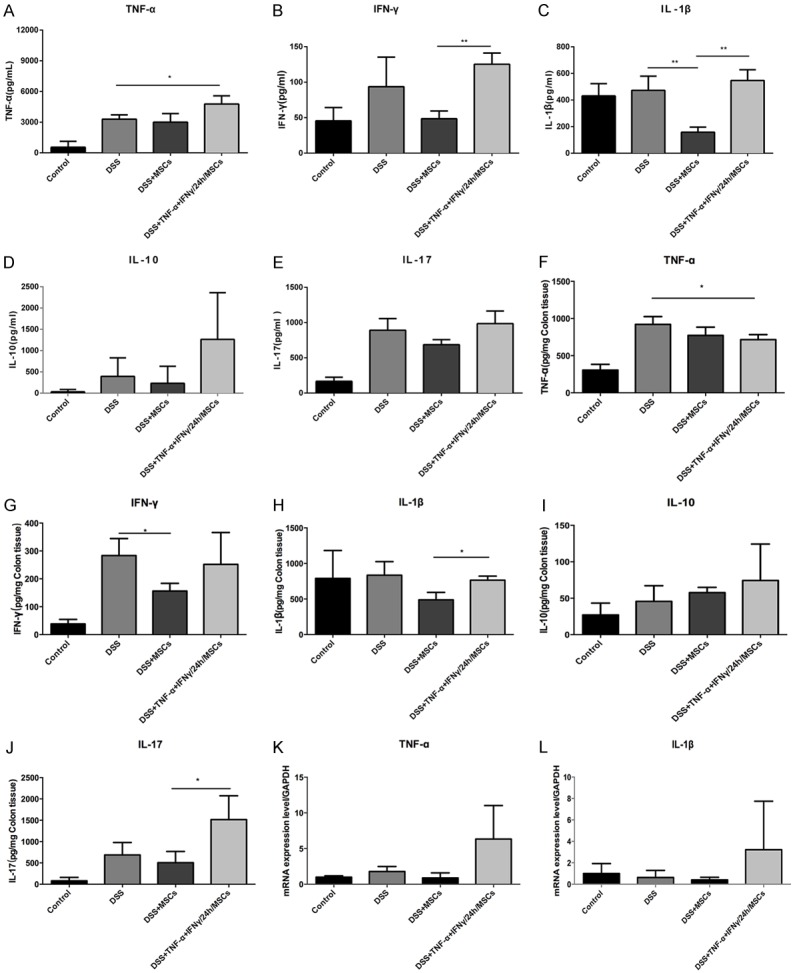
TNF-α and IFN-γ synergistically stimulate MSCs impacting the cytokines expression in each group. A-E. The expressions of IL-17, TNF-α, IL-1β, IFN-γ and IL-10 were detected in each group from mice serum. F-J. The expressions of IL-17, TNF-α, IL-1β, IFN-γ and IL-10 were also detected in each group from mice colon tissue. K, L. The mRNA levels of TNF-α and IL-1β were showed in mice plasma (Data were presented with mean ± SD of three independent experiments, *P<0.05, **P<0.01).
TNF-α and IFN-γ pretreated MSCs exacerbate mice colon cancer
As shown in Figure 4A, an azoxymethane (AOM)/dextransulfate sodium (DSS)-induced mice models were established and administered with or without MSCs, MSCs pretreated with TNF-α and IFN-γ or without, and for 6 h, 24 h and 48 h, to examine macroscopically and histologically, the severity of CAC. Significant body weight loss was observed in AOM/DSS induced mice when compared with the control group (Figure 4B), and most of the injected groups. Moreover, the body weight loss was lower in the MSCs group and the 48 h TNF-α and IFN-γ pretreated group, when compared to body weight loss in the AOM/DSS model group (*P<0.05).
Macroscopically, MSCs markedly decreased the incidence, number, and size of the tumor. However, cytokines treated groups revealed positive effects on tumor formation (Figure 4C). We next evaluated the histopathological characteristics of tumor tissue samples from each group by Hematoxylin and eosin (H&E) staining. In comparison with the AOM/DSS model group, cytokines treated groups exhibited more irregular tubular structures, multiple lumens and a reduced stroma (“back to back” aspect) (Figure 4C). Consequently, treatment with MSCs alleviated colon cancer tumorigenesis, consistent with previous studies using animal models, however, the combined treatment with TNF-α and IFN-γ has the opposite effect on MSCs.
The pretreated MSCs enhance angiogenesis and cell proliferation in mice colon cancer
To determine the effects of the pretreated MSCs on tumorigenesis and cell proliferation, we investigated the expression of several markers which are closely related with these processes. MSCs had a remarkable inhibition of MMP9 and VEGF expression (Figure 5A and 5B). However, cytokines treated groups sharply impair their effects. Besides, high expression of CD34 in these groups reinforced and confirmed the crucial role of pre-treatments with inflammatory factors (Figure 5C). Further evidence based on the enhanced expression of PCNA and Ki67 after cytokines stimulation, confirmed our conclusions that the combination of TNF-α and IFN-γ increases angiogenesis and cell proliferation in CAC mice (Figure 5D).
Figure 5.
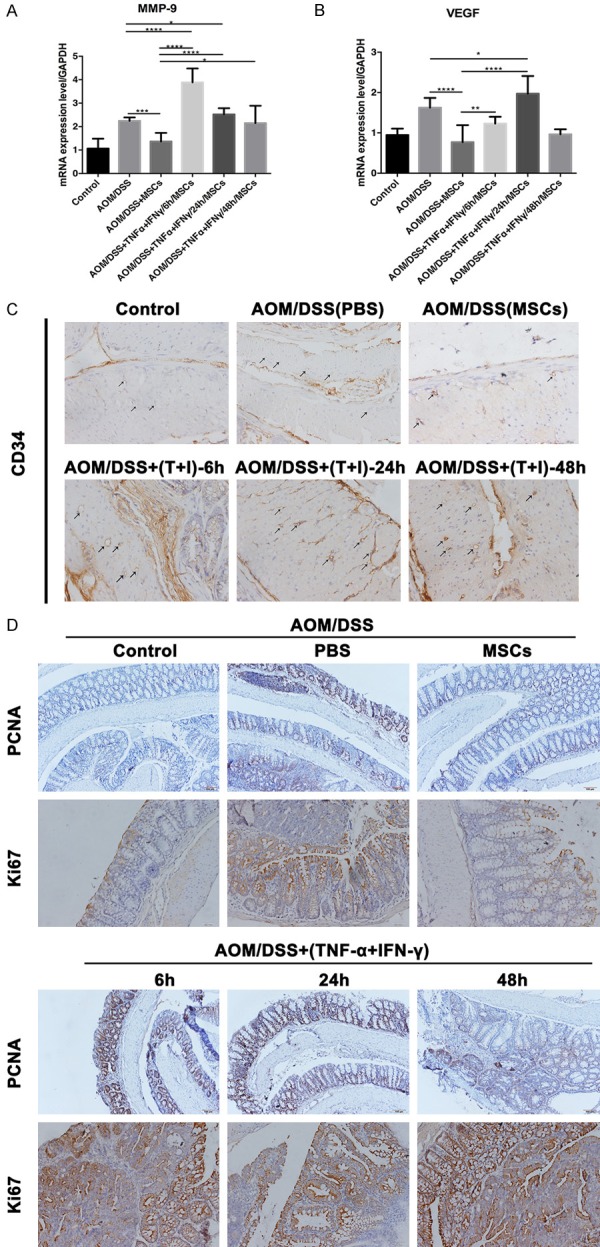
The pretreated MSCs enhance the angiogenesis and cell proliferation in mice colon cancer. A and B. The higher expression of MMP9 and VEGF was observed in cytokines treatment groups from mice blood. C. The black arrows pointed out more presence of CD34 in the pretreated MSCs groups than the other groups by immunohistochemistry. D. Immunohistochemistry of PCNA and Ki67 in murine intestine. Strong positive staining in the intestine of MSCs pre-treatment with inflammatory factors mice. (Data were presented with mean ± SD of three independent experiments, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Influence of MSCs pretreatment with TNF-α and IFN-γ on the immune regulation of mice colon cancer
It has been shown that MSCs have the ability to migrate into tumors and injury sites by sensing signaling molecules, which are released from distant damaged tissues, due to the increase of inflammatory mediators [29]. To investigate this, mRNA expression changes of several related cytokines in mice were determined, and we found that weaker expression of all detected cytokines, especially TGF-β, COX-2, IL-6 and IL-8, in the MSCs group when compared to the AOM/DSS-induced CAC group (Figure 6A-F). Additionally, cytokines release in the pre-treated MSCs was time independent. For instance, the highest expression of TNF-α occurred in the 48 h group, while the strongest expression of COX-2 was observed in the 24 h group, and the maximum expression of IL-6 and IL-8 were noticed in the 6 h group. We attribute these differences to the diversity in cytokines synthesis and secretion. Taken together, our results indicate that MSCs regulate the immune system by suppressing the release of inflammatory factors in mice, to promote wound healing, whereas, the use of TNF-α and IFN-γ synergistically weakens their repair function.
Figure 6.

Influence of MSCs treatment with TNF-α and IFN-γ on the immune regulation of mice colon cancer. A-F. The mRNA level of TNF-α, TGF-β, COX-2, IL-6, IL-8 and IL-12 were weaker in MSCs group than in AOM/DSS-induced CAC model group and the release of cytokines in the pre-treated MSCs was independent of the treatment time from mice blood (Data were presented with mean ± SD of three independent experiments, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Discussion
The multilineage differentiation capacity of MSCs, and their immunomodulatory activity, implied that these somatic progenitor cells are highly versatile for a wide range of therapeutic applications [30]. In the etiology and progression of human IBD, including CD and UC are multifactorial, uncontrolled immune responses to intestinal microbes are one of the critical players [31]. Both CD and UC are progressively fatal without curative treatment. In recent years, an increasing number of reports consistently showed that MSCs could be applied for the treatment of inflammation-mediated diseases. For example, a study based on a new model for chronic IBD, which was established by an alternative administration periods of DSS, showed a great potential in improving the stool condition, weight gain, the normal histopathologic picture, and in decreasing gene expression of inflammatory markers such as IL-23, TNF-α and IFN-γ associated with the therapeutic use of MSCs in treating IBD [32]. Furthermore, multi-mechanisms involving the efficacy of MSCs treatment have been proved, such as the induction of IL-10 secreting regulatory T cells [33], the down-regulation of IL-23/IL-17 regulated inflammatory reactions and the modulation of Treg/Th17 cells [34]. It was also found that MSCs had tumor suppressive properties in CAC models and from different species with bone marrow or cord blood origin [35].
In our study, similar results were obtained on the positive effects of MSCs in the DSS and AOM/DSS models in mice. These effects significantly delayed body weight changes, improved colon tissue damage, as assessed by visual observation and microscopy, and reduced the expression of pro-inflammatory factors (IL-1β, TNF-α, IL-6, IL-12, and COX-2) when compared to the PBS groups (Figure 6). Meanwhile, the lower expression of KI67, PCNA, CD34, VEGF and MMP9 in the MSC group may infer that MSCs have negative effects on the development of colon carcinoma by decreasing tumor cell proliferation and angiogenesis (Figure 5). Furthermore, we also discovered that the potential mechanisms associated with MSCs effects may rely on exerting forward influences on colitis and CAC through the inhibition of the NF-κB/STAT3 pathway (Figure 2).
In addition, previous reports on TNF-α and IFN-γ synergistically inducing MSC deficiency and tumorigenesis in OVX-induced osteoporotic mice, are consistent with our findings on the exposure of MSCs to TNF-α and IFN-γ via the activation of the NF-κB signaling in colitis mice (Figure 2) [19]. Moreover, we demonstrated that pretreated MSCs possess the ability to promote the tumor progression in CAC mice (Figure 5). It was previously described that pretreated MSCs with TNF-α and IFN-γ, promote tumor angiogenesis through upregulating the expression of VEGF, which is associated to the hypoxia-inducible factor 1α (HIF-α)-dependent signaling [17]. Similarly, we confirmed that the stimulation of MSCs with two cytokines not only upregulated the level of VEGF expression, but also MMP9 and CD34 expression levels. However, the mechanism behind MSCs angiogenic ability is not completely understood. In a most recent study, it has been well established that TNF-α preactivated-hMSCs exert a much stronger tumor-promoting effect via the CCL5/β-catenin/Slug pathway in CRC progression [36]. Accordingly, we can suppose that MSCs, pre-activated with TNF-α and IFN-γ, promote CAC proliferation that involves the CCL5/β-catenin/Slug pathway.
The Lixin Wei group demonstrated that the combined use of TNF-α and IFN-γ caused a significant up-regulation of TGF-β expression in MSCs, which induced autophagy and chemoresistance in hepatocellular carcinoma cells (HCC) [20]. Afterwards, a research showed that TNF-α and IFN-γ synergistically promote the ability of human placenta-derived MSCs to enhance the expression of programmed death ligand-2 and CD4+IL-10+ and CD8+IL-10+ Treg subsets from T cells [21]. Recently more studies were performed in vitro, such as the one from Haibin Zhou group which showed that pre-stimulated MSCs produce more IL-6, HGF, VEGF and TGF-β but not IL-2, IL-4 and IL-10 [15]. Ping, et al. confirmed that stimulation with TNF-α and IFN-γ polarized MSCs to a Th1 phenotype, resulting in the release of IL4, IL-10, CD274/PD-L1 and IDO [22]. Besides, it was also reported that TNF-α and IFN-γ synergistically induced the expression of IGF-1, TGF-β, Qa2 and pluripotency genes by significantly promoting the proliferation of murine adipose tissue-derived MSCs [37]. Interestingly, these studies correlate with ours, in that TNF-α together with IFN-γ increased the immuno-modulatory effects of MSCs on colitis and CAC mice by expressing more cytokines including TNF-α, IFN-γ, IL-10, IL-1β, IL-17, TGF-β, COX-2, IL-8 and IL-12. Therefore, our study confirmed that the pre-stimulated MSCs with TNF-α and IFN-γ can exacerbate mice colitis and CAC by decreasing body weight, worsening colon tissues damage, facilitating the expression of pro-inflammatory factors, and in contrast with what was observed in the PBS and MSCs groups. Consequently, we concluded that the pre-treated MSCs negatively affected colitis-related disease in mice via activating NF-κB/STAT3 pathway. In the CAC study, we also found, that the two factors TNF-α and IFN-γ, possessed the ability to promote proliferation and angiogenesis stimulated by MSCs in CAC mice.
It should be noted that this study only examined, in vivo, the effects of pretreated MSCs, with two pro-inflammatory cytokines, and did not focus on exploring, in vitro, possible mechanisms affecting the immunophenotype and immuno-modulatory actions of combining TNF-α and IFN-γ on MSCs. Overall, our study investigated, for the first time, the effects of TNF-α and IFN-γ pre-treated MSCs in colitis and CAC model, and demonstrated that the two pro-inflammatory cytokines efficiently reversed the repairing function of MSCs in colitis. It also suggests that these effects are potentially associated with the sensitization of the NF-κB/STAT3 pathway. Undoubtedly, these findings would lay a solid foundation for further investigations.
Acknowledgements
This work was supported by the grants from the national natural science foundation of China (grant no. 81872045, 81470793), and the special fund for public welfare research institutes of Fujian Province (grant no. 2017R1036-1, 2018R1036-4).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Vecchi Brumatti L, Marcuzzi A, Tricarico PM, Zanin V, Girardelli M, Bianco AM. Curcumin and inflammatory bowel disease: potential and limits of innovative treatments. Molecules. 2014;19:21127–21153. doi: 10.3390/molecules191221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomasello G, Tralongo P, Damiani P, Sinagra E, Di Trapani B, Zeenny MN, Hussein IH, Jurjus A, Leone A. Dismicrobism in inflammatory bowel disease and colorectal cancer: changes in response of colocytes. World J Gastroenterol. 2014;20:18121–18130. doi: 10.3748/wjg.v20.i48.18121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Szylberg L, Janiczek M, Popiel A, Marszalek A. Large bowel genetic background and inflammatory processes in carcinogenesis--systematic review. Adv Clin Exp Med. 2015;24:555–563. doi: 10.17219/acem/31239. [DOI] [PubMed] [Google Scholar]
- 5.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Merga Y, Campbell BJ, Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis. 2014;32:475–483. doi: 10.1159/000358156. [DOI] [PubMed] [Google Scholar]
- 7.Sales-Campos H, Basso PJ, Alves VB, Fonseca MT, Bonfa G, Nardini V, Cardoso CR. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz J Med Biol Res. 2015;48:96–107. doi: 10.1590/1414-431X20143774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He XW, He XS, Lian L, Wu XJ, Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci. 2012;57:3136–3144. doi: 10.1007/s10620-012-2290-5. [DOI] [PubMed] [Google Scholar]
- 9.Sun T, Gao GZ, Li RF, Li X, Li DW, Wu SS, Yeo AE, Jin B. Bone marrow-derived mesenchymal stem cell transplantation ameliorates oxidative stress and restores intestinal mucosal permeability in chemically induced colitis in mice. Am J Transl Res. 2015;7:891–901. [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HJ, Oh SH, Jang HW, Kwon JH, Lee KJ, Kim CH, Park SJ, Hong SP, Cheon JH, Kim TI, Kim WH. Long-term effects of bone marrow-derived mesenchymal stem cells in dextran sulfate sodium-induced murine chronic colitis. Gut Liver. 2016;10:412–419. doi: 10.5009/gnl15229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, He X, He X, Chen X, Lin X, Zou Y, Wu X, Lan P. Bone marrow mesenchymal stem cells ameliorate colitis-associated tumorigenesis in mice. Biochem Biophys Res Commun. 2014;450:1402–1408. doi: 10.1016/j.bbrc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Nasuno M, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nakagaki S, Watanabe S, Idogawa M, Yamashita K, Naishiro Y, Adachi Y, Suzuki H, Fujimiya M, Imai K, Shinomura Y. Mesenchymal stem cells cancel azoxymethane-induced tumor initiation. Stem Cells. 2014;32:913–925. doi: 10.1002/stem.1594. [DOI] [PubMed] [Google Scholar]
- 13.Tang RJ, Shen SN, Zhao XY, Nie YZ, Xu YJ, Ren J, Lv MM, Hou YY, Wang TT. Mesenchymal stem cells-regulated Treg cells suppress colitis-associated colorectal cancer. Stem Cell Res Ther. 2015;6:71. doi: 10.1186/s13287-015-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pourgholaminejad A, Aghdami N, Baharvand H, Moazzeni SM. The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine. 2016;85:51–60. doi: 10.1016/j.cyto.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Li G, Liu M, Zhou T, Zhou H. Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. J Biosci Bioeng. 2016;121:213–219. doi: 10.1016/j.jbiosc.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Romieu-Mourez R, Francois M, Abate A, Boivin MN, Birman E, Bailey D, Bramson JL, Forner K, Young YK, Medin JA, Galipeau J. Mesenchymal stromal cells expressing ErbB-2/neu elicit protective antibreast tumor immunity in vivo, which is paradoxically suppressed by IFN-gamma and tumor necrosis factor-alpha priming. Cancer Res. 2010;70:7742–7747. doi: 10.1158/0008-5472.CAN-10-0296. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX, Wang CY, Sun K, Jiang GC, Zhao X, Li R, Gao L, Zhao QD, Wu MC, Wei LX. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem. 2011;286:25007–25015. doi: 10.1074/jbc.M110.213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Zhao Y, Liu Y, Akiyama K, Chen C, Qu C, Jin Y, Shi S. IFN-gamma and TNF-alpha synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFkappaB signaling. Stem Cells. 2013;31:1383–1395. doi: 10.1002/stem.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Z, Jing Y, Xia Y, Zhang S, Hou J, Meng Y, Yu F, Liu X, Wu M, Zhang P, Wei L. Mesenchymal stem cells contribute to the chemoresistance of hepatocellular carcinoma cells in inflammatory environment by inducing autophagy. Cell Biosci. 2014;4:22. doi: 10.1186/2045-3701-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Wang W, Wang G, Hou Y, Xu F, Liu R, Wang F, Xue J, Hu T, Luan X. Interferon-gamma and tumor necrosis factor-alpha promote the ability of human placenta-derived mesenchymal stromal cells to express programmed death ligand-2 and induce the differentiation of CD4 interleukin-10 and CD8 interleukin-10 Treg subsets. Cytotherapy. 2015;17:1560–1571. doi: 10.1016/j.jcyt.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Jin P, Zhao Y, Liu H, Chen J, Ren J, Jin J, Bedognetti D, Liu S, Wang E, Marincola F, Stroncek D. Interferon-gamma and tumor necrosis factor-alpha polarize bone marrow stromal cells uniformly to a Th1 phenotype. Sci Rep. 2016;6:26345. doi: 10.1038/srep26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karaoz E, Okcu A, Gacar G, Saglam O, Yuruker S, Kenar H. A comprehensive characterization study of human bone marrow mscs with an emphasis on molecular and ultrastructural properties. J Cell Physiol. 2011;226:1367–1382. doi: 10.1002/jcp.22468. [DOI] [PubMed] [Google Scholar]
- 24.Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, Kaps C, Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 25.Zhang SJ, Song XY, He M, Yu SB. Effect of TGF-beta1/SDF-1/CXCR4 signal on BM-MSCs homing in rat heart of ischemia/perfusion injury. Eur Rev Med Pharmacol Sci. 2016;20:899–905. [PubMed] [Google Scholar]
- 26.Eseonu OI, De Bari C. Homing of mesenchymal stem cells: mechanistic or stochastic? Implications for targeted delivery in arthritis. Rheumatology Oxford. 2015;54:210–218. doi: 10.1093/rheumatology/keu377. [DOI] [PubMed] [Google Scholar]
- 27.Bouguen G, Chevaux JB, Peyrin-Biroulet L. Recent advances in cytokines: therapeutic implications for inflammatory bowel diseases. World J Gastroenterol. 2011;17:547–556. doi: 10.3748/wjg.v17.i5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammam O, Mahmoud O, Zahran M, Sayed A, Salama R, Hosny K, Farghly A. A possible role for TNF-alpha in coordinating inflammation and angiogenesis in chronic liver disease and hepatocellular carcinoma. Gastrointest Cancer Res. 2013;6:107–114. [PMC free article] [PubMed] [Google Scholar]
- 29.Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta. 2013;1836:321–335. doi: 10.1016/j.bbcan.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK, Yen BL. Human mesenchymal stem cells for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23:76. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 32.Abdel Salam AG, Ata HM, Salman TM, Rashed LA, Sabry D, Schaalan MF. Potential therapeutic utility of mesenchymal stem cells in inflammatory bowel disease in mice. Int Immunopharmacol. 2014;22:515–521. doi: 10.1016/j.intimp.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Liu S, Xu Y, Zhang A, Jiang J, Tan W, Xing J, Feng G, Liu H, Huo F, Tang Q, Gu Z. Human umbilical cord-derived mesenchymal stem cells downregulate inflammatory responses by shifting the Treg/Th17 profile in experimental colitis. Pharmacology. 2013;92:257–264. doi: 10.1159/000354883. [DOI] [PubMed] [Google Scholar]
- 35.Prakash MD, Miller S, Randall-Demllo S, Nurgali K. Mesenchymal stem cell treatment of inflammation-induced cancer. Inflamm Bowel Dis. 2016;22:2694–2703. doi: 10.1097/MIB.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 36.Chen K, Liu Q, Tsang LL, Ye Q, Chan HC, Sun Y, Jiang X. Human MSCs promotes colorectal cancer epithelial-mesenchymal transition and progression via CCL5/beta-catenin/Slug pathway. Cell Death Dis. 2017;8:e2819. doi: 10.1038/cddis.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammadpour H, Pourfathollah AA, Nikougoftar Zarif M, Hashemi SM. Increasing proliferation of murine adipose tissue-derived mesenchymal stem cells by TNF-alpha plus IFN-gamma. Immunopharmacol Immunotoxicol. 2016;38:68–76. doi: 10.3109/08923973.2015.1115519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


