Abstract
Long non-coding RNAs (lncRNAs) act important roles in several tumors including cholangiocarcinoma. However, the expression pattern and function of PCAT6 in intrahepatic cholangiocarcinoma remains unknown. In our research, we showed that the PCAT6 expression level was upregulated in intrahepatic cholangiocarcinoma cell lines. The expression of PCAT6 in intrahepatic cholangiocarcinoma tissues than that in noncancerous samples and the higer expression of PCAT6 was associated with advanced stage. Ectopic expression of PCAT6 induced cell proliferation and invasion in cholangiocarcinoma cell. Moreover, we demonstrated that PCAT6 interacts with miR-330-5p by directly targeting and PCAT6 overexpression inhibited the expression of miR-330-5p in the ICC-9810 cell. We also showed that the expression level of miR-330-5p in intrahepatic cholangiocarcinoma samples was downregulated compared to noncancerous tissues. Interesting, we proved that the miR-330-5p expression was negative correlated with PCAT6 expression in intrahepatic cholangiocarcinoma. Ectopic expression of miR-330-5p suppressed cell proliferation and invasion. Finally, we showed that PCAT6 induced cell proliferation and invasion by decreasing miR-330-5p in cholangiocarcinoma cell. Taken together, these data suggested that lncRNA PCAT6 was an oncogenic player in the development of intrahepatic cholangiocarcinoma.
Keywords: Intrahepatic cholangiocarcinoma, PCAT6, miR-330-5p, oncogene
Introduction
Cholangiocarcinoma is one epithelial tumor of biliary tree and is the 2nd most frequent primary hepatic malignancy [1-4]. Despite the incidence of extrahepatic cholangiocarcinoma has maintained stable, the rate of intrahepatic cholangiocarcinoma has risen [5-7]. Although amelioration in therapy has been developed, the survival of these patients remains dissatisfied [8-11]. The majority of cholangiocarcinoma patients are diagnosed at adavanced stages and surgery resection is not a viable alternative [1,12-16]. Therefore, it is necessary to study the molecular mechanisms of cholangiocarcinoma and find some new biomarkers for diagnosis of cholangiocarcinoma.
Long non-coding RNAs (lncRNAs), one novel member of non-coding RNAs (ncRNAs), are longer than two hundreds nucleotides with no or limited protein-coding ability [17-21]. Previous studies showed that dysregulated lncRNAs were found to be linked with several diseases such as tumors, immunological disease and infection [22-25]. Increasing evidences suggested that lncRNAs participated in diverse cell biological processes including cell migration, apotosis, proliferation, invasion and differentiation [26-29]. Recently, a novel lncRNA PCAT6 (prostate cancer-associated transcript 6) was initially found to be induced colony formation and keratinocyte proliferation of prostate tumor cells [30]. Moreover, Wan et al. [31] indicated that PCAT6 expression was increased in the lung cancer tissues and cells and knockdown of PCAT6 suppressed lung cancer cell metastasis and proliferation. Shi et al. [32] reported that PCAT6 expression was also upregulated in lung cancer tissues and ectopic expression of PCAT6 induced cell growth, invasion and migration. So far, the function role of PCAT6 in cholangiocarcinoma has not been studied.
In our research, we showed that the PCAT6 expression level was upregulated in intrahepatic cholangiocarcinoma cell lines. The expression of PCAT6 in intrahepatic cholangiocarcinoma tissues than that in noncancerous samples and the higer expression of PCAT6 was associated with advanced stage of intrahepatic cholangiocarcinoma. PCAT6 overexpression induced cell proliferation and invasion in cholangiocarcinoma cell.
Materials and methods
Tissue samples
Human intrahepatic cholangiocarcinoma tissues and non-tumorous tissues were collected from Guangdong Provincial Cardiovascular Institute, Guangdong General Hospital, Guangdong Academy of Medical Sciences (Guangdong, China). These samples were frozen in liquid nitrogen until use. This research was approved by the Ethics Committee of Guangdong General Hospital, Guangdong Academy of Medical Sciences (Guangdong, China). Informed consent was written by each patient.
Cell lines cultured and transfection
Human cholangiocarcinoma cell line (ICC-9810) was purchased from ATCC (American Type Culture Collection, VA, USA) and cultured in the RPMI-1640 medium containing FBS (fetal bovine serum, Gibco, USA). pcDNA-PCAT6 and control vector, miR-330-5p mimic and scramble were purchased from RiboBi company (Guangzhou, China). ICC-9810 cell was transfected by using Lipofectamine-2000 (Invitrogen, USA) following to the manufacturer’s information.
RNA extraction and qRT-PCR analyses
Total cell and tissues’ RNA was isolated using Trizol kit (Invitrogen, VA, USA) and cDNAs were composed with the Superscript II reverse kit following to manufacturer’s description. Quantitative real-time PCR (qRT-PCR) assay was performed to measure the expression of PCAT6 and miR-330-5p using SYBR Green kit (TaKaRa, Dalian) on the LightCycler 480 system (Roche Applied Science, Switzerland). GAPDH and U6 were exploited as the internal control reference for mRNA and miRNA respectively. The primers were shown as following: U6 (forward: CTCGCTTCGGCAGCACA, reverse: AACGCTTCACGAATTTGCGT), PCAT6 (forward: 5’-TCCTCATTCGGTCCATCCAACTCC-3’, reverse: 5’-GAAGCACGAGCAAGGCAGAGAC-3’), GAPDH (forward: 5’-GGAGCGAGATCCCTCCAAAAT-3’, reverse: 5’-AGTCCTTCCACGATACCA-3’).
Cell proliferation and invasion
Cell growth was analyzed with Cell Counting Kit-8 (CCK-8, Japan) according to instruction. These cells were plated in the 96-well culture dish and cultured for 24, 48 and 72 hours. Ten μl CCK-8 liquid was added into the each well and the absorbance at the 450 nm was detected by using Thermo reader (Thermo Fisher Scientific). For cell invasion, Transwell chambers with Matrigel were used. ICC-9810 cells were plated in the upper chambers with no-serum RPMI-1640 medium and FBS was added to the lower chamber. After 48 hours, the invasive cells were fixed with mechanol and then stained with the crystal violet. Cells were visualized and photographed under microscope.
Luciferase reporter assay
The pGL3-PCAT6 mutant (PCAT6-mut) and pGL3-PCAT6 wild (PCAT6-wt) vectors were synthetized by PCAT6 cDNA fragment insertion which contained mutated or wild binding sites of miR-330-5p into the pGL3 luciferase reporter vectors. ICC-9810 cells were co-transfected with PCAT6-mut or PCAT6-wt and miR-330-5p mimic or scramble with Lipofectamine-2000 (Invitrogen, USA). The luciferase activities were analyzed with Dual Luciferase Reporter (Promega, USA).
Statistical analysis
Statistical assay was calculated using SPSS (version 18, Chicago, USA) and P value <0.05 was deemed to statistically significant difference. Data were showed as mean ± Standard Deviation (SD). Difference between 2 groups was compared by using Two-way student’s t-test. One-way ANOVA was exploiting to explore the significant differences between multiple groups.
Results
PCAT6 was upregulated in intrahepatic cholangiocarcinoma tissues and cell lines
First, we investigated the expression level of PCAT6 in intrahepatic cholangiocarcinoma samples and noncancerous tissues. As shown in the Figure 1A, the PCAT6 level in intrahepatic cholangiocarcinoma cell lines (ICC-9810, CCLP1, HuCC-T1 and QBC939) was higher than that of nonmalignant cholangiocyte cell (BEC). The expression level of PCAT6 in intrahepatic cholangiocarcinoma samples was upregulated compared with noncancerous tissues (Figure 1B). The PCAT6 expression level was upregulated in 76.7% of (23/30) tumor tissues comared to noncancerous samples (Figure 1C). Moreover, we showed that the PCAT6 expression was higher in the stage III and IV than that in stage I and II of intrahepatic cholangiocarcinoma (Figure 1D).
Figure 1.
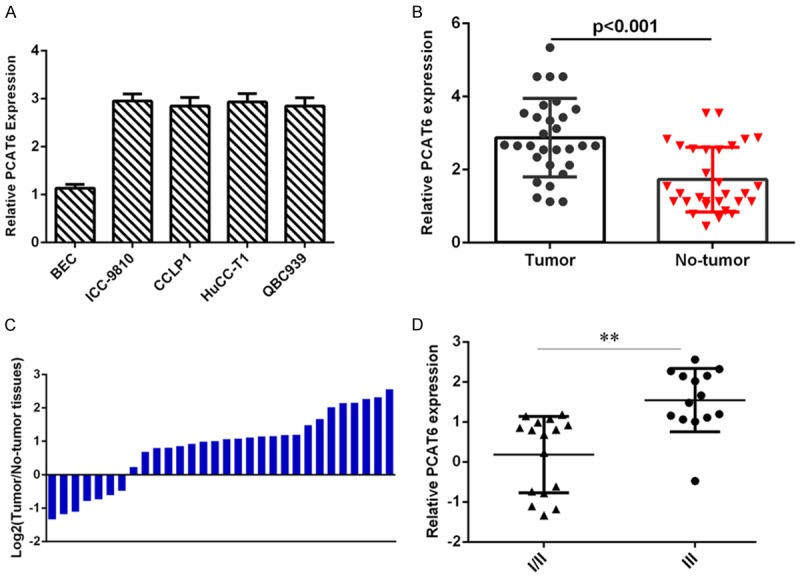
PCAT6 was upregulated in intrahepatic cholangiocarcinoma tissues and cell lines. A. The expression of PCAT6 in four intrahepatic cholangiocarcinoma cell lines (ICC-9810, CCLP1, HuCC-T1 and QBC939) and nonmalignant cholangiocyte cell (BEC) was analyzed by qRT-PCR analysis. B. The expression level of PCAT6 in intrahepatic cholangiocarcinoma samples was upregulated compared with noncancerous tissues. C. The PCAT6 expression level in intrahepatic cholangiocarcinoma samples and noncancerous tissues was shown as log2. D. The PCAT6 expression was higher in the stage III and IV than that in stage I and II of intrahepatic cholangiocarcinoma. **P<0.01.
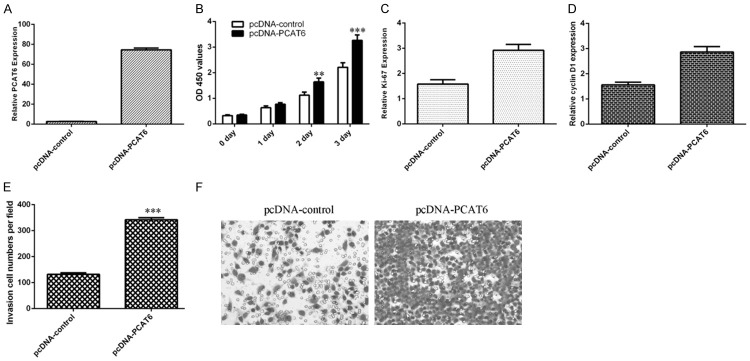
PCAT6 overexpression induced cell proliferation and invasion in cholangiocarcinoma cells
Ecotpic expression of PCAT6 using pcDNA- PCAT6 and the PCAT6 expression level was measured to validate the efficiency of this vector (Figure 2A). Elevated expression of PCAT6 promoted cell growth of CCA cell line ICC-9810 (Figure 2B). Further, we proved that PCAT6 increased the expression of ki-67 and cyclin D1 in the ICC-9810 cell (Figure 2C and 2D). In addition, we demonstrated that the invasive ability was markedly increased in the PCAT6 overexpressing cells (Figure 2E and 2F).
Figure 2.
PCAT6 overexpression induced cell proliferation and invasion in cholangiocarcinoma cells. A. The expression of PCAT6 in the ICC-9810 cell after treated with pcDNA-PCAT6 was measured by qRT-PCR assay. B. Elevated expression of PCAT6 promoted cell growth of CCA cell line ICC-9810. C. The expression of ki-67 was analyzed by qRT-PCR analysis and GAPDH was used as the internal control. D. The expression of cyclin D1 was analyzed by qRT-PCR analysis and GAPDH was used as the internal control. E. The invasive ability was markedly increased in the PCAT6 overexpressing cells. F. The relative invasive cells were shown. **P<0.01 and ***P<0.001.
PCAT6 interacts with miR-330-5p by directly targeting
Bioinformatics assay targetscan (http://www.targetscan.org/vert_72/) was used to predict that there are putative binding sites between miR-330-5p and PCAT6 (Figure 3A). Ectopic expression of PCAT6 suppressed the expression of miR-330-5p in the ICC-9810 cell (Figure 3B). In addition, we validated that the miR-330-5p expression was significantly upregulated in the ICC-9810 cell after treating miR-330-5p mimic (Figure 3C). The luciferase activity of wt-PCAT6 transfected cell was suppressed by overexpression of miR-330-5p; however there is no change of mut-PCAT6 (Figure 3D). Overexpression of miR-330-5p decreased the PCAT6 expression in the ICC-9810 cell (Figure 3E).
Figure 3.
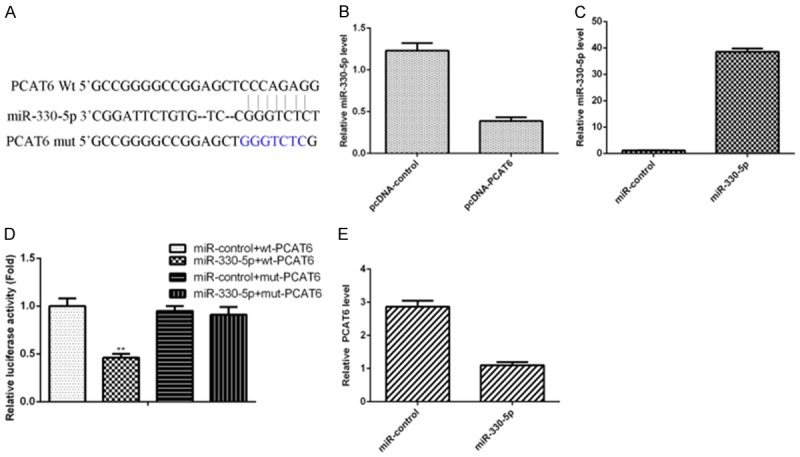
PCAT6 interacts with miR-330-5p by directly targeting. A. There are putative binding sites between miR-330-5p and PCAT6 by using Bioinformatics assay targetscan (http://www.targetscan.org/vert_72/). B. Ectopic expression of PCAT6 suppressed the expression of miR-330-5p in the ICC-9810 cell. C. miR-330-5p expression was detected by qRT-PCR analysis. D. The luciferase activity of wt-PCAT6 transfected cell was suppressed by overexpression of miR-330-5p; however there is no change of mut-PCAT6. E. Overexpression of miR-330-5p decreased the PCAT6 expression in the ICC-9810 cell. **P<0.01.
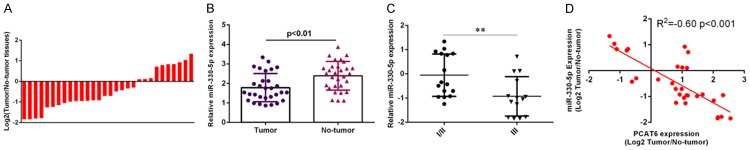
miR-330-5p was downregulated in intrahepatic cholangiocarcinoma tissues
Then, we studied the expression level of miR-330-5p in intrahepatic cholangiocarcinoma samples and noncancerous tissues. The expression level of miR-330-5p in intrahepatic cholangiocarcinoma samples was downregulated compared with noncancerous tissues (Figure 4A). The miR-330-5p expression level was downregulated in 66.7% of (20/30) tumor tissues comared to noncancerous samples (Figure 4B). Moreover, we showed that the miR-330-5p expression was lower in the stage III and IV than that in stage I and II of intrahepatic cholangiocarcinoma (Figure 4C). Interesting, we indicated that the miR-330-5p expression was negative correlated with PCAT6 expression in intrahepatic cholangiocarcinoma (Figure 4D).
Figure 4.
miR-330-5p was downregulated in intrahepatic cholangiocarcinoma tissues. A. The expression level of miR-330-5p in intrahepatic cholangiocarcinoma samples was downregulated compared with noncancerous tissues. B. The miR-330-5p expression level in intrahepatic cholangiocarcinoma samples and noncancerous tissues was shown as log2. C. The miR-330-5p expression was lower in the stage III and IV than that in stage I and II of intrahepatic cholangiocarcinoma. D. The miR-330-5p expression was negative correlated with PCAT6 expression in intrahepatic cholangiocarcinoma. **P<0.01.
Ectopic expression of miR-330-5p inhibited cell proliferation and invasion
Elevated expression of miR-330-5p inhibited cell growth of CCA cell line ICC-9810 (Figure 5A). Further, we proved that miR-330-5p decreased the expression of ki-67 and cyclin D1 in the ICC-9810 cell (Figure 5B and 5C). In addition, we indicated that the invasive ability was markedly decreased in the miR-330-5p overexpressing cells (Figure 5D and 5E).
Figure 5.
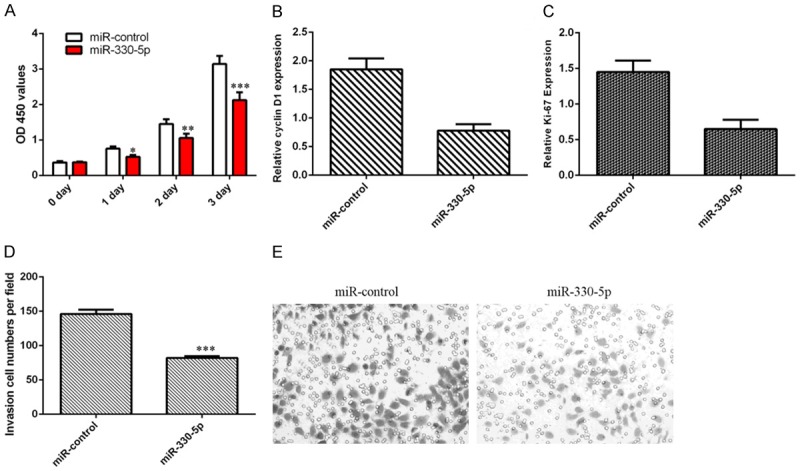
Ectopic expression of miR-330-5p inhibited cell proliferation and invasion. A. Elevated expression of miR-330-5p inhibited cell growth of CCA cell line ICC-9810. B. The expression of ki-67 was analyzed by qRT-PCR analysis and GAPDH was used as the internal control. C. The expression of cyclin D1 was analyzed by qRT-PCR analysis and GAPDH was used as the internal control. D. The invasive ability was markedly decreased in the miR-330-5p overexpressing cells. E. The relative invasive cells were shown. *P<0.05, **P<0.01 and ***P<0.001.
PCAT6 enhanced cell proliferation and invasion through decreasing miR-330-5p in cholangiocarcinoma cells
Next, we studied the impact role of miR-330-5p on PCAT6-induced proliferation and invasion of cholangiocarcinoma cell. As indicated in the Figure 6A, overexpression of miR-330-5p suppressed the cell proliferation in the ICC-9810 cell that was transfected with pcDNA-PCAT6. Moreover, elevated expression of miR-330-5p inhibited the ki-67 and cyclin D1 expression in the PCAT6-overexpressing ICC-9810 cell (Figure 6B and 6C). In addition, we showed that ectopic expression of miR-330-5p suppressed the cell invasion in the PCAT6-overexpressing ICC-9810 cell (Figure 6D and 6E).
Figure 6.
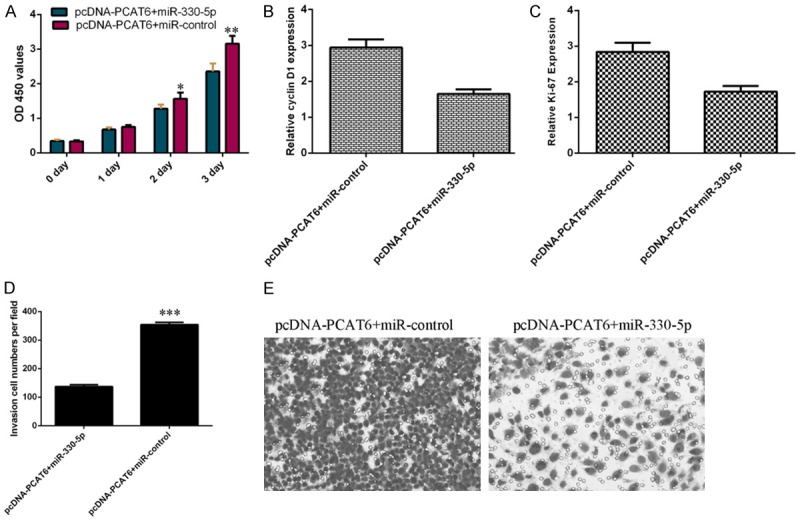
PCAT6 enhanced cell proliferation and invasion through decreasing miR-330-5p in cholangiocarcinoma cells. A. Overexpression of miR-330-5p suppressed the cell proliferation in the ICC-9810 cell that was transfected with pcDNA-PCAT6. B. The expression of ki-67 was analyzed by qRT-PCR analysis and GAPDH was used as the internal control. C. The expression of cyclin D1 was analyzed by qRT-PCR analysis and GAPDH was used as the internal control. D. Ectopic expression of miR-330-5p suppressed the cell invasion in the PCAT6-overexpressing ICC-9810 cell. E. The relative invasive cells were shown. *P<0.05, **P<0.01 and ***P<0.001.
Dicsussion
The current research showed that the PCAT6 expression level was upregulated in intrahepatic cholangiocarcinoma cell lines. The expression of PCAT6 in intrahepatic cholangiocarcinoma tissues than that in noncancerous samples and the higer expression of PCAT6 was associated with advanced stage of intrahepatic cholangiocarcinoma. PCAT6 overexpression induced cell proliferation and invasion in cholangiocarcinoma cell. Moreover, we showed that PCAT6 interacts with miR-330-5p by directly targeting and ectopic expression of PCAT6 suppressed the expression of miR-330-5p in the ICC-9810 cell. We also demonstrated that the expression level of miR-330-5p in intrahepatic cholangiocarcinoma samples was downregulated compared with noncancerous tissues. Interesting, we indicated that the miR-330-5p expression was negative correlated with PCAT6 expression in intrahepatic cholangiocarcinoma. Ectopic expression of miR-330-5p inhibited cell proliferation and invasion. Finally, we showed that PCAT6 enhanced cell proliferation and invasion through decreasing miR-330-5p in cholangiocarcinoma cell. Taken together, these data suggested that lncRNA PCAT6 was an oncogenic player in the development of intrahepatic cholangiocarcinoma.
Growing evidences indicated that LncRNA PCAT6 was upregulated and played as oncogenic roles in several cancers including lung cancer, gastric cancer and colon cancer [32-34]. Aberrant upregulated PCAT6 was found to be in lung cancer and knockdown of PCAT6 suppressed lung cancer cell metastasis and proliferation and induced cell apoptosis [31]. Xu et al. [33] demonstrated that PCAT6 expression was upregulated in gastric tumor tissues and ectopic expression of PCAT6 induced cell invasion, proliferation and migration partly through regulating miR-30 expression. Huang et al. [34] reported that PCAT6 expression level was over-expressed in colon cancer samples and higher expression of PCAT6 was correlated with worse survival status and poorer clinical stage. Overexpression of PCAT6 enhanced cell proliferation and suppressed cell apoptosis. However, the expression pattern of PCAT6 in intrahepatic cholangiocarcinoma remains unknown. First, we investigated the expression level of PCAT6 in intrahepatic cholangiocarcinoma samples and noncancerous tissues. We also found that PCAT6 expression level was upregulated in intrahepatic cholangiocarcinoma cell lines and tissues. In addition, we demonstrated that PCAT6 overexpression induced cell proliferation and invasion in cholangiocarcinoma cells.
The molecular mechanism via which PCAT6 induces tumor cell growth and invasion alters from differenct tumor species. Previous study indicated that PCAT6 induced lung tumor cell growth, invasion and migration via modulating miR-330-5p expression [35]. In line with this, we found that PCAT6 interacts with miR-330-5p by directly targeting and ectopic expression of PCAT6 suppressed the expression of miR-330-5p in the ICC-9810 cell. MiR-330-5p has been indicated to play suppressive roles in some cancers including glioblastoma, ovarian cancer, pancreatic cancer and cutaneous malignant melanoma [29,36-38]. In line with this, we also found that expression level of miR-330-5p in intrahepatic cholangiocarcinoma samples was downregulated compared with noncancerous tissues. Interesting, we indicated that the miR-330-5p expression was negative correlated with PCAT6 expression in intrahepatic cholangiocarcinoma. Ectopic expression of miR-330-5p inhibited cell proliferation and invasion. Furthermore, we showed that PCAT6 overexpression enhanced cell proliferation and invasion through decreasing miR-330-5p in cholangiocarcinoma cell.
Taken together, we demonstrated that the expression of PCAT6 was overexpressed in intrahepatic cholangiocarcinoma tissues and cell and ectopic expression of PCAT6 induced intrahepatic cholangiocarcinoma cell proliferation and invasion partly through regulating miR-330-5p expression. These data suggested that lncRNA PCAT6 was an oncogenic player in the development of intrahepatic cholangiocarcinoma.
Acknowledgements
This work was supported by National Key Research and Development Program of China (No. 2017YFA0205200), National Natural Science Foundation of China (No. 81571785, 81801811, 81771957, 81502642), and Natural Science Foundation of Guangdong Province, China (No. 2016A030311055, 2016A030313770, 2015A030310108, 2018A030313074), Medical Research Foundation of Guangdong Province, China (No. A2017427), Youth Science Foundation of Guangdong Second Provincial General Hospital (No. YQ2016-001).
Disclosure of conflict of interest
None.
References
- 1.Tan XY, Huang ZG, Li XG. Long non-coding RNA MALAT1 interacts with miR-204 to modulate human hilar cholangiocarcinoma proliferation, migration, and invasion by targeting CXCR4. J Cell Biochem. 2017;118:3643–3653. doi: 10.1002/jcb.25862. [DOI] [PubMed] [Google Scholar]
- 2.Huang Q, Liu L, Liu CH, You H, Shao F, Xie F, Lin XS, Hu SY, Zhang CH. MicroRNA-21 regulates the invasion and metastasis in cholangiocarcinoma and may be a potential biomarker for cancer prognosis. Asian Pac J Cancer Prev. 2013;14:829–834. doi: 10.7314/apjcp.2013.14.2.829. [DOI] [PubMed] [Google Scholar]
- 3.Yamanaka S, Campbell NR, An F, Kuo SC, Potter JJ, Mezey E, Maitra A, Selaru FM. Coordinated effects of microRNA-494 induce G(2)/M arrest in human cholangiocarcinoma. Cell Cycle. 2012;11:2729–2738. doi: 10.4161/cc.21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu C, Huang F, Deng G, Nie W, Huang W, Zeng X. miR-31 promotes oncogenesis in intrahepatic cholangiocarcinoma cells via the direct suppression of RASA1. Exp Ther Med. 2013;6:1265–1270. doi: 10.3892/etm.2013.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li B, Han Q, Zhu Y, Yu Y, Wang J, Jiang X. Down-regulation of miR-214 contributes to intrahepatic cholangiocarcinoma metastasis by targeting twist. FEBS J. 2012;279:2393–2398. doi: 10.1111/j.1742-4658.2012.08618.x. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Tian F, Li D, Chen J, Jiang P, Zheng S, Li X, Wang S. MiR-605 represses PSMD10/Gankyrin and inhibits intrahepatic cholangiocarcinoma cell progression. FEBS Lett. 2014;588:3491–3500. doi: 10.1016/j.febslet.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Zeng B, Li Z, Chen R, Guo N, Zhou J, Zhou Q, Lin Q, Cheng D, Liao Q, Zheng L, Gong Y. Epigenetic regulation of miR-124 by hepatitis C virus core protein promotes migration and invasion of intrahepatic cholangiocarcinoma cells by targeting SMYD3. FEBS Lett. 2012;586:3271–3278. doi: 10.1016/j.febslet.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 8.Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Han C, Wu T. MicroRNA-26a promotes cholangiocarcinoma growth by activating beta-catenin. Gastroenterology. 2012;143:246–256. e248. doi: 10.1053/j.gastro.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haga H, Yan I, Takahashi K, Wood J, Patel T. Emerging insights into the role of microRNAs in the pathogenesis of cholangiocarcinoma. Gene Expr. 2014;16:93–99. doi: 10.3727/105221614X13919976902174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olaru AV, Ghiaur G, Yamanaka S, Luvsanjav D, An F, Popescu I, Alexandrescu S, Allen S, Pawlik TM, Torbenson M, Georgiades C, Roberts LR, Gores GJ, Ferguson-Smith A, Almeida MI, Calin GA, Mezey E, Selaru FM. MicroRNA down-regulated in human cholangiocarcinoma control cell cycle through multiple targets involved in the G1/S checkpoint. Hepatology. 2011;54:2089–2098. doi: 10.1002/hep.24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013;52:297–303. doi: 10.1002/mc.21864. [DOI] [PubMed] [Google Scholar]
- 13.Kunkeaw N, Jeon SH, Lee K, Johnson BH, Tanasanvimon S, Javle M, Pairojkul C, Chamgramol Y, Wongfieng W, Gong B, Leelayuwat C, Lee YS. Cell death/proliferation roles for nc886, a non-coding RNA, in the protein kinase R pathway in cholangiocarcinoma. Oncogene. 2013;32:3722–3731. doi: 10.1038/onc.2012.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Yin J, Li T, Yuan L, Wang D, He J, Du X, Lu J. Upregulated circulating miR-150 is associated with the risk of intrahepatic cholangiocarcinoma. Oncol Rep. 2015;33:819–825. doi: 10.3892/or.2014.3641. [DOI] [PubMed] [Google Scholar]
- 15.Iwaki J, Kikuchi K, Mizuguchi Y, Kawahigashi Y, Yoshida H, Uchida E, Takizawa T. MiR-376c down-regulation accelerates EGF-dependent migration by targeting GRB2 in the HuCCT1 human intrahepatic cholangiocarcinoma cell line. PLoS One. 2013;8:e69496. doi: 10.1371/journal.pone.0069496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chusorn P, Namwat N, Loilome W, Techasen A, Pairojkul C, Khuntikeo N, Dechakhamphu A, Talabnin C, Chan-On W, Ong CK, Teh BT, Yongvanit P. Overexpression of microRNA-21 regulating PDCD4 during tumorigenesis of liver fluke-associated cholangiocarcinoma contributes to tumor growth and metastasis. Tumour Biol. 2013;34:1579–1588. doi: 10.1007/s13277-013-0688-0. [DOI] [PubMed] [Google Scholar]
- 17.Chen XJ, Dong H, Liu S, Yu L, Yan DX, Yao XW, Sun WJ, Han DZ, Gao GZ. Long noncoding RNA MHENCR promotes melanoma progression via regulating miR-425/489-mediated PI3K-Akt pathway. Am J Transl Res. 2017;9:90–102. [PMC free article] [PubMed] [Google Scholar]
- 18.Bian DH, Shi W, Shao Y, Li PL, Song GD. Long non-coding RNA GAS5 inhibits tumorigenesis via miR-137 in melanoma. Am J Transl Res. 2017;9:1509–1520. [PMC free article] [PubMed] [Google Scholar]
- 19.Ma X, Qi S, Duan Z, Liao H, Yang B, Wang W, Tan J, Li Q, Xia X. Long non-coding RNA LOC554202 modulates chordoma cell proliferation and invasion by recruiting EZH2 and regulating miR-31 expression. Cell Prolif. 2017;50 doi: 10.1111/cpr.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang JK, Ma L, Song WH, Lu BY, Huang YB, Dong HM, Ma XK, Zhu ZZ, Zhou R. LncRNA-MALAT1 promotes angiogenesis of thyroid cancer by modulating tumor-associated macrophage FGF2 protein secretion. J Cell Biochem. 2017;118:4821–4830. doi: 10.1002/jcb.26153. [DOI] [PubMed] [Google Scholar]
- 21.Wang XM, Lu XB, Geng ZS, Yang GY, Shi Y. LncRNA PTCSC3/miR-574-5p governs cell proliferation and migration of papillary thyroid carcinoma via Wnt/-catenin signaling. J Cell Biochem. 2017;118:4745–4752. doi: 10.1002/jcb.26142. [DOI] [PubMed] [Google Scholar]
- 22.Zhang DW, Li HY, Xie JP, Jiang DC, Cao LQ, Yang XW, Xue P, Jiang XF. Long noncoding RNA LINC01296 promotes tumor growth and progression by sponging miR-5095 in human cholangiocarcinoma. Int J Oncol. 2018;52:1777–1786. doi: 10.3892/ijo.2018.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang C, Wu K, Wang S, Wei G. Long non-coding RNA XIST promotes osteosarcoma progression by targeting YAP via miR-195-5p. J Cell Biochem. 2018;119:5646–5656. doi: 10.1002/jcb.26743. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Jeang KT. Long non-coding RNAs (lncRNAs) and viral infections. Biomed Pharmacother. 2013;3:34–42. doi: 10.1016/j.biomed.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imamura K, Imamachi N, Akizuki G, Kumakura M, Kawaguchi A, Nagata K, Kato A, Kawaguchi Y, Sato H, Yoneda M, Kai C, Yada T, Suzuki Y, Yamada T, Ozawa T, Kaneki K, Inoue T, Kobayashi M, Kodama T, Wada Y, Sekimizu K, Akimitsu N. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell. 2014;53:393–406. doi: 10.1016/j.molcel.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Song ZW, Feng C, Lu YL, Zhou Y, Lin Y, Dong CY. The long non-coding RNA SUMO1P3 facilitates breast cancer progression by negatively regulating miR-320a. Am J Transl Res. 2017;9:5594–5602. [PMC free article] [PubMed] [Google Scholar]
- 27.Lian YF, Li ZH, Fan YY, Huang QW, Chen JM, Liu WM, Xiao CX, Xu HZ. The lncRNA-HOXA-AS2/EZH2/LSD1 oncogene complex promotes cell proliferation in pancreatic cancer. Am J Transl Res. 2017;9:5496–5506. [PMC free article] [PubMed] [Google Scholar]
- 28.Li QH, Shen W, Li XY, Zhang LL, Jin X. The lncRNA n340790 accelerates carcinogenesis of thyroid cancer by regulating miR-1254. Am J Transl Res. 2017;9:2181–2194. [PMC free article] [PubMed] [Google Scholar]
- 29.Fu X, Zhang L, Dan L, Wang K, Xu Y. LncRNA EWSAT1 promotes ovarian cancer progression through targeting miR-330-5p expression. Am J Transl Res. 2017;9:4094–4103. [PMC free article] [PubMed] [Google Scholar]
- 30.Du Z, Fei T, Verhaak RG, Su Z, Zhang Y, Brown M, Chen YW, Liu XS. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat Struct Mol Biol. 2013;20:908–913. doi: 10.1038/nsmb.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan L, Zhang L, Fan K, Cheng ZX, Sun QC, Wang JJ. Knockdown of long noncoding RNA PCAT6 inhibits proliferation and invasion in lung cancer cells. Oncol Res. 2016;24:161–170. doi: 10.3727/096504016X14618564639178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi XF, Liu ZL, Liu ZC, Feng XR, Hua F, Hu XX, Wang B, Lu KH, Nie FQ. Long noncoding RNA PCAT6 functions as an oncogene by binding to EZH2 and suppressing LATS2 in non-small-cell lung cancer. EBioMedicine. 2018;37:177–187. doi: 10.1016/j.ebiom.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Sun JY, Jin YF, Yu H. PCAT6 participates in the development of gastric cancer through endogenously competition with microRNA-30. Eur Rev Med Pharmacol Sci. 2018;22:5206–5213. doi: 10.26355/eurrev_201808_15718. [DOI] [PubMed] [Google Scholar]
- 34.Huang W, Su G, Huang X, Zou A, Wu J, Yang Y, Zhu Y, Liang S, Li D, Ma F, Guo L. Long noncoding RNA PCAT6 inhibits colon cancer cell apoptosis by regulating anti-apoptotic protein ARC expression via EZH2. Cell Cycle. 2019;18:69–83. doi: 10.1080/15384101.2018.1558872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui LH, Xu HR, Yang W, Yu LJ. lncRNA PCAT6 promotes non-small cell lung cancer cell proliferation, migration and invasion through regulating miR-330-5p. Onco Targets Ther. 2018;11:7715–7724. doi: 10.2147/OTT.S178597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng LS, Ma JH, Ji HM, Liu YC, Hu WX. miR-330-5p suppresses glioblastoma cell proliferation and invasiveness through targeting ITGA5. Biosci Rep. 2017;37 doi: 10.1042/BSR20170019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Trehoux S, Lahdaoui F, Delpu Y, Renaud F, Leteurtre E, Torrisani J, Jonckheere N, Van Seuningen I. Micro-RNAs miR-29a and miR-330-5p function as tumor suppressors by targeting the MUC1 mucin in pancreatic cancer cells. Biochim Biophys Acta. 2015;1853:2392–403. doi: 10.1016/j.bbamcr.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Su BB, Zhou SW, Gan CB, Zhang XN. MiR-330-5p regulates tyrosinase and PDIA3 expression and suppresses cell proliferation and invasion in cutaneous malignant melanoma. J Surg Res. 2016;203:434–40. doi: 10.1016/j.jss.2016.03.021. [DOI] [PubMed] [Google Scholar]