Abstract
Background and objectives: Pancreatic cancer is one of the most lethal cancer types. Pancreatic cancer is highly malignant and characterized by rapid and uncontrolled growth. This study was designed to investigate the effect of baicalin on proliferation and apoptosis in pancreatic cancer cells. Methods: CCK-8 assay and Clone formation assay were performed to detect the effect of baicalin on proliferation in pancreatic cancer cells. Cell invasion and migration were all assessed with Wound healing assay and Transwell invasion assay. Flow Cytometry Analysis and DAPI staining were performed to detect the effect of baicalin on apoptosis in pancreatic cancer cells. Furthermore, proliferation-associated protein and apoptosis-related protein were detected to evaluate the cell proliferation and apoptosis levels. P-JNK protein, t-JNK protein, Foxo1 protein and BIM protein were examined by western blot to verify whether baicalin could regulate the proliferation and apoptosis via the JNK/Foxo1/BIM signaling pathway in pancreatic cancer cells. Results: The cell proliferation level was significantly decreased while the cell apoptosis level was significantly increased in pancreatic cancer SW1990 cells treated with baicalin. As the same, baicalin downregulated the ability of invasion and migration in pancreatic cancer SW1990 cells. Conclusion: Baicalin might inhibit cell proliferation and promote cell apoptosis via JNK/Foxo1/BIM signaling pathway in pancreatic cancer SW1990 cells.
Keywords: Baicalin, proliferation, apoptosis, pancreatic cancer cell
Introduction
Pancreatic cancer is a digestive system tumor with high degree of malignancy and poor prognosis, accounting for the fourth leading cause of cancer-related deaths worldwide [1]. The incidence and mortality rates of pancreatic cancer show a sustained increase recently, with the overall 5-year relative survival rate from 5% to 6% [2]. According to the GLOBOCAN 2012, pancreatic cancer accounts for more than 0.33 million deaths per year [3]. It is characterized by rapid disease progression and metastatic ability, high resistance to chemotherapy and radiotherapy, and difficult early diagnosis so that most patients have no chance to cure it [4]. Therefore, exploration of new strategies for the treatment of pancreatic cancer is urgently needed.
Apoptosis and proliferation, as two major mechanisms which regulates pathways to control cell state, are suggested for consideration as the targeted therapy strategies for tumor cells [5]. Previous studies demonstrated that CDK2 and cyclinD1 genes are the most important regulators in controlling the proliferation of cells [6]. CDK2 forms a complex with cyclinE1 to promote G1/S transition and forms a complex with cyclinA to bypass the S phase and the G2/M phase [7]. P16 is known to be an important tumor suppressor gene functioning as cell cycle regulator [8], thus regulation of the function of CDK2, cyclinD1 and p16 is an appropriate strategy to inhibit the proliferation of cancer cells. The p53 tumor suppressor protein can inhibit tumor formation, in part by inducing apoptosis. It has been demonstrated that p53 is able to regulate the transcription and expression of proapoptotic and anti-apoptotic proteins, including Bax, Bcl-2 and caspase-3, resulting in cellular apoptosis [9]. It is known that JNK phosphorylates c-Jun at Ser63 and Ser73 regions to activate the pro-apoptotic effects of c-Jun [10]. Also, JNK enhances expression of FOXO-regulated genes and FOXO can regulate cell apoptosis, cycle and ROS resistance by affecting Bim [11].
In recent years, many phytochemicals, with low toxicity and adverse side effects, have been observed to exhibit anti-cancer activities and thus may represent potential alternative medicine to conventional cytotoxic chemotherapy [12]. Baicalin is an active flavone isolated from Scutellaria baicalensis Georgi, with a chemical formula of C21H18O11 and has been demonstrated to have anti-proliferation and antitumor effects [13]. It has been reported that we can inhibit the proliferation of various cancer cells through inhibition of migration and invasion and induction of apoptosis [14]. However, the effects of baicalin on pancreatic cancer cells and the underlying molecular mechanisms are still not clear. So, the present study aims to evaluate whether baicalin can exert antitumor effects on pancreatic cancer cells and investigate the underlying molecular mechanisms. In conclusion, the findings in our study suggest that baicalin may be identified as a novel regulator in inhibiting pancreatic cancer progress by JNK/Foxo1/BIM pathway. Therefore, in-depth study on the molecular mechanisms will provide new strategies for the treatment.
Materials and methods
Cell culture
Pancreatic cancer SW1990 cell lines were obtained from the Second Military Medical University (Shanghai, China). SW1990 cells was grown in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS, Gibco) and antimycotic agents (100 units/ml penicillin, 100 μg/ml streptomycin; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in an incubator with standard culture conditions (37°C, 5% CO2 and 95% humidity).
Cell Counting Kit-8 (CCK-8) assay
Cell viability was measured by CCK-8 assay following the instructions of manufacturer. In brief, cells were digested, counted, and prepared into the cell suspension at a concentration of 5×103 cells/ml in 96-well plates cultured in an incubator with standard culture conditions (37°C, 5% CO2 and 95% humidity). After treatments, 10 μL of CCK-8 solution was added to each 96-well plates and the mixture was incubated for 3 hours in the incubator at 37°C. λ=450 nm, and the OD value of each well was read by a microplate reader (Bio-Rad, Hercules, CA, USA).
Clone formation assay
Colony-formation assays were conducted to evaluate the Cell proliferation ability. Suspensions containing 5000 cells per well were seeded into the six-well plates and individually exposed to doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. After treatments, the cells were incubated for 48 hours in an incubator with standard culture conditions (37°C, 5% CO2 and 95% humidity). Next, the colonies were fixed with carbinol for 15 minutes and stained with 0.1% Giemsa for 30 minutes. Clones were counted under an inverted microscope and clones containing >50 cells were counted for statistical analysis. Cells in each group were plated in three duplicate wells and all experiments were conducted at least for three times.
Wound healing assay
Stable cells were plated into the 6-well cell culture plates and grown to near 100% confluence. Then, the cells were scratched with a 200 μL pipette tip and the detached cells were washed with phosphate-buffered saline (PBS). Images of cells migrating at the corresponding wound sites were taken at 0 and 24 h with an inverted microscope and the wound areas were determined using Image J software. Cell migration was expressed as the percentage of wound closure/initial wound area. All experiments were performed at least for three times.
Transwell invasion assay
Transwell invasion assays were performed to evaluate the cell invasive ability. Sw1990 cells were seeded on Matrigel-coated transwell inserts with 500 μL of serum free medium. The lower chamber was filled with 500 μL of medium containing 10% FBS. After incubation for 24 h, the cells remaining on upper surface of transwell inserts were wiped with cotton wool. The invaded cells were stained with crystal violet for 5 min and counted under an inverted microscope. Data were collected from three independent experiments.
Flow cytometry analysis of cell apoptosis
A cell apoptosis experiment was performed to determine the apoptosis rate with a FITC Annexin V Apoptosis Detection Kit according to the manufacturer’s instructions. In brief, cells (5×105) were seeded in 6-well plates and treated with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. The cell samples cultured for 48 h after treatment were harvested with 0.25% trypsin, then washed twice with ice-cold PBS and re-suspended in binding buffer. The cells were double-stained with FITC-conjugated Annexin V and propidium iodide (FITC-Annexin V/PI) (BD Biosciences, San Diego, CA, USA) for 20 minutes in the dark. The mixture was then analyzed on a flow cytometer (FACSCalibur; BD Biosciences) with the excitation wavelength of Ex=488 nm and emission wavelength of Em=530 nm according to the manufacturer’s protocol. The experiments were performed in triplicate and repeated at least for three times.
Detection of apoptosis via DAPI staining
After treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin respectively, the cells were harvested, washed with a phosphate-buffered saline (PBS) twice, and fixed in 4% formaldehyde prepared with PBS. The cells were stained with 1.0 mg/mL of 4’, 6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich Chemical Co.) solution for 15 min at 37°C. The stained cells were transferred to a glass slide and morphology changes in the nucleus were observed with a fluorescence microscope (Olympus BX51, Tokyo, Japan).
Measurement of reactive oxygen species (ROS) level
2’, 7’-dichlorodihyrofluorecein diacetate (DCHF-DA) is a dye that can be oxidized by ROS to fluorescent compound dichlorofluorescein (DCF), which is monitored by fluorescence microplate spectrophotometry. ROS level is quantified by the fluorescence intensity of DCF with a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA). In briefly, the cells were washed twice with phosphate-buffered saline (PBS) and then stained with DCFH-DA (10 μmol/l) at 37°C for 20 min according to the manufacturer’s protocol. ROS levels of cell samples were assessed by a commercial ROS assay kit (Nanjing Jancheng Bioengineering Institute, Nanjing, China).
Western blot analysis
Western blot analysis was used to detect the relative protein expression level including CDK2, cyclinE1, p15, bcl-2, bax, cleaved caspase3, caspase3, p53, p-JNK, JNK, Foxo1 and BIM, as described. The total protein in cell samples was extracted from pancreatic cancer SW1990 cells with a mixture of RIPA buffer (10 mM Tris-HCl, pH 7.4, 1% Triton X-100, 0.1% SDS), protease inhibitors and phosphatase inhibitor, which protect proteins from degradation by an endogenous protease. The total protein concentration was determined with an Enhanced BCA Protein Assay kit (P0010; Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s protocol. Equal amounts (20 μg) of total protein lysate per lane was used for Western blot analysis with the following method: Proteins were separated by a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (5%-10%) gel and then transferred to a polyvinylidene fluoride (PDVF) membrane (Sigma-Aldrich; Merck KGaA). Non-specific protein interactions were blocked by 5% fat-free milk in 1X TBST buffer at room temperature for 1 h. Following this treatment, the PVDF membranes were incubated with primary antibodies against CDK2 (1:1000, ab32147, abcam, UK), cyclinE1 (1:2000, ab33911, abcam, UK), p15 (1:2000, ab53034, abcam, UK), bcl-2 (1:2000, ab185002, abcam, UK), bax (1:1000, ab32503, abcam, UK), cleaved caspase3 (1:500, ab49822, abcam, UK), caspase3 (1:500, ab13847, abcam, UK), p53 (1:1000, ab32389, abcam, UK), p-JNK (1:500, ab47337, abcam, UK), JNK (1:2500, ab199380, abcam, UK), Foxo1 (1:5000, ab70382, abcam, UK), BIM (1:1000, ab32158, abcam, UK) and GAPDH (1:10000, ab181602, abcam, UK) at 4°C overnight. On second day, the PVDF membranes were incubated in a solution of goat anti-rabbit (1:20000, ab205718, abcam, UK) or anti-mouse IgG (1:10000, ab6708, abcam, UK) at room temperature for 1 h. GAPDH was used as an endogenous control. Protein bands were visualized by an enhanced chemiluminescence (ECL) kit (sc-2048, Sigma-Aldrich, Merck KGaA) following the manufacturer’s instructions. Data were analyzed with Image Pro Plus v.6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) for densitometry.
Statistical analysis
Data are processed using SPSS 12.0 software (SPSS, Inc., Chicago, IL, USA) and presented as the mean ± standard deviation with Graph-PAD prism software. All the experiments were performed at least for three times. A Student t-test was used for statistical comparison between means where applicable. Differences among more than two groups in the above assays were estimated using one-way ANOVA. Differences were considered significant when P-value less than 0.05.
Results
Baicalin inhibit proliferation of pancreatic cancer SW1990 cells
To further elucidate the biological role of baicalin on pancreatic cancer, we investigate the effects of baicalin on pancreatic cancer cell proliferation. Pancreatic cancer SW1990 cells were incubated for 24, 48, 72 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. CCK-8 assay data showed that baicalin could significantly inhibit pancreatic cancer cell proliferation in a dose-dependent manner (P<0.01, P<0.05, P<0.001, Figure 1A) and in a time-dependent manner (P<0.05, P<0.01, Figure 1B). Clone formation assay showed that baicalin could significantly inhibit pancreatic cancer cell proliferation in a dose-dependent manner (P<0.01, P<0.05, P<0.001, Figure 1C and 1D).
Figure 1.
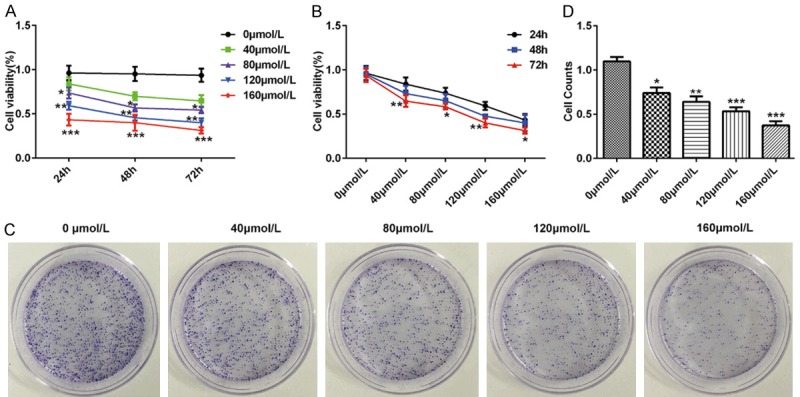
Baicalin inhibited proliferation of pancreatic cancer SW1990 cells. Pancreatic cancer SW1990 cells were incubated for 24, 48, 72 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. A. The time-dependent cell viability of pancreatic cancer SW1990 cells determined by CCK8; B. The dose-dependent cell viability of pancreatic cancer SW1990 cells determined by CCK8; C. The cell clone number of pancreatic cancer SW1990 cells determined by clone formation assay; D. The cell clone number of pancreatic cancer SW1990 cells determined by clone formation assay. *P<0.05, **P<0.01, ***P<0.001.
Baicalin influenced cell cycle of pancreatic cancer SW1990 cells
To explore whether baicalin was associated with cell cycle, we detected protein levels of CDK2, cyclinE1 and p15 by western blot. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. Baicalin obviously decreased the protein levels of CDK2 (P<0.01, P<0.05, P<0.001, Figure 2A and 2B) and cyclinE1 (P<0.01, P<0.05, P<0.001, Figure 2A and 2C) and increased the protein level of p15 (P<0.01, P<0.05, P<0.001, Figure 2A and 2D) in a dose-dependent manner.
Figure 2.
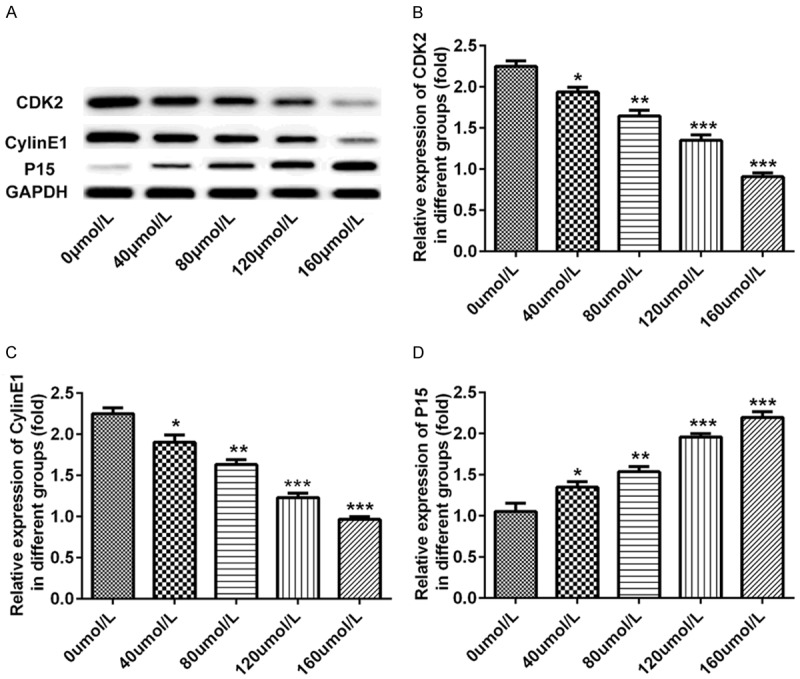
Baicalin influenced cell cycle of pancreatic cancer SW1990 cells. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. A. The dose-dependent protein band of CDK2, cyclinE1 and p15 determined by western blot; B. The dose-dependent protein level of CDK2 determined by western blot; C. The dose-dependent protein level of cyclinE1 determined by western blot; D. The dose-dependent protein level of p15 determined by western blot. *P<0.05, **P<0.01, ***P<0.001.
Baicalin decreased migratory and invasive ability of pancreatic cancer SW1990 cells
To determine whether baicalin can influence the migration and invasion of pancreatic cancer SW1990 cells, wound-healing assay and trans-well assay were performed. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. Wound-healing assay showed that baicalin could suppress the migration of pancreatic cancer SW1990 cells in a dose-dependent manner (P<0.01, P<0.05, P<0.001, Figure 3A and 3B). Similar results were obtained in trans-well assay. Trans-well assay verified that baicalin could suppress the invasion of pancreatic cancer SW1990 cells in a dose-dependent manner (P<0.01, P<0.05, P<0.001, Figure 3C and 3D).
Figure 3.
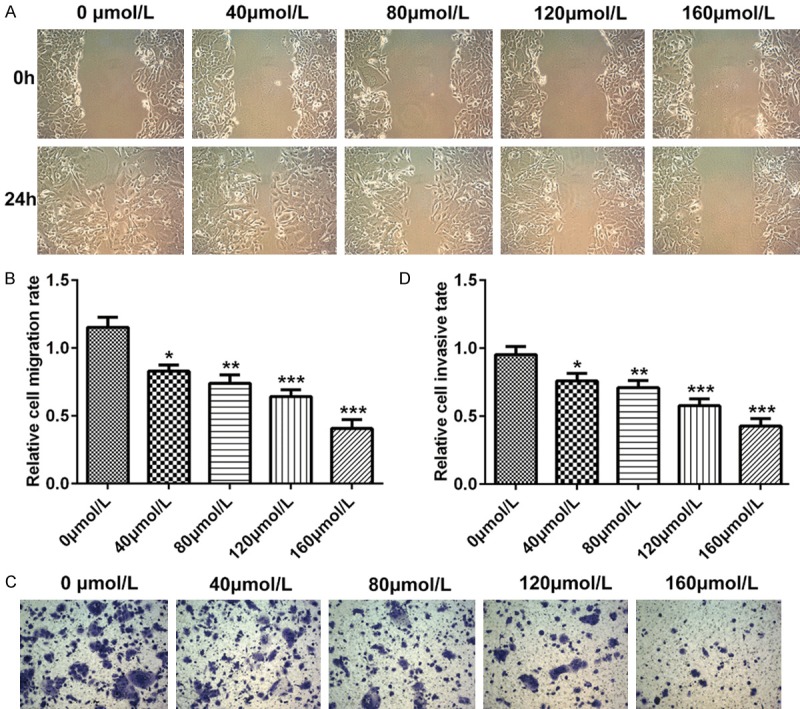
Baicalin decreased migratory and invasive ability of pancreatic cancer SW1990 cells. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. A. The dose-dependent migratory ability of pancreatic cancer SW1990 cells determined by wound-healing assay; B. The dose-dependent cell counts of pancreatic cancer SW1990 cells determined by wound-healing assay; C. The dose-dependent invasive ability of pancreatic cancer SW1990 cells determined by transwell assay; D. The dose-dependent cell counts of pancreatic cancer SW1990 cells determined by transwell assay. *P<0.05, **P<0.01, ***P<0.001.
Baicalin promoted the apoptosis of pancreatic cancer SW1990 cells
To determine whether baicalin contributed to the apoptosis of pancreatic cancer SW1990 cells, we analyzed cell apoptosis of SW1990 cells by flow cytometry, DAPI staining and western blot. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. The result of flow cytometry showed that the apoptosis levels of SW1990 cells incubated with baicalin increased in a dose-dependent manner (P<0.01, P<0.001, Figure 4A and 4B). Also, DAPI staining assay showed that Baicalin promoted the apoptosis of SW1990 cells in a dose-dependent manner (P<0.05, P<0.01, P<0.001, Figure 4C and 4D). Besides, Baicalin obviously decreased the protein levels of Bcl-2 (P<0.01, P<0.001, Figure 5A and 5B) and increased the levels of Bax (P<0.05, P<0.01, P<0.001, Figure 5A and 5C), Cleaved caspase-3 (P<0.01, P<0.001, Figure 5A and 5D) and p53 (P<0.05, P<0.01, P<0.001, Figure 5A and 5E) in a dose-dependent manner.
Figure 4.
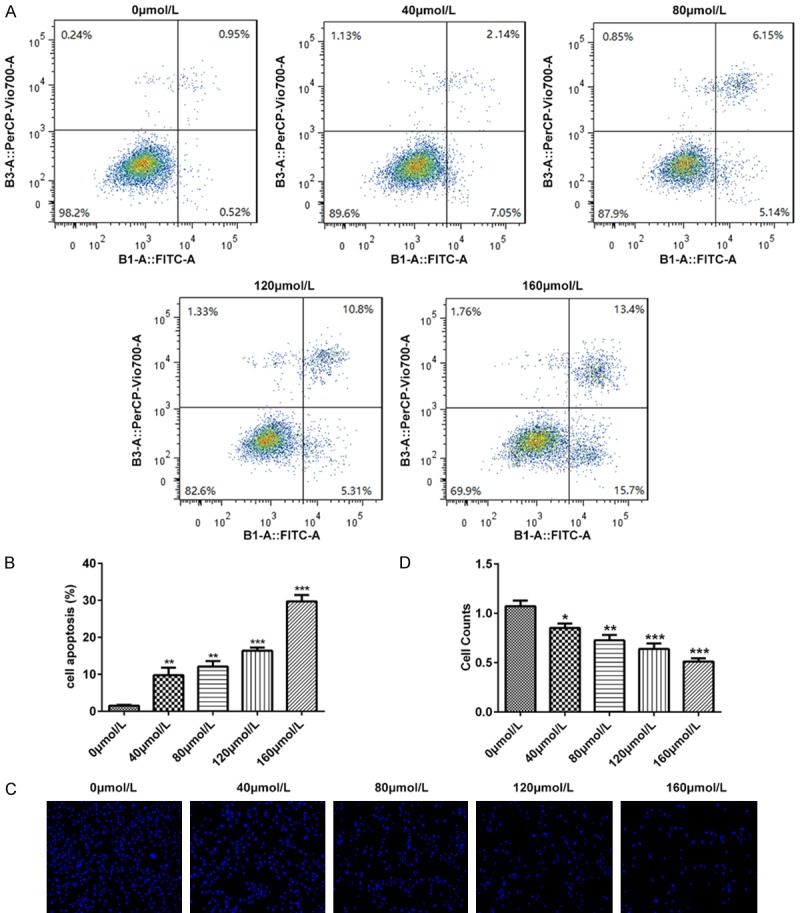
Baicalin promoted the apoptosis of pancreatic cancer SW1990 cells. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. A. The pancreatic cancer SW1990 cell apoptosis determined by flow cytometry; B. The dose-dependent cell apoptosis counts of pancreatic cancer SW1990 cells determined by flow cytometry; C. The pancreatic cancer SW1990 cell apoptosis determined by DAPI staining; D. The dose-dependent cell apoptosis counts of pancreatic cancer SW1990 cells determined by DAPI staining. *P<0.05, **P<0.01, ***P<0.001.
Figure 5.
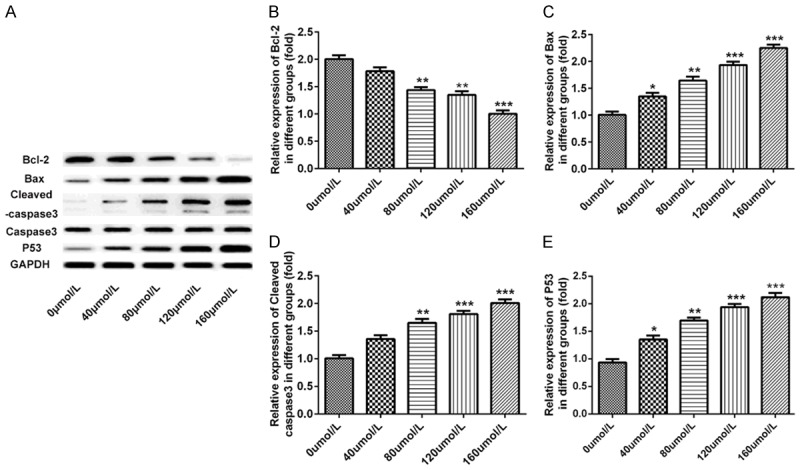
Baicalin significantly promoted apoptosis of pancreatic cancer SW1990 cells. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. A. The dose-dependent protein band of Bcl-2, Bax, Cleaved caspase-3, caspase-3 and p53 determined by western blot; B. The dose-dependent protein level of Bcl-2 determined by western blot; C. The dose-dependent protein level of Bax determined by western blot; D. The dose-dependent protein level of Cleaved caspase-3 determined by western blot; E. The dose-dependent protein level of p53 determined by western blot. *P<0.05, **P<0.01, ***P<0.001.
Baicalin increased the ROS level of pancreatic cancer SW1990 cells
To analyze whether Baicalin would affect the ROS level in pancreatic cancer SW1990 cells, we detected the ROS level with DCFH-DA ROS assay. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. The result showed that baicalin could increase the ROS level of pancreatic cancer SW1990 cells in a dose-dependent manner, and NAC could obviously decrease the ROS level (P<0.001, Figure 6).
Figure 6.
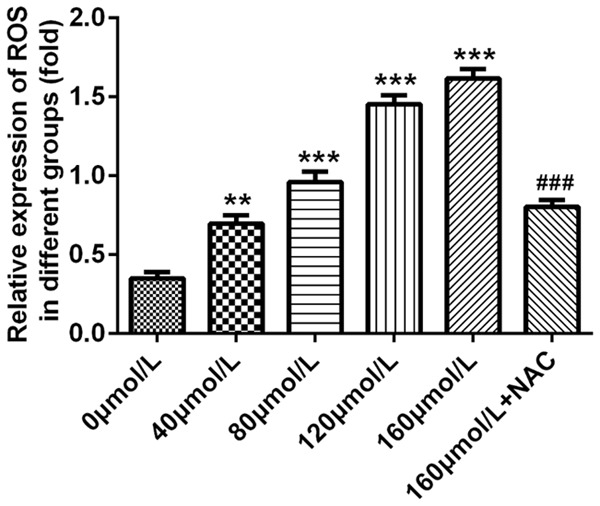
Baicalin increased the ROS level of pancreatic cancer SW1990 cells. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. The ROS level of pancreatic cancer SW1990 cells determined by DCFH-DA ROS assay. **P<0.01, ***P<0.001, ###P<0.001.
Baicalin activated JNK/Foxo1/BIM pathway
To investigate the influence of baicalin on JNK/Foxo1/BIM pathway in pancreatic cancer SW1990 cells, we assessed the protein levels of JNK, p-JNK, Foxo1 and BIM. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. As expected, baicalin obviously increased the protein levels of p-JNK (P<0.05, P<0.01, P<0.001, Figure 7A and 7B), Foxo1 (P<0.05, P<0.01, P<0.001, Figure 7A and 7C) and BIM (P<0.01, P<0.001, Figure 7A and 7D) in a dose-dependent manner. However, free radical scavenger (NAC) reversed the effect of baicalin. NAC significantly decreased the protein levels of p-JNK (P<0.01, Figure 7A and 7B), Foxo1 (P<0.001, Figure 7A and 7C) and BIM (P<0.001, Figure 7A and 7D).
Figure 7.
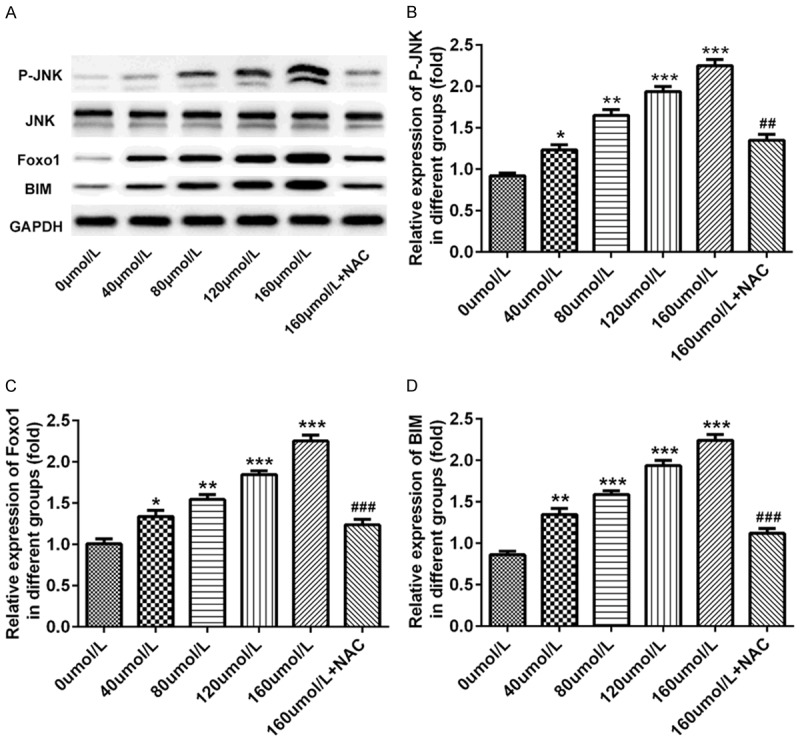
Baicalin activated JNK/Foxo1/BIM pathway. Pancreatic cancer SW1990 cells were incubated for 48 hours after treatment with doses of 0, 40, 80, 120 and 160 μmol/L baicalin, respectively. A. The dose-dependent protein band of JNK, p-JNK, Foxo1 and BIM determined by western blot; B. The dose-dependent protein level of p-JNK determined by western blot; C. The dose-dependent protein level of Foxo1 determined by western blot; D. The dose-dependent protein level of BIM determined by western blot. *P<0.05, **P<0.01, ***P<0.001, ###P<0.001.
Discussion
Recently, the use of plant extracts to prevent the progress of cancer is getting more and more attention [15]. In our study, we revealed that baicalin could significantly inhibit pancreatic cancer cell proliferation and influence cell cyc0le in a dose-dependent manner. Besides, we demonstrated that baicalin was a positive regulator of pancreatic cancer cell apoptosis. We also evaluated that the ROS level of pancreatic cancer cells increased after incubating with baicalin. Finally, we find that baicalin influenced pancreatic cancer cells through JNK/Foxo1/BIM pathway.
It has been well reported that baicalin have extensive antitumor activity [16]. A research showed that baicalin can inhibit the viability and proliferation of hepatic cancer cell lines in a dose-dependent manner [17]. In the present study, we found that baicalin inhibit proliferation and clone of pancreatic cancer cells. It has been reported that baicalin suppresses human osteosarcoma cells’ migration, invasion, and anoikis [18]. Results revealed that baicalin markedly increased apoptosis of pancreatic cancer by influence cell cycle and apoptosis proteins. Also, we found that baicalin could inhibit pancreatic cancer cell’ migration and invasion. Recently, it has been reported in the literature that baicalin triggered a significant generation of reactive oxygen species (ROS) [19]. Previous studies demonstrated that baicalin can increase the ROS level in pancreatic cancer cells. JNK/Foxo1/BIM pathway has been reported to regulate cell apoptosis, cycle and ROS resistance [13,20]. And our research proved that baicalin can activate JNK/Foxo1/BIM pathway. Here, results confirmed that baicalin influence pancreatic cancer cells by activating JNK/Foxo1/BIM pathway.
In conclusion, baicalin was identified as a novel regulator in inhibiting pancreatic cancer progress by JNK/Foxo1/BIM pathway. Therefore, this study provided new insights into the possibility of baicalin being a potential therapeutic target in pancreatic cancer.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Cai J, Du S, Guo Z, Xin B, Wang J, Wei W, Shen X. Fractalkine/CX3CR1 induces apoptosis resistance and proliferation through the activation of the AKT/NF-κB cascade in pancreatic cancer cells. Cell Biochem Funct. 2017;35:315–326. doi: 10.1002/cbf.3278. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 5.Roshanravan N, Asgharian P, Dariushnejad H, Mesri Alamdari N, Mansoori B, Mohammadi A, Alipour S, Barati M, Ghavami A, Ghorbanzadeh V, Aamazadeh F, Ostadrahimi A. Eryngium billardieri induces apoptosis via bax gene expression in pancreatic cancer cells. Adv Pharm Bull. 2018;8:667–674. doi: 10.15171/apb.2018.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etemadmoghadam D, Au-Yeung G, Wall M, Mitchell C, Kansara M, Loehrer E, Batzios C, George J, Ftouni S, Weir BA, Carter S, Gresshoff I, Mileshkin L, Rischin D, Hahn WC, Waring PM, Getz G, Cullinane C, Campbell LJ, Bowtell DD. Resistance to CDK2 inhibitors is associated with selection of polyploid cells in CCNE1-amplified ovarian cancer. Clin Cancer Res. 2013;19:5960–5971. doi: 10.1158/1078-0432.CCR-13-1337. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Zhao Y, Wang C, Ju H, Liu W, Zhang X, Miao S, Wang L, Sun Q, Song W. Rhomboid domain-containing protein 1 promotes breast cancer progression by regulating the p-Akt and CDK2 levels. Cell Commun Signal. 2018;16:65. doi: 10.1186/s12964-018-0267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012;130:1715–1725. doi: 10.1002/ijc.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry H, Thomas A, Shen Y, White E. Regulation of the mitochondrial checkpoint in p53-mediated apoptosis confers resistance to cell death. Oncogene. 2002;21:748–760. doi: 10.1038/sj.onc.1205125. [DOI] [PubMed] [Google Scholar]
- 10.Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim Biophys Acta. 2010;1804:463–475. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 11.La Colla A, Vasconsuelo A, Milanesi L, Pronsato L. 17β-Estradiol protects skeletal myoblasts from apoptosis through P53, BCL-2 and FoxO families. J Cell Biochem. 2017;118:104–115. doi: 10.1002/jcb.25616. [DOI] [PubMed] [Google Scholar]
- 12.Gao C, Zhou Y, Li H, Cong X, Jiang Z, Wang X, Cao R, Tian W. Antitumor effects of baicalin on ovarian cancer cells through induction of cell apoptosis and inhibition of cell migration in vitro. Mol Med Rep. 2017;16:8729–8734. doi: 10.3892/mmr.2017.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CZ, Zhang CF, Chen L, Anderson S, Lu F, Yuan CS. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int J Oncol. 2015;47:1749–1758. doi: 10.3892/ijo.2015.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Pei M, Li L. Baicalin induces apoptosis in hepatic cancer cells in vitro and suppresses tumor growth in vivo. Int J Clin Exp Med. 2015;8:8958–8967. [PMC free article] [PubMed] [Google Scholar]
- 15.Tao Y, Zhan S, Wang Y, Zhou G, Liang H, Chen X, Shen H. Baicalin, the major component of traditional Chinese medicine scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci Rep. 2018;8:14477. doi: 10.1038/s41598-018-32734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong WY, Zhao ZX, Liu BJ, Lu LW, Dong JC. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur J Med Chem. 2017;126:844–852. doi: 10.1016/j.ejmech.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 17.Yu Y, Pei M, Li L. Baicalin induces apoptosis in hepatic cancer cells in vitro and suppresses tumor growth in vivo. Int J Clin Exp Med. 2015;8:8958–67. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wang H, Zhou R, Zhong W, Lu S, Ma Z, Chai Y. Baicalin inhibits human osteosarcoma cells invasion, metastasis, and anoikis resistance by suppressing the transforming growth factor-β1-induced epithelial-to-mesenchymal transition. Anticancer Drugs. 2017;28:581–587. doi: 10.1097/CAD.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 19.Wan D, Ouyang H. Baicalin induces apoptosis in human osteosarcoma cell through ROS-mediated mitochondrial pathway. Nat Prod Res. 2018;32:1996–2000. doi: 10.1080/14786419.2017.1359173. [DOI] [PubMed] [Google Scholar]
- 20.Choi Y, Park J, Choi Y, Ko YS, Yu DA, Kim Y, Pyo JS, Jang BG, Kim MA, Kim WH, Lee BL. c-Jun N-terminal kinase activation has a prognostic implication and is negatively associated with FOXO1 activation in gastric cancer. BMC Gastroenterol. 2016;16:59. doi: 10.1186/s12876-016-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


