Abstract
Background: Thyroid carcinoma is the most common malignant endocrine tumor; the anaplastic thyroid cancer subtype is aggressive and has a poor prognosis. However, there is no effective treatment for this disease. Methods: This study was analyzed using the Surveillance, Epidemiology, and End Results (SEER) database. Joinpoint regression models, linear regression models, Kaplan-Meier survival curves and Cox regression models were used to study the trends in incidence, survival rate and median survival time and to detect the risk factors affecting prognosis in patients with anaplastic thyroid cancer. Results: While the incidence rate and truncated incidence rate fluctuated slightly over the past 30 years, they were relatively stable and had no obvious upward trend (APC = -0.22 and 0.24, respectively, P>0.05). The median survival was 3.16 months, and the survival rate did not improve significantly (the APC values of the 3-, 6-, 9-, and 12-month survival rates were 0.44, 0.35, -0.23 and -0.86, respectively, P>0.05). After subgroup analysis and survival analysis, it was concluded that the prognosis of the patients might be related to their metastatic stage, surgical status, chemotherapy treatment, age and socioeconomic status at the time of diagnosis (P<0.05). Total thyroidectomy is superior to other methods and is beneficial in prolonging the life of patients and improving the overall survival rate (the median survival was 10 months, and the 6-month survival rate was 59.26%). Conclusion: The incidence trend for anaplastic thyroid cancer over the last 30 years was stable, and the survival rate and median survival time were not significantly improved. The prognosis of the patients may be related to their metastatic stage, age, socioeconomic status, surgical status and chemotherapy treatment.
Keywords: Anaplastic thyroid cancer, SEER database, epidemiology, prognosis
Introduction
Thyroid cancer is the most common endocrine malignancy, accounting for 5% of new malignant tumors. Its incidence has increased annually, especially among women. Thyroid cancer is currently ranked as the fourth highest malignant tumor in terms if incidence [1-3]. Although differentiated thyroid cancer has a favorable prognosis, the prognosis of anaplastic thyroid cancer (ATC) is poor [4-6]. Anaplastic thyroid cancer not only has high morbidity but also acquires chemotherapy resistance easily [4-6]. Because of its aggressive characteristics, surgery cannot achieve satisfactory outcomes. Furthermore, losing I-131-uptake ability limits the main treatment modalities to local external beam radiation treatment and systemic chemotherapy [6,7]. Various targeted drugs have been attempted [8-14].
At present, research on ATC is becoming more urgent. The lack of cases makes it difficult for clinicians to accumulate treatment experience data. As a result, it is particularly important to obtain epidemiological information for ATC from resources such as large databases.
In this study, we aim to use the Surveillance, Epidemiology, and End Results (SEER) database to discuss the incidence and survival status of anaplastic thyroid cancer and to delineate the prognostic factors.
Materials and methods
SEER cohort
Using the Surveillance, Epidemiology, and End Results (SEER) database, we reviewed patients diagnosed with ATC within the United States from 1986 to 2015; the data acquisition date was January 22, 2019.
ATC patients were selected from the SEER database based on histology type according to the International Classification of Diseases for Oncology, third edition, ICD-O-3, using following codes: 8012, 8020, 8021, 8030, 8031, and 8032. A total of 1567 cases (from the SEER 18 region) were included in our study. Clinical information, such as year of diagnosis, age, sex, race/ethnicity, economic status, M staging, treatment (surgical method, radiotherapy and chemotherapy), follow-up time, and survival status, were downloaded as well. We excluded patients with missing information, as well as those who were diagnosed at autopsy or who had multiple primary cancers without the first discharge diagnosis of ATC. Patients who suffered from recurrent ATC or aberrant thyroid cancer were also excluded.
Statistical methods
Patient age was summarized by the median. The survival rate was expressed as the mean ± standard error (SE). Enumeration data (including percentages) were used for the other variables. Annual percent change (APC) values of standardized incidence, truncated incidence, and disease-related survival rates were calculated by multiphase regression to describe the trends in incidence and survival rates over time, which were fitted using the Joinpoint Regression Program (version 4.5.0.1; Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute), with P<0.05 indicating a significant difference. Incidence rates were analyzed with SEER*Stat Software version 8.3.5 (Surveillance Research Program, National Cancer Institute, seer.cancer.gov/seerstat). The linear regression model was used to describe the changing trend in disease-related median survival. In addition, a stratified analysis was performed on subgroups based on (1) age (0-59, 60-79 vs ≥80), (2) sex (male vs female), (3) race (white, black vs others), (4) year of onset (1986-1995, 1996-2005 vs 2006-2015), (5) socioeconomic status (high income vs low income), (6) metastatic (M) stage (M0 vs M1), (7) radiotherapy (yes vs no), (8) chemotherapy (yes vs no) and (9) surgical procedure (no surgery/biopsy, partial resection, gland lobectomy/subtotal/near total resection, total resection without lymph node dissection, total resection + selective lymph node dissection, total resection + radical lymph node dissection, total resection + the extent of lymph node dissection is unknown). The multivariate Cox proportional hazard model was used to evaluate the hazard ratios (HRs) and the 95% confidence intervals (CIs) to screen for prognostic factors. Survival analysis (overall survival, OS) and the comparison of survival time among different variables were performed using Kaplan-Meier estimates and the log-rank test. Statistical analyses and graphing were performed using Stata 12.0 and GraphPad 6 with P<0.05 indicating statistically significant.
Results
Patient demographics
The SEER database was used to obtain incidence data from 1986 to 2015. To avoid regional differences, the incidence data were obtained from SEER 9, which covers 9.4% of the United States population (based on the 2010 US Census). The more recent SEER databases (SEER 18, SEER 21) could not be used since the new registries that joined these databases only had newer regional clinical information from as early as 2000.
The SEER database was also used for the survival analysis data. Differences in regions, however, were not believed to affect the results; thus, SEER 18 was used. The SEER 18 region data cover nearly 27.8% of the US population (based on the US 2010 census).
The SEER 18 database included 1567 ATC patients between 1986 and 2015; data from 717 patients were used to calculate the morbidity and survival rates from the SEER 9 database. These patients had a median (range) age of 71 years (23-100 years). The age standardized rate between 1986 and 2015 was 0.09/100,000, and the truncated age incidence rate (35-64 years) was 0.07/100,000. Although the average incidence of Caucasians and African American patients was slightly lower than that of other ethnic groups, the truncated rate of Caucasians was the highest in the population. The incidence rate and truncated rate of the high-income group were higher than those of the low-income group (Table 1). As of January 1, 2015, the standardized prevalence of ATC in the past 30 years was 0.16/100,000, and the number of people who were alive was 57. This means that the low prevalence is partly due to its low incidence and partly due to its low survival rate.
Table 1.
The incidence of ATC, 1986-2015
| Items (number of cases) | Standardized incidence* | Truncated incidence between 35 to 64 years old* |
|---|---|---|
| SEX | ||
| Male (296) | 0.87 | 0.75 |
| Female (421) | 0.95 | 0.70 |
| Race# | ||
| White (578) | 0.90 | 0.72 |
| Black (53) | 0.81 | 0.65 |
| Other (83) | 1.14 | 0.76 |
| SES | ||
| High (614) | 0.93 | 0.73 |
| Low (103) | 0.89 | 0.70 |
| Total (717) | 0.92 | 0.72 |
Rates are per 1,000,000 and age-adjusted to the 2000 US Std Population standard.
3 patients’ data of race unavailable.
SES: Socioeconomic status.
The trend of ATC
We made the joinpoint regression model fitting curve of standardized incidence and truncated incidence, with change based on the data from SEER 9; these curves showed that the change in ATC incidence was not significant (Figure 1). From Figures 2, 3, we analyzed the survival rate and median survival time. There was no significant improvement in the last 10 years compared with 30 years ago, regardless of the survival rate (P>0.05) or the median survival time (F test P = 0.304). These results suggest that there has been no effective treatment to improve the prognosis and prolong the survival time of ATC patients in the past 30 years.
Figure 1.
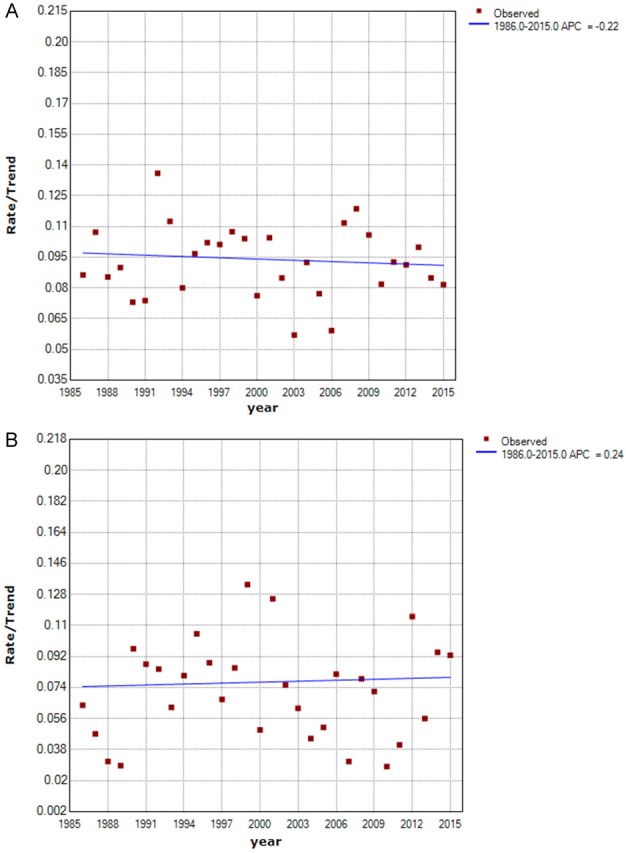
Curve of the Standardized Incidence from 1986 to 2015. A. Curve of Standardized Incidence. B. Curve of Truncated Incidence. The truncated incidence was counted among 35-64-year-old patients. The points indicate the observed incidence (per 100,000 person-years). The fitting curve shows the annual percentage change in standardized incidence from 1986 to 2015. Rates are age-adjusted to the 2000 US standard population.
Figure 2.
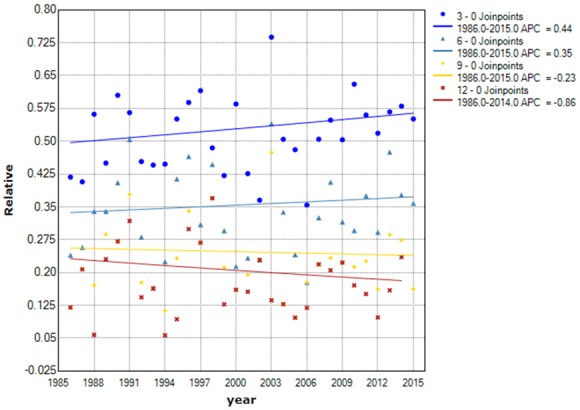
Curve of disease-related survival rates from 1986 to 2015. The points represent observed disease-related relative survival rates. The fitting curve shows the annual percentage change in the survival rate from 1986 to 2015. Because the survival rate occurring above 1 year is 0, this method is not suitable for fitting it. Abbreviations: 3-0 joinpoint, 3-month survival rate; 6-0 joinpoint, 6-month survival rate; 9-0 joinpoint, 9-month survival rate. ^The APC had statistical significance, P<0.05.
Figure 3.
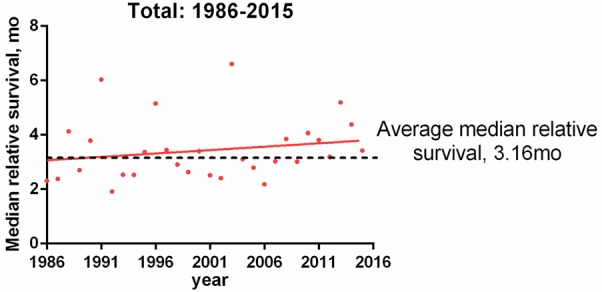
Curve of disease-related median survival time from 1986 to 2015. Points represent median disease-related survival times. Trend line for calculating median survival time by linear regression (R square = 0.04, F test P = 0.304).
Survival analysis
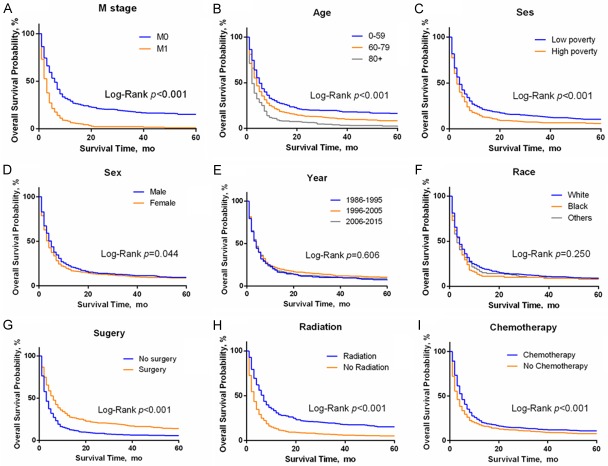
We further analyzed the survival data of all eligible ATC patients downloaded from the SEER 18 region database. A total of 1567 cases were obtained. We found that the Kaplan-Meier survival curves for overall survival and DFS (disease-free survival) (Figure 4) nearly coincide. Figure 5A-I shows that the prognosis of patients with M0 stage (HR = 0.50, 95% CI: 0.31-0.44, P<0.001) was significantly better than that of patients with M1 stage, which is consistent with the AJCC TNM stage. The total survival time of patients who received a surgical intervention was significantly improved (HR = 0.63, 95% CI: 0.52-0.66, P<0.001). In addition, young patients (P<0.001), male patients (HR = 0.89, 95% CI: 0.78-0.99, P = 0.044) and those with higher income levels (HR = 0.79, 95% CI: 0.66-0.86, P<0.001) also had longer survival times. From the guidelines, we know that radiotherapy and chemotherapy are the common choices for ATC. The Kaplan-Meier survival curves showed that patients who received radiotherapy (HR = 0.59, 95% CI: 0.48-0.60, P<0.001) and chemotherapy (HR = 0.79, 95% CI: 0.67-0.85, P<0.001) had a significant survival advantage.
Figure 4.
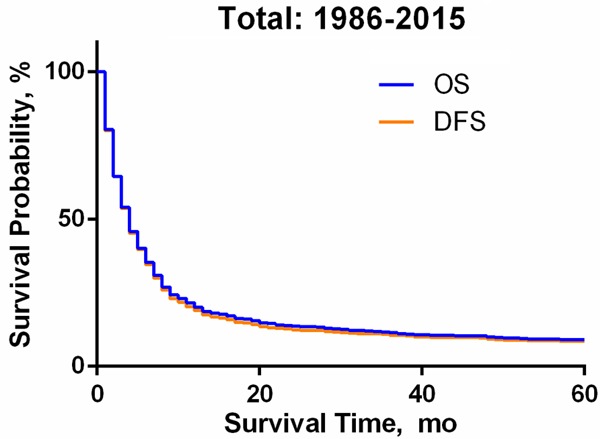
Kaplan-Meier Survival Curve of Anaplastic Thyroid Cancer from 1986 to 2015. Abbreviations: OS, Overall Survival; DFS, Disease-Free Survival.
Figure 5.
Total survival subset analysis of anaplastic thyroid cancer patients from 1986 to 2015. Abbreviation: SES, socioeconomic status.
Multivariate COX regression analysis indicated that age, socioeconomic status, M stage, chemotherapy treatment and surgical status were independent risk factors for overall survival (Table 2).
Table 2.
Cox regression analysis of prognosis for ATC
| Analysis | Variable | Hazard Ratio (95% CI) | p-value |
|---|---|---|---|
| Univariate analysis | Year of diagnosis | 1.01 (0.99-1.04) | 0.356 |
| Age | 1.02 (1.01-1.02) | <0.001* | |
| Sex | |||
| Male | 1 | ||
| Female | 1.07 (0.91-1.25) | 0.407 | |
| Race | |||
| White | 1 | ||
| Black | 1.24 (0.95-1.62) | 0.115 | |
| Others | 1.09 (0.97-1.23) | 0.154 | |
| SES | |||
| Low poverty | 1 | ||
| High poverty | 1.32 (1.12-1.55) | 0.001* | |
| M stage | |||
| M0 | 1 | ||
| M1 | 2.13 (1.81-2.51) | <0.001* | |
| Radiation | |||
| No | 1 | ||
| Yes | 0.50 (0.42-0.58) | <0.001* | |
| Chemotherapy | |||
| No | 1 | ||
| Yes | 0.65 (0.56-0.76) | <0.001* | |
| Surgery | |||
| No | 1 | ||
| Yes | 0.53 (0.45-0.62) | <0.001* | |
| Multivariate analysis | Age | 1.02 (1.01-1.02) | <0.001* |
| SES | |||
| Low poverty | 1 | ||
| High poverty | 1.26 (1.07-1.48) | 0.005* | |
| M stage | |||
| M0 | 1 | ||
| M1 | 2.04 (1.73-2.41) | <0.001* | |
| Radiation or not | |||
| No | 1 | ||
| Yes | 1.04 (0.73-1.46) | 0.841 | |
| Chemotherapy or not | |||
| No | 1 | ||
| Yes | 0.79 (0.67-0.93) | 0.005 | |
| Surgery or not | |||
| No | 1 | ||
| Yes | 0.56 (0.39-0.80) | 0.001 |
Abbreviation: 95% CI, 95% confidence interval; SES, socioeconomic status.
p value has statistical significance.
In a previous analysis, it was found that surgery had a greater impact on the overall survival, which means that the choice of treatment affects the prognosis. By drawing Kaplan-Meier survival curves (Figure 6A and 6B) and calculating the median survival time and 6-month survival rate (Table 3), we observed that different surgical methods also impacted the survival time. The prognosis of patients undergoing total thyroidectomy (HR = 0.6105, 95% CI: 0.4158-0.6804, P<0.0001) was significantly better than those undergoing partial thyroidectomy/lobectomy/subtotal thyroidectomy/subtotal thyroidectomy. The median survival time was also significantly improved in those who had a total thyroidectomy. There was, however, no significant difference in overall survival between patients undergoing total thyroidectomy with or without lymph node dissection (HR = 0.745, 95% CI: 0.4797-1.076, P = 0.129). In addition, the 6-month survival rate of patients who underwent glandular lobectomy or more was significantly better than that of patients who did not undergo surgery or underwent partial excision only. Most patients in the nonoperative group had distant metastasis, i.e., M1 stage (58.04%), and most patients in the operative group had M0 stage disease (68.07%).
Figure 6.
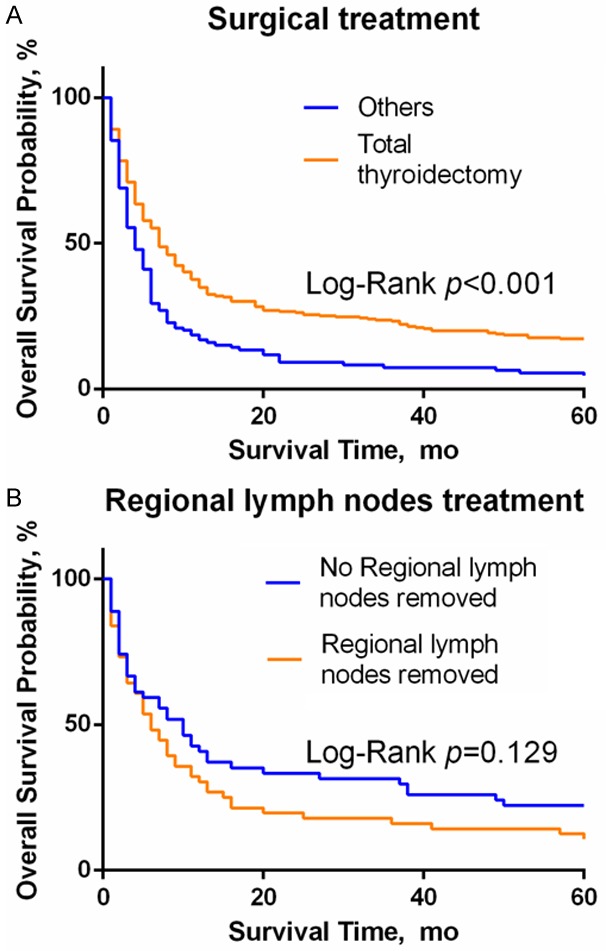
The influence of the choice of surgical methods on OS. A. The prognosis of patients with glandular lobe/subtotal/subtotal resection alone is worse than that of patients with bilateral total resection, P<0.0001. B. In patients undergoing bilateral total lobectomy, regional lymph node dissection had no effect on OS, P = 0.129.
Table 3.
The influence of surgical methods on survival time
| Operative methods (number of cases/person) | Median survival time/month | 6-mon survival rate/% |
|---|---|---|
| Total (1567) | 4 | 35.23±1.37 |
| No operation/biopsy only (1001) | 3 | 27.26±1.67 |
| Partial resection (12) | 5 | 14.29±13.23 |
| Lobectomy/subtotal/near total resection# (133) | 4 | 30.32±4.33 |
| Total resection without lymph node dissection* (57) | 10 | 59.26±6.69 |
| Total resection + selective lymph node dissection* (42) | 7 | 51.28±8.00 |
| Total resection + radical lymph node dissection* (21) | 6 | 41.18±11.94 |
| Total resection + lymph node dissection scope is unknown* (228) | 7 | 55.91±3.41 |
| Type of operation is unknown (73) | 3.5 | 35.19±6.50 |
Total/subtotal/subtotal unilateral lobectomy or total unilateral lobectomy and subtotal contralateral lobectomy.
Total resection refers to total excision of bilateral glandular lobes.
Discussion
In this study, the data from ATC cases were collected from the SEER database between 1986 and 2015. The age of peak incidence was 71 years old. While the incidence rate and truncated rate fluctuated over the past 30 years, there was no obvious escalating or descending trend. The survival rate was not improved significantly. Subgroup analysis and survival analysis showed that the prognosis of patients may be related to M stage, age at diagnosis, choice of operation and socioeconomic status. In accordance with preliminary studies [15], the incidence of ATC was higher in women than in men, which may be related to the biological characteristics of the cancer itself.
Moreover, the incidence rate of high-income people was higher than that of low-income people. This may be due to the high-income people paying more attention to their health and the popularization of routine physical examination. In addition, the proportion of M0 patients in the high-income patients (55.94%) was higher than that in the low-income patients (49.70%), which may indicate that the higher incidence in the high-income population could be due to early detection. Unfortunately, the long-term survival of ATC has not improved. According to the literature, there is no effective treatment for ATC. The survival prognosis may be affected by BMI (height and body mass index) [14,16], sex, age, T stage or M stage [17-19]. In contrast, we found that the year of diagnosis, sex and race seem to have no impact on overall survival.
Next, this analysis showed that the Kaplan-Meier survival curves for overall survival and disease-free survival overlapped. This indicates that ATC is a tumor with a high mortality rate, with most patients dying from it within a short period after diagnosis. According to the multivariate analysis, age, socioeconomic status, M stage, chemotherapy and surgical methods were independent prognostic factors. It is interesting that subgroup analysis showed that patients could benefit from radiotherapy, while multivariate analysis did not. In other series, the benefit of radiotherapy was primarily seen in patients with simultaneous adjuvant or neoadjuvant chemotherapy and surgery. Radiotherapy can provide a good impact on loco-regional control but does not increase the survival rate [11,20,21]. We also thought a selection bias that shunted earlier stage patients into the radiotherapy groups may have been introduced; only 32.41% of patients in the radiotherapy group were M1 stage, but 52.37% of patients in the no radiotherapy group were M1 stage. This bias has been corrected effectively by the multivariate analysis.
Total thyroidectomy was found to be better than partial thyroidectomy, lobectomy, subtotal thyroidectomy or subtotal thyroidectomy alone. Patients who underwent total thyroidectomy had a greatly reduced short-term relapse rate. This may be related to the fact that the most important factor related to mortality of ATC is the invasion of surrounding tissues and local compression. Most patients who were able to undergo total thyroidectomy had earlier stages and better timing of their surgery than those who could only undergo a partial thyroidectomy. With early diagnosis and proper surgical treatment, survival time can be prolonged, and local recurrence can be reduced [22].
The prognosis of patients undergoing lymph node dissection was not better than that of patients without lymph node dissection. We believe this is because patients who need lymph node dissection may have more clinically visible lymph node metastasis, and the staging is relatively backward. It is also possible that even with surgical treatment, removal of all metastatic nodes is difficult. For this reason, expanding the extent of surgery may not achieve better oncological outcomes; instead, it may increase operative trauma and affect recovery. Therefore, local control and systemic treatment may be more meaningful than extensive surgery.
Overall, the incidence and rate of mortality for ATC has been relatively stable, and oncological outcomes did not improve significantly in the past 30 years. A survival advantage was observed in younger, low poverty and M0 stage patients. Furthermore, active surgical treatment was beneficial for ATC patients, but the benefits of extensive surgery were limited.
Acknowledgements
We acknowledge the support received from the Guangdong Provincial Science and Technology Department Research Projects (grant No. 2017A010105029 and grant No. 2016A040403049). In addition, we want to thank, in particular, Dr. Gregory W. Randolph (MEEI) and Dr. Dipti Kamani (MEEI) for the support of the study design and paper revision.
Disclosure of conflict of interest
None.
References
- 1.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12:646–653. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris LG, Tuttle RM, Davies L. Changing trends in the incidence of thyroid cancer in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142:709–711. doi: 10.1001/jamaoto.2016.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388:2783–2795. doi: 10.1016/S0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 5.Besic N, Auersperg M, Us-Krasovec M, Golouh R, Frkovic-Grazio S, Vodnik A. Effect of primary treatment on survival in anaplastic thyroid carcinoma. Eur J Surg Oncol. 2001;27:260–264. doi: 10.1053/ejso.2000.1098. [DOI] [PubMed] [Google Scholar]
- 6.Sherman EJ, Lim SH, Ho AL, Ghossein RA, Fury MG, Shaha AR, Rivera M, Lin O, Wolden S, Lee NY, Pfister DG. Concurrent doxorubicin and radiotherapy for anaplastic thyroid cancer: a critical re-evaluation including uniform pathologic review. Radiother Oncol. 2011;101:425–430. doi: 10.1016/j.radonc.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal MS, Shah MH, Shaha AR, Tuttle RM American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 8.Arora S, Christos P, Pham A, Desai P, Wernicke AG, Nori D, Chao KS, Parashar B. Comparing outcomes in poorly-differentiated versus anaplastic thyroid cancers treated with radiation: a surveillance, epidemiology, and end results analysis. J Cancer Res Ther. 2014;10:526–530. doi: 10.4103/0973-1482.138207. [DOI] [PubMed] [Google Scholar]
- 9.Ezaki H, Ebihara S, Fujimoto Y, Iida F, Ito K, Kuma K, Izuo M, Makiuchi M, Oyamada H, Matoba N, et al. Analysis of thyroid carcinoma based on material registered in Japan during 1977-1986 with special reference to predominance of papillary type. Cancer. 1992;70:808–814. doi: 10.1002/1097-0142(19920815)70:4<808::aid-cncr2820700415>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Hanley JP, Jackson E, Morrissey LA, Rizzo DM, Sprague BL, Sarkar IN, Carr FE. Geospatial and temporal analysis of thyroid cancer incidence in a rural population. Thyroid. 2015;25:812–822. doi: 10.1089/thy.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini A, Torregrossa L, Basolo F, Vitti P, Elisei R. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. 2017;13:644–660. doi: 10.1038/nrendo.2017.76. [DOI] [PubMed] [Google Scholar]
- 12.Hsu KT, Yu XM, Audhya AW, Jaume JC, Lloyd RV, Miyamoto S, Prolla TA, Chen H. Novel approaches in anaplastic thyroid cancer therapy. Oncologist. 2014;19:1148–1155. doi: 10.1634/theoncologist.2014-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raue F, Frank-Raue K. Thyroid cancer: risk-stratified management and individualized therapy. Clin Cancer Res. 2016;22:5012–5021. doi: 10.1158/1078-0432.CCR-16-0484. [DOI] [PubMed] [Google Scholar]
- 14.Kitahara CM, McCullough ML, Franceschi S, Rinaldi S, Wolk A, Neta G, Olov Adami H, Anderson K, Andreotti G, Beane Freeman LE, Bernstein L, Buring JE, Clavel-Chapelon F, De Roo LA, Gao YT, Gaziano JM, Giles GG, Hakansson N, Horn-Ross PL, Kirsh VA, Linet MS, MacInnis RJ, Orsini N, Park Y, Patel AV, Purdue MP, Riboli E, Robien K, Rohan T, Sandler DP, Schairer C, Schneider AB, Sesso HD, Shu XO, Singh PN, van den Brandt PA, Ward E, Weiderpass E, White E, Xiang YB, Zeleniuch-Jacquotte A, Zheng W, Hartge P, Berrington de Gonzalez A. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid. 2016;26:306–318. doi: 10.1089/thy.2015.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukas J, Drabek J, Lukas D, Dusek L, Gatek J. The epidemiology of thyroid cancer in the Czech Republic in comparison with other countries. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:266–275. doi: 10.5507/bp.2012.086. [DOI] [PubMed] [Google Scholar]
- 16.Kapiteijn E, Schneider TC, Morreau H, Gelderblom H, Nortier JW, Smit JW. New treatment modalities in advanced thyroid cancer. Ann Oncol. 2012;23:10–18. doi: 10.1093/annonc/mdr117. [DOI] [PubMed] [Google Scholar]
- 17.Hannequin P, Liehn JC, Delisle MJ. Multifactorial analysis of survival in thyroid cancer. Pitfalls of applying the results of published studies to another population. Cancer. 1986;58:1749–1755. doi: 10.1002/1097-0142(19861015)58:8<1749::aid-cncr2820580828>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Paunovic IR, Sipetic SB, Zoric GV, Diklic AD, Savic DV, Marinkovic J, Zivaljevic VR. Survival and prognostic factors of anaplastic thyroid carcinoma. Acta Chir Belg. 2015;115:62–67. [PubMed] [Google Scholar]
- 19.Tan RK, Finley RK 3rd, Driscoll D, Bakamjian V, Hicks WL Jr, Shedd DP. Anaplastic carcinoma of the thyroid: a 24-year experience. Head Neck. 1995;17:41–47. doi: 10.1002/hed.2880170109. discussion 47-48. [DOI] [PubMed] [Google Scholar]
- 20.Huang NS, Shi X, Lei BW, Wei WJ, Lu ZW, Yu PC, Wang Y, Ji QH, Wang YL. An update of the appropriate treatment strategies in anaplastic thyroid cancer: a population-based study of 735 patients. Int J Endocrinol. 2019;2019:8428547. doi: 10.1155/2019/8428547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Tward JD, Shrieve DC, Hitchcock YJ. Surgery and radiotherapy improves survival in patients with anaplastic thyroid carcinoma: analysis of the surveillance, epidemiology, and end results 1983-2002. Am J Clin Oncol. 2008;31:460–464. doi: 10.1097/COC.0b013e31816a61f3. [DOI] [PubMed] [Google Scholar]
- 22.Sugino K, Ito K, Mimura T, Nagahama M, Fukunari N, Kubo A, Iwasaki H, Ito K. The important role of operations in the management of anaplastic thyroid carcinoma. Surgery. 2002;131:245–248. doi: 10.1067/msy.2002.119936. [DOI] [PubMed] [Google Scholar]