Abstract
Neuregulin 4 (Nrg4) play important roles in the pathogenesis of obesity-associated disorders, including type 2 diabetes mellitus (T2DM) and nonalcoholic fatty liver disease (NAFLD). This study aims to investigate roles of Nrg4 in the pathophysiologic mechanism underlying the progression of tubulointerstitial fibrosis (TIF) in diabetic nephropathy (DN). In present study, Nrg4 is under-expression in serum and renal tissue of diabetic nephropathy rats. In present study, Nrg4 attenuate renal function injury, tubulointerstitial fibrosis, inflammation and suppress the expression levels of advanced glycosylation end products (AGEs) in vivo and vitro. Furthermore, the results reveal that Nrg4 ameliorates fibrosis and attenuate the expression of AGEs and inflammation via TNF-R1 signaling instead of TNF-R2 signaling in HK-2 cells. In conclusion, these results revealed that Nrg4 may effectively ameliorates TIF and attenuate the expression of AGEs in DN through TNF-R1 signaling instead of TNF-R2 signaling. We have provided evidence indicating that Nrg4 possesses therapeutic effect on TIF in DN.
Keywords: Neuregulin 4, tubulointerstitial fibrosis, diabetic nephropathy, TNF-R1
Introduction
Diabetic nephropathy (DN), identified as a cause of end-stage renal disease (ESRD), is a major micro-vascular complication with the chronic loss of kidney function occurring in diabetes mellitus (DM) [1]. With the increased incidence of DM, DN plays a critical role in increasing mortality and morbidity worldwide. Multiple mechanisms contribute to the occurrence and progression of DN, including excessive generation of advanced glycosylation end products (AGEs) and the interaction with their receptors, oxidative stress and renal hemodynamic alterations. It is well known tubulointerstitial fibrosis (TIF) always occur in the early stage of DN. TIF, characterized by excessive deposition of extracellular matrix (ECM) components, is considered as the main cause of renal disease worldwide [2]. When renal epithelial cells undergo heavy and prolonged insults, they can release multiple cytokines, involved in pathogenesis of interstitial fibroblasts and ECM production [3]. Fibronectin (FN) and collagen accumulation were reported to be linked in the development of TIF [4]. Furthermore, Li H et al. demonstrate autophagy-related gene 5 (ATG5) prevent the progression of renal fibrosis depend on its autophagic activity in proximal epithelial cells [2]. Previous studies have demonstrated that autophagy have renal protective effects on acute kidney injury, obstructive nephropathy, diabetic nephropathy, and other renal diseases [5]. Liu N et al. reported that activation of autophagy in podocytes may be a potential therapy to prevent the development of DN [6].
Phosphatase and tension homologue (PTEN), identified as inhibitor PI3K (phosphatidylinositol 3-kinase)/Akt (protein kinase B) signal pathway, was reported to be a potential target to prevent renal extracellular matrix secretion of DM [7]. PTEN was also reported to play important roles in cardiac fibroblast proliferation and phenotypic switch [8]. At present, increased evidences demonstrated oxidative stress and overexpression of tumor necrosis factor-α (TNFα) is related to excessive deposition of ECM, tubulointerstitial cell proliferation and apoptosis, eventually accelerating the DN progress [9,10]. The elevated levels of TNF receptors 1 and 2 (TNFR1 and TNFR2) in serum and urine were associated with the severity of renal interstitial fibrosis in nephropathy patients [11]. On binding of TNFα, TNFR1 recruits the adaptor protein, TNFR associated death domain (TRADD), directly to its cytoplasmic death domain. It is reported that both ubiquitination and degradation of TRADD were elevated in unilateral ureteral obstruction (UUO) kidneys, followed by TIF. Moreover, degradation of TRADD was stimulated by TNFα in proximal tubule cells [12].
Neuregulin 4 (Nrg4), identified as an adipokine, play important roles in regulating systemic energy metabolism and the pathogenesis of obesity-associated disorders, including type 2 diabetes mellitus (T2DM) and nonalcoholic fatty liver disease (NAFLD) [13]. Nrg4 was reported to be a member of the epidermal growth factor (EGF) family of extracellular ligands and has been found highly expressed in adipose tissues, enriched in brown adipose tissue (BAT) and markedly increased during brown adipocyte differentiation [14]. Previous study reported that the T2DM patients with the highest high-sensitivity C-reactive protein (hs-CRP) levels showed higher atherogenic coefficients and atherogenic index of plasma (AIP) levels, but lower levels of plasma Nrg4, as compared to those with the lowest hs-CRP levels [15]. Yan PJ et al. reported that decreased plasma Nrg4 levels is associated with augmented oxidative stress, inflammation [16], and dyslipidemia, and plasma Nrg4 levels were positively correlated with concentrations of high-density lipoprotein cholesterol (HDL-C) and apolipoprotein A and negatively correlated with triglyceride and hs-CRP [15]. BAT exert modulation effect on liver lipogenesis via secretion of the growth factor Nrg4 [17], Wang GX et al. further demonstrated that Nrg4 has potential therapeutic effect on obesity-associated disorders, including T2DM and NAFLD [14].
However, the role of Nrg4 in DN has not been studied, therefore this study aims to investigate the roles of Nrg4 in the pathophysiologic mechanism underlying the progression in TIF of DN.
Materials and methods
Animal model preparation
Male Sprague-Dawley (SD) rats (160-180 g) were obtained from Slaccas-Shanghai Lab Animal Ltd. All rats were kept in standard cages on a 12-hour light/dark cycle at 22-24°C with 55%-65% relative humidity and had ad libitum access to chow and tap water. All the rats received humane care in accordance with the Guidelines established by the Care and Use of Laboratory Animals of the National Institutes of Health. Procedures involving animals and their care were approved by Min Dong Hospital of Ning De City. After three-day acclimatization, the rats were allocated into two dietary regimens consisting of 7 and 28 rats by feeding either NPD or HFD (60% fat, as a percentage of total kcal) ad libitum, respectively. After 4 weeks (W), the rats were fast for overnight, subsequently, these rats were intraperitoneally injected freshly prepared streptozotocin (STZ) (35 mg·kg-1, dissolved in 0.1 mol·L-1 of cold citrate buffer, pH 4.5) to induce diabetes. One week later, postprandial blood glucose levels were detected by blood glucose test strips (Roche, Mannheim, Germany). The rats with blood glucose levels higher than 16.7 mmol·L-1 were defined as diabetic rats and chosen for the next experiments. Age-matched normal rats were given an equal volume of vehicle as the control. All rats used in the study were weighted once a week since feeding HFD or NPD, 24 h-urine samples were collected and recorded weekly and blood was obtained from the tail vein to measure non-fasting blood glucose.
Treatment with neuregulin 4
The diabetic rats were randomly allocated 4 groups of 7 animals each, including one non-treated diabetic group (model group) and three neuregulin 4 (Nrg4) treated diabetic groups (35, 70, 105 g/kg). The Nrg4 was intraperitoneally injected into the diabetic rats once daily for 4 weeks. Normal non-diabetic rats received normal saline orally as control group (n = 5).
Western blot analysis
Renal tissue or cultured cells were homogenized in lysis buffer (Sigma, R0278) supplemented with protease inhibitors. Total protein (25 μg) was separated by SDS-PAGE and transferred to membranes. Protein concentration was measured with the Bradford assay. The membranes were blocked with 5% nonfat milk powder and incubated with appropriate primary antibodies at 4°C overnight. Rabbit anti-PTEN antibody (1:1000), Rabbit anti-FN antibody (1:1000), Rabbit anti-Collagen I antibody (1:1000), Rabbit anti-Collagen IV antibody (1:1000), Rabbit anti-S100B Rabbit (1:1000), anti-TNF-R1 antibody (1:1000), Rabbit anti-TNF-R2 antibody (1:1000), Rabbit anti-TRADD antibody (1:1000) were purchased from Cell Signaling Technology (Danvers, USA), Mouse anti-AGEs antibody (1:500), Mouse anti-RAGES antibody (1:500) were purchased from Merck (Darmstadt, USA), Mouse anti-Neuregulin 4 antibody (1:1000) was purchased from Santa Cruz Biotechnology (California, USA). After washing with PBS, subsequently the membranes were probed with appropriate horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. Protein expression levels were visualized using Tanon-5200 Chemiluminescence Imager (Tanon, Shanghai, China) with ECL western blotting substrate (Millipore).
Quantitative real-time PCR (RT-qPCR)
Total RNA from kidney tissue, blood or cultured cells was extracted using the Trizol agent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. Then, Reverse Transcription regents (TaKaRa, Otsu, Shiga, Japan) was used to synthesize cDNAs. Quantitative real-time PCR was measured by using a System 7500 instrument (Applied Biosystems, Carlsbad, CA, USA). The relative expression was calculated by using the 2-ΔΔCt method. RNA U6 was performed as the internal control for miRNA and GAPDH was used for mRNA expression. The primer sequences for qPCR were as follows (Table 1):
Table 1.
The primer sequences
| Neuregulin 4 | Forward | 5’-CACCATGCCAACAGATCACGAAGAGCC-3’ |
| Reverse | 5’-GTGTTGTTCATGACTGTGGTGG-3’ | |
| TNF-R1 | Forward | 5’-CGGAAAGAAATGTTCCAGGT-3’ |
| Reverse | 5’-CACTGGAAATGCGTCTCACT-3’ | |
| GAPDH | Forward | 5’-CTCACCGGATGCACCAATGTT-3’ |
| Reverse | 5’-CGCGTTGCTCACAATGTTCAT-3’ |
Immunohistochemistry
The fixed renal tissues in rat were frozen and cut into 5 μm thick sections. Slides were immunostained by the streptavidin-biotin-peroxidase complex (SABC) method on paraffin sections. Slides were deparaffinized, rehydrated and immersed in 3% hydrogen peroxide to block endogenous peroxidase activity. After being blocked with 5% Bovine Serum Albumin (BSA), Slides were incubated primary antibody overnight. Mouse anti-Neuregulin 4 antibody (1:200) and Mouse anti-collagen IV (1:100) Santa Cruz Biotechnology (California, USA), Mouse anti-AGEs antibody (1:100) was purchased from Merck (Darmstadt, USA). All slides were incubated with biotinylated secondary antibodies and SABC reagent. The detection was visualized using chromogen and counterstaining with 3, 3’-diaminobenzidine-tetrahydrochloride followed with hematoxylin and examined under a light microscope at the 400× magnification.
Kidney histology analyses
The tissue samples were fixed with 4% paraformaldehyde, dehydrated in graded alcohols and embedded in paraffin. The paraffin-embedded kidneys tissues were sectioned into 2-µm-thick sections. Hematoxylin and eosin (H&E) and Masson staining was performed for the visualization of general histology. The stained tissues were observed using an optical microscope (Nikon Eclipse Ti; Nikon Corporation, Tokyo, Japan) at the 200× magnification, the renal fibrosis and collage deposition were estimated, respectively.
Cell culture and treatments of HK-2 cells
Immortalized tubular epithelial cells (HK-2) was purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in DMEM/F-12 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Gibco, MA, USA), 1% penicillin and streptomycin and maintained at 37°C in a humidified atmosphere containing 5% CO2.
The HK-2 cells were seeded into a 6-well plates, grown overnight and transfected with TNF-R1 plasmid to investigate the regulatory role of Neuregulin 4. All transfections were conducted with Lipofectamine 3000 in accordance with the manufacturer’s instructions. A blank vector pcDNA was used as a negative control (NC). TNF-R1 expression in cells following transfection was detected by RT-qPCR and western blot. Subsequently, High glucose stimulation was employed by culturing cells in DMEM medium containing 30 mM D-glucose (high glucose (HG)) for 48 h, followed by cell harvest for further experiments.
Immunofluorescence
Immunofluorescence was performed to visualize intracellular collagen IV and AGEs expression. The slides with HK-2 cells were washed with cold PBS for 5 min each time, followed by fixation with 4% paraformaldehyde (20 min, room temperature). Subsequently, the cells were permeabilized with 0.2% TritonX-100 (20 min, room temperature), and blocked with 3% BSA (40 min, room temperature). Then, the cells were probed with primary antibody with the following primary antibodies: Mouse anti-collagen IV (1:100) Santa Cruz Biotechnology (California, USA), Mouse anti-AGEs antibody (1:100) was purchased from Merck (Darmstadt, USA) in a humidified chamber at 4°C overnight, followed by secondary antibody for 1 h in a dark. Finally, Cells’ nuclei were counterstained with DAPI dye for 5 minutes and mounted with 95% glycerol. The images were observed and collected using a conventional light microscope (Olympus PD70) with a 400× magnification. Pictures were analyzed using the ImageJ software.
Biochemical assays
After mashing and homogenizing, the renal tissues were centrifuged at 4500 g for 10 min at 4°C, the supernatants were then collected as well as the supernatants of HK-2 cells. The levels of urine creatinine, IL-6, TNF-α and COX2 levels were measured using appropriate enzyme-linked immunoassay (ELISA) kits in accordance with the manufacturer’s instructions. Cytokine concentrations in samples were interpolated from recombinant IL-6, IL-1β TNF-α and COX2 standard curves. The levels of nitric oxide (NO) and inducible nitric oxide synthase (iNOS) were measured using commercial test kits according to the manufacturer’s protocols. Serum glucose, blood urea nitrogen (BUN) and serum creatinine (Scr) levels were measured using an automatic biochemistry analyzer (Hitachi, Tokyo, Japan).
Statistical analysis
Statistical data analysis was presented as the mean ± SEM with SPSS 19.0 and GraphPad Prism 5.0, and all the experiments were performance three times independently. Unpaired two tailed student’s t-test was employed to compare the difference between the two groups. The one-way analysis of variance (ANOVA) test followed by the Dunnett’s post hoc test was performed for comparisons in the multiple groups. Differences at P < 0.05 were considered statistically significant.
Results
Decreased level of neuregulin 4 in serum and renal tissue of diabetic nephropathy rats
To assess the levels of neuregulin 4 in serum and renal tissue, western blotting, RT-qPCR and immunohistochemistry were carried out. All data showed Nrg4 is under-expression in serum and renal tissue of diabetic nephropathy (DN) rats compared with control group, as showed in Figure 1 (P < 0.001).
Figure 1.
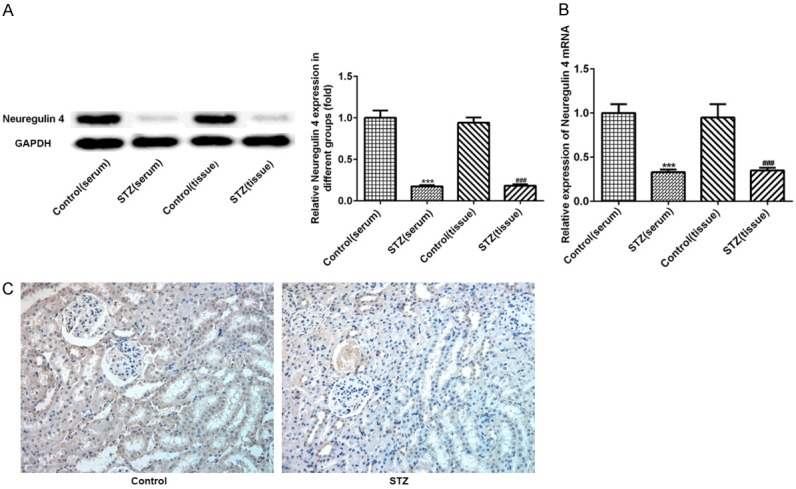
Decreased level of Nrg4 in serum and renal tissue of DN rats. Total protein and DNA was extracted from the renal tissue and serum. The level of Nrg4 was detected by Western blot analysis (A), RT-qPCR (B), Immunohistochemistry (C). The data are presented as the means ± SEM, n = 3. ***P < 0.001, vs. Control (serum) group. ###P < 0.001, vs. Control (tissure) group.
Neuregulin 4 attenuate renal function injury of diabetic nephropathy rats
To measure the roles of neuregulin 4 on renal functions, body weight and blood glucose were collected once week. Furthermore, serum creatinine (Scr), blood urea nitrogen (BUN) and urine creatinine, as the markers of renal functions, were also detected. As expected, there were no significant differences in body weight and blood glucose at baseline. Streptozocin (STZ) induced DN rats had significant weight gain and higher blood glucose, compared with control group. Notably, Nrg4 blocked the increment in body weight and blood glucose in a dose dependent manner (P < 0.05, P < 0.01, P < 0.001) (Figure 2A, 2B). Scr, BUN and urine creatinine levels strikingly elevated in serum of DN rats. In comparation with model group, Nrg4 suppressed this increment in a dose dependent manner (P < 0.05, P < 0.01, P < 0.001) (Figure 2C-E). In addition, hematoxylin and eosin (H&E) staining showed the obvious tubular epithelial disruption and hypertrophy of glomeruli, the results further demonstrated neuregulin 4 attenuate pathological damage in STZ-induced DN rats.
Figure 2.
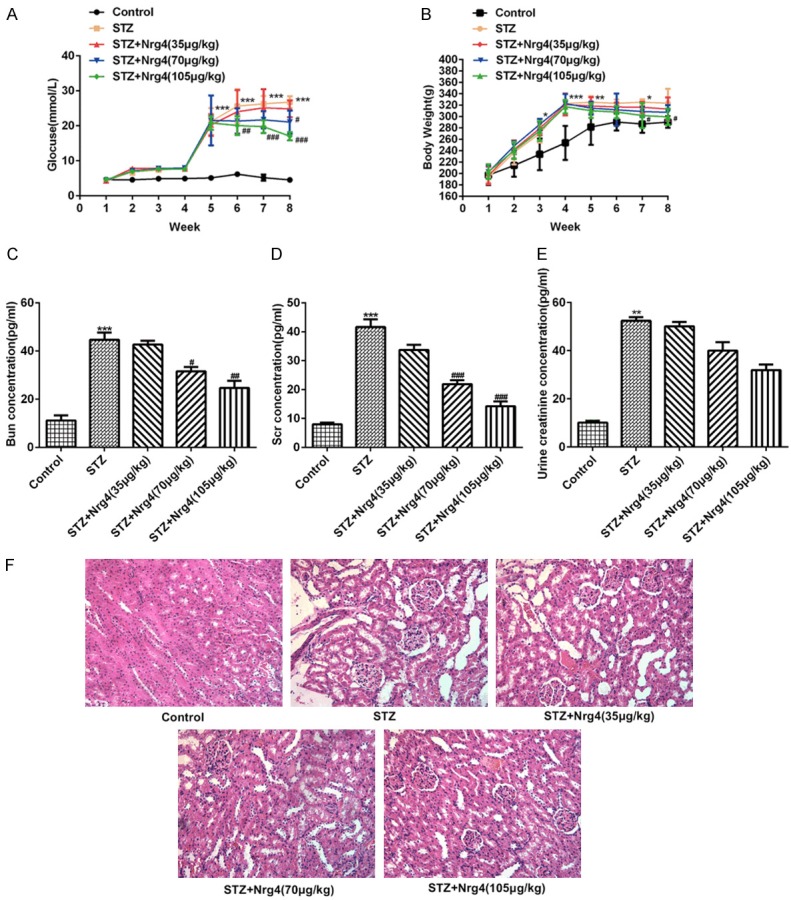
Influence of Nrg4 on body weight, blood glucose and renal function of DN rats (n = 7 for each group). A, B. Weight and blood glucose gain after combination of high-fat diet and STZ treatment, respectively. C. Blood urea nitrogen (BUN); D. Serum creatinine (Scr); E. Urine creatinine were measured using an automatic biochemistry analyzer. F. H&E staining of representative sections from kidney (×200). The data are presented as the means ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, vs. Control group. #P < 0.05, ##P < 0.01, ###P < 0.001, vs. STZ group.
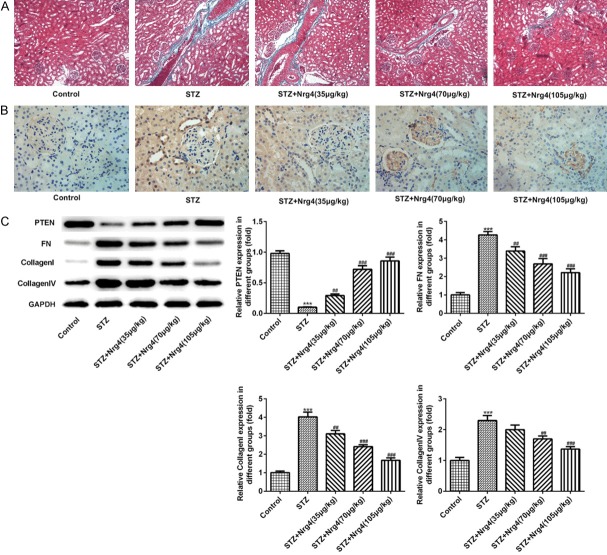
Neuregulin 4 ameliorates tubulointerstitial fibrosis
Masson staining indicated DN rats had higher levels of tubulointerstitial fibrosis (TIF) compared with normal rats, which was attenuated by Nrg4 treatment, as showed in Figure 3A. To further verify the amelioration of TIF by Nrg4 treatment, the western blotting and immunohistochemistry technique were used. The results showed that the expression levels of collagen I, collagen IV and fibronectin (FN) were markedly increased in DN rats. In contrast, the expression level of PTEN was decreased significantly in STZ group compared with control group (P < 0.001). Interestingly, Nrg4 treatment can suppress above abnormal expression notably (P < 0.01, P < 0.001), which showed in Figure 3B, 3C.
Figure 3.
Nrg4 ameliorates tubulointerstitial fibrosis. A. Masson staining of renal tissues. B. Immunohistochemistry was used to measure the level of Collagen IV. C. The levels of PTEN, FN, Collagen I and Collagen IV were detected by Western blot analysis. The columns and error bars represent the mean and SEM (n = 3). ***P < 0.001, vs. Control group. ##P < 0.01, ###P < 0.001, vs. STZ group.
Neuregulin 4 suppresses the expression of advanced glycosylation end products in the kidney
In the present study, western blotting and immunohistochemistry were performed to examine the expression levels of advanced glycosylation end products (AGEs). The AGE accumulation in the kidney was observed in DN rats, as shown by using both western blotting and immunohistochemical analysis (Figure 4A, 4B). Furthermore, RAGE and S100B were highly enhanced in renal tissue of DN rats (P < 0.001), which were attenuated by the Nrg4 in a dose dependent manner (P < 0.01, P < 0.001) (Figure 4A).
Figure 4.
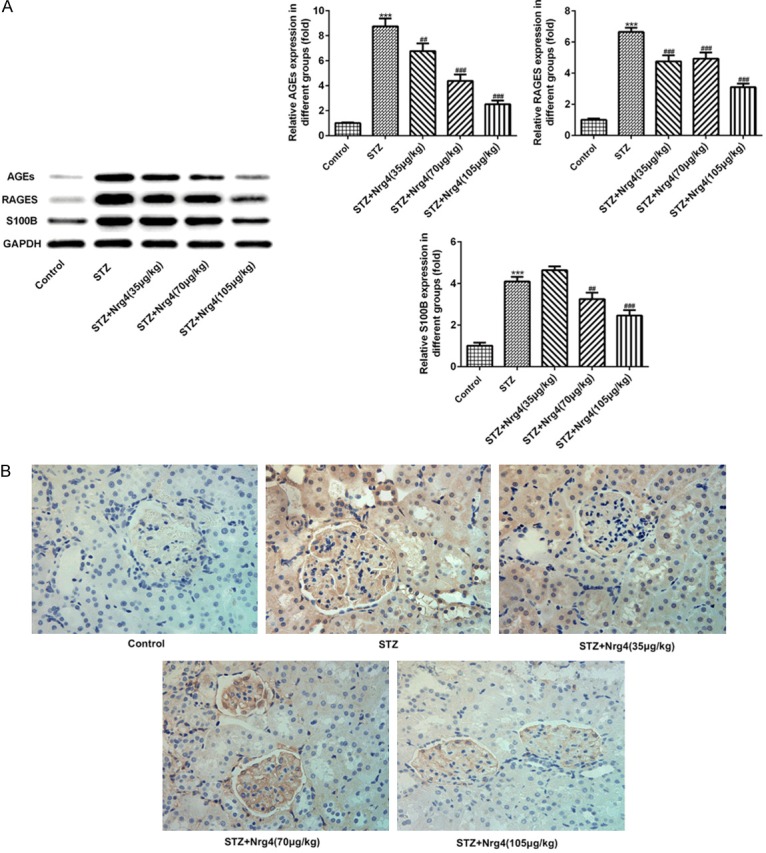
Nrg4 suppresses the expression of advanced glycosylation end products in the kidney. A. The levels of AGEs, RAGEs and S100B were detected by Western blot analysis. B. Immunohistochemistry was used to measure the level of AGEs. The columns and error bars represent the mean and SEM (n = 3). ***P < 0.001, vs. Control group. ##P < 0.01, ###P < 0.001, vs. STZ group.
Neuregulin 4 attenuate inflammation in kidney of diabetic nephropathy rats
Biochemical assays were used to detect COX2, NO, iNOS and inflammatory factor including IL-6 and tumor necrosis factor-α (TNFα). As shown in Figure 5A-E, compared with normal rats, the levels of COX2, IL-6, TNFα, NO, and iNOS were significantly enhanced in STZ-induced DN rats (P < 0.001), Nrg4 reversed the abnormal levels of levels of these proteins in a dose dependent manner (P < 0.01, P < 0.001).
Figure 5.
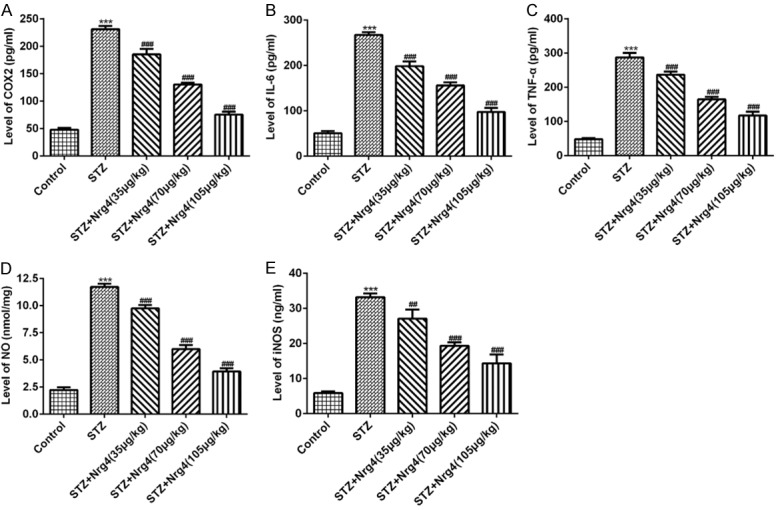
Nrg4 attenuate inflammation in kidney of diabetic nephropathy rats. The levels of COX2 (A) and IL-6 (B), TNF-α (C) were measured using appropriate enzyme-linked immunoassay (ELISA) kits. The levels NO (D) and iNOS (E) were using commercial test kits. The columns and error bars represent the mean and SEM (n = 3). ***P < 0.001, vs. Control group. #P < 0.05, ##P < 0.01, ###P < 0.001, vs. STZ group.
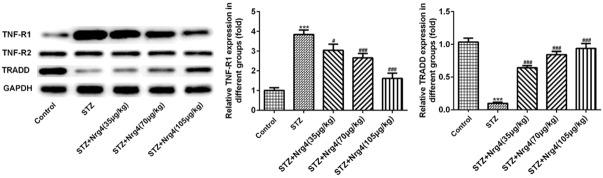
Neuregulin 4 may attenuate renal dysfunction via TNF-R1 signaling instead of TNF-R2 signaling
To analyze the expression levels of TNFα binds to TNF receptor 1 (TNF-R1), TNF-R2, TNFR associated death domain (TRADD) protein, Western blotting was performed. Compared with control group, significantly increased expression level of TNF-R1 protein was observed in the STZ group (P < 0.001), which can be reversed by Nrg4 treatment in a dose manner (P < 0.05, P < 0.001). In contrast, the expression level of TRADD protein of STZ group was decreased (P < 0.001), Nrg4 increase the expression level of TRADD protein (P < 0.001). Interestingly, the expression level of TNF-R2 protein had no change compared with control group, as shown in Figure 6.
Figure 6.
Nrg4 may attenuate renal dysfunction via TNF-R1 signaling instead of TNF-R2 signaling. The levels of TNF-R1, TNR-R2, TRADD were detected by Western blot analysis. The columns and error bars represent the mean and SEM. ***P < 0.001, vs. Control group. #P < 0.05, ###P < 0.001, vs. STZ group.
Neuregulin 4 ameliorates fibrosis in HK-2 cell via TNF-R1 signaling
The HK-2 cells were transfected with TNF-R1 plasmid to investigate the regulatory role of Nrg4. Western blotting and RT-qPCR were used to detect the expression level of TNF-R1 protein. The expression level of TNF-R1 protein significantly increased in pcDNA-TNF-R1 group, compared with pcDNA group (P < 0.001) (Figure 7A, 7B). Consistent with the western blotting and immunohistochemistry results in vivo, the expression levels of collagen I, collagen IV and FN, analyzed by western blotting and immunofluorescence, were notably increased in HK-2 cells with high glucose (HG) stimulation (P < 0.001), which can be decreased by Nrg4 treatment (P < 0.01, P < 0.001). In contrast, the expression level of PTEN was decreased significantly in HK-2 cells of HG group compared with control group (P < 0.001). As expected, the corrective effect of Nrg4 treatment is reversed by TNF-R1 overexpression (P < 0.05, P < 0.001) (Figure 7C, 7D).
Figure 7.
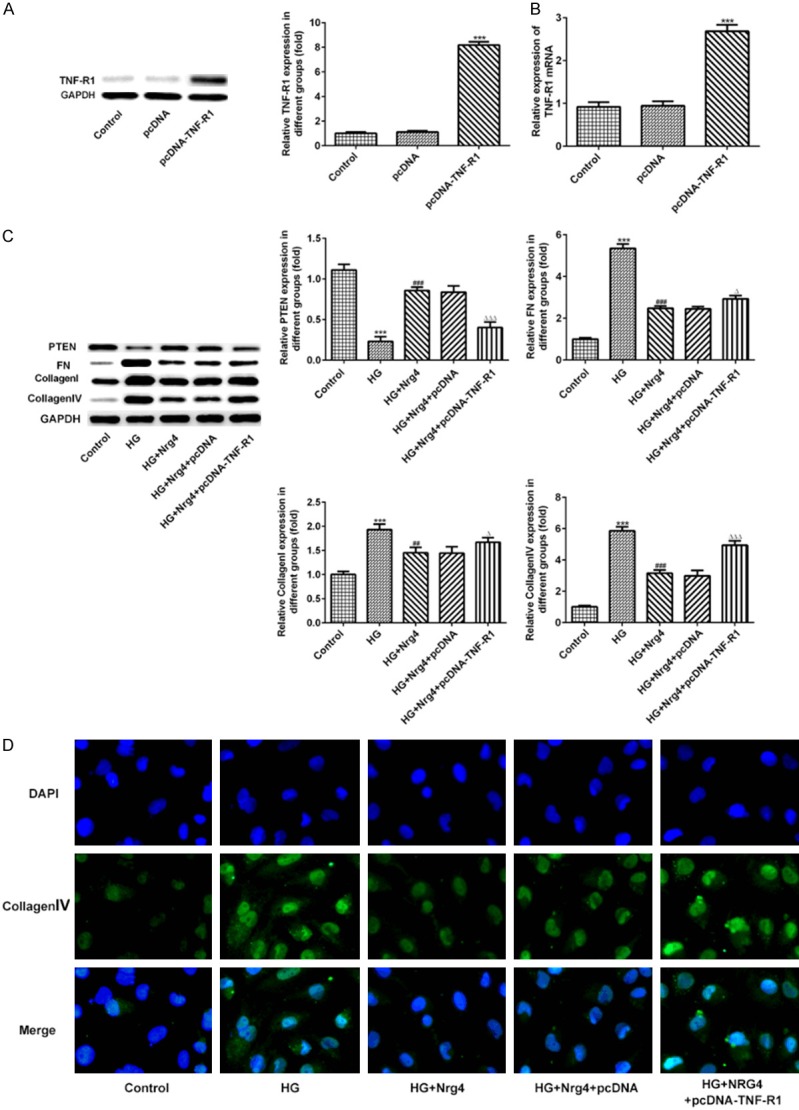
Nrg4 ameliorates fibrosis in HK-2 cell via TNF-R1 signaling. A, B. The TNF-R1 expression level was analyzed by Western blot and RT-qPCR. C. The expression levels of PTEN, FN, Collagen I and Collagen IV protein were detected by Western blot analysis. D. Immunofluorescence was used to measure the Collagen IV levels. The columns and error bars represent the mean and SEM. ***P < 0.001, vs. Control group. ##P < 0.01, ###P < 0.001, vs. HG group. ΔP < 0.05, ΔΔΔP < 0.001, vs. HG+Nrg4+pcDNA group.
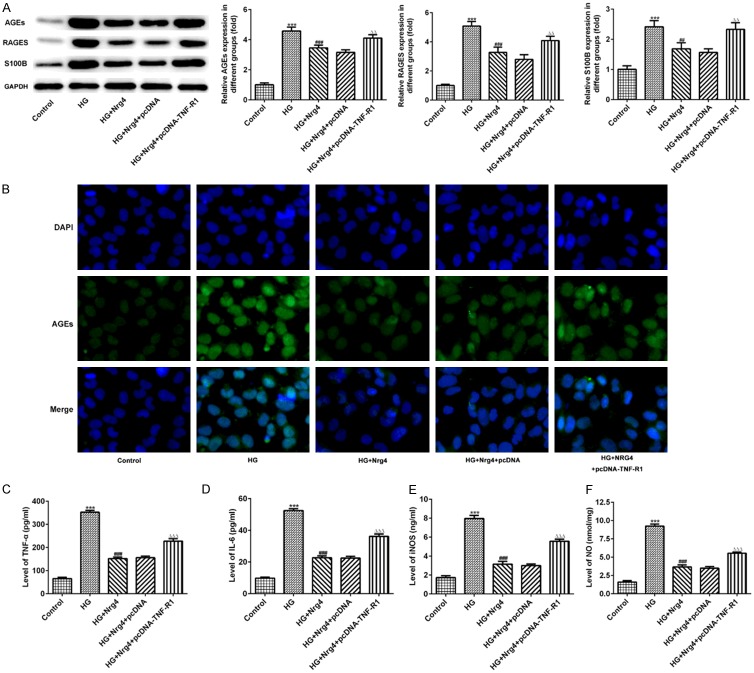
Neuregulin 4 attenuate the expression of advanced glycosylation end products and inflammation in HK-2 cell via TNF-R1 signaling
To further confirm the regulatory role of Nrg4 in vivo, AGEs, RAGE and S100B were detected with western blotting or immunofluorescence, the expressions of these protein were markedly increased in HG group (P < 0.001), compared with control group. TNF-R1 overexpression blocked the suppressive effect of Nrg4 treatment (P < 0.01) (Figure 8A, 8B). COX2, NO, iNOS and inflammatory factor including IL-6 and TNFα were examined by appropriate commercial kits. The data showed COX2, NO, iNOS, IL-6 and TNFα were elevated by HG condition (P < 0.001). Nrg4 treatment suppress the abnormal expression, which can be reversed by TNF-R1 overexpression (P < 0.001) (Figure 8C-F).
Figure 8.
Nrg4 attenuate the expression of advanced glycosylation end products and inflammation in HK-2 cell via TNF-R1 signaling. (A) The levels of AGEs, RAGEs and S100B were detected by Western blot analysis. (B) Immunofluorescence was used to measure the AGEs levels. The levels of TNF-α (C), IL-6 (D) were measured using appropriate enzyme-linked immunoassay (ELISA) kits. The levels of iNOS (E) and NO (F) were using commercial test kits. The data are presented as the means ± SEM. ***P < 0.001, vs. Control group. ##P < 0.01, ###P < 0.001, vs. HG group. ΔΔP < 0.01, ΔΔΔP < 0.001, vs. HG+Nrg4+pcDNA group.
Discussion
Diabetic nephropathy (DN) is a most common and severe diabetic microvascular complication worldwide [18]. Multiple physiological factors, including high glucose environment, inflammatory cytokines, oxidative stress, play important roles in the pathogenesis of DN [19]. Tubulointerstitial fibrosis (TIF) is a key event in DN development. In this study, rat model of DN were established successfully by intraperitoneal injections of streptozotocin (STZ). STZ-induced rats showed notably weight gain, overt hyperglycemia and injured renal function. Furthermore, extracellular matrix (ECM) accumulation, mesangial expansion, glomerular hypertrophy, and thickened tubular and basement membrane were also observed on the renal histopathological images of DN rats. More importantly, the present study demonstrated that neuregulin 4 (Nrg4) treatment ameliorates the defect of renal function and attenuate renal pathological changes in DN rats. Taken together, our findings provide novel insights into the onset and progression of renal fibrosis in DN.
In the current study, decreased neuregulin 4 (Nrg4) was detected in serum and tissue of DN rats, which is consistent with the change of circulating Nrg4 levels in T2DM patients [20]. Nrg4 attenuate renal function injury and ameliorates tubulointerstitial fibrosis of DN rats in a dose dependent manner. To further investigate the underlying mechanism of Nrg4 effect on TIF, inflammatory cytokines, advanced glycosylation end products (AGEs), proteins involved in oxidative stress and fibrosis were detected. Higher expression of collagen I, collagen IV and fibronectin (FN) were reported to be involved with renal fibrosis progression [21], which is consistent with our data. Our results indicated that Nrg4 treatment decreased the aberrant expression of collagen I, collagen IV and FN in DN rats. Phosphatase and tension homologue (PTEN) have protective effect on renal fibrosis. Liu et al. found the activation of cardiac fibroblasts can be inhibited by regulating PTEN signaling pathway [22]. Interestingly, Nrg4 treatment enhanced expression levels of PTEN protein of DN rats. In addition, Nrg4 treatment suppress the advanced glycation end-products (AGEs) accumulation and decreased the expression levels of RAGE (receptor for AGE) and S100B protein. RAGE, as a multiligand, transmembrane receptor, play important roles in activating major pro-inflammatory and pro-oxidative signaling pathways [23]. S100B was shown to interact with RAGE and to trigger inflammatory signaling [24]. The inflammatory cytokines IL-6, TNF-α, COX2, NO and iNOS are upregulated in DN rats. This result is consistent with other research, such as Zhang et al., who reported that AGEs stimulation significantly increased the section of IL-1β and TNF-α in mouse tubular epithelial cells [25]. In present study, the inflammatory cytokines IL-6, TNF-α, COX2, NO and iNOS are downregulated by Nrg4 treatment in a dose dependent manner. Collectively, the data presented here support potential roles and a novel function of Nrg4 in the TIF of DN process.
TRADD, identified as an assembly platform to diverge TNFR1 signaling, was reported to have lower expression in rat unilateral ureteral obstruction (UUO) kidneys [12]. To put forward a possible molecular mechanism for Nrg4 action in the TIF, the expression levels of TNF-R1, TNF-R2 and TRADD protein were further detected by Western blot analysis. In accordance with previous studies, the expression level of TRADD protein was significantly decreased in DN rats, Nrg4 treatment enhanced the TRADD levels. In contrast, the expression level of TNF-R1 protein was increased, which can be reversed by Nrg4 treatment. However, the expression level of TNF-R2 protein have no change. The data suggest that Nrg4 attenuate inflammation, AGEs accumulation and ameliorates fibrosis through TNF-R1 signaling, instead of TNF-R2 signaling. To further confirm our hypothesis, the HK-2 cells were transfected with TNF-R1 plasmid to investigate the regulatory role of Nrg4. As expected, all the corrective effects of Nrg4 treatment were blocked by TNF-R1 overexpression. The results revealed that Nrg4 may effectively ameliorates TIF and attenuate the expression of AGEs in DN through TNF-R1 signaling instead of TNF-R2 signaling. We have provided evidence indicating that Nrg4 possesses therapeutic effect on TIF in DN.
In conclusion, this comprehensive approach to the study of the function of Nrg4 in TIF provides novel insight into the onset and development of DN and assistance in defining alternative renoprotective strategies in diabetes.
Disclosure of conflict of interest
None.
References
- 1.Li SY, Huang PH, Tarng DC, Lin TP, Yang WC, Chang YH, Yang AH, Lin CC, Yang MH, Chen JW, Schmid-Schonbein GW, Chien S, Chu PH, Lin SJ. Four-and-a-Half LIM domains protein 2 is a coactivator of Wnt signaling in diabetic kidney disease. J Am Soc Nephrol. 2015;26:3072–3084. doi: 10.1681/ASN.2014100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H, Peng X, Wang Y, Cao S, Xiong L, Fan J, Wang Y, Zhuang S, Yu X, Mao H. Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis. Autophagy. 2016;12:1472–1486. doi: 10.1080/15548627.2016.1190071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canaud G, Bonventre JV. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant. 2015;30:575–583. doi: 10.1093/ndt/gfu230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han R, Hu S, Qin W, Shi J, Zeng C, Bao H, Liu Z. Upregulated long noncoding RNA LOC105375913 induces tubulointerstitial fibrosis in focal segmental glomerulosclerosis. Sci Rep. 2019;9:716. doi: 10.1038/s41598-018-36902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim WY, Nam SA, Song HC, Ko JS, Park SH, Kim HL, Choi EJ, Kim YS, Kim J, Kim YK. The role of autophagy in unilateral ureteral obstruction rat model. Nephrology (Carlton) 2012;17:148–159. doi: 10.1111/j.1440-1797.2011.01541.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu N, Xu L, Shi Y, Zhuang S. Podocyte autophagy: a potential therapeutic target to prevent the progression of diabetic nephropathy. J Diabetes Res. 2017;2017:3560238. doi: 10.1155/2017/3560238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, Zhao S, Liu S, Liu Q, Li F, Hao J. PTEN regulates renal extracellular matrix deposit via increased CTGF in diabetes mellitus. J Cell Biochem. 2016;117:1187–1198. doi: 10.1002/jcb.25402. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Chu M, Hong J, Shang J, Xu D. Hypoxia induces cardiac fibroblast proliferation and phenotypic switch: a role for caveolae and caveolin-1/PTEN mediated pathway. J Thorac Dis. 2014;6:1458–1468. doi: 10.3978/j.issn.2072-1439.2014.08.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni WJ, Ding HH, Tang LQ. Berberine as a promising anti-diabetic nephropathy drug: an analysis of its effects and mechanisms. Eur J Pharmacol. 2015;760:103–112. doi: 10.1016/j.ejphar.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Tziomalos K, Athyros VG. Diabetic nephropathy: new risk factors and improvements in diagnosis. Rev Diabet Stud. 2015;12:110–118. doi: 10.1900/RDS.2015.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonoda Y, Gohda T, Suzuki Y, Omote K, Ishizaka M, Matsuoka J, Tomino Y. Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PLoS One. 2015;10:e0122212. doi: 10.1371/journal.pone.0122212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misaki T, Yamamoto T, Suzuki S, Fukasawa H, Togawa A, Ohashi N, Suzuki H, Fujigaki Y, Oda T, Uchida C, Kitagawa K, Hattori T, Kitagawa M, Hishida A. Decrease in tumor necrosis factor-alpha receptor-associated death domain results from ubiquitin-dependent degradation in obstructive renal injury in rats. Am J Pathol. 2009;175:74–83. doi: 10.2353/ajpath.2009.080884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang GX, Zhao XY, Lin JD. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol Metab. 2015;26:231–237. doi: 10.1016/j.tem.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, Li S, Bluher M, Lin JD. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20:1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan PJ, Xu Y, Wan Q, Feng J, Li H, Gao CL, Yang J, Zhong HH, Zhang ZH. Decreased plasma neuregulin 4 concentration is associated with increased high-sensitivity C-reactive protein in newly diagnosed type 2 diabetes mellitus patients: a cross-sectional study. Acta Diabetol. 2017;54:1091–1099. doi: 10.1007/s00592-017-1044-4. [DOI] [PubMed] [Google Scholar]
- 16.Yan P, Xu Y, Wan Q, Feng J, Li H, Yang J, Zhong H, Zhang Z. Plasma neuregulin 4 levels are associated with metabolic syndrome in patients newly diagnosed with type 2 diabetes mellitus. Dis Markers. 2018;2018:6974191. doi: 10.1155/2018/6974191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeifer A. NRG4: an endocrine link between brown adipose tissue and liver. Cell Metab. 2015;21:13–14. doi: 10.1016/j.cmet.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Tromp J, Lim SL, Tay WT, Teng TK, Chandramouli C, Ouwerkerk W, Wander GS, Sawhney JPS, Yap J, MacDonald MR, Ling LH, Sattar N, McMurray JJV, Richards AM, Anand I, Lam CSP ASIAN-HF Investigators. Microvascular disease in patients with diabetes with heart failure and reduced ejection versus preserved ejection fraction. Diabetes Care. 2019;42:1792–1799. doi: 10.2337/dc18-2515. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Zhang ZP, Xin GD, Guo LH, Jiang Q, Wang ZX. miR-192 prevents renal tubulointerstitial fibrosis in diabetic nephropathy by targeting Egr1. Eur Rev Med Pharmacol Sci. 2018;22:4252–4260. doi: 10.26355/eurrev_201807_15420. [DOI] [PubMed] [Google Scholar]
- 20.Yan P, Xu Y, Zhang Z, Gao C, Zhu J, Li H, Wan Q. Decreased plasma neuregulin 4 levels are associated with peripheral neuropathy in Chinese patients with newly diagnosed type 2 diabetes: a cross-sectional study. Cytokine. 2019;113:356–364. doi: 10.1016/j.cyto.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 21.You YK, Luo Q, Wu WF, Zhang JJ, Zhu HJ, Lao L, Lan HY, Chen HY, Cheng YX. Petchiether A attenuates obstructive nephropathy by suppressing TGF-beta/Smad3 and NF-kappaB signalling. J Cell Mol Med. 2019;23:5576–5587. doi: 10.1111/jcmm.14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu HL, Chen CH, Sun YJ. Overexpression of lncRNA GAS5 attenuates cardiac fibrosis through regulating PTEN/MMP-2 signal pathway in mice. Eur Rev Med Pharmacol Sci. 2019;23:4414–4418. doi: 10.26355/eurrev_201905_17949. [DOI] [PubMed] [Google Scholar]
- 23.Teissier T, Quersin V, Gnemmi V, Daroux M, Howsam M, Delguste F, Lemoine C, Fradin C, Schmidt AM, Cauffiez C, Brousseau T, Glowacki F, Tessier FJ, Boulanger E, Frimat M. Knockout of receptor for advanced glycation end-products attenuates age-related renal lesions. Aging Cell. 2019;18:e12850. doi: 10.1111/acel.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793:993–1007. doi: 10.1016/j.bbamcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Zhang YY, Tan RZ, Zhang XQ, Yu Y, Yu C. Calycosin ameliorates diabetes-induced renal inflammation via the NF-kappaB pathway in vitro and in vivo. Med Sci Monit. 2019;25:1671–1678. doi: 10.12659/MSM.915242. [DOI] [PMC free article] [PubMed] [Google Scholar]