Abstract
Because radiotherapy (RT) can induce diaphragm dysfunction, this study investigated the protective effect of inspiratory muscle training (IMT) on RT-induced diaphragm damage in patients with esophageal cancer during concurrent chemoradiotherapy (CCRT) in a preclinical setting, and an animal model was designed to confirm and explore the underlying mechanism. Six subjects who underwent CCRT were randomly enrolled in the control or concurrent-IMT group (n=3 per group). The training intensity was set to 30% maximal effort. The diaphragmatic function and functional exercise capacity were assessed weekly during the course of CCRT. Furthermore, Sprague-Dawley (SD) rats were randomly assigned to receive IMT using the tracheal banding method over a 1-week period (n=6) or the sham group (n=6). After training was completed, 5-Gy RT was applied to the diaphragm. All the rats were sacrificed 24 h following RT, and their diaphragms were removed and examined for contractile function, antioxidant capacity, and oxidative injury. In patients receiving IMT, the diaphragm activation efficiency and fatigability and the functional exercise capacity were improved during the CCRT course. The animals belonging to the training group demonstrated significantly higher peak twitch (P<0.01) and tetanus tension (P<0.001), less fatigue (P=0.04), lower protein carbonyl levels (P<0.01) and higher Cu/Zn-SOD and Mn-SOD mRNA expression levels (both P<0.05) compared with those belonging to the control group. Preclinical human and animal models show that the IMT-conditioned diaphragm exhibits better resistance to off-target irradiation damage, but studies with a larger patient sample size are warranted to confirm the applicability of this concept in clinical practice.
Keywords: Inspiratory muscle training (IMT), diaphragm, antioxidant, irradiation, contractile function
Introduction
Radiation therapy (RT) is a common treatment for patients with cancer. During this treatment, normal tissues surrounding the targeted treatment area are exposed to low but significant doses of radiation, which increases the risk for the development of normal tissue toxicity [1]. Irradiation not only damages deoxyribonucleic acid (DNA) directly but also increases oxidative stress, which can result in oxidative damage to proteins, lipids, and DNA [2]. Skeletal muscle is generally considered resistant to radiation due to its postmitotic state [3]. However, Caiozzo and colleagues showed that a radiation dose of 5 Gy can cause significant damage to myogenic stem cells in skeletal muscle [4]. Recently, Hsieh et al. showed that off-target low-dose irradiation can induce acute contractile dysfunction of the diaphragm in a rodent model [5].
The diaphragm is the primary muscle of the inspiratory pump and accounts for 70% of alveolar ventilation [6], and impaired diaphragm function can thus lead to severe ventilatory compromise. Previous studies have shown that the preoperative respiratory muscle strength among patients with cancer is an important indicator for predicting the prevalence of postoperative pulmonary complications [7]. Inspiratory muscle training can increase the pressure-generating capacity and improve the fatigue-resistance capacity of the inspiratory pump [8]. Tracheal banding has been used in animal models to evaluate the effect of resistive training on diaphragm function, and the results have shown that this training significantly increases the percentage of type I fibers [9]. Additionally, tracheal banding training could resemble the IMT training used for patients in the preoperative phase [10,11].
Anticancer therapy is often associated with unique and varying degrees of adverse effects on normal tissue function. Previous studies have shown that the forced expiratory volume in 1 s (FEV1) and the diffusion capacity of the lung for carbon monoxide (Dlco) decreased significantly after preoperative CCRT in patients with esophageal cancer [12,13]. Two previous studies have shown that IMT at the preoperative phase could increase the preoperative respiratory muscle strength of patients undergoing esophageal resection, but the effect on reducing postoperative pulmonary complications remains inconclusive [14,15]. The adverse effect of anticancer therapy on respiratory function might occur simultaneously during therapy, and thus, to examine the protective effect of IMT, the timing of the training is of critical importance. Both previous studies have started IMT at the recovering phase after CCRT, and whether starting IMT simultaneously with CCRT would better preserve or improve diaphragm function and thereby prevent the imbalance between ventilator demand and ventilatory capacity, which would result in a reduction in postoperative pulmonary complications, remained to be determined.
The purpose of the present study was to examine whether IMT concurrent with CCRT for patients with esophageal cancer could reduce RT-induced diaphragm contractile dysfunction. Additionally, the effect of IMT training on the contractile function of the diaphragm after RT challenge was further tested in an animal model.
Materials and methods
Patient characteristics
Six patients with esophageal cancer who underwent CCRT at a single medical center from March 2015 to May 2016 were enrolled. None of the patients had a history of disease recurrence or had previously received radiotherapy with or without concurrent chemotherapy. All the patients provided written informed consent before participating in the study (trial registration: NCT03099629). A flow diagram summarizing the processes used in the present study is shown in Figure 1.
Figure 1.
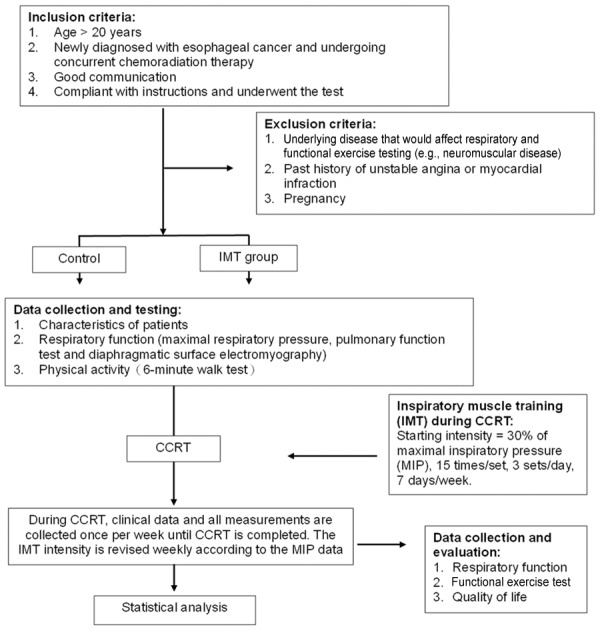
Flowchart of the patient selection process in this study. Abbreviations: CCRT, concurrent chemoradiation therapy; IMT, inspiratory muscle training; MIP, maximal inspiratory pressure.
Inspiratory muscle function
Maximum static inspiratory (MIP) and pulmonary function tests were performed to assess the global inspiratory function according to the recommendations from the American Thoracic Society [16-18]. The MIP was measured using a manometer (Inspiratory Force Meter, Model 4103; Boehringer, Norristown, PA, USA). Spirometry was performed using a MicroLab® spirometer (CareFusion, Basingstoke, UK), and the FEV1 was determined. The normal predicted values were derived using the equations developed by Kundson [19].
Functional exercise capacity
The functional exercise capacity was assessed through a 6-min walk test (6MWT), which was conducted using a standardized protocol [16]. The distance covered (6MWD) was expressed in absolute and percentage predicted values. The percent predicted 6MWD was calculated based on the reference equations developed by Troosters et al. [17].
Surface electromyography (EMG) recordings for the diaphragm
The surface EMG signal of the diaphragm was detected using a pair of Ag/AgCl electrodes (Kendall™ 100 Foam Electrodes, Conductive Adhesive Hydrogel 31118733, Mansfield, MA, USA), which were placed at an inter-electrode distance of 2 cm in the 7th or 8th intercostal spaces on the right side of the body between the mid-clavicular line and the anterior axillary line (Figure 2) [18]. The EMG signals from the modular amplifiers were recorded at a sampling frequency of 1000 Hz by a data acquisition system (MP150, Biopac Systems), filtered through a bandpass from 10 to 500 Hz, displayed and stored in a computer for future analysis. MATLAB (v. R2010a, Natick, MA, USA) software was used for all analyses of the EMG amplitude and frequency characteristics.
Figure 2.
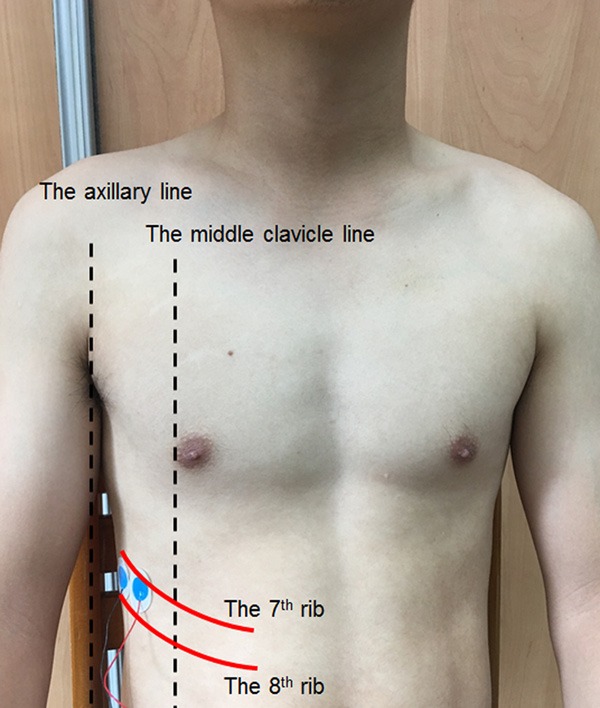
The surface EMG signal of the diaphragm was detected using a pair of Ag/AgCl electrodes (Kendall™ 100 Foam Electrodes, Conductive Adhesive Hydrogel 31118733, Mansfield, MA, USA), which were placed at an inter-electrode distance of 2 cm in the 7th or 8th intercostal spaces on the right side of the body between the mid-clavicular line and the anterior axillary line.
The patients were instructed to breathe from their residual volume to their total lung capacity in order to assess their maximal voluntary muscle activation. The data were analyzed off-line. The EMG signal was analyzed in the time domain as the root mean square (RMS) amplitude with a time constant of 25 ms. Computer-aided analysis was performed over a 1.5-s window initiated at the point of peak pressure during the maximal inspiratory effort (EMGmax). The diaphragm activation (expressed as %EMGmax) was calculated using the mean RMS values from 10 IMT breaths and normalized to the EMGMIP [18]. During window periods within each diaphragm EMG burst while performing IMT, the power spectral density (PSD) of the EMG signal was calculated using fast Fourier transform, and the median frequency (fm) was computed [20].
Animals and sample preparation
A total of 12 Sprague-Dawley (SD) rats weighing between 250 and 350 g were utilized in this study (Bio-LASCO Taiwan Co., Taiwan). The animals were maintained in an environmentally controlled room (25±1°C; 12-h light/dark cycle) at the Laboratory Animal Center at Mackay Memorial Hospital (Taipei, Taiwan) and were fed standard rat chow and water ad libitum. The study was approved by the Institutional Animal Care and Use Committee at Mackay Memorial Hospital, Taiwan (MMH-A-S-102-02), and was performed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Experimental protocol
The rats were randomly assigned to the tracheal banding training group or the sham training group. All the rats were first administered an injection of atropine (0.1 mg/kg) to reduce bronchospasm due to tracheal manipulation and were then anesthetized with Zoletil 50 (20 mg/kg BW) and Rompun (5 mg/kg BW). Tracheal banding was then performed using sterile techniques as previously described [9]. A band (a 3-mm-long, 2.5-mm-ID piece of plastic tubing) was secured with a 4-0 silk suture around the trachea, and the incision wound was closed. The wound in the rats belonging to the control group was closed immediately after the midline ventral cervical incision was made and the trachea was exposed.
After surgical management, the rats received a single radiation dose of 5 Gy to the hemi-diaphragm [5]. The rats in both groups were sacrificed 24 h after irradiation (or sham irradiation), and the diaphragms were removed en bloc with their rib cage origin intact as previously described [5]. Diaphragm strips measuring approximately 2 mm in width were dissected from the anterolateral portion of the diaphragm parallel to the long axis of the muscle fibers, and the attachments to the central tendon and rib cage were left intact. The remaining diaphragms were fixed in formalin for subsequent immunohistochemical analysis or frozen in liquid nitrogen and stored at -80°C for subsequent biochemical analysis.
Irradiation field
The rats were anesthetized and immobilized on a board before undergoing computed tomography. The field was applied according to a previous study design [5]. Briefly, considering the respiratory motion, the craniocaudal margin of irradiation was set to 1.5 cm above and below the dome of the diaphragm. The width of the irradiation field was opened to the right and left thoracic cage with a 5-mm bilateral expansion. Radiation was delivered using a conventional radiotherapy technique to the anterior-posterior (AP) and posterior-anterior (PA) fields, and 6-MV X-ray beams were delivered at 600 MU/min for a total dose of 5 Gy using a Varian 600CD linear accelerator (Varian Medical Systems, Palo Alto, CA, USA).
Assessment of diaphragm strip contractility
The contractile function of the diaphragm was assessed as previously reported [21]. Briefly, intact diaphragm strips were dissected from the left costal diaphragm and mounted vertically in water-jacketed organ baths (37°C, bubble 95% O2/5% CO2) containing Tyrode solution (137 mM NaCl, 4 mM KCl, 0.5 mM MgCl2, 0.5 mM NaH2PO4, 11.9 mM NaHCO3, 5.6 mM glucose, and 2.7 mM CaCl2). The rib end of the strips was attached to the bottom of the bath using silk ties, and the central tendon end was tied to a force transducer (XDFT200, Diagnostic & Research Instruments Co., Taiwan). Platinum field electrodes were placed around the strips and connected to a Grass S88 stimulator (Grass Technologies, Warwick, RI, USA). All the data were recorded and analyzed using an XctionView II Data Acquisition System recorder (Diagnostic & Research Instruments Co., Taiwan).
The twitch tension (Pt) was obtained using 1-ms supramaximal square wave pulses, and the tetanic tension (Po) was obtained by applying a train of supramaximal stimuli for 400 ms at optimal length. A force-frequency curve was constructed by stimulating the strips with trains of supramaximal stimuli at 1, 15, 30, 50, 80, 100 and 120 Hz with a 1-min rest period between adjacent stimulus trains. The fatigue characteristics were subsequently measured by giving the muscle a series of 300-ms tetanic stimulations every 3 s at a frequency that was adjusted to produce 50% of Po for 10 min.
Measurement of protein carbonyls
Several methods have been developed to evaluate the expression of protein carbonyl groups, and these include commercial enzyme-linked immunoassay (ELISA) and mass spectrophotometric and western blot methods [22]. Augustyniak compared these methods and found that western blotting is less quantitative than the other methods, the LC-MS/MS method does not provide quantitative information but does identify carbonylated proteins, and ELISA has a greater degree of robustness in the determination of protein carbonyl groups [22]. As mentioned above, the concentration of protein carbonyl groups in the diaphragm was assessed using ELISA and a protein carbonyl content assay kit (Abcam®, Cambridge, UK) according the manufacturer’s instructions. The absorbance was determined at 375 nm, and the results are expressed as nmol carbonyl per mg protein.
Primer design for quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) and sequence analysis
Oligonucleotide primers specific for the hamster (Mesocricetus auratus) Cu/Zn-SOD, Mn-SOD, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, used as an endogenous control) mRNA sequences were designed based on sequences in GenBank [23]. The primer sequences are as follows: Cu/Zn-SOD, forward: (5’-AGGACCTCATTTTAATCCTCACTCT-3’), reverse (5’-TTGTACTTTCTTCATTTCCACCTTT-3’); Mn-SOD, forward (5’-CCGAGGAGAAGTACCACGAG-3’), reverse (5’-GCTTGATAGCCTCCAGCAAC-3’); and GAPDH, forward (5’-AGAAGACTGTGGATGGCCCC-3’), reverse (5’-TGACCTTGCCCACAGCCTT-3’). The PCR products were confirmed after cloning into in-house constructed T-vectors and sequencing using the respective Cy5-labeled gene-specific primers (Applied Biosystems, Foster City, CA, USA) with the MegaBACE™ 1000 DNA Analysis System (Pharmacia, Piscataway, NJ, USA). The DNA sequences were assembled and analyzed using BioEdit software (http://www.mbio.ncsu.edu/BioEdit). The BLAST network service was used to search the nucleotide and protein database maintained by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov./BLASTn or BLASTX).
Preparation of RNA from the diaphragms of rats with and without tracheal banding
Total RNA was isolated from the diaphragms of SD rats with and without tracheal banding training using a commercial kit in accordance with the manufacturer’s recommended protocol [23]. Approximately 150 mg of diaphragm was rapidly dissected and dipped into TRIzol (Invitrogen, Carlsbad, CA, USA). Total RNA was treated with 5 units of DNase (Promega, Madison, WI, USA) and 119 units of ribonuclease inhibitor (Promega) in a buffer containing 400 mM Tris-HCl, 100 mM NaCl, 60 mM MgCl2, and 20 mM dithiothreitol at pH 7.5. Total RNA was extracted with phenol/chloroform, precipitated with ethanol, and dissolved in RNase-free water. Total RNA (3 μg) was reverse-transcribed into cDNA using Oligo (dT) 15 primers (Promega) following the suggested protocol for transcription by Moloney murine leukemia virus reverse transcriptase (Promega). The resulting cDNA was used for real-time RT-PCR analysis.
SYBR green real-time RT-PCR analysis
Real-time RT-PCR for Cu/Zn-SOD, Mn-SOD, and endogenous control GAPDH mRNA expression was performed using a SYBR green assay. The PCR cycling conditions were as follows: 95°C for 10 min followed by 40 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min and 72°C for 10 min. During each cycle, the accumulated PCR products were detected by monitoring the increase in fluorescence of the reporter dye obtained from the binding of dsDNA to SYBR green. All the data were analyzed using Rotor Gene 5 software (Corbett Research, Sydney, New South Wales, Australia). Validation experiments were performed in triplicate. The relative expression levels of Cu/Zn-SOD, Mn-SOD, and GAPDH mRNA were calculated using the comparative cycle threshold method, as described previously [24]. The values for Cu/Zn-SOD and Mn-SOD were normalized to the levels of the GAPDH gene.
Data and statistical analyses
Statistical analyses were performed using SPSS version 17.0 (IBM Corporation, Armonk, NY, USA). The results are presented as the means ± standard errors (SEs) of the means. Differences between continuous variables (i.e., specific twitch and tetanic tension, TPT, ½RT, fatigue index and level of protein carbonyl groups) were tested using Student’s t test. The generalized estimating equation (GEE) regression model was used with an exchangeable correlation matrix to consider the repeated measurements of tension during these tests. A p value of <0.05 was considered to indicate statistical significance.
Results
Concurrent IMT for patients with esophageal cancer undergoing CCRT preserves diaphragm function
The clinical characteristics of the patient population are shown in Table 1. The mean ages of the patients in the control and training groups were 63.3 and 64.8 years, respectively. The patients in both groups were all male, and their histological type was squamous cell carcinoma. At the conclusion of CCRT, the MIP of the patients in the training group had increased 32% from baseline, whereas almost no change was observed in the control group (Figure 3A). The FEV1 decreased in both groups after 1 week of CCRT and slowly recovered during the rest of the CCRT course in the training group but not the control group (Figure 3B). At the end of the CCRT course, the diaphragm activation (%EMGmax) of the control patients increased from 61% at baseline to 71%, whereas the training group exhibited a 19% decrease (from 73% down to 54%) (Figure 4A). The fm decreased in both groups after 1 week of CCRT and slowly recovered during the rest of the CCRT course in the training group but continued to decrease in the control group (Figure 4B). The distance covered in the 6MWT decreased from 405 m at baseline to 363 m (-42 m) and increased from 385 m to 457 m (+72 m) at the conclusion of CCRT in the control and training groups, respectively (Table 2). The above-mentioned data were tested using the GEE regression model.
Table 1.
Baseline characteristics of the patients
| Control n=3 | Training n=3 | |
|---|---|---|
| Gender | ||
| Male | 3 (100) | 3 (100) |
| Female | 0 (0) | 0 (0) |
| Age (mean ± SD) | 57.7±9.5 | 48.7±5.7 |
| Body weight (mean ± SD) | 63.3±11.2 | 64.8±7.4 |
| Body mass index (kg/m2) | 21.9±4.3 | 23.2±2.9 |
| Tumor histology (%) | ||
| Adenocarcinoma | 0 (0) | 0 (0) |
| Squamous cell carcinoma | 3 (100.0) | 3 (100.0) |
| Clinical stage (%) | ||
| IB | 0 (0) | 0 (0) |
| IIB | 1 (33.3) | 0 (0) |
| IIIA | 0 (0) | 0 (0) |
| IIIB | 1 (33.3) | 1 (33.3) |
| IIIC | 0 (0) | 2 (66.7) |
| IV | 1 (33.3) | 0 (0) |
| Smoking history | ||
| Current or former smoker | 3 (100.0) | 3 (100.0) |
| Never smoked | 0 (0) | 0 (0) |
Figure 3.
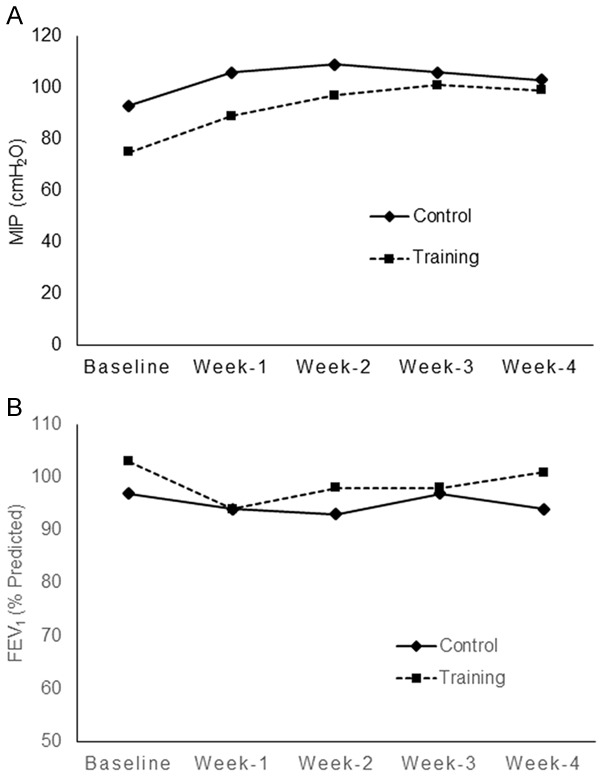
Maximum static inspiratory (MIP) and pulmonary function tests were performed to assess the global inspiratory function in accordance to the recommendations provided by the American Thoracic Society. The MIP was measured using a manometer (Inspiratory Force Meter, Model 4103; Boehringer, Norristown, PA, USA). Spirometry was performed using a MicroLab® spirometer (CareFusion, Basingstoke, UK), and the forced expiratory volume in 1 s (FEV1) was determined. Comparison of the (A) MIP and (B) FEV1 at baseline and weekly over the course of concurrent chemoradiation therapy (CCRT) in the control (-♦-) and training groups (--■--).
Figure 4.
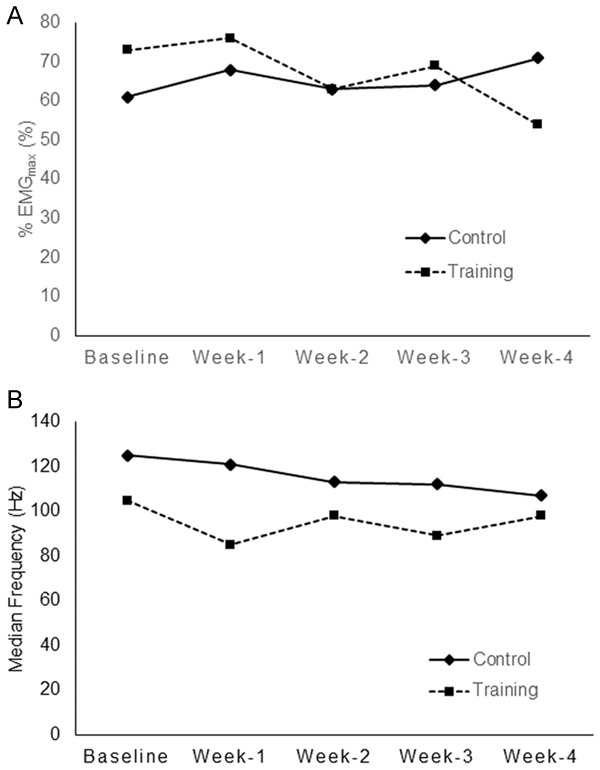
Patients were instructed to breathe from their residual volume to their total lung capacity for assessment of their maximal voluntary muscle activation. The surface EMG signal of the diaphragm was detected using a pair of Ag/AgCl electrodes (Kendall™ 100 Foam Electrodes, Conductive Adhesive Hydrogel 31118733, Mansfield, MA, USA). Comparison of (A) diaphragm activation based on the %EMGmax and (B) fm during inspiratory muscle training (IMT) breaths at baseline and weekly over the course of concurrent chemoradiation therapy (CCRT) in the control (-♦-) and training groups (--■--).
Table 2.
Results from the 6-min walking test for the control and training groups
| Control group (n=3) | Training group (n=3) | |
|---|---|---|
| Six-minute walk distance (6MWD, m) | ||
| Baseline (pre-CCRT) | 405.0±17.7 | 385.3±36.1 |
| First week | 378.0±24.7 | 384.1±76.9 |
| Second week | 417.5±15.2 | 390.3±45.4 |
| Third week | 388.0±5.7 | 449.9±21.6 |
| After CCRT | 363.0±0.0 | 457.4±25.4 |
The data are expressed as the means ± SDs (n=3).
Resistive breathing training overcomes the damage to the diaphragm caused by low-dose irradiation
The animals in the training group weighed an average of 296.7±3.3 g and 318.3±8.3 g at baseline and after 1 week of tracheal banding training, respectively, and the animals in the control group weighed an average of 295±5 g and 332.5±6.3 g at baseline and after one week of no training, respectively. The groups showed o significant differences in body weight at either time point, as demonstrated using Student’s t test.
The effects of low-dose radiation on diaphragm contractile properties are shown in Figure 5A. After 5-Gy single-dose irradiation, the peak twitch (training: 8.7±0.8 N/cm2 vs. control: 6.7±0.7, P<0.01) and tetanus tension (training: 38.5±3.7 N/cm2 vs. control: 24.7±3.4, P<0.001) were significantly higher in the training group than in the control group, as demonstrated using Student’s t test. The Pt/Po ratios of the two groups were similar (P=0.3).
Figure 5.
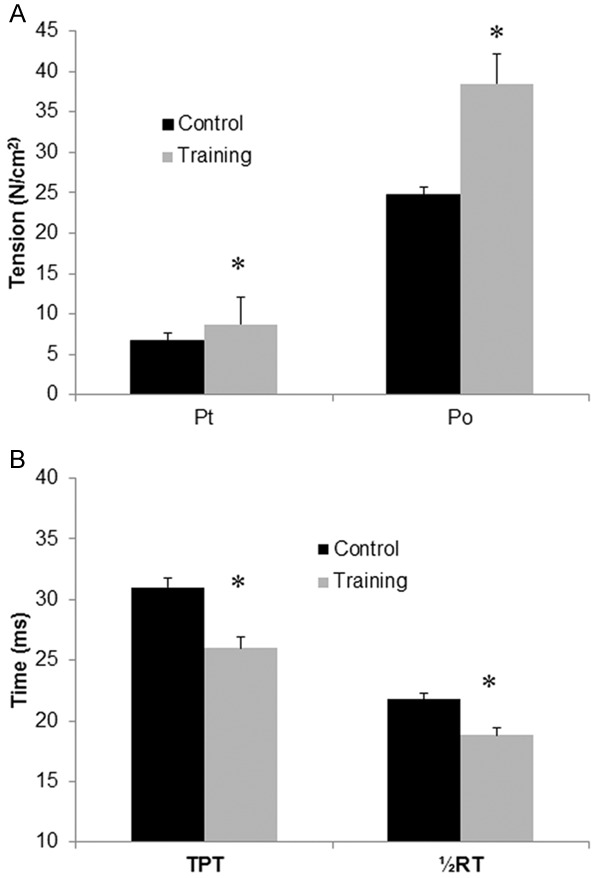
Expression of twitch and tetanic tension (N/cm2), time to peak tension (TPT) and half-relaxation time (½RT) of diaphragm strips in the rats belonging to the control and training groups 24 h after irradiation. The twitch tension (Pt) was obtained using 1-ms supramaximal square wave pulses, and the tetanic tension (Po) was obtained by applying a train of supramaximal stimuli for 400 ms at optimal length. A. Specific twitch and tetanic tension (N/cm2) of diaphragm strips. B. Time to peak tension (TPT) and half-relaxation time (½RT) of diaphragm strips in the control (black bars) and training (white bars) groups 24 h after irradiation. The data are presented as the means ± SEs. *indicates a significant difference between the two groups (P<0.05).
Compared with the control group, the TPT (training: 26.2±0.9 N/cm2 vs. control: 31.4±0.8) and ½RT (training: 18.8±0.6 N/cm2 vs. control: 21.8±0.5, P<0.01) were significantly shorter in the training group (both P<0.001) after 5-Gy irradiation (Figure 5B). The mean Pt/TPT ratio was significantly lower in the control group than in the training group (P<0.01). These data were tested using Student’s t test.
The obtained force-frequency curves are presented in Figure 6A. Compared with the control group, the force-frequency curve for the training group was significantly upwardly shifted, i.e., there was more diaphragmatic force with increased frequency in the training group than in the control group after irradiation challenge. At stimulation frequencies of 30 Hz and above (all P<0.05, Student’s t test), specific tensions in the training group were significantly higher than those in the control group (Figure 6A).
Figure 6.
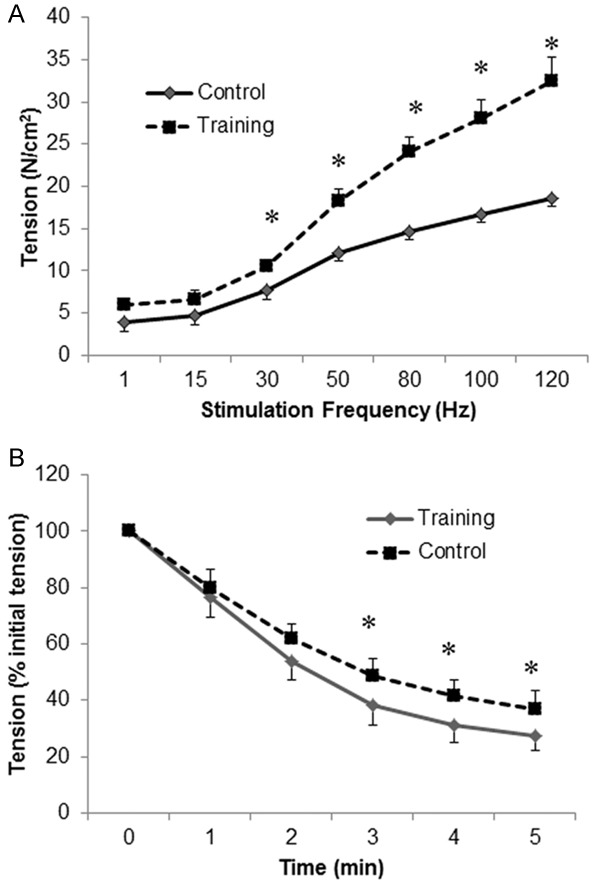
A force-frequency curve was constructed by stimulating the rats’ strips with trains of supramaximal stimuli at 1, 15, 30, 50, 80, 100 and 120 Hz with a 1-min rest period between adjacent stimulus trains. The fatigue characteristics were measured by giving the muscle a series of 300-ms tetanic stimulations every 3 s at a frequency that was adjusted to produce 50% of Po for 10 min. (A) Diaphragmatic force (N/cm2)-frequency (Hz) curves and (B) mean changes in peak tetanic tension during the repetitive stimulation of diaphragmatic strips of the control (-♦-) and training (--■--) groups 24 h after irradiation. The data are presented as the means ± SEs. *indicates a significant difference between the two groups (P<0.05).
The relative force (force as a percentage of its initial value)-over-time curves for repetitive fatiguing electrical stimulation trials performed using the diaphragm strips from both groups are displayed in Figure 6B. The relative force-over-time curve of the training group showed a slower rate of decrease in the force over time, which indicated that the training group exhibited increased resistance to fatigue. The fatigue index of the training group (61.7±5.2) was significantly higher than that of the control group (53.7±6.5; P=0.04, Student’s t test).
According to our previous study [5], the protein carbonyl concentration of the irradiated group was three-fold higher than that of the control group 24 h after the radiation procedure. In the current study, the protein carbonyl concentration was significantly lower in the training group (1.4±0.2 nmol/mg) than in the control group (2.4±0.1 nmol/mg, P<0.01) 24 h after 5-Gy irradiation. Tracheal banding training did not induce alterations in Cu/Zn-SOD mRNA expression but significantly enhanced Mn-SOD mRNA expression (P=0.03, Student’s t test). Compared with the control group, the expression levels of Cu/Zn-SOD mRNA and Mn-SOD mRNA were significantly higher in the training group 24 h after 5-Gy irradiation (Figure 7A and 7B).
Figure 7.
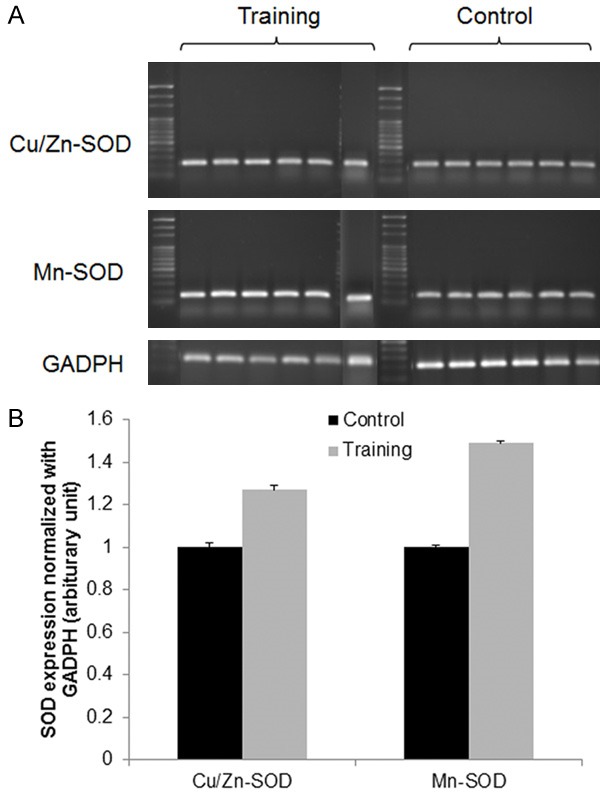
Cu/Zn-SOD and Mn-SOD mRNA expression in rat diaphragms. Real-time RT-PCR for Cu/Zn-SOD, Mn-SOD, and endogenous control GAPDH mRNA expression was performed using a SYBR green assay. All the data were analyzed using Rotor Gene 5 software (Corbett Research, Sydney, New South Wales, Australia). The values for Cu/Zn-SOD and Mn-SOD were normalized to the levels of the GAPDH gene. Diaphragm samples were assayed for Cu/Zn-SOD and Mn-SOD mRNA levels by (A) western blot analysis and (B) real-time quantitative PCR analysis (n=6 in each group). The bars represent the means ± SEs, and *indicates significance at the P<0.05 level compared with the control group.
Discussion
The diaphragm is the most important muscle for ventilation, and diaphragm contractile dysfunction is associated with the progression of respiratory failure. Human and animal studies have shown that ventilator-induced diaphragmatic dysfunction can lead to prolonged mechanical ventilator use and progressive respiratory failure [25]. Chemotherapy and radiotherapy have been confirmed to cause diaphragm contractile dysfunction in rodent models [5,26], and doxorubicin could induce diaphragm weakness in C57BL/6 mice [26]. Additionally, low-dose irradiation also damages the diaphragm and causes contractile dysfunction in Sprague-Dawley rats [5]. A human study showed that CCRT decreases pulmonary function in patients with esophageal cancer; specifically, the carbon monoxide diffusion capacity and total lung capacity were significantly reduced at a median of 15.5 days after CCRT [12], and the vital capacity and FEV1 were reduced after the conclusion of CCRT in these patients [13]. Similarly, the present study showed that the FEV1 started to decline after 1 week of CCRT, and no significant recovery of the FEV1 was observed throughout the rest of the CCRT course in the nontraining group. However, the impact of CCRT on diaphragm function in humans had not been previously studied. The adverse effect of CCRT on the pressure-generating capacity of the inspiratory muscle was not obvious in the current study. Nevertheless, in another study (n=33), we found an average reduction of 11% in the IMP in patients with esophageal cancer after CCRT (data not shown). Therefore, the prevention of diaphragm dysfunction caused by anticancer therapy is an important issue.
The effects of IMT on general respiratory performance have been extensively studied in both healthy and diseased populations. A recent meta-analysis showed that respiratory muscle training improves respiratory muscle endurance in a non-athlete population [27]. Additionally, IMT could improve the MIP by 18% and the respiratory resistance to fatigue during exhaustive exercise in healthy subjects [28]. Moreover, preoperative IMT for an average of 25.4 days significantly increases the median MIP by 32% in patients with esophageal cancer [14]. Similarly, at the end of the CCRT course, the MIP was increased 32% from baseline in patients with esophageal cancer receiving concurrent IMT during CCRT (Figure 3A). Additionally, the animal experiment performed in this study revealed that the diaphragm contractility and its force production efficiency (Pt/TPT ratio, data not shown) were significantly higher in the tracheal banding training group compared with the control group. Furthermore, the preconditioned diaphragm group showed significantly improved contractility and fatigue-resisted properties after irradiation challenge.
Increased diaphragm activation during quiet breathing has been observed in patients with severe COPD using an esophageal electrode [29,30]. However, the diaphragm activation during loaded breathing conditions (e.g., threshold loading) has not yet been examined in patient populations. Notably, under the same loaded breathing condition (30% MIP), we noted that the diaphragm activation was increased 10% and decreased 19% in the control and training groups, respectively. The results demonstrate that the diaphragm has to work increasingly harder to accomplish the same work demand during the course of CCRT and is able to perform the same work with less effort if IMT is applied concurrent to CCRT. As mentioned previously, the current study indicates that diaphragm activation could be impaired by CCRT, and this detrimental side effect could be reversed by the application of IMT concurrent with CCRT. However, it is also important to note that in a healthy population, the diaphragm activation ranges from 29% to 33% while breathing at 40% MIP [31,32]. In contrast, in our study, the baseline diaphragm activation in patients with esophageal cancer ranged from 61% to 73% while breathing at 30% MIP; therefore, whether the commonly prescribed training intensity for IMT (30% MIP) requires modification for this patient population warrants further investigation.
Anticancer therapy is also known to impair physical function [33] and causes fatigue [34]. Interestingly, compared with the baseline, both the respiratory function and the functional exercise capacity and fatigue were improved in the patients receiving concurrent IMT during CCRT. The 6MWD increased 19% (from 385.3 m to 457.4 m) in the IMT group but declined 10% (from 405.0 m to 363.0 m) in the control group. Moreover, the measurement of fatigue using the EORTC questionnaire showed that the fatigue score from baseline to CCRT completion remained unchanged in the patients receiving IMT and increased an average of 33 points in the patients not administered IMT (data not shown). This finding indicates that IMT for esophageal cancer patients who undergo CCRT not only preserves respiratory function but also improves or maintains the functional exercise capacity and quality of life at optimal levels during anticancer treatment.
Immunohistochemistry for γH2AX foci can be used to identify the number, location and repair deficiencies of double-strand breaks (DSBs) [35]. In our previous study, the number of nuclei in diaphragm cells in the irradiation group that stained positive for γH2AX at 24 h was 30% higher than that found in the control group, which suggests that exposure to radiation results in a high degree of DSB repair deficiency in the diaphragm [5]. Mn-SOD overexpression in the mitochondria plays a critical role in protecting HeLa cells against ionizing radiation (5.5 Gy) [36]. Moreover, preconditioning skeletal muscle through exercise training enhances the response of antioxidant and mitochondrial enzymes to radiation [37]. Additionally, whole-body aerobic training can enhance the antioxidant capacity in the diaphragm [38,39]. However, whether targeting training for the inspiratory muscle protects the diaphragm against anticancer therapies is less understood. In the rodent model used in the present study, the expression of Mn-SOD in the diaphragm was upregulated after resistive breathing training.
By increasing the generation of reactive oxygen species (ROS), muscle contraction is known to activate the transcription factor nuclear factor kappa B (NF-κB) to enhance the transcription of genes encoding antioxidative enzymes [40]. Moreover, mitochondria are the main source of ROS production during skeletal muscle contraction [41]. These observations might explain the upregulation of Mn-SOD mRNA but not Cu/Zn-SOD mRNA after resistive respiratory training. In other words, using an animal model, we demonstrated that diaphragm Mn-SOD mRNA expression was enhanced after resistive breathing training and explained the superior resistance of the preconditioned diaphragm to radiation challenge. In addition, the impaired function of the diaphragm caused by CCRT could be reversed by the administration of IMT concurrent with CCRT, in agreement with the results obtained in the preclinical human study.
To apply the results of this study to clinical settings, several factors should be considered. First, the IMT method used in our animal model represents a continuous chronic training mode that is different from the interval training mode used in human studies. However, the human data support the positive impacts of concurrent IMT to preserve diaphragm and pulmonary function in patients with esophageal cancers during CCRT. Second, animal models have the advantage of allowing the direct assessment of diaphragm contractile function. In patients, the effects of IMT on diaphragm function can only be indirectly assessed, and patient motivation might interfere with their performance in these tests. Third, the current study shows the benefit provided by IMT against a single dose of a low level of irradiation. However, patients with esophageal carcinoma are continuously exposed to off-target doses during radiotherapy in daily practice. Finally, the timing for the application of IMT differed between the human and animal studies. In the clinical setting, the time from cancer diagnosis to anticancer therapy initiation is usually very short; therefore, it is difficult to precondition the diaphragm, and adjustment of the timing of IMT application is thus needed. In addition, although a positive effect of concurrent IMT with CCRT was observed in this study, the limited sample size of patients makes any statistical conclusions very preliminary.
Conclusion
In patients with esophageal cancer, concurrent IMT during CCRT shows beneficial effects on diaphragm function, functional exercise capacity, and fatigue. Moreover, in a rodent model, preconditioning of the diaphragm though training can upregulate the antioxidant capacity and protect the diaphragm from oxidative damage caused by low-dose off-target irradiation. These data warrant further evaluation of the application of IMT in clinical settings for patients under CCRT through a prospective study with a larger sample size.
Acknowledgements
This study was partly supported by a grant from Far Eastern Memorial Hospital (FEMH-2016-D-018, FEMH-2018-C-010, and FEMH 107-2314-B-418-007). We wish to acknowledge all the patients who participated in this study. We also thank Hung-Chi Tai, Chin-Ping Lin and Jia-Hui Gao for the technical support provided. The results of the study are presented clearly, honestly, and without any fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute any endorsement by ACSM.
Disclosure of conflict of interest
None.
References
- 1.Shueng PW, Lin SC, Chang HT, Chong NS, Chen YJ, Wang LY, Hsieh YP, Hsieh CH. Toxicity risk of non-target organs at risk receiving low-dose radiation: case report. Radiat Oncol. 2009;4:71. doi: 10.1186/1748-717X-4-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135–1141. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]
- 3.Khan MY. Radiation-induced changes in skeletal muscle. An electron microscopic study. J Neuropathol Exp Neurol. 1974;33:42–57. doi: 10.1097/00005072-197401000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Caiozzo VJ, Giedzinski E, Baker M, Suarez T, Izadi A, Lan M, Cho-Lim J, Tseng BP, Limoli CL. The radiosensitivity of satellite cells: cell cycle regulation, apoptosis and oxidative stress. Radiat Res. 2010;174:582–589. doi: 10.1667/RR2190.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh CH, Lin YC, Chen YJ, Wu HD, Wang LY. Diaphragm contractile dysfunction causes by off-target low-dose irradiation. Am J Transl Res. 2016;8:1510–1517. [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley RC, Ferreira LF. Diaphragm abnormalities in heart failure and aging: mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail Rev. 2017;22:191–207. doi: 10.1007/s10741-016-9549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uramoto H, Nakanishi R, Fujino Y, Imoto H, Takenoyama M, Yoshimatsu T, Oyama T, Osaki T, Yasumoto K. Prediction of pulmonary complications after a lobectomy in patients with non-small cell lung cancer. Thorax. 2001;56:59–61. doi: 10.1136/thorax.56.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37:416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 9.Supinski G, Nethery D, Stofan D, Hirschfield W, DiMarco A. Diaphragmatic lipid peroxidation in chronically loaded rats. J Appl Physiol (1985) 1999;86:651–658. doi: 10.1152/jappl.1999.86.2.651. [DOI] [PubMed] [Google Scholar]
- 10.Inoue J, Ono R, Makiura D, Kashiwa-Motoyama M, Miura Y, Usami M, Nakamura T, Imanishi T, Kuroda D. Prevention of postoperative pulmonary complications through intensive preoperative respiratory rehabilitation in patients with esophageal cancer. Dis Esophagus. 2013;26:68–74. doi: 10.1111/j.1442-2050.2012.01336.x. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez-Sarmiento A, Orozco-Levi M, Guell R, Barreiro E, Hernandez N, Mota S, Sangenis M, Broquetas JM, Casan P, Gea J. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med. 2002;166:1491–1497. doi: 10.1164/rccm.200202-075OC. [DOI] [PubMed] [Google Scholar]
- 12.Gergel TJ, Leichman L, Nava HR, Blumenson LE, Loewen GM, Gibbs JE, Khushalani NI, Leichman CG, Bodnar LM, Douglass HO, Smith JL, Kuettel MR, Proulx GM. Effect of concurrent radiation therapy and chemotherapy on pulmonary function in patients with esophageal cancer: dose-volume histogram analysis. Cancer J. 2002;8:451–460. doi: 10.1097/00130404-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 13.von Dobeln GA, Nilsson M, Adell G, Johnsen G, Hatlevoll I, Tsai J, Lundell L, Lund M, Lind P. Pulmonary function and cardiac stress test after multimodality treatment of esophageal cancer. Pract Radiat Oncol. 2016;6:e53–59. doi: 10.1016/j.prro.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Dettling DS, van der Schaaf M, Blom RL, Nollet F, Busch OR, van Berge Henegouwen MI. Feasibility and effectiveness of pre-operative inspiratory muscle training in patients undergoing oesophagectomy: a pilot study. Physiother Res Int. 2013;18:16–26. doi: 10.1002/pri.1524. [DOI] [PubMed] [Google Scholar]
- 15.van Adrichem EJ, Meulenbroek RL, Plukker JT, Groen H, van Weert E. Comparison of two preoperative inspiratory muscle training programs to prevent pulmonary complications in patients undergoing esophagectomy: a randomized controlled pilot study. Ann Surg Oncol. 2014;21:2353–2360. doi: 10.1245/s10434-014-3612-y. [DOI] [PubMed] [Google Scholar]
- 16.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 17.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–274. doi: 10.1034/j.1399-3003.1999.14b06.x. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society/European Respiratory Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 19.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 20.Moxham J, Edwards RH, Aubier M, De Troyer A, Farkas G, Macklem PT, Roussos C. Changes in EMG power spectrum (high-to-low ratio) with force fatigue in humans. J Appl Physiol Respir Environ Exerc Physiol. 1982;53:1094–1099. doi: 10.1152/jappl.1982.53.5.1094. [DOI] [PubMed] [Google Scholar]
- 21.Oishi PE, Cholsiripunlert S, Gong W, Baker AJ, Bernstein HS. Myo-mechanical analysis of isolated skeletal muscle. J Vis Exp. 2011 doi: 10.3791/2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Augustyniak E, Adam A, Wojdyla K, Rogowska-Wrzesinska A, Willetts R, Korkmaz A, Atalay M, Weber D, Grune T, Borsa C, Gradinaru D, Chand Bollineni R, Fedorova M, Griffiths HR. Validation of protein carbonyl measurement: a multi-centre study. Redox Biol. 2015;4:149–157. doi: 10.1016/j.redox.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinlaor S, Prakobwong S, Hiraku Y, Kaewsamut B, Dechakhamphu S, Boonmars T, Sithithaworn P, Pinlaor P, Ma N, Yongvanit P, Kawanishi S. Oxidative and nitrative stress in opisthorchis viverrini-infected hamsters: an indirect effect after praziquantel treatment. Am J Trop Med Hyg. 2008;78:564–573. [PubMed] [Google Scholar]
- 24.Gerard CJ, Olsson K, Ramanathan R, Reading C, Hanania EG. Improved quantitation of minimal residual disease in multiple myeloma using real-time polymerase chain reaction and plasmid-DNA complementarity determining region III standards. Cancer Res. 1998;58:3957–3964. [PubMed] [Google Scholar]
- 25.Matecki S, Dridi H, Jung B, Saint N, Reiken SR, Scheuermann V, Mrozek S, Santulli G, Umanskaya A, Petrof BJ, Jaber S, Marks AR, Lacampagne A. Leaky ryanodine receptors contribute to diaphragmatic weakness during mechanical ventilation. Proc Natl Acad Sci U S A. 2016;113:9069–9074. doi: 10.1073/pnas.1609707113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilliam LA, Moylan JS, Ann Callahan LA, Sumandea MP, Reid MB. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve. 2011;43:94–102. doi: 10.1002/mus.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sales AT, Fregonezi GA, Ramsook AH, Guenette JA, Lima IN, Reid WD. Respiratory muscle endurance after training in athletes and non-athletes: a systematic review and meta-analysis. Phys Ther Sport. 2016;17:76–86. doi: 10.1016/j.ptsp.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Segizbaeva MO, Timofeev NN, Donina ZhA, Kur’yanovich EN, Aleksandrova NP. Effects of inspiratory muscle training on resistance to fatigue of respiratory muscles during exhaustive exercise. Adv Exp Med Biol. 2015;840:35–43. doi: 10.1007/5584_2014_20. [DOI] [PubMed] [Google Scholar]
- 29.Druz WS, Sharp JT. Electrical and mechanical activity of the diaphragm accompanying body position in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1982;125:275–280. doi: 10.1164/arrd.1982.125.3.275. [DOI] [PubMed] [Google Scholar]
- 30.Wanke T, Formanek D, Lahrmann H, Brath H, Wild M, Wagner C, Zwick H. Effects of combined inspiratory muscle and cycle ergometer training on exercise performance in patients with COPD. Eur Respir J. 1994;7:2205–2211. doi: 10.1183/09031936.94.07122205. [DOI] [PubMed] [Google Scholar]
- 31.Jung JH, Kim NS. Relative activity of respiratory muscles during prescribed inspiratory muscle training in healthy people. J Phys Ther Sci. 2016;28:1046–1049. doi: 10.1589/jpts.28.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsook AH, Koo R, Molgat-Seon Y, Dominelli PB, Syed N, Ryerson CJ, Sheel AW, Guenette JA. Diaphragm recruitment increases during a bout of targeted inspiratory muscle training. Med Sci Sports Exerc. 2016;48:1179–1186. doi: 10.1249/MSS.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 33.Hatakenaka M, Yonezawa M, Nonoshita T, Nakamura K, Yabuuchi H, Shioyama Y, Nagao M, Matsuo Y, Kamitani T, Higo T, Nishikawa K, Setoguchi T, Honda H. Acute cardiac impairment associated with concurrent chemoradiotherapy for esophageal cancer: magnetic resonance evaluation. Int J Radiat Oncol Biol Phys. 2012;83:e67–73. doi: 10.1016/j.ijrobp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Ream E, Richardson A. Fatigue: a concept analysis. Int J Nurs Stud. 1996;33:519–529. doi: 10.1016/0020-7489(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 35.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosoki A, Yonekura S, Zhao QL, Wei ZL, Takasaki I, Tabuchi Y, Wang LL, Hasuike S, Nomura T, Tachibana A, Hashiguchi K, Yonei S, Kondo T, Zhang-Akiyama QM. Mitochondria-targeted superoxide dismutase (SOD2) regulates radiation resistance and radiation stress response in HeLa cells. J Radiat Res. 2012;53:58–71. doi: 10.1269/jrr.11034. [DOI] [PubMed] [Google Scholar]
- 37.De Lisio M, Kaczor JJ, Phan N, Tarnopolsky MA, Boreham DR, Parise G. Exercise training enhances the skeletal muscle response to radiation-induced oxidative stress. Muscle Nerve. 2011;43:58–64. doi: 10.1002/mus.21797. [DOI] [PubMed] [Google Scholar]
- 38.Oh-ishi S, Kizaki T, Ookawara T, Sakurai T, Izawa T, Nagata N, Ohno H. Endurance training improves the resistance of rat diaphragm to exercise-induced oxidative stress. Am J Respir Crit Care Med. 1997;156:1579–1585. doi: 10.1164/ajrccm.156.5.96-11035. [DOI] [PubMed] [Google Scholar]
- 39.Vincent HK, Powers SK, Stewart DJ, Demirel HA, Shanely RA, Naito H. Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur J Appl Physiol. 2000;81:67–74. doi: 10.1007/PL00013799. [DOI] [PubMed] [Google Scholar]
- 40.Hemmrich K, Suschek CV, Lerzynski G, Kolb-Bachofen V. iNOS activity is essential for endothelial stress gene expression protecting against oxidative damage. J Appl Physiol (1985) 2003;95:1937–1946. doi: 10.1152/japplphysiol.00419.2003. [DOI] [PubMed] [Google Scholar]
- 41.Michaelson LP, Shi G, Ward CW, Rodney GG. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve. 2010;42:522–529. doi: 10.1002/mus.21724. [DOI] [PMC free article] [PubMed] [Google Scholar]


