Abstract
Background: Diabetic nephropathy (DN) is one of the most important microvascular complications of diabetes mellitus. The present study aims to explore whether angiopoietin-like protein 2 (ANGPTL2) can promote renal tissue fibrosis in DN. Materials and methods: Models includes diabetic SD rats induced by streptozotocin (STZ) and high glucose (HG)-stimulated HK-2 cells. qRT-PCR, western blot and immunohistochemical analysis were performed to explore ANGPTL2 expression. The renal injury and fibrosis were assessed using hematoxylin-eosin staining (H&E) and Masson trichrome staining. Immunofluorescence was conducted to detect the expression of collagen IV and LC3II. The levels of pro-inflammatory factors IL-6, -1β, TNF-α and ANGPTL2 were assessed by an ELISA, and nitric oxide (NO) production was determined using Griess method. Protein levels of iNOS, PTEN, fibronectin (FN), collagen I, IV, p62, beclin1 and MEK/ERK/Nrf-1 pathway in DN rats and HK-2 cells were determined, respectively. Results: When compared with normal rats, DN rats experienced severe renal injury and fibrosis and showed decreased LC3II and beclin1, increased PTEN, FN, collagen I and IV, p62, NO, iNOS and ANGPTL2 in kidney. The pro-inflammatory factors and ANGPTL2 were markedly elevated. Again, knockdown of ANGPTL2 caused an increase in MEK, p-ERK, Nrf-1, LC3II, beclin1, and a decrease in PTEN, FN, collagen I and IV, p62, NO, iNOS and pro-inflammatory factors of HK-2 cells. Furthermore, knockdown of MEK/ERK reversed these changes. Conclusion: ANGPTL2 may serve an important role in the autophagy of DN and activate MEK/ERK/Nrf-1 pathway, which may therefore have potential as a treatment to prevent renal fibrosis in DN.
Keywords: Diabetes mellitus, diabetic nephropathy, angiopoietin-like protein 2, autophagy, fibrosis, MEK/ERK
Introduction
Diabetic nephropathy is one of the most severe complications among the various types of diabetes related complications all through the world [1]. In the past, DN was considered to be primarily a glomerular disease and tubule interstitial fibrosis (TIF) always occur in the early stage of DN [2]. TIF is prominent in glomerular diseases that progress to chronic renal failure, which is characterized by tubular atrophy and excessive accumulation of extracellular matrix components such as collagen I and IV and fibronectin (FN). In addition, the accumulation of renal extracellular matrix in early diabetic patients usually leads to renal glomerular sclerosis and interstitial fibrosis [3]. The extracellular matrix is mainly composed of fibronectin and collagen. FN attaches cells to the extracellular matrix components and collagen is the most important structural protein in the extracellular matrix. So far, 44 different collagen genes have been identified and more than 28 different types of collagen have been created. Among them, the types 1, 2, 3 collagens are the most abundant [4,5]. Beside, PTEN is a phosphatase closely related to renal fibrosis [6,7]. Except insulin and nutrition starvation, intracellular metabolism alternations are also related to the pathogenesis of DN, involving the increase in reactive oxygen species (ROS).
Recent studies have shown that activation of autophagy can inhibit fibrosis and prevent the progression of DN and autophagy is a highly conserved cellular process in which proteins and damaged cell organelles are delivered to lysosomes for degradation and recycling to maintain intracellular homeostasis [8]. In addition, LC3-II formation is recognized as a marker of the existence of autophagosomes in cell or animal experiments [9,10]. A recent study showed that berberine could suppress the degree of oxidative stress by inhibiting the signal pathway of nuclear factor erythroid-2-related factor-2 (Nrf2) [11]. It has been reported that the increase of inflammatory cytokines usually occurs in the process of DN [12]. Subsequently, after the treatment of berberine, the levels of the corresponding inflammatory factor and cytokines decreased, the inhibitory effect of GLUT-4 was improved, and the utilization rate of glucose was increased, which also indirectly improved insulin resistance [13]. Meanwhile, the level of IL-6 is related to the process of insulin resistance in diabetes, and the decrease of IL-6 promote the tyrosine phosphorylation of insulin receptor substrate 1 and stimulate the insulin pathway, which can directly improve insulin resistances [14]. In addition, iNOS is also related to insulin resistances [15], which is an important mediator of DN.
Angiopoietin-like proteins (ANGPTLs) are a family of secreted proteins structurally similar to angiopoietin. Different ANGPTLs have different functions. Among them, ANGPTL2 has the function of physiological tissue remodeling [16] and plays important roles in the pathological conditions related to chronic non-infectious inflammation, such as the adipose tissue inflammation induced by obesity, rheumatoid arthritis, atherosclerosis and chemically induced cancers [17-20]. In many pathologies, tissue fibrosis is closely associated with chronic inflammation, and the association of ANGPTL2 with chronic inflammatory diseases leads us to hypothesize that ANGPTL2 may also play a role in fibrogenic responses of several organs [21]. ANGPTL2 may play an important role in the pathogenesis of DN.
In the present study, we address the questions whether ANGPTL2 can promote renal tissue fibrosis through autophagy. The purpose of the present study was to explore the molecular mechanism of ANGPTL2 affecting in DN rats, that is, whether it plays a role by regulating autophagy process and the MEK/ERK/Nrf-1 pathway, in order to provide a new idea for the treatment of diabetic nephropathy in clinical practice.
Materials and methods
Animals
SD rats (male, 200-250 g) were obtained from the experiment animal center. After housed on a 12 h-light/12-dark circle for 1 week adjusting, rats were randomly divided into the normal group (n = 9) and the diabetic group (n = 9). The rats in the normal group were fed standard diet and rats in diabetic group were fed with high-fat diet for 4 weeks following by intraperitoneal one-time injection with STZ (35 mg/kg body weight, 0.1 mmol/L sodium citrate solution, pH 4.4). Tail vein blood glucose was measured every week and those with blood glucose higher than 11.0 mmol/L were considered to be diabetic models. 4 weeks later, after assessment of renal function, rats were sacrificed by cervical dislocation. Subsequently, renal tissue was removed and cleaned. Then, one part of tissue was immediately saved in 4% formaldehyde and embedded in paraffin for immunohistochemistry, immunofluorescence and Masson staining, and the other part was saved in liquid nitrogen for extraction of RNA and protein.
All rats were managed according to the guidelines of Zhongda Hospital affiliated with Southeast University. Experimental protocols were approved by the Institutional Animal Care and Use Committee of Zhongda Hospital affiliated with Southeast University.
Assessment of renal function
Before the sacrifice of the rats, after fasting for 12 hours, venous blood and urine was collected from DN rats in the morning of the next day. The fasting blood glucose (FBG) and urine protein levels were determined using an automatic biochemistry analyzer (Japan). DN model was successfully prepared with blood glucose greater than 11 mmol/L and urine protein level at 24 h greater than 50% before modeling.
H&E and Masson trichrome staining
The paraffin blocks of renal tissue were cut in 5 mm thickness, and the sections were deparaffinized in xylene and dehydrated in alcohol. Then, the sections were stained in hematoxylin and eosin (H&E) and Masson’s trichrome method. HE staining was performed as follows: hematoxylin solution for 5 min before bluing in 0.2% ammonia water or saturated lithium carbonate solution for 30 s. After rinsing in 70% and 90% alcohol, the sections were counterstained in eosin-phloxine solution for 2 min. Masson trichrome staining was conducted as following: After deparaffinized and rehydrated, the sections were stained in hematoxylin solution for 8 min. Then, ponceau acid fuchsin solution for 5 min after rinsed in running tap water for 8 min. After differentiated in phosphomolybdic-phosphotungstic acid for 5 min, sections were transferred into aniline blue solution for 5 min. Then sections were differentiated in 0.2% acetic acid for 2 min and followed by dehydration, clearing, and mounting. Finally, Images were all captured by a Nikon microscope (Japan).
Immunohistochemistry
After dehydration, transparence, embedding, and the renal tissue sections were deparaffinized and rehydrated. Antigen retrieve was applied to perform immunohistochemistry by heating sections immersed in EDTA solution (PH 9.0) with pressure cooker for 2 min and left to cool down at room temperature for 20 min. After washed by phosphate-buffer saline (PBS), sections were incubated with specific primary antibody (rabbit anti-ANGPTL2 antibody, ab199133, 1:50) at 4°C overnight, sections were washed by phosphate-buffer saline for three times for 5 min each. Then, sections were incubated with goat anti-rabbit secondary antibody labeled with biotin for 30 min at 37°C. After rinsing three times with phosphate-buffered saline, staining was produced by DAB before counterstaining with haematoxylin. Brownish yellow granules in cytoplasm and nuclei were interpreted as positive region.
Cells and transfection
Human proximal tubular cell (HK-2 cells), purchased from ATCC, were cultured with DMEM medium containing 5.5 mmol/L glucose supplemented with 10% serum, 1% penicillin, and streptomycin. Then, cells of normal glucose group were cultured with normal glucose medium whereas cells of other groups were treated with high glucose medium for 48 h followed by related analysis. Briefly, HK-2 cells seeded in a 24-well plates were grown to 90% confluence and mixtures of 4 µg DNA and 10 µl Lipofectamine 2000 were added into serum-free medium. Cell transfection was performed using Lipofectamine 2000 (Invitrogen). First, high glucose stimulation was performed by culturing cells in DMEM medium containing 25 mmol/L glucose for 48 h. Then, for cell transfection experiment of siRNA-ANGPTL2-1, siRNA-ANGPTL2-2 and siRNA-NC vectors, HK-2 cells were randomly divided into five groups: normal glucose group (control), high glucose treatment group (HG), siRNA-NC group (NC), siRNA-ANGPTL2-1 group and siRNA-ANGPTL2-2 group. Subsequently, the MEK/ERK inhibitor U0126 (10 μM/L) was added to co-culture for 1 h. HK-2 cells were randomly divided into four groups: control normal glucose group (control), high glucose treatment group (HG), high glucose plus siRNA-ANGPTL2-1 vector-transfected group, and high glucose plus siRNA-ANGPTL2-1 vector-transfected plus U0126 group.
Enzyme-linked immunosorbent assay (ELISA)
Serum was isolated from rats’ blood. Additionally, after treatment, HK-2 cell culture supernatants from different groups were collected and centrifuged at 3000 r/min for 15 min, and supernatant was extracted at 4°C for later use. Then the serum supernatants were used to assess the contents of IL-6, TNF-α and IL-1β using the ELISA kits (Abcam, USA). Each sample was assayed in triplicate and experiment was repeated three times to lessen inter and intra-assay coefficients of variation.
NO measurement
NO concentration in renal tissue was quantified using the Griess reaction. The specific steps were performed according to the instructions of the kit (E1030, APPLYGEN, Beijing, China).
Immunofluorescence
For renal tissue, antigen retrieve was performed by the method of pressure cooker after deparaffinization and rehydration of slides. After blockage of goat serum, slides were incubated with rabbit anti-Collagen IV antibody (ab6586, 1:250) and rabbit anti-LC3-II antibody (ab48394, 1:200) at 4°C overnight. After rinsed with PBS for three times, slides were incubated with conjugated secondary antibody for 2 h at 37°C and stained with DAPI for 1 h. Slides were observed by fluorescence microscope. For HK-2 cells cultured on chamber slides, then cells were fixed with 4% paraformaldehyde and permeabilized with PBS containing 0.1% Triton X-100. Cells were incubated with primary antibody anti-Collagen IV antibody (ab6586, 1:250) and anti-LC3-II antibody (ab48394, 1:200) at 4°C overnight followed by incubation of secondary antibody for 2 h at 37°C. DAPI (Life Technologies Corporation, Gaithersburg, MD, USA) was used as counterstain for 1 h. Slides were examined by fluorescence microscope and analyzed with Image J software.
Western blot analysis
Renal tissue or HK-2 cells were lysed with cold RIPA lysis buffer including protease inhibitors and total protein were extracted after centrifuge (12000 r/min) at 4°C for 15 min. Protein concentration was quantified by BCA method. Then protein was denatured by storing in a water of 100°C for 5 min after mixed with loading buffer and the supernatant was used for electrophoresis. Subsequently, equal amounts of total protein (30 µg) was loaded per lane and electrophoresed in the denatured SDS-PAGE gel at 80 V for 15 min and the gel was separated at 120 V until bromophenol blue ran to the bottom of the gel. After proteins were transferred to PVDF membrane, 5% skimmed milk powder was sealed at room temperature for 2 h. Then, blots were incubated with primary antibody obtained from Abcam company, such rabbit anti-PTEN antibody (ab170941, 1:1000), mouse anti-fibronectin antibody (ab6328, 1:500), rabbit anti-Collagen I antibody (ab34710, 1:1000), rabbit anti-Collagen IV antibody (ab6586, 1:1500), rabbit anti-Beclin1 antibody (ab62557, 1:1000), rabbit anti-p62 antibody (ab155686, 1:500), rabbit anti-iNOS antibody (ab3523, 1:500), rabbit anti-ANGPTL2 antibody (ab199133, 1:500), rabbit anti-GAPDH antibody (ab9485, 1:2000) at 4°C overnight. The next day, blots were incubated with goat anti-rabbit antibody (ab205718, 1:5000) and goat anti-mouse (ab205719, 1:5000) for 2 h at room temperature. After washed with TTBS, blots were placed in a gel imager and treated with ECL super-sensitive solution for different time. Whereafter, exposure and visualization were performed, and bands were evaluated with Gel-pro analyzer software for quantitative analysis, normalized for GAPDH.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from renal tissue and HK-2 cells using the Trizol reagent kit (Invitrogen). The cDNA was synthesized with the RNA and the reverse transcription kit (Takara Bio Inc., Japan). To investigate the level of ANGPTL2 mRNA, real-time PCR was performed with real-time PCR reagents according to the following protocol: 95°C for 5 min, 95°C for 10 s, 55°C for 30 s, 72°C for 10 s, 40 cycles. The specific primers for ANGPTL2 (Thermo Fisher Scientific) were 5’-CGCCTGGATGGCTCTGTC-3’ (forward) and 5’-GTTTGTAGTTGCCTTGGTTCGTG-3’ (reverse). GAPDH, used as a control, was amplified using the following specific primers: Forward, 5’-CGTGCCGCCTGGAGAAACCTG-3’; and reverse, 5’-AGAGTGGGAGTTGCTGTTGAAGTCG-3’ (Thermo Fisher Scientific, Inc.). ANGPTL2 mRNA level in the renal tissue of DN rats and HK-2 cells were calculated with the 2-ΔΔCq method [22].
Statistical analysis
Result is shown as means ± standard deviation (SD). Data were expressed using ANOVA and Tukey’s test. SPSS (version 17.0) statistical software was used for all calculations, with P < 0.05 as the difference being statistically significant.
Results
Levels of blood glucose and urine protein in DN rats
In order to evaluate the success of the establishment of the DN rat model, the FBG level and 24 h urine protein level were measured using an automatic biochemical analyzer. The results revealed that FBG and 24 h urine protein present higher level in the rats after STZ treatment for 1-3 days while low level in rats before STZ treated (Figure 1A), which indeed proves that the DN rat model has been successfully established.
Figure 1.
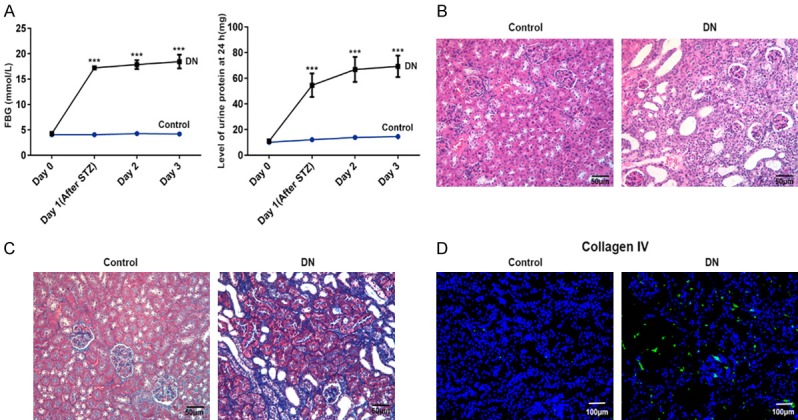
Increased fasting blood-glucose (FBG), 24-hour urine protein level, renal tissue injury, renal fibrosis, and collagen IV were demonstrated in kidney of DN rats in comparison with control normal rats by methods of an automatic biochemistry analyzer, HE staining, Masson staining and immunofluorescence. A. The broken line graph of FBG and 24-hour urine protein level in kidney of DN rats and control normal rats. B. HE staining for renal tissue injury in kidney of DN rats and control normal rats (magnification, ×200). C. Masson staining for renal fibrosis in kidney of DN rats and control normal rats (magnification, ×200). D. Immunofluorescence of collagen IV (indicated in green) with DAPI counterstaining in control normal rats and DN rats. n = 9. ***P < 0.001 versus control group.
Renal fibrosis level in kidney of DN rats
HE staining and Masson staining were chosen to detect the pathological and renal fibrosis level in kidney of DN rats. It can be seen in Figure 1B and 1C that there was no evident change in glomerular volume or structure and renal fibrosis in kidney of normal rats (control). However, the glomerular basement membrane thickened, mesangial hyperplasia and glomerular volume was revealed in renal interstitium of DN rats. Again, significant renal fibrils accumulation (stained with blue) was found in kidney of DN rats.
Furthermore, collagen IV known as fibrotic protein was detected by immunofluorescence to prove to be significantly up-regulated in kidney of DN rats (Figure 1D). Meanwhile, the results of western blot showed that the fibrosis-associated proteins, PTEN, FN, collagen I and IV, are elevated in renal tissue significantly when compared with that in the control group (Figure 2A).
Figure 2.
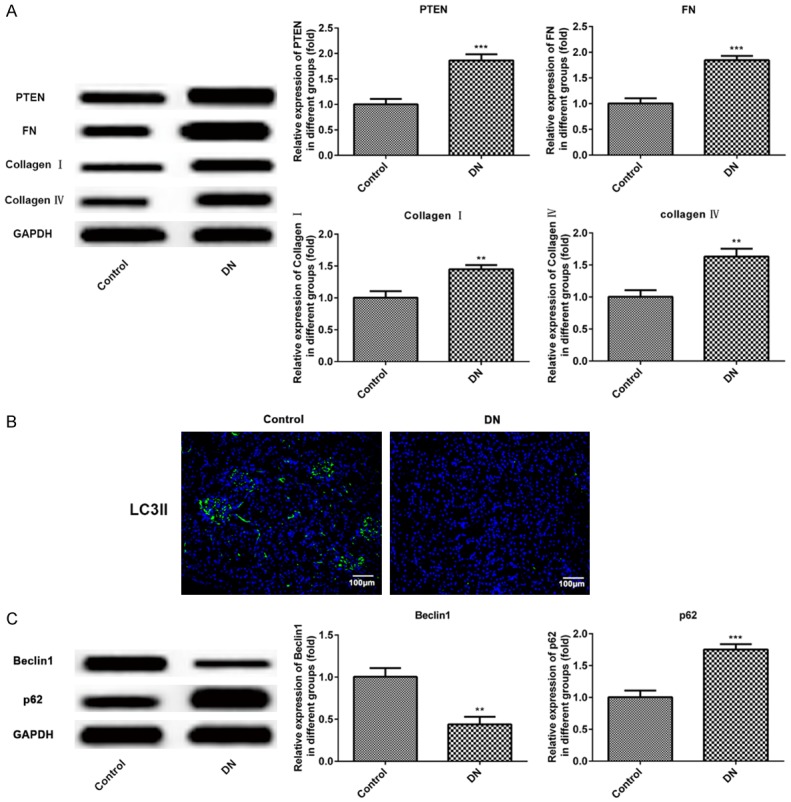
Increased PTEN, FN, collagen I, IV, p62, decreased LC3II and beclin1 were revealed in DN rats and control normal rats. A. Western blot detection and statistical analysis for PTEN, FN, collagen I and IV proteins in kidney of control normal rats and DN rats. B. Immunofluorescence of LC3II (indicated in green) with DAPI counterstaining in control normal rats and DN rats. C. Western blot of beclin1 and p62 proteins expression in kidney of control normal rats and DN rats. n = 9. **P < 0.01, ***P < 0.001 versus control group.
The level of autophagy in renal tissue of DN rats
Immunofluorescence analysis revealed that LC3II was low expressed in renal tissue of DN rats comparing to the control group (Figure 2B). Then, we analyzed the protein levels of autophagy-associated proteins, p62 and beclin1, using western blot assay. The results indicated that beclin1 of autophagy promoting protein was down-regulated and p62 autophagy inhibiting protein was up-regulated, both dramatically in DN rats when compared with the control group (Figure 2C). These results indicated that the autophagy level was significantly decreased in diabetic nephropathy rats.
The levels of inflammatory factors in serum and ANGPTL2 expression in renal tissue of DN rats
Serum levels of inflammatory factors, IL-6, IL-1β and TNF-α, were particularly elevated in DN rats comparing to the control group (Figure 3A). What’s more, the level of NO and the iNOS expression were all up-regulated in renal tissue of DN rats compared with the control group (Figure 3B and 3C). The oxidative stress may play an important role in the pathogenesis of diabetic nephropathy. To investigate the role of ANGPTL2 in DN rats, RT-qPCR and western blot were performed to detect the ANGPTL2 expression. As a result, both the ANGPTL2 mRNA level and its protein concentration were significantly increased in the renal tissue of DN rats (Figure 3D and 3E). Significant increase in serum ANGPTL2 concentration in DN rats when compared with normal rats (Figure 3F). Meanwhile, the results of immunohistochemistry were consistent with that of the RT-qPCR and western blot, ANGPTL2 expression was increased in renal tissue of DN rats. Specifically, brown-yellow granules were obviously increased in the DN group, and were concentrated around the renal tubular epithelial cells (Figure 3G). These findings establish ANGPTL2 is a bioactive molecular that may has a role in DN diseases.
Figure 3.
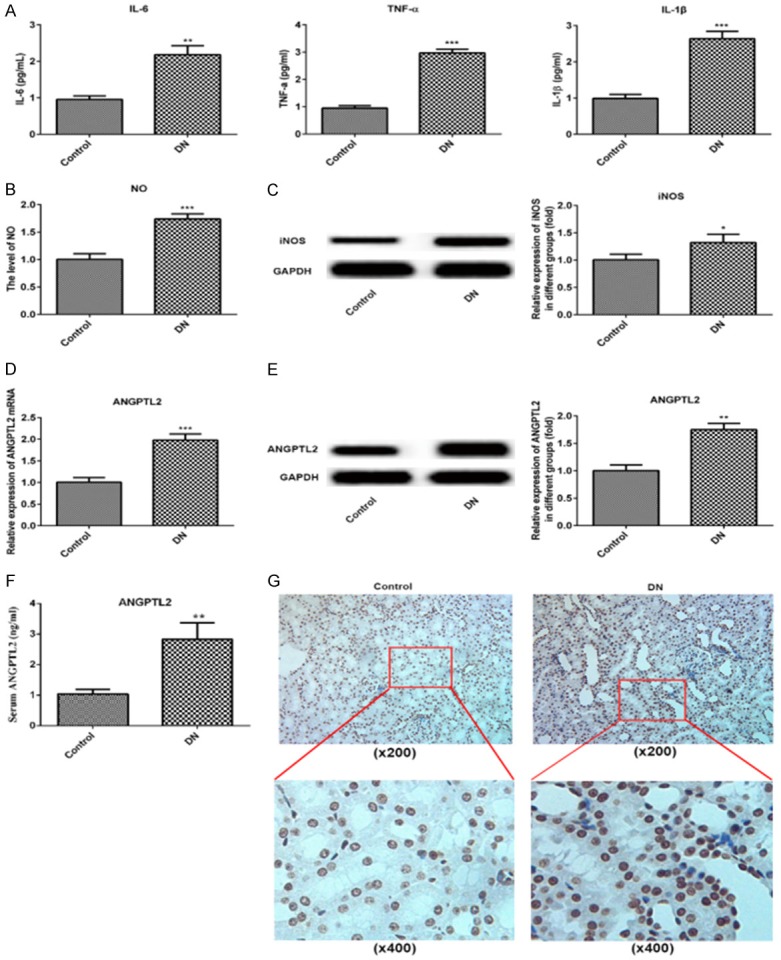
Increased IL-6, TNF-α, IL-1β, NO, iNOS and ANGPTL2 were showed in in DN rats and control normal rats. A. ELISA detection and statistical analysis for serum IL-6, TNF-α and IL-1β in control normal rats and DN rats. B. Griess reaction for NO concertration in in kidney of control normal rats and DN rats. C. Western blot detection and statistical analysis for iNOS in kidney of control normal rats and DN rats. D. Real-time qPCR of ANGPTL2 mRNA with an internal control of GAPDH in kidney of control normal rats and DN rats. E. Western blot of ANGPTL2 protein expression in kidney of control normal rats and DN rats. F. The level of serum ANGPTL2 was detected by method of ELISA in normal or DN rats. G. Immunohistochemistry of ANGPTL2 expression in kidney of control normal rats and DN rats. N = 9. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group.
ANGPTL2 affects autophagy and inflammatory changes of HK-2 cells induced by high glucose via activating MEK/ERK/Nrf-1 signal pathway
After two different interfering plasmids of si-ANGPTL2-1 and si-ANGPTL2-2 were constructed and transfected into the HK-2 cells, and the transfection efficiency of them was verified by RT-qPCR and western blot assays. As shown in Figure 4A and 4B, ANGPTL2 overexpressed in the HG-induced HK-2 cells, and the level in protein and mRNA of ANGPTL2 were significantly decreased in both si-ANGPTL2-1 and si-ANGPTL2-2 groups and the interference efficiency was the most obvious in the si-ANGPTL2-1 group. Therefore, the si-ANGPTL2-1 was selected for further experimental study.
Figure 4.
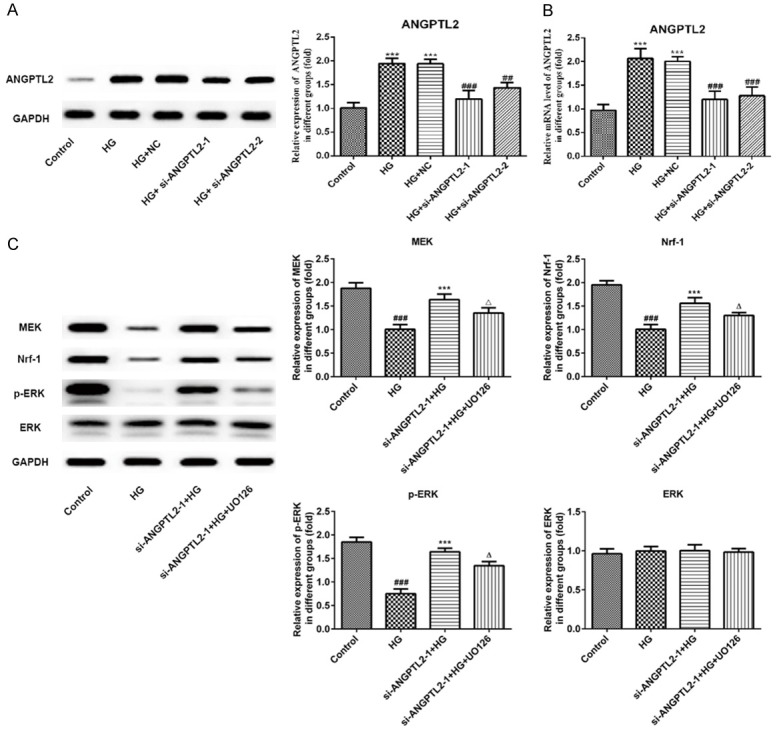
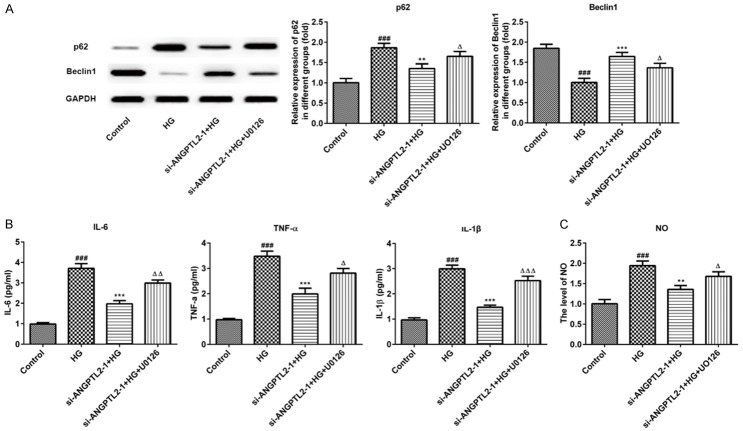
The effects of knocking down of ANGPTL2 and U0126 on MEK, ERK, phospho-ERK, and Nrf-1 in HG-induced HK-2 cells. (A) Western blot and statistical analysis and (B) real-time qPCR of ANGPTL2 protein and mRNA with an internal control of GAPDH in HK-2 cells of normal glucose group (control), high glucose group (HG), control vector-transfected group (NC), si-ANGPTL2-1 vector-transfected group (si-ANGPTL2-1), and si-ANGPTL2-2 vector-transfected group (si-ANGPTL2-2). Each experiment was repeated three times. ***P < 0.001 versus control group; ##P < 0.01, ###P < 0.001 versus HG+NC group. (C) The expression and statistical analysis of MEK, ERK, phospho-ERK, and Nrf-1 by Western blot in HK-2 cells of control normal group (control), high glucose group (HG), high glucose plus transfected with si-ANGPTL2-1 vector group (si-ANGPTL2-1+HG), and high glucose plus transfected with si-ANGPTL2-1 vector plus U0126 group (si-ANGPTL2-1+HG+U0126). Each experiment was repeated three times. ###P < 0.001 versus control group; ***P < 0.001 versus HG group; ΔP < 0.05 versus si-ANGPTL2-1+HG group.
To further investigate the effect of ANGPTL2 on regulating autophagy process via regulating MEK/ERK/Nrf-1 pathway, the MEK/ERK inhibitor (U0126) was added to HK-2 cells to block the MEK/ERK/Nrf-1 signaling pathway. Then, we performed western blotting analysis of the MEK/ERK/Nrf-1 signaling pathway. First, we found that MEK, P-ERK and Nrf-1 expressions in HK-2 cells were significantly decreased after HG induction comparing to control group, indicating that HG induction could inhibit the activation of the MEK/ERK/Nrf-1 pathway (Figure 4C). Next, knockdown of ANGPTL2 with si-ANGPTL2-1 obviously up-regulated the protein levels of MEK, P-ERK and Nrf-1 compared with the HG group in HK-2 cells. Rather, U0126 reversed the promoting effect of si-ANGPTL2-1 on the MEK/ERK/Nrf-1 signaling pathway and down-regulated the MEK, P-ERK and Nrf-1 expressions when compared with that in the si-ANGPTL2-1+HG group (Figure 4C). These results suggested that ANGPTL2 may play a role in inhibiting autophagy and promoting inflammatory responses by activating MEK/ERK/Nrf-1 signaling pathway.
As shown in Figure 5, the immunofluorescence results in HK-2 cells showed that LC3II expression was significantly decreased after HG induction and autophagy was inhibited. In addition, si-ANGPTL2-1 could up-regulate the level of LC3II and promote autophagy, and then U0126 could decrease the expression of LC3II and inhibit autophagy, thus reversing the effect of si-ANGPTL2-1 on HK-2 cells. Consistently, there was increased level of p62 and decreased level of beclin1 in HK-2 cells after HG induction (Figure 6A). Additionally, ANGPTL2 downregulation inhibit p62 expression and promote beclin1 expression, and then U0126 upregulated p62 expression and downregulated beclin2 expression comparing to the si-ANGPTL2-1+HG group (Figure 6A).
Figure 5.
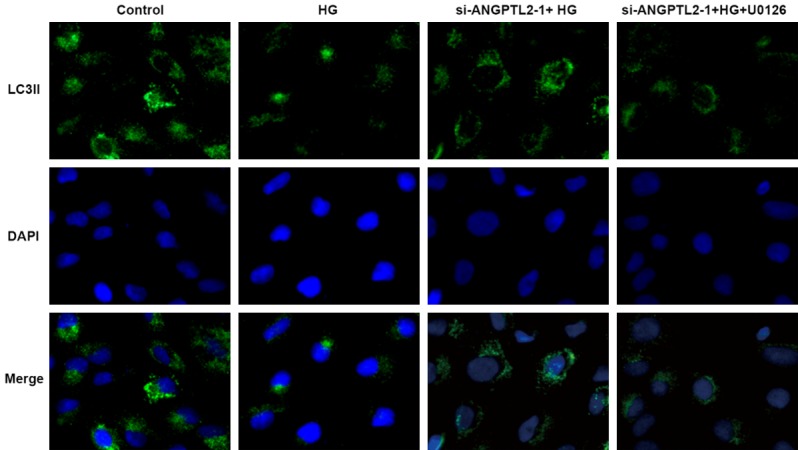
Immunofluorescence of LC3II counterstained by DAPI in HK-2 cells of control normal group (control), high glucose group (HG), high glucose plus transfected with si-ANGPTL2-1 vector group (si-ANGPTL2-1+HG), and high glucose plus transfected with si-ANGPTL2-1 vector plus U0126 group (si-ANGPTL2-1+HG+U0126). Each experiment was repeated three times.
Figure 6.
The effects of knocking down of ANGPTL2 and U0126 on p62, beclin1, IL-6, TNF-α, IL-1β, and NO in HG-induced HK-2 cells. A. Expression of p62 and beclin1 were determined by Western blot and quantified by densitometry in HK-2 cells of control normal group (control), high glucose group (HG), high glucose plus transfected with si-ANGPTL2-1 vector group (si-ANGPTL2-1+HG), and high glucose plus transfected with si-ANGPTL2-1 vector plus U0126 group (si-ANGPTL2-1+HG+U0126). B. ELISA for IL-6, TNF-α and IL-1β in in HK-2 cells of control normal group (control), high glucose group (HG), high glucose plus transfected with si-ANGPTL2-1 vector group (si-ANGPTL2-1+HG), and high glucose plus transfected with si-ANGPTL2-1 vector plus U0126 group (si-ANGPTL2-1+HG+U0126). C. Griess reaction for NO concertration in HK-2 cells of control normal group (control), high glucose group (HG), high glucose plus transfected with si-ANGPTL2-1 vector group (si-ANGPTL2-1+HG), and high glucose plus transfected with si-ANGPTL2-1 vector plus U0126 group (si-ANGPTL2-1+HG+U0126). Each experiment was repeated three times. ###P < 0.001 versus control group; **P < 0.01, ***P < 0.001 versus HG group; ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001 versus si-ANGPTL2-1+HG group.
To verify the role of ANGPTL2 in the inflammatory process, we examined the expression of inflammatory cytokines (IL-6, TNF-α and IL-1β) in the HK-2 cells using an ELISA assay. Meantime, the levels of NO and iNOS were measured. As shown in Figure 6B, the levels of IL-6, TNF-α and IL-1β were all increased in high glucose-treated HK-2 cells. However, si-ANGPTL2-1 inhibited these inflammatory cytokines and U0126 promoted inflammation by upregulating the expression of IL-6, TNF-α and IL-1β. Interestingly, the levels of NO (Figure 6C) and iNOS (Figure 7A) were consistent with trends in above inflammatory cytokines.
Figure 7.
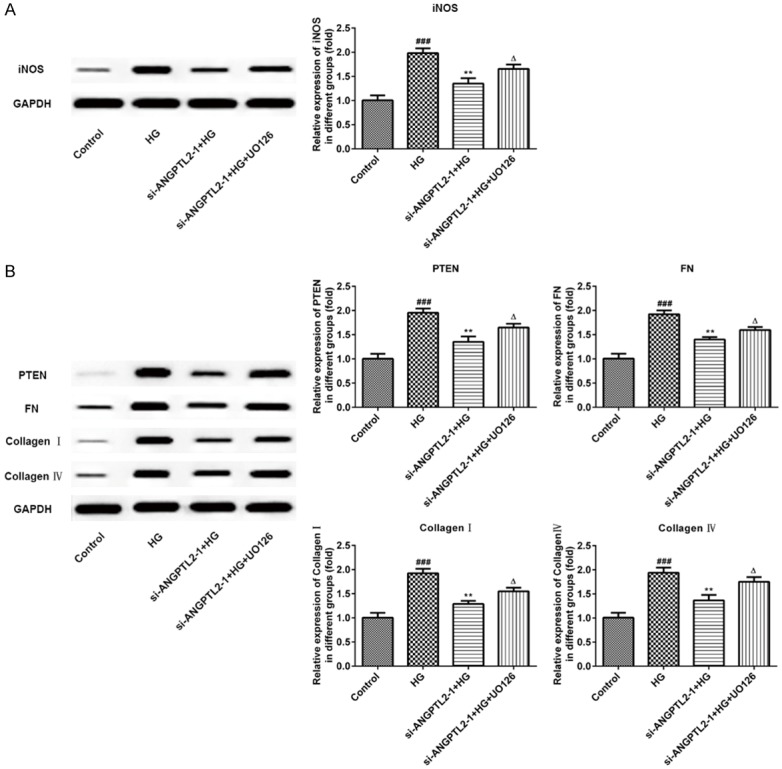
The effects of knocking down of ANGPTL2 and U0126 on iNOS, PTEN, FN, collagen I and IV in HG-induced HK-2 cells. A. Western blot detection and statistical analysis for iNOS in HK-2 cells of control normal group (control), high glucose group (HG), high glucose plus transfected with si-ANGPTL2-1 vector group (si-ANGPTL2-1+HG), and high glucose plus transfected with si-ANGPTL2-1 vector plus U0126 group (si-ANGPTL2-1+HG+U0126). B. Protein level of PTEN, FN, collagen I and IV were determined by Western blot and quantified by densitometry in HK-2 cells of control normal group (control), high glucose group (HG), high glucose plus transfected with si-ANGPTL2-1 vector group (si-ANGPTL2-1+HG), and high glucose plus transfected with si-ANGPTL2-1 vector plus U0126 group (si-ANGPTL2-1+HG+U0126). Each experiment was repeated three times. ###P < 0.001 versus control group; **P < 0.01 versus HG group; ΔP < 0.05 versus si-ANGPTL2-1+HG group.
ANGPTL2 affects fibrosis-associated protein levels of HK-2 cells induced by high glucose via activating MEK/ERK/Nrf-1 signal pathway
Finally, we determined whether knockdown of ANGPTL2 decreased the fibrosis in HK-2 cells. Western blotting and immunofluorescence were performed to detect the expressions of fibrosis-associated protein, including PTEN, FN, collagen I and IV. As shown in Figure 7B, HG induction upregulated the protein levels of PTEN, FN, collagen I and IV comparing to the control group, while these protein were all inhibited in the si-ANGPTL2-1+HG group comparing to the HG group. The treatment of U0126 increased PTEN, FN, collagen I and IV expression when compared with that in the si-ANGPTL2-1+HG group. Lastly, the results of immunofluorescence again confirmed this point, and the fluorescence expression level of collagen IV was consistent with the results of western blotting above (Figure 8). These results indicated ANGPTL2 may be a new target for reducing renal fibrosis in diabetic nephropathy.
Figure 8.
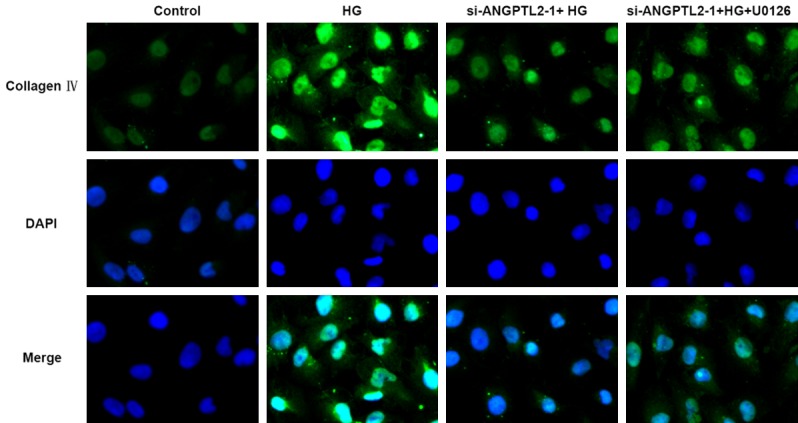
Immunofluorescence staining for collagen IV with DAPI counterstaining in HK-2 cells of control normal group (control), high glucose group (HG), high glucose plus transfected with si-ANGPTL2-1 vector group (si-ANGPTL2-1+HG), and high glucose plus transfected with si-ANGPTL2-1 vector plus U0126 group (si-ANGPTL2-1+HG+U0126). Each experiment was repeated three times.
Discussion
We demonstrated that ANGPTL2, a novel DN-associated growth factor identified in the gene chip screening, is a key mediator of renal fibrosis and autophagy in diabetic nephropathy.
The overexpression of ANGPTL2 in DN shows a close relationship to abnormal microvasculature and endothelial inflammation. A recent report demonstrated that in renal disease, increased albuminuria and decreased glomerular filtration rate were positively correlated with increased serum ANGPTL2 levels in patients with type 2 diabetes [23]. Besides, ANGPTL2 may be involved in the inflammatory mechanisms of adipose tissue and may contribute to the insulin resistance related to obesity [17]. Studies have shown that PTEN has a protective effect on myocardial fibrosis, liver fibrosis, pulmonary fibrosis and renal fibrosis, and it is a marker of fibrosis [24]. The protein p62, known as sequestosome 1, localized at the autophagosomes through interaction with LC3 and degraded by the autophagy-lysosome system [25].
In our present study, we also revealed that ANGPTL2 was upregulated in the renal tissue of DN rats with severe renal injury and renal fibrosis, and high levels of inflammation, NO and iNOS, which was accompanied by a significant decrease in autophagy in vivo compared with that of normal rats. Again, it was proved in the present research high glucose could promote ANGPTL2 expression in DN rats and in vitro cultured HK-2 cells. Knock down of ANGPTL2 reversed HG-induced increased fibrosis and inflammation levels and decreased autophagy level in HK-2 cells. These above data suggested strongly that ANGPTL2 played an important role in regulating renal fibrosis and autophagy process.
However, the exact mechanism involved in HG-induced change of ANGPTL2, fibrosis and autophagy is still unknown. Prolonged hyperglycemia induces insulin resistance and oxidative stress, which in turn activates the P38 and extracellular signal-regulated kinase (ERK) signaling pathways, thereby increasing the expression of transforming growth factor-β (TGF-β), FN, and collagen I and IV in glomerular mesangial cells. Previous study have shown that ROS are the most common factors in activating podocyte autophagy and podocytes exposed to angiotensin II (ANGII) also increased the generation of ROS and promoted autophagy activation [26]. Excessive oxidative stress activates a variety of intracellular signaling pathways and stimulates the production of a large number of transcription factors, thereby increasing the deposition of ECM and eventually leading to renal fibrosis [27]. Notably, DN is a complex metabolic disease characterized by decreased insulin sensitivity, leading to insulin resistance, which is an important indicator of hyperglycemia and hyperinsulinemia, which leads to glucose metabolic disturbance, inflammation and the stress response, resulting in the further damage of islet β-cells [27]. Another research also shows that the ameliorative effects of berberine on renal injury and proliferation of glomerular mesangial cells may be explained by reducing the expression of the c-Fos and EGR-1 belonging to MEK/ERK-dependent transcription factors, which play regulatory roles in the activation of growth factors and re-entry process of cell cycle [28]. Our results showed that knocking down of ANGPTL2 in HG-induced HK-2 cells caused directly activated MEK/ERK/Nrf-1 signaling, increased autophagy level and less fibrosis. However, inhibition of MEK/ERK with U0126 followed by down-regulation of autophagy level, and up-regulation of renal fibrosis and inflammation level, were revealed in HG-induced HK-2 cells. The above data suggested MEK/ERK/Nrf-1 pathway participated ANGPTL2 expression and the progression of renal fibrosis and autophagy in kidney of diabetic nephropathy.
Conclusion
In summary, increased ANGPTL2 suppress renal autophagy and mediates renal fibrosis through inhibition of MEK/ERK/Nrf-1 pathway, overexpression of PTEN, FN, collagen I, IV, and secretion of inflammatory cytokines in renal tissue of DN rats and HK-2 cells. Knockdown of ANGPTL2 is suggested to be a potential way to lessen the development of renal fibrosis in kidney of diabetic nephropathy.
Disclosure of conflict of interest
None.
References
- 1.Liu W, Hao J, Zhu L, Li F, Liu Q, Liu S, Zhao S, Li H, Duan H. Phospho-GSK-3beta is involved in the high-glucose-mediated lipid deposition in renal tubular cells in diabetes. Int J Biochem Cell Biol. 2013;45:2066–2075. doi: 10.1016/j.biocel.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Ina K, Kitamura H, Tatsukawa S, Takayama T, Fujikura Y, Shimada T. Transformation of interstitial fibroblasts and tubulointerstitial fibrosis in diabetic nephropathy. Med Electron Microsc. 2002;35:87–95. doi: 10.1007/s007950200011. [DOI] [PubMed] [Google Scholar]
- 3.Kolset SO, Reinholt FP, Jenssen T. Diabetic nephropathy and extracellular matrix. J Histochem Cytochem. 2012;60:976–986. doi: 10.1369/0022155412465073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker AE, Boutell J, Carr A, Maciewicz RA. Novel cartilage-specific splice variants of fibronectin. Osteoarthritis Cartilage. 2002;10:528–534. doi: 10.1053/joca.2002.0792. [DOI] [PubMed] [Google Scholar]
- 5.Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs. 2011;194:25–37. doi: 10.1159/000322399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, Gregorevic P, Kantharidis P, Cooper ME. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond) 2015;129:1237–1249. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- 7.Zhu L, Zhao S, Liu S, Liu Q, Li F, Hao J. PTEN regulates renal extracellular matrix deposit via increased CTGF in diabetes mellitus. J Cell Biochem. 2016;117:1187–1198. doi: 10.1002/jcb.25402. [DOI] [PubMed] [Google Scholar]
- 8.Liu N, Xu L, Shi Y, Zhuang S. Podocyte autophagy: a potential therapeutic target to prevent the progression of diabetic nephropathy. J Diabetes Res. 2017;2017:3560238. doi: 10.1155/2017/3560238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu YY, Chen CS, Wu SN, Jong YJ, Lo YC. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur J Pharm Sci. 2012;46:415–425. doi: 10.1016/j.ejps.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Tziomalos K, Athyros VG. Diabetic nephropathy: new risk factors and improvements in diagnosis. Rev Diabet Stud. 2015;12:110–118. doi: 10.1900/RDS.2015.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi BH, Ahn IS, Kim YH, Park JW, Lee SY, Hyun CK, Do MS. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp Mol Med. 2006;38:599–605. doi: 10.1038/emm.2006.71. [DOI] [PubMed] [Google Scholar]
- 14.Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology. 2005;146:3417–3427. doi: 10.1210/en.2004-1468. [DOI] [PubMed] [Google Scholar]
- 15.Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 16.Kadomatsu T, Endo M, Miyata K, Oike Y. Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol Metab. 2014;25:245–254. doi: 10.1016/j.tem.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, Kaikita K, Miyashita K, Iwawaki T, Shimabukuro M, Sakaguchi K, Ito T, Nakagata N, Yamada T, Katagiri H, Kasuga M, Ando Y, Ogawa H, Mochizuki N, Itoh H, Suda T, Oike Y. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10:178–188. doi: 10.1016/j.cmet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Okada T, Tsukano H, Endo M, Tabata M, Miyata K, Kadomatsu T, Miyashita K, Semba K, Nakamura E, Tsukano M, Mizuta H, Oike Y. Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am J Pathol. 2010;176:2309–2319. doi: 10.2353/ajpath.2010.090865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, Ninomiya T, Horiguchi H, Endo M, Tabata M, Tazume H, Tian Z, Takahashi O, Terada K, Takeya M, Hao H, Hirose N, Minami T, Suda T, Kiyohara Y, Ogawa H, Kaikita K, Oike Y. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler Thromb Vasc Biol. 2014;34:790–800. doi: 10.1161/ATVBAHA.113.303116. [DOI] [PubMed] [Google Scholar]
- 20.Aoi J, Endo M, Kadomatsu T, Miyata K, Nakano M, Horiguchi H, Ogata A, Odagiri H, Yano M, Araki K, Jinnin M, Ito T, Hirakawa S, Ihn H, Oike Y. Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011;71:7502–7512. doi: 10.1158/0008-5472.CAN-11-1758. [DOI] [PubMed] [Google Scholar]
- 21.Morinaga J, Kadomatsu T, Miyata K, Endo M, Terada K, Tian Z, Sugizaki T, Tanigawa H, Zhao J, Zhu S, Sato M, Araki K, Iyama K, Tomita K, Mukoyama M, Tomita K, Kitamura K, Oike Y. Angiopoietin-like protein 2 increases renal fibrosis by accelerating transforming growth factor-beta signaling in chronic kidney disease. Kidney Int. 2016;89:327–341. doi: 10.1016/j.kint.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Chung AB, Stepien G, Haraguchi Y, Li K, Wallace DC. Transcriptional control of nuclear genes for the mitochondrial muscle ADP/ATP translocator and the ATP synthase beta subunit. Multiple factors interact with the OXBOX/REBOX promoter sequences. J Biol Chem. 1992;267:21154–21161. [PubMed] [Google Scholar]
- 24.Gao Y, Chu M, Hong J, Shang J, Xu D. Hypoxia induces cardiac fibroblast proliferation and phenotypic switch: a role for caveolae and caveolin-1/PTEN mediated pathway. J Thorac Dis. 2014;6:1458–1468. doi: 10.3978/j.issn.2072-1439.2014.08.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 26.Yadav A, Vallabu S, Arora S, Tandon P, Slahan D, Teichberg S, Singhal PC. ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol. 2010;299:C488–496. doi: 10.1152/ajpcell.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni WJ, Ding HH, Tang LQ. Berberine as a promising anti-diabetic nephropathy drug: an analysis of its effects and mechanisms. Eur J Pharmacol. 2015;760:103–112. doi: 10.1016/j.ejphar.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Liang KW, Ting CT, Yin SC, Chen YT, Lin SJ, Liao JK, Hsu SL. Berberine suppresses MEK/ERK-dependent Egr-1 signaling pathway and inhibits vascular smooth muscle cell regrowth after in vitro mechanical injury. Biochem Pharmacol. 2006;71:806–817. doi: 10.1016/j.bcp.2005.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]