Abstract
Colorectal cancer (CRC) is one of the leading causes of cancer deaths worldwide, CRC was estimated to be the third most commonly diagnosed cancer and a leading cause of cancer deaths in developed countries due to therapy resistance and metastasis. Cancer stem cells (CSCs) were found in a variety of malignant tumors, including colorectal cancer. miR-27b play pivotal roles in the acquisition of CSC properties such as tumor initiation, drug resistance and asymmetric cell division. The aim of the present study was to investigate the underlying mechanisms that miR-27b inhibits proliferation, invasion and migration of CSCs. In present study, miR-27b were found to be significantly upregulated in CSCs. Overexpression of miR-27b inhibit CSCs proliferation and migration. In addition, overexpression of miR-27b suppress the character expression of CSCs, including of CD44, CD133, Sox2 and Oct4. Furthermore, it has been demonstrated that miR-27b is directly targeted by PIK3CA and miR-27b overexpression can effectively attenuate the expression of Phosphor-PI3K p110α and phosphor-Akt. In conclusion, these results reveal that PIK3CA is significantly downregulated by miR-27b expression in CSCs. Thus, we presume that miR-27b may be a therapeutic anti-tumor agent for CRC via targeting PI3K p110α.
Keywords: MiR-27b, PI3K p110α, colorectal cancer, proliferation, migration
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer deaths worldwide, associated with a high-fat, low-fiber diet [1]. Currently, CRC estimated to be the third most commonly diagnosed cancer and the third leading cause of cancer deaths in developed countries [2,3] due to therapy resistance and metastasis. More than half of patients suffered from colorectal cancer develop hepatic metastases. Unfortunately, Surgical resection of hepatic metastases remains the only curative treatment capable of providing prolonged survival. Furthermore, Five-year survival after resection of hepatic colorectal metastases is less than 44% [4,5]. Therefore, understanding the molecular mechanism underlying colorectal carcinogenesis is vital for diagnosis and treatment of CRC in clinically.
Cancer stem cell (CSCs), which are operationally defined as cells that are capable of tumor initiation and propagation and their presence are involved with a more aggressive tumor phenotype, show self-renewal and drug tolerance [6,7]. At present, many evidences confirmed that CSCs are found in a variety of malignant tumors, including colorectal cancer, breast cancer, prostate cancer, lung cancer, liver cancer, ovarian cancer [8,9]. CD133+ colon cancer cells were first reported it process the CSC-like phenotype in 2007 [10,11], and their existence suggests that therapeutic strategies, targeted the cancer stem cells, could be effective on colorectal cancer. CD44 has recently been recognized as another signature that promotes the maintenance of cell self-renewal for cancer stem cells. Nuclear CD44 are functionally critical to CSCs for sphere formation, and CD44 ligation eliminates the high tumor seeding ability [12]. Engaged CD44 is internalized and could elicit STAT3 acetylation, CD44/acetylated-STAT3 regulate the expression of downstream proteins included octamer-binding transcription factor 4 (Oct-4) and sex determining region Y-box 2 (Sox-2), whose expression are related to reprogramming of somatic cells to a stem cell phenotype [13].
MicroRNAs (miRNAs), a class of small, endogenous and noncoding RNA, are emerging as regulators of genes expression by targeting mRNA at the 3’-untranslated region (3’UTR) that leads to translational repression or mRNA degradation. In cancer, miRNAs perform radically different functions on cancer therapeutic strategies, as evidenced by miR-126-3p inhibiting the development of pancreatic cancer through the downregulation of ADAM9 [14], miR-7 inhibiting tumorigenesis and reversing metastasis through the phosphoinositoide-3 kinase (PI3K)/AKT pathway in hepatocellular carcinoma [15], miR155 promoting lymphoma progression via modulating PD-1/PD-L1-mediated interaction with CD8+ T cells of tumor microenvironment [16]. Growing studies demonstrated that miRNAs play pivotal roles in the acquisition of CSC properties such as tumor initiation, drug resistance and asymmetric cell division.
The phosphatidylinositol 3-kinases (PI3Ks) belong to a unique and conserved family of intracellular lipid kinases that phosphorylate the 3’-hydroxyl group of phosphatidylinositol and phosphoinositides [17]. Mammalian cells contain multiple isoforms of PI3Ks which are subdivided into three classes. Class IA PI3Ks are heterodimers comprised a p110α catalytic subunit and a regulatory adaptor subunit. Activating mutations in PIK3CA gene, encoding the p110α catalytic subunit of PI3K, have been reported in human cancers via PI3K/AKT signaling [18]. Interestingly, it was found that PIK3CA is the potential target genes of miR-27b by searching on the targetscan database. Thus, the aim of the present study was to investigate the underlying mechanisms that miR-27b inhibits proliferation, invasion and migration of CSCs.
Materials and methods
Culture of HT29 and tumor spheres
Human colorectal cell line (HT29) was purchased from the Pasteur Institute (Tehran, Iran). Cells were cultured in RPMI1640 (Gibco-Invitrogen, Karlsruhe, Germany) supplement by 10% fetal bovine serum (Gibco-Invitrogen) and 200 IU/mL penicillin and streptomycin (Sigma-Aldrich, St. Louis, MO, USA). HT29 cells were incubated with a humidified atmosphere at 37°C with 5% CO2. The cells’ medium was changed every 2 days. Tumor spheres were derived by placing HT29 cells grown as monolayers into a defined serum-free DMEM/F12 medium (SFM) on collagen type I coated plates (Col/SFM) supplemented with 20 μg/ml both EGF (Peprotech, Rocky Hill, NJ) and bFGF (Peprotech, Rocky Hill, NJ), 1 × B-27 supplement (Gibco, Grand Island, NY), BSA (0.4%), insulin (4 mg/L), and 200 IU/mL both penicillin and streptomycin. The morphological changes of HT29 cells and formation of spheroid in Col/SFM at each time point was observed with an inverted microscopy (Nikon TS100, Tokyo, Japan). Single-cell suspensions was added to each well of a 96-well plate after clonal assay sphere counting. To isolate cancer stem-like cells (CSLCs) with the ability to form spheroid-like structures, each well was examined for single cells, and only the wells that contained single cells were marked and analyzed for tumor spheres that contained at least 15 cells.
Transfection of miRNAs
The CSCs were transfected in six-well plates with either mimic or inhibitor miRNAs (100 pmol) for modulating miRNA expression levels. MiRNA mimics are synthetic double-stranded RNAs that act as functional equivalents to endogenous human miRNAs and causes an overexpression of a miRNA of interest. On the other hand, miRNA inhibitors are single-stranded, modified RNAs which specifically inhibit endogenous miRNA molecules and causes a down-regulation of miRNA activity. MiRNA-27b mimics and inhibitors (hsa-miRNA-27b mimic (#MH10750), hsa-miRNA-27b inhibitor (#MC10750)) were purchased from Ambion (Austin, TX). CyTM3-labeled pre-miRNA (#AM17120; Ambion) was used as a negative transfection control. The reactions were performed with the TransIT-X2 Dynamic Delivery System reagent (Mirus, Wisconsin, USA), following the manufacturer’s instructions.
Colony formation assay
To evaluate the ability of proliferative activity and tumorigenicity of HT29 stem cells, colony formation assay was performed in 6-well plates. 1 × 103 cells of HT29 cells and HT29 stem cells were seeded onto 6-well sterile plates. After adhesion, the cells were transfected with miR-27b mimic and miR-27b inhibitor for 72 h respectively, HT29 stem cells as control, stem cells transfected with miR-mimic-NC as negative control. After the treatment period media was aspirated, fresh media was added and incubated for 2 weeks at 37°C incubator supplied with 5% CO2, then cell colonies formed in the dish. 4% paraformaldehyde for fixation and 0.1% crystal violet solution was used to staining. Colonies whose diameters were larger than 0.5 mm were counted by a microscope (Nikon, Chiyoda, Japan) at the 400 × magnification.
Cell proliferation assay
Cell Counting Kit-8 assay (Dojindo Laboratories, Kumamoto, Japan) was carried out to detected cell viability. HT29 stem cells were seeded in 96-well plates (5 × 103 cells/well) and incubated at 37°C in a 5% CO2 humidified incubator. At the indicated time points (24, 48 and 72 h) after transfection, 10 μl of CCK-8 solution were separately added to each well. Following incubation at 37°C for 1 h, the absorbance was measured with a microplate reader at 450 nm.
Wound-healing assay
Wound-healing assay was performed to detect the migration ability of HT29 stem cells with slight modifications. Briefly, cells were seeded in 6-well plates for 24 h. The cells were transfected with miR-27b mimic and miR-27b inhibitor for 72 h respectively, HT29 stem cells as control, stem cells were transfected with miR-mimic-NC as negative control. The monolayers were then scratched vertically using 200 μl sterile pipette tip and the floating cells were washed off with PBS. Later, cells were photographed at the indicated time points (0, 6, 12 and 24 h) using Leica DMIL LED equipped with an Integrated 5.0 Mega-Pixel MC 170 HD camera (Wetzlar, Germany).
Western blotting
Cells were harvested and lysed in RIPA buffer supplied with protease/phosphatase inhibitor cocktail. Protein concentration was determined by the BCA assay (Pierce). Then the protein (25 μg) was separated by SDS-PAGE and transferred to membranes. Membranes were blocked for 1 h and then incubated overnight at 4°C with the appropriate antibodies. Rabbit anti-CD44 antibody (1:1000, cat. no. 37259S), Rabbit anti-CD133 antibody (1:1000, cat. no.64326S), rabbit anti-Sox2 antibody (1:1000, cat. no. 3579S), rabbit anti-Oct4 antibody (1:1000, cat. no. 2750S), rabbit anti-Akt antibodies (1:1000; cat. no. 9272S), rabbit anti-phospho-Akt antibodies (1:1000; cat. no. 4060S), rabbit anti-PI3K p110α antibody (1:1000, cat. no. 4255S) were purchased from Cell Signaling Technology (Danvers, USA), mouse anti-β-actin antibodies was from Millipore (Billerica, MA), After washing three times, the membranes were incubated with at 4°C overnight, and then incubated with HRP-conjugated goat anti-rabbit IgG (Millipore, Billerica, MA) or goat anti-mouse IgG (Millipore, Billerica, MA) for 2 h. The membranes were visualized using Tanon-5200 Chemiluminescence Imager (Tanon, Shanghai, China) with ECL western blotting substrate (Millipore, Billerica, MA).
Quantitative real-time PCR (RT-qPCR)
The cells were washed with ice-cold phosphate buffer, and total RNA was extracted using the Trizol agent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. Then, Reverse Transcription regents (TaKaRa, Otsu, Shiga, Japan) was used to synthesize cDNAs. Quantitative real-time PCR was measured by using a System 7500 instrument (Applied Biosystems, Carlsbad, CA, USA). The relative expression was calculated by using the 2-ΔΔCt method. RNA U6 was performed as the internal control for miRNA and GAPDH was used for mRNA expression. The primer sequences for qPCR were as follows (Table 1):
Table 1.
The primer sequences
| miR-27b | Forward | 5’-CGGCGGTTCACAGTGGCTAA-3’ |
| Reverse | 5’-GTGCAGGGTCCGAGGT-3’ | |
| CD44 | Forward | 5’-CCAATGCCTTTGATGGACCA-3’ |
| Reverse | 5’-TGTGAGTGTCCATCTGATTC-3’ | |
| CD133 | Forward | 5’AGTGGCATCGTGCAAACCTG-3’ |
| Reverse | 5’CTCCGAATCCATTCGACGATAGTA-3’ | |
| Sox2 | Forward | 5’GCTAGTCTCCAAGCGACGAA-3’ |
| Reverse | 5’GCAAGAAGCCTCTCCTTGAA-3’ | |
| Oct4 | Forward | 5’TCGAGAACCGAGTGAGAGG-3’ |
| Reverse | 5’GAACCACACTCGGACCACA-3’ | |
| GAPDH | Forward | 5’CTCACCGGATGCACCAATGTT-3’ |
| Reverse | 5’CGCGTTGCTCACAATGTTCAT-3’ |
Luciferase reporter assay
For the luciferase reporter assay, the 3’UTR of the PIK3CA cDNA containing wildtype (WT) and mutant (mut) miR-27b binding sites were amplified and cloned into the pGL3 vector (Promega, Madison, WI, USA) to construct reporter plasmids. The day before transfection, A375 cells were seeded in 24-well plates and cultured at 37°C. Subsequently, cells were transfected with wild-type (WT) or mutated (mut) PIK3CA reporter plasmids and miR-27b mimics (miR-27b) or negative control (miR-NC) using Lipofectamine 3000 (Invitrogen). After 48 h, the firefly luciferase activity was measured and normalized to that of Renilla using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Statistical analysis
Statistical data analysis was performed with SPSS 19.0 and GraphPad Prism 5.0. The group data were expressed as the means ± standard error (SEM). Differences among the means were analyzed using One-way ANOVA. For comparison of two groups, a student’s t-test was used. Differences at P < 0.05 were considered statistically significant.
Results
The identification of cancer stem cells
To determine whether the successful HT29 stem cells were isolated and cultured, the Colony formation assay, Western blotting and RT-qPCR were employed in the present study. The cells were photographed to observe the cultured cell morphology (Figure 1A). Numbers of surface markers can be detected for CSCs sorting, including CD24, CD44, and CD133 [19]. In the present study, we evaluated the expression levels of genes and proteins of CD44 and CD133. As shown in Figure 1B, CD44 and CD133 expression of stem cell group were significantly increased, as comparison with HT29 group. On the other hand, stem cells proliferation is higher than HT29 cells, further confirming that sphere-forming populations, HT29 stem cells, were successful enriched.
Figure 1.
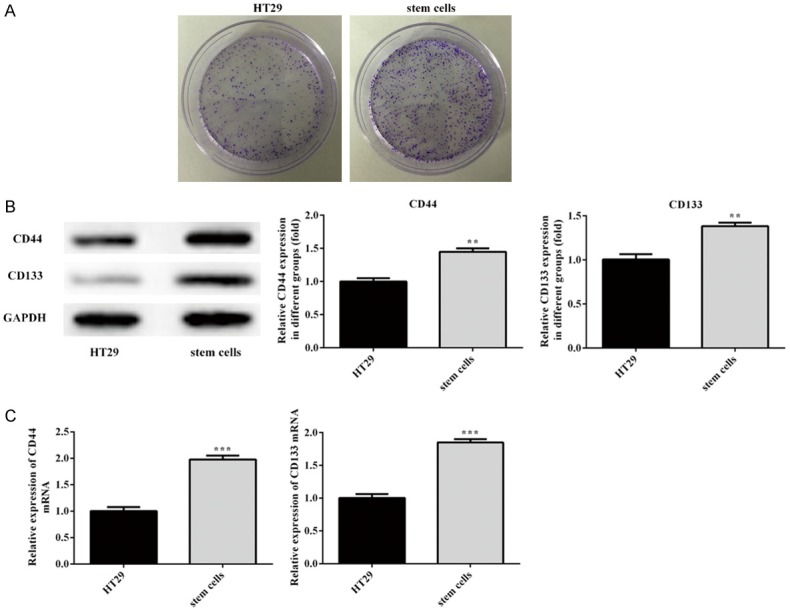
The identification of cancer stem cells. A. Colony formation assay was employed to identify HT29 stem cells. B, C. The expression of CDK2, P21, PCNA was analysed by Western blotting and RT-qPCR, respectively. Error bars represent the mean ± SEM from three independent experiments. **P < 0.01, ***P < 0.001 vs. HT29 cell.
Overexpression of miR-27b inhibit cancer stem cells proliferation
Both miR-27b mimic and miR-27b inhibitor stable CSCs was established to study the biological function of miR-27b by determining proliferation and colony formation in vitro. Stem cells were transfected miR-27b mimic and miR-27b inhibitor for 72 h, miR-mimic NC and miR-inhibitor NC as the negative control, respectively. Then the miR-27b expression level was detected by RT-qPCR (Figure 2A). Compared with the negative control, qPCR results confirmed miR-27b expression is elevated significantly in miR-27b mimic group, whereas miR-27b expression of miR-27b inhibitor is decreased. More importantly, after transfection with miR-27b mimic and miR-27b inhibitor for 24, 48, 72 h, we found that miR-27b could effective suppress CSCs proliferation in CCK8, while anti-miR-27b promoted cell proliferation (Figure 2B). Consistent with CCK8 results, colony formation assay results further demonstrated miR-27b inhibition on CSCs proliferation (Figure 2C).
Figure 2.
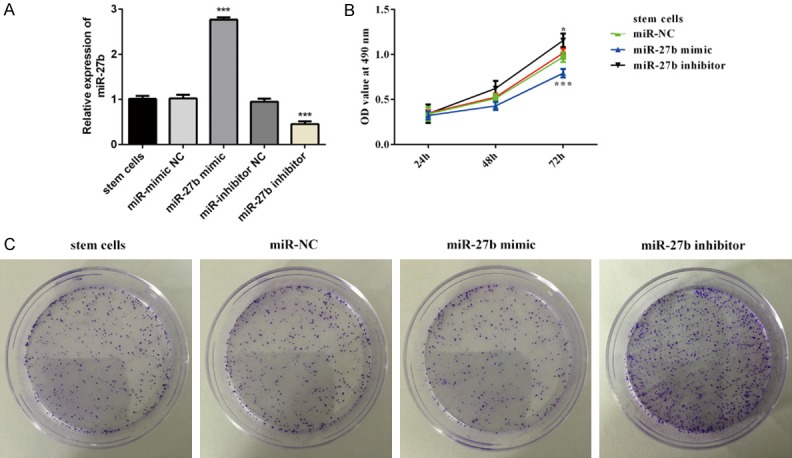
Overexpression of miR-27b inhibit cancer stem cells proliferation. Stem cells were transfected with miR-mimic NC, miR-27b mimics, miR-inhibitor NC and miR-27b inhibitor, respectively. A. The expression of miR-27b were evaluated by RT-qPCR. B. Cell Counting Kit-8 assay was used to detect viability of stem cells. C. Colony formation assay was performed to evaluate the ability of proliferative activity of HT29 stem cells. *P < 0.05, ***P < 0.001 vs. miR-NC.
Overexpression of miR-27b inhibit cancer stem cells migration
After stem cells were transfected miR-27b mimic and miR-27b inhibitor for 72 h, cell scratch test was employed to assess miR-27b impact on CSCs migration and photographed at 0 h, 12 h, 24 h. Compared with negative control, the rate of scar closure in miR-27b group was significantly reduced, whereas transfection with miR-27b inhibitor could significantly reverse the suppression (Figure 3A, 3B).
Figure 3.
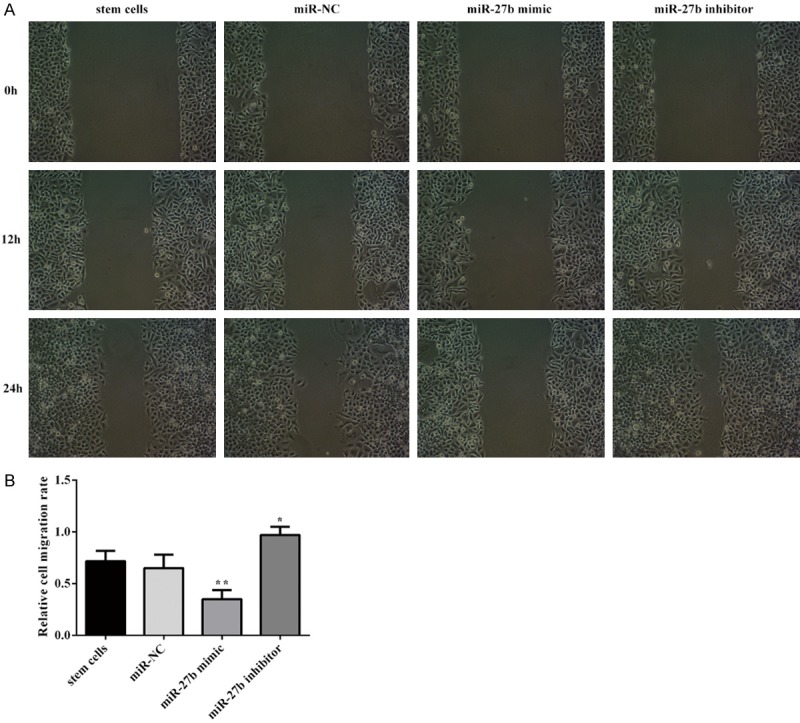
Overexpression of miR-27b inhibit cancer stem cells migraton. A, B. The effect of miR-27b on stem cell migration was determined via wound scratch assay. Error bars represent the mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01 vs. Control or miR-NC.
Overexpression of miR-27b suppress the expression of CD44, CD133, Sox2 and Oct4
Cluster of differentiation (CD)44 and CD133 is the primary character of CSCs, another feature of CSCs is overexpression of stem cell-associated genes, including Oct-4 and Sox-2 [20]. After stem cells were transfected miR-27b mimic and miR-27b inhibitor for 72 h, the expression levels of genes and proteins of CD44, CD133, Sox2 and Oct4 were measured by western blotting and RT-qPCR, as showed in Figure 4. Compared with negative control, expression of CD44, CD133, Sox2 and Oct4 in miR-27b group is decreased, which were all reversed by miR-27b inhibitor.
Figure 4.
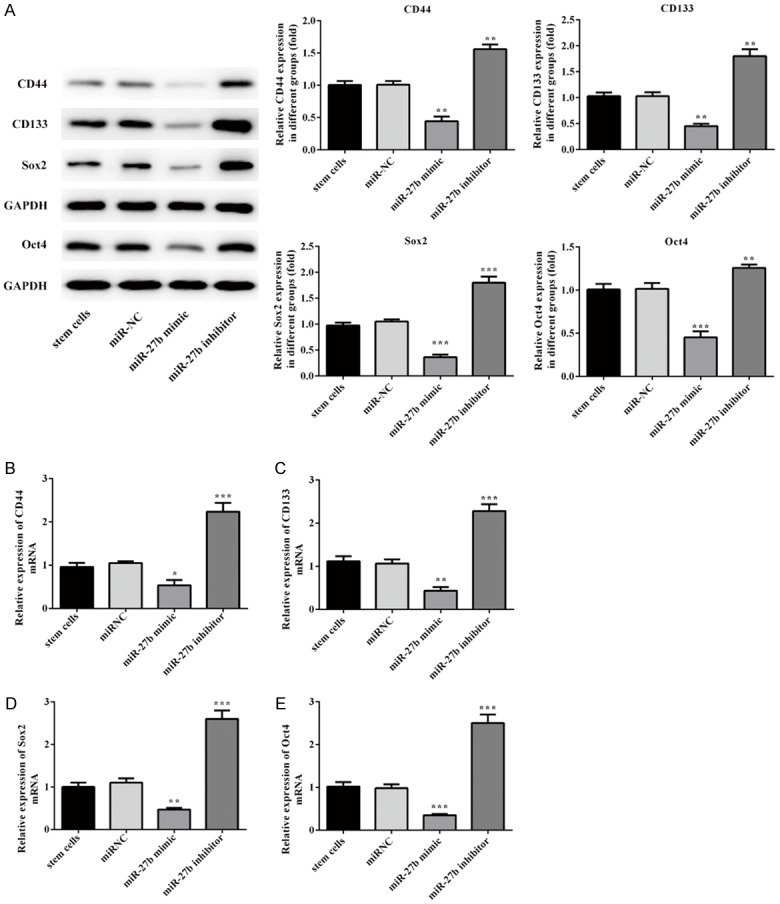
Overexpression of miR-27b suppress the expression of CD44, CD133, Sox2 and Oct4. The expression of CD44, CD133, Sox2 and Oct4 was analysed by Western blotting. Error bars represent the mean ± SEM from three independent experiments. **P < 0.05, **P < 0.01, ***P < 0.001 vs. Control or miR-NC.
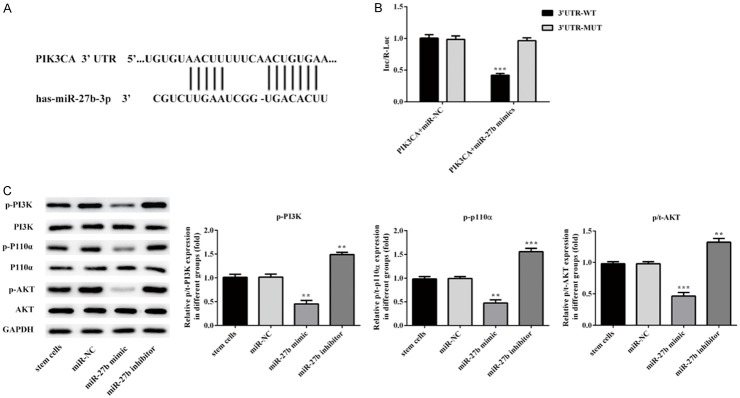
MiR-27b inhibit cancer stem cell proliferation and migration by binding PIK3CA
Luciferase reporter assay was used to investigate whether PIK3A is a direct target of miR-27b. The putative miR-27b binding sites was showed in Figure 5A. Compared to NC, the relative luciferase activity was significantly lower in A375 cells co-transfected with wild-type luciferase vector and miR-27b mimic (Figure 5B). These results suggest that miR-27b specifically binds the 3’UTR of the PIK3CA gene to inhibit its expression. To further demonstrated that miR-27b inhibit PIK3CA expression, PI3K p110α, AKT and phospho-AKT protein level were measured by western blot, phospho-PI3K p110α and phospho-AKT level were significantly lower in CSCs with transfected with miR-27b. taken together, the results reveals that miR-27b could negatively regulates phosphor-PI3K p110α expression (Figure 5C).
Figure 5.
MiR-27b inhibit cancer stem cell proliferation and migration by binding PIK3CA. PIK3CA is a target gene of miR-27b. A. 3’-UTR regions of PIK3CA mRNA is partially complementary to miR-27b. B. The relative luciferase activities in cells transfected with wild-type (WT) or mutated (mut) PIK3CA reporter plasmids and miR-27b mimics (miR-27b) or negative control (miR-NC). Firefly luciferase activity was normalized to Renilla luciferase. C. Western blotting results demonstrated transfection of miR-27b suppressed the phosphorylation of PI3K, p110α, AKT protein in stem cells. Error bars represent the mean ± SEM from three independent experiments. **P < 0.01, ***P < 0.001 vs. Control or miR-NC.
Discussion
Colorectal cancer is one of the highest fatal malignant tumors related to the existence of tumor stem cells (CSCs) closely. Because of limitation of traditional therapy, it is urgent to find the effective therapy for colorectal cancer. CSC, characterized by overexpression of CD44, CD133, Sox2 and Oct4, has features of self-renewal, high oncogenicity, differentiation potential and drug resistance [20]. MicroRNA, binding to the 3’-untranslated regions (3’-UTR) of their target mRNAs, regulate gene expression post-transcriptionally. MiR-27b is associated with a variety of biologic processes, including insulin resistance, type 2 diabetes and tumor suppressor. The present investigation reveals miR-27b can affect colorectal cancer stem cell by targeting PI3K p110α, which provide more knowledge for elucidating the metastasis mechanism of colorectal cancer and offer new direction for clinical treatment strategy. The CSCs were successful to isolated and enriched and produce consistent phenotypes as previous researches [21]. Cell proliferation rate of the cultured HT29 stem cells is greater than normal HT29 cells.
Increasing evidence has suggested that miRNAs have been highlighted because of their negatively regulate the expression of genes and play an important role in the processes of tumorigenesis and various physiological and pathological developments including cell proliferation, invasion and metastasis. Ye J et al. provide evidence demonstrating the anti-tumor effect of miR-27b in vitro and in vivo, suggesting miR-27b mediated gene silencing was potential therapeutic agent [22]. We sought to investigate the function and underlying mechanism of miR-27b in Colorectal cancer (CRC). We found that miR-27b mimics transfection dramatically repressed cell proliferation and migration, whereas miR-27b inhibitor promoted cell proliferation and migration. Consistent with our study, Li J et al. reported that overexpression of miR-27b remarkably inhibited proliferation and migration of retinal pigment epithelium cells through regulation of Nox2 and its downstream P13K/AKT/mTOR signaling [23]. In addition, Overexpression of miR-27b suppress the expression of CD44, CD133, Sox2 and Oct4 and demonstrated that it could significantly repress self-renewal in vitro.
To further understand the underlying mechanism of miR-27b suppression, we next investigated relations between miR-27b and phosphatidylinositol-3 kinase/Protein kinase B (PI3K/AKT) signaling. Aberrant activation of PI3K/AKT signaling pathway, involved in cell growth and migration, is associated with tumorigenesis. Recent study indicated that miR-107 was identified as a tumor inhibitor that suppress the cell proliferation and metastasis of Gastric cancer via inhibiting the PI3K/AKT signaling pathway [24]. Yan T et al. reported that miR-328-3p inactivated PI3K/AKT pathway to suppressed cell proliferation, migration and invasion in bladder cancer through targeting integrin α5 [25]. Moreover, Ralitsa R Madsen et al. demonstrated that cancer cells benefit from additional PI3K pathway activation, and the marked allele dose-dependent effects of PIK3CA, which encodes the p110α catalytic subunit of PI3K, may have implications for understanding of PI3K-associated cancers [26]. It was found that PIK3CA is the potential target genes of miR-27b by searching on the targetscan database. It is well known that microRNAs always play their roles by binding to the 3’-untranslated regions (UTR) and repressing the expression of target mRNAs [27]. Our study found wild type miR-27b mimic strikingly reduced PIK3CA activity, and western blot results reveals miR-27b mimic also suppressed the PI3K p110α protein and its downstream phosphor-AKT expression, while miR-27b inhibitor could abolish the suppression of miR-27b mimics. Consistent with our data, Chen D et al. uncovered miR-27b enhanced the antitumor effect of paclitaxel by directly targeting CBLB and GRB2 genes to downregulating both PI3K/Akt and MAPK/Erk signaling pathways [28]. Thus, all data suggested that miR-27b overexpression played suppressive role on CSCs proliferation.
Taken together, our results demonstrated that miR-27b inhibits proliferation and migration by directly targeting and suppressing PI3K p110α in CSCs. Silencing PIK3CA may be a novel therapeutic strategy in the treatment of CRC. Thus, we presume that miR-27b may be a therapeutic anti-tumor agent for CRC via targeting PI3K p110α.
Acknowledgements
This work was supported by Medical Health Technology Project of Zhejiang Province, China (grant number: 2018ZD015).
Disclosure of conflict of interest
None.
References
- 1.Choi IS, Choi EY, Jin JY, Park HR, Choi JI, Kim SJ. Kaempferol inhibits P. intermedia lipopolysaccharide-induced production of nitric oxide through translational regulation in murine macrophages: critical role of heme oxygenase-1-mediated ROS reduction. J Periodontol. 2013;84:545–555. doi: 10.1902/jop.2012.120180. [DOI] [PubMed] [Google Scholar]
- 2.Khelwatty SA, Essapen S, Bagwan I, Green M, Seddon AM, Modjtahedi H. Co-expression and prognostic significance of putative CSC markers CD44, CD133, wild-type EGFR and EGFRvIII in metastatic colorectal cancer. Oncotarget. 2019;10:1704–1715. doi: 10.18632/oncotarget.26722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Pessaux P, Chenard MP, Bachellier P, Jaeck D. Consequences of chemotherapy on resection of colorectal liver metastases. J Visc Surg. 2010;147:e193–201. doi: 10.1016/j.jviscsurg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 6.Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. J. Clin. Oncol. 2008;26:2795–2799. doi: 10.1200/JCO.2008.17.7436. [DOI] [PubMed] [Google Scholar]
- 7.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 11.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 12.Su YJ, Lai HM, Chang YW, Chen GY, Lee JL. Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 2011;30:3186–3199. doi: 10.1038/emboj.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JL, Wang MJ, Chen JY. Acetylation and activation of STAT3 mediated by nuclear translocation of CD44. J Cell Biol. 2009;185:949–957. doi: 10.1083/jcb.200812060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu DM, Wen X, Han XR, Wang S, Wang YJ, Shen M, Fan SH, Zhang ZF, Shan Q, Li MQ, Hu B, Lu J, Chen GQ, Zheng YL. Bone marrow mesenchymal stem cell-derived exosomal microRNA-126-3p inhibits pancreatic cancer development by targeting ADAM9. Mol Ther Nucleic Acids. 2019;16:229–245. doi: 10.1016/j.omtn.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Fang Y, Xue JL, Shen Q, Chen J, Tian L. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55:1852–1862. doi: 10.1002/hep.25576. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Z, Sun R, Zhao HJ, Fu D, Zhong HJ, Weng XQ, Qu B, Zhao Y, Wang L, Zhao WL. MiR155 sensitized B-lymphoma cells to anti-PD-L1 antibody via PD-1/PD-L1-mediated lymphoma cell interaction with CD8+ T cells. Mol Cancer. 2019;18:54. doi: 10.1186/s12943-019-0977-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikenoue T, Kanai F, Hikiba Y, Obata T, Tanaka Y, Imamura J, Ohta M, Jazag A, Guleng B, Tateishi K, Asaoka Y, Matsumura M, Kawabe T, Omata M. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 2005;65:4562–4567. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 18.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 20.Lin H, Wang B, Yu J, Wang J, Li Q, Cao B. Protein arginine methyltransferase 8 gene enhances the colon cancer stem cell (CSC) function by upregulating the pluripotency transcription factor. J Cancer. 2018;9:1394–1402. doi: 10.7150/jca.23835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira DM, Gomes SE, Borralho PM, Rodrigues CMP. MEK5/ERK5 activation regulates colon cancer stem-like cell properties. Cell Death Discov. 2019;5:68. doi: 10.1038/s41420-019-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z, Chen Z, Qiu F, Xu J, Huang J. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis in colorectal cancer. PLoS One. 2013;8:e60687. doi: 10.1371/journal.pone.0060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Hui L, Kang Q, Li R. Down-regulation of microRNA-27b promotes retinal pigment epithelial cell proliferation and migration by targeting Nox2. Pathol Res Pract. 2018;214:925–933. doi: 10.1016/j.prp.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Li K, Wang C, Shi X, Yang H. miR-107 regulates growth and metastasis of gastric cancer cells via activation of the PI3K-AKT signaling pathway by down-regulating FAT4. Cancer Med. 2019;8:5264–5273. doi: 10.1002/cam4.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan T, Ye XX. MicroRNA-328-3p inhibits the tumorigenesis of bladder cancer through targeting ITGA5 and inactivating PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2019;23:5139–5148. doi: 10.26355/eurrev_201906_18178. [DOI] [PubMed] [Google Scholar]
- 26.Madsen RR, Knox RG, Pearce W, Lopez S, Mahler-Araujo B, McGranahan N, Vanhaesebroeck B, Semple RK. Oncogenic PIK3CA promotes cellular stemness in an allele dose-dependent manner. Proc Natl Acad Sci U S A. 2019;116:8380–8389. doi: 10.1073/pnas.1821093116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu LP, Jin S, Xu RC, Zhang J, Geng YC, Shao XY, Qin LB. Long non-coding RNA PCAT-1 promotes tumor progression by inhibiting miR-129-5p in human ovarian cancer. Arch Med Sci. 2019;15:513–521. doi: 10.5114/aoms.2018.75534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen D, Si W, Shen J, Du C, Lou W, Bao C, Zheng H, Pan J, Zhong G, Xu L, Fu P, Fan W. miR-27b-3p inhibits proliferation and potentially reverses multi-chemoresistance by targeting CBLB/GRB2 in breast cancer cells. Cell Death Dis. 2018;9:188. doi: 10.1038/s41419-017-0211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]