Abstract
The angiopoietin-1 (Ang-1)/Tie-2 signaling pathway plays a crucial role in the maintenance of vascular stabilization and permeability. In this study, we evaluated the protective effect of a designed Ang-1 variant (COMP-Ang-1) on peritoneal vascular permeability and peritoneal transport function in a uremic peritoneal dialysis (PD) model. Compared to the sham controls, uremic rats were characterized by decreased pericyte coverage and downregulated endothelial junction expression. The permeability of the peritoneal vasculature to FITC-BSA and FITC-dextran in uremic rats was also higher than that in the sham controls, as well as increased levels of proinflammatory adhesion molecules and cytokines, increased D/Pcr and decreased ultrafiltration. Such changes were more marked in uremia+PD rats after exposure to glucose-based peritoneal dialysis fluid (PDF) for 4 weeks. Peritoneal Ang-1 protein expression and Tie-2 phosphorylation were significantly lower in uremic rats than in control rats and were further significantly reduced in uremia+PD rats. After COMP-Ang-1 administration, phosphorylation of the Tie-2 receptor was significantly increased. Treatment with COMP-Ang-1 also significantly enhanced pericyte coverage, upregulated endothelial junction protein expression and inhibited leakage of FITC-BSA and FITC-dextran from the peritoneal vasculature induced during PD therapy; these changes were accompanied by reduced peritoneal tissue levels of proinflammatory adhesion molecules and cytokines, decreased D/Pcr and increased ultrafiltration. These findings suggest that COMP-Ang-1 may exert a protective effect against glucose-based PDF-induced peritoneal vascular permeability and inflammation, at least in part, by enhancing pericyte coverage and endothelial junction protein expression, which subsequently significantly improves peritoneal transport function.
Keywords: Peritoneal dialysis, COMP-angiopoietin-1, vascular permeability, peritoneal transport function
Introduction
Peritoneal dialysis (PD) is a well-established renal replacement therapy for end-stage renal disease (ESRD) patients. PD relies on the peritoneal membrane (PM) as a semipermeable barrier for ultrafiltration and diffusion [1]. However, over time, PD can cause progressive injury to the PM, and the most common functional alteration during long-term PD is an increased peritoneal solute transport rate (PSTR), leading to impaired ultrafiltration and, ultimately, discontinuation of PD treatment [2,3]. Encapsulating peritoneal sclerosis is a rare but serious complication that is also associated with an increased PSTR [4]. The peritoneal vascular endothelium plays a pivotal role in constituting the transport barrier during PD [5]. Therefore, protecting peritoneal vascular endothelial integrity may be essential for preventing a PSTR increase during long-term PD.
Angiopoietin-1 (Ang-1) is known to be a ligand of the Tie-2 tyrosine kinase receptor expressed on endothelial cells [6]. Ang-1/Tie-2 signaling is involved in the branching and remodeling of the primitive vascular network and in the recruitment of mural cells during development [7]. Ang-1 also decreases vascular permeability [8] and has anti-inflammatory [9] and antiapoptotic [10] properties. Thus, the Ang-1 protein may have potential therapeutic application in preserving vascular endothelial integrity. However, large-scale production of Ang-1 is hindered by the aggregation and insolubility of this protein. Cartilage oligomeric matrix protein (COMP)-Ang-1, a chimeric form of native Ang-1 containing a minimal coiled-coil domain of the cartilage oligomeric matrix protein sufficient for oligomerization, has thus been synthesized [11]. COMP-Ang-1 is more potent than native Ang-1 in phosphorylating Tie-2 and signaling via Akt in primary cultured endothelial cells and overcomes problems associated with the aggregation and insolubility of native Ang-1 over time [11]. Cho et al. [12] demonstrated that i.v. administration of COMP-Ang-1 protected against radiation-induced apoptosis in microcapillary endothelial cells of the intestinal villi and prolonged cell survival. Studies have also reported that treatment with COMP-Ang-1 can preserve the renal microvasculature and exert anti-inflammatory effects in different kidney disease models (e.g., the unilateral ureteral obstruction model, the cyclosporine-induced renal injury model, the type 2 diabetic db/db mouse model and a mouse model of acute ischemia reperfusion injury), ultimately ameliorating the progression of renal fibrosis [13-16]. However, the role of Ang-1/Tie-2 signaling in regulating peritoneal vascular permeability during PD has not been studied.
In the present study, we examined the effects of uremia and bioincompatible PD fluid exposure on peritoneal vascular endothelial integrity and investigated whether COMP-Ang-1 treatment could alleviate these injuries and ameliorate peritoneal transport function in a uremic PD model.
Materials and methods
Animal experiments
The protocol of the animal study was approved by the Animal Care and Use Committee of School of Medicine, Shanghai Jiao Tong University (HKDL [2018] 38), and all experiments were performed in accordance with the relevant guidelines and regulations. A twelve-hour light-dark cycle and climate control were maintained. Access to food and water was allowed ad libitum. First, thirty-six male Sprague-Dawley (SD) rats (Chinese Academy of Sciences, Shanghai, China), weighing 120-160 g, were randomly allocated into three groups: the sham operation group (control, n = 12), uremia group (n = 12) and uremia+PD group (n = 12). Uremia was induced by subtotal nephrectomy. Rats undergoing sham operation were subjected to bilateral flank incisions. Four weeks after subtotal nephrectomy, uremia+PD rats were dialyzed with 3 ml/100 g body weight 4.25% Dianeal® (Baxter Healthcare Corporation, Deerfield, IL, USA) twice a day for 4 weeks. For COMP-Ang-1 treatment, twenty additional uremic rats were established and randomly assigned into the Ade-COMP-Ang-1 group (n = 10) and Ade-vehicle group (Ade-control, n = 10) followed by PD for 4 weeks. COMP-Ang-1 adenovirus or vehicle adenovirus was injected at an optimized dose (1 × 1010 pfu/ml per injection) via the tail vein 1 d before PD and 10 d and 21 d after PD. All surgical procedures were carried out under general anesthesia induced by 9% chloral hydrate (0.3 ml/100 g, i.m.).
Peritoneal microcirculation permeability
After PD for 4 weeks, rats were re-anesthetized with 9% chloral hydrate. FITC-BSA (69 kDa, 10 mg/100 g, 2 ml/100 g, Sigma-Aldrich, St. Louis, MO) or FITC-dextran (4 kDa, 10 mg/100 g, 2 ml/100 g, Sigma-Aldrich, St. Louis, MO) was injected via the tail vein. After 20 min, systemic blood vessels were lavaged with saline to wash out intravascular fluorescent substances. After rats were euthanized, mesenteric samples were separated and trimmed to create a flat surface followed by digestion with 0.25% trypsin (Thermo Fisher Scientific) for 5 min at 37°C. After washing 3 × 5 min with phosphate-buffered saline (PBS), slides were submerged in ice-cold acetone for 30 min. Following three washes with PBS, blocking solution (10% donkey serum and 0.5% Triton X-100 in PBS) was applied for 2 h at room temperature, and sections were then incubated with primary antibodies (CD31, 1:50, BD, America) in blocking solution for 36 h at 4°C. After three wash steps with PBS, sections were incubated with Cy3-goat anti-mouse IgG (1:150, Jackson, America). The microscopic images were focused on “networks” including arterioles, venules and capillaries at × 100 magnification. ImageJ software (National Institutes of Health, Bethesda, MD) was used to calculate the total green fluorescence intensity from the leakage of FITC-BSA or FITC-dextran into the peritoneal tissue.
Pericyte coverage rate
After processing as described above, mesenteric samples were stained for immunofluorescence analysis with a mixture of primary antibodies against CD31 (1:50, BD, America) and Desmin (1:50, Abcam, America) at 4°C. After 36 h, sections were incubated with FITC-goat anti-rabbit IgG (1:150, Jackson, America) and Cy3-goat anti-mouse IgG (1:150, Jackson, America). Microscopic images were focused on a “network” including arterioles, venules and capillaries at × 40 magnification and × 100 magnification. ImageJ software was used to calculate the pericyte coverage rate (the fluorescence intensity ratio of Desmin/CD31).
Western blot analysis
Total protein was extracted from visceral peritoneal membranes using RIPA buffer (Invitrogen, Carlsbad, CA, USA) on ice, and the protein concentration was measured using a BCA protein assay kit (Beyotime, Shanghai, China). Equal amounts of protein from each sample were loaded onto 10% SDS-PAGE gels and transferred to PVDF membranes. After blocking in 5% nonfat dry milk in Tris-buffered saline-Tween 20 (TBST) for 2 h, membranes were incubated with primary antibodies at 4°C overnight. After washing with TBST, membranes were incubated with a secondary antibody diluted 1:5,000 in TBST at room temperature for 1 h. Signals were detected by enhanced chemiluminescence (ECL) reagent (Amersham, Little Chalfont, Bucks, UK) according to the manufacturer’s instructions. Densitometric analysis was performed using Quantity One software (Bio-Rad, Hercules, CA, USA). The following primary antibodies were used: anti-Ang-1 (1:5000) and anti-occludin (1:5000) from Abcam; anti-Tie-2 (1:100), anti-Claudin (1:5000) and anti-ZO-1 (1:2000) from Millipore; anti-Phospho-Tie-2 (1:1000) from Cell Signaling; anti-VE-Cadherin (1:500) from LSBio; and anti-β-actin (1:200) from Santa Cruz.
Real-time PCR
Total RNA was extracted from mesenteric tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed using a Transcriptor First Strand cDNA Synthesis Kit (Takara, Japan) according to the manufacturer’s instructions. RT-PCR was performed using SYBR Green Master Mix on an ABI Prism 7500 Sequence Detection System (Foster City, USA). The comparative 2-ΔΔCt method was used to determine the relative quantification of the target genes, with GAPDH serving as the internal reference. The primer sequences used for PCR amplification are shown in Table 1.
Table 1.
RT-PCR primer sequences
| Gene | Forward primer sequence (5’-3’) | Reverse primer sequence (5’-3’) |
|---|---|---|
| VCAM-1 | GCTGCTGTTGGCTGTGACTCTC | GCTCAGCGTCAGTGTGGATGTAG |
| ICAM-1 | TGTCGGTGCTCAGGTATCCATCC | TTCGCAAGAGGAAGAGCAGTTCAC |
| MCP-1 | GCAGGTCTCTGTCACGCTTCTG | GAATGAGTAGCAGCAGGTGAGTGG |
| IL-6 | TCCTACCCCAACTTCCAATGCTC | TTGGATGGTCTTGGTCCTTAGCC |
| TNF-α | GCCACCACGCTCTTCTGTC | GCTACGGGCTTGTCACTCG |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
Peritoneal equilibration test
The peritoneal equilibration test (PET) was performed as previously described [17,18]. Briefly, each rat was injected with 21 ml of 2.5% Dianeal® (Baxter Healthcare Corporation, Deerfield, IL, USA), and 1 ml of dialysate was obtained immediately after infusion for a glucose test. After injection, rats were allowed free access to water. Two hours later, rats were anesthetized with 9% chloral hydrate (0.3 ml/100 g, i.m.). The remaining dialysate was completely drained and measured to calculate the net ultrafiltration (nUF). The peritoneal nUF was defined as the volume of peritoneal effluent recovered 2 h after intraperitoneal injection minus the volume recovered at 0 h. In addition, blood samples were obtained for plasma analysis. The peritoneal transport characteristic was determined from the dialysate-to-plasma (D/P) ratio of creatinine. To exclude peritonitis, we also cultured the peritoneal effluents to determine the presence of bacteria.
Statistical analysis
Data are expressed as the means ± standard deviations (SDs) and were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni’s comparison test. Results with P < 0.05 were considered to be statistically significant. The IBM SPSS Statistics 21 program was used for statistical analyses.
Results
COMP-Ang-1 increases peritoneal Tie-2 phosphorylation after uremia and bioincompatible PD fluid exposure
As shown in Figure 1A, the expression of peritoneal Ang-1 protein in the uremia group rats was significantly decreased compared to that in the control group rats and was further significantly reduced in the uremia+PD group rats. Consistent results were also obtained for phosphorylation of Tie-2 (Figure 1B). However, phosphorylation of peritoneal Tie-2 was significantly increased after COMP-Ang-1 treatment compared with that in both the uremia and uremia+PD groups (1.4-fold and 2.7-fold, respectively) but was still lower than that in control rats. Furthermore, there was no significant difference in peritoneal Tie-2 phosphorylation between the Ade-control group and the uremia+PD group (P > 0.05).
Figure 1.
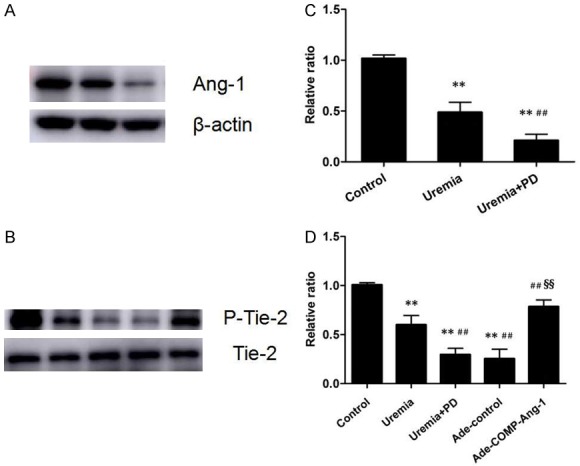
Western blot analysis of peritoneal angiopoietin-1 (Ang-1) after uremia and bioincompatible PD fluid exposure and the effect of COMP-Ang-1 treatment on the phosphorylation of the Tie-2 receptor. A. Ang-1 protein expression in the visceral peritoneal membranes of the control, uremia nondialysis and uremia+PD groups, respectively. B. Peritoneal Tie-2 phosphorylation after COMP-Ang-1 treatment. C, D. Relative expression of Ang-1 normalized to the expression of β-actin and the level of phosphor-Tie-2 normalized to the level of total Tie-2. Data are presented as the means ± SDs (n = 6 per group). **P < 0.01 versus the control group; ##P < 0.01 versus the uremia group; §§P < 0.01 versus the uremia+PD group.
COMP-Ang-1 ameliorates uremia and bioincompatible PD fluid-induced peritoneal microvascular hyperpermeability
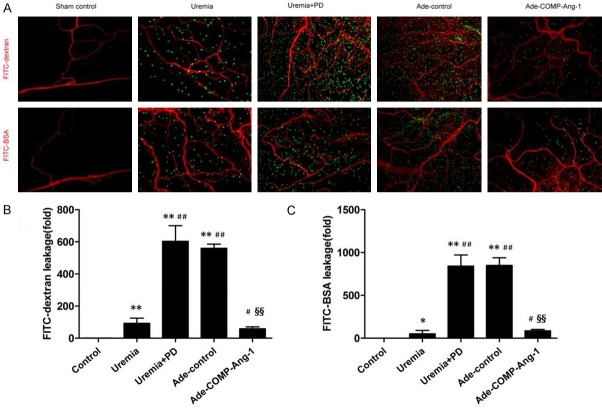
Peritoneal microcirculation permeability was examined by assessing the leakage of FITC-dextran and FITC-BSA with immunofluorescence staining. As shown in Figure 2A, in sham-operated rats, minimal FITC-dextran was leaked from the blood vessels, and there was almost no FITC-BSA leakage. The permeability of the peritoneal microcirculation to FITC-dextran (92.1-fold) and FITC-BSA (51.2-fold) was significantly higher in uremic nondialyzed rats than in control group rats. After infusion of PD fluid for 4 weeks, the permeability of the peritoneal microcirculation to FITC-dextran and FITC-BSA were further markedly increased. The mean increases in FITC-dextran and FITC-BSA leakage were 6.6- and 16.4-fold, respectively, in the uremia+PD group relative to the leakage in the uremia nondialysis group (Figure 2B, 2C). After COMP-Ang-1 treatment, FITC-dextran and FITC-BSA leakage was significantly decreased by 85% and 80%, respectively, in the Ade-COMP-Ang-1 group compared with the leakage in the uremia+PD group.
Figure 2.
COMP-Ang-1 ameliorates uremia and bioincompatible PD fluid-induced peritoneal microvascular hyperpermeability. After PD for 4 weeks, rats were anesthetized with 9% chloral hydrate. FITC-BSA (69 kDa, 10 mg/100 g, 2 ml/100 g, Sigma-Aldrich, St. Louis, MO) or FITC-dextran (4 kDa, 10 mg/100 g, 2 ml/100 g, Sigma-Aldrich, St. Louis, MO) was injected via the tail vein. Systemic blood vessels were lavaged with saline to wash out intravascular fluorescent substances after 20 min. After rats were euthanized, mesenteric samples were separated and trimmed to create a flat surface and stained with the immunofluorescent antibody against CD31 (a marker of endothelial cells). A. Immunofluorescence staining for FITC-dextran and FITC-BSA in visceral peritoneal membranes (magnification, × 100). Red indicates the endothelial cell marker CD31. Green indicates FITC-conjugated dextran or BSA. B, C. Semiquantitative analysis of peritoneal vessel permeability to FITC-dextran and FITC-BSA in the control, uremia nondialysis, uremia+PD, Ade-control and Ade-COMP-Ang-1 groups. Data are presented as the means ± SDs (n = 6 per group). *P < 0.05 versus the control group; **P < 0.01 versus the control group; #P < 0.05 versus the uremia group; ##P < 0.01 versus the uremia group; §§P < 0.01 versus the uremia+PD group.
COMP-Ang-1 preserves peritoneal pericyte attachment after uremia and bioincompatible PD fluid exposure
Ang-1 is known to be a potent growth factor for pericyte recruitment to nascent endothelial cells during vasculogenesis under physiological and pathological conditions [7,8,19]. To determine the effect of COMP-Ang-1 on pericyte attachment in the peritoneum, we stained slides with immunofluorescent antibodies against CD31 (a marker of endothelial cells, red) and Desmin (a specific marker of pericytes, green). The fold changes in double-positive fluorescence intensity are presented in Figure 3C. The pericyte coverage of uremic nondialyzed rats was significantly reduced (approximately 46%, P < 0.0001) and further decreased in uremia+PD rats (approximately 87%, P < 0.0001) compared with that in sham-operated rats. COMP-Ang-1 treatment attenuated the decrease in pericyte coverage by approximately 22% and 59%, respectively, compared with the uremia nondialysis and uremia+PD group rats. Treatment with Ade-vehicle did not affect the density of Desmin-positive pericytes in the peritoneum compared to that in uremia+PD group rats (P > 0.05).
Figure 3.
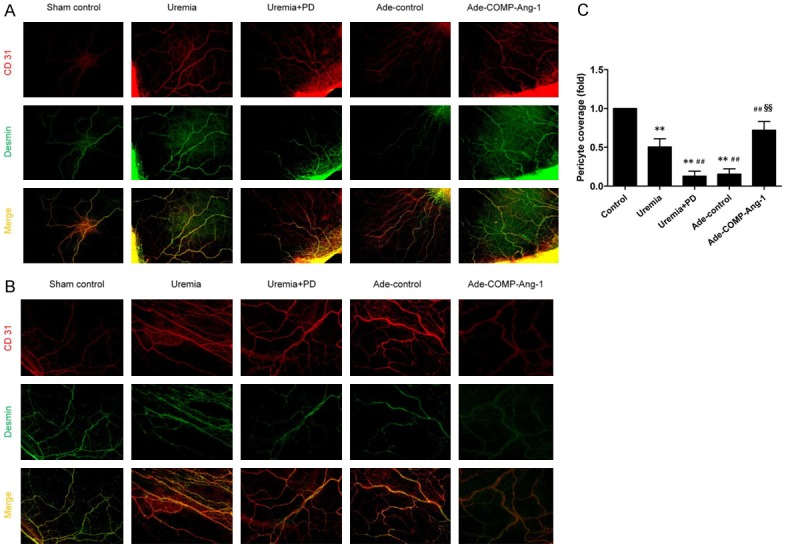
COMP-Ang-1 preserves peritoneal pericyte attachment after uremia and bioincompatible PD fluid exposure. After being processed as described in the Materials and Methods, mesenteric samples were stained with immunofluorescent antibodies against CD31 (a marker of endothelial cells, red) and Desmin (a specific marker of pericytes, green). A, B. CD31/Desmin double immunofluorescence staining in visceral peritoneal membrane sections (magnification, × 40 and × 100, respectively). Red indicates the endothelial cell marker CD31. Green indicates the pericyte marker Desmin. C. Semiquantitative analysis of pericyte coverage measured in visceral peritoneal membrane sections from six rats in each group. Data are presented as the means ± SDs. **P < 0.01 versus the control group; ##P < 0.01 versus the uremia group; §§P < 0.01 versus the uremia+PD group.
COMP-Ang-1 increases peritoneal endothelial junction protein expression after uremia and bioincompatible PD fluid exposure
Given that both tight junction (TJ) proteins and adherens junction (AJ) proteins are important for preserving the integrity of the vascular endothelium, we examined the effects of COMP-Ang-1 treatment on the expression levels of claudin, ZO-1, occludin and VE-cadherin. As shown in Figure 4, the expression levels of these junction proteins as measured by Western blotting were significantly lower in the uremia nondialysis group than in the control group and were further markedly reduced after 4 weeks of PD. However, compared to the uremia+PD group, the COMP-Ang-1 treatment group exhibited increases in claudin, ZO-1, occludin and VE-cadherin expression of approximately 4.2-, 1.7-, 2.0- and 2.9-fold, respectively.
Figure 4.
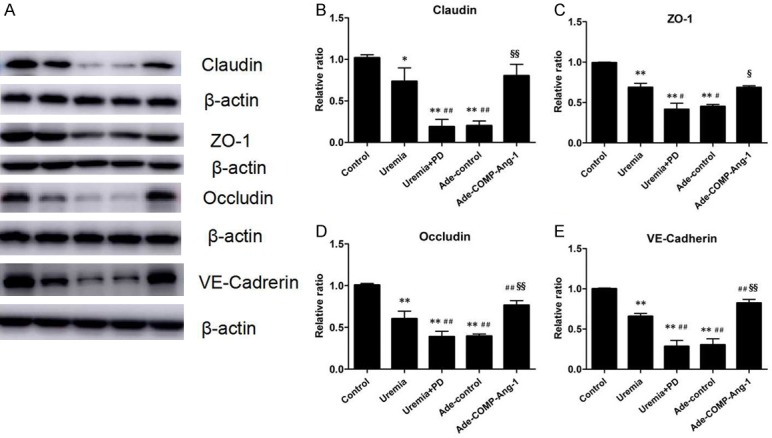
Western blot analysis of peritoneal endothelial junction proteins after COMP-Ang-1 treatment. A. Western blot analysis of claudin, ZO-1, occludin and VE-cadherin. B-E. Relative expression of claudin, ZO-1, occludin and VE-cadherin normalized to β-actin expression. Data are presented as the means ± SDs (n = 6 per group). *P < 0.05 versus the control group; **P < 0.01 versus the control group; #P < 0.05 versus the uremia group; ##P < 0.01 versus the uremia group; §P < 0.05 versus the uremia+PD group; §§P < 0.01 versus the uremia+PD group.
COMP-Ang-1 inhibits the upregulation of peritoneal proinflammatory adhesion molecules and cytokines
The expression of peritoneal proinflammatory adhesion molecules and cytokines was evaluated by real-time PCR, and the results are shown in Figure 5. The peritoneal VCAM-1, ICAM-1, MCP-1, TNF-α and IL-6 mRNA levels were significantly increased in the uremia nondialysis group (2.0-fold, 2.3-fold, 2.2-fold, 2.1-fold and 1.7-fold, respectively) and were further significantly increased in the uremia+PD group (4.5-fold, 5.9-fold, 4.7-fold, 3.0-fold and 5.9-fold, respectively) compared to those in the control group. However, these effects were significantly suppressed by COMP-Ang-1 treatment. Indeed, the VCAM-1, ICAM-1, MCP-1, TNF-α and IL-6 mRNA levels were significantly lower in the Ade-COMP-Ang-1 group than in the uremia+PD group.
Figure 5.
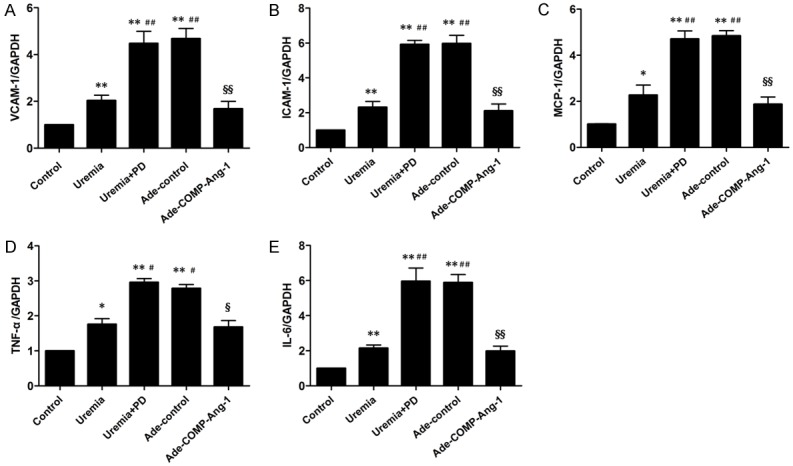
Expression of peritoneal proinflammatory adhesion molecules and cytokines after COMP-Ang-1 treatment. Semiquantitative analysis of peritoneal VCAM-1 (A), ICAM-1 (B), MCP-1 (C), TNF-α (D) and IL-6 (E) mRNA expression normalized to GAPDH mRNA expression, as measured by real-time PCR. The data are presented as the means ± SDs (n = 6 per group). *P < 0.05 versus the control group; **P < 0.01 versus the control group; #P < 0.05 versus the uremia group; ##P < 0.01 versus the uremia group; §P < 0.05 versus the uremia+PD group; §§P < 0.01 versus the uremia+PD group.
COMP-Ang-1 preserves peritoneal transport function after uremia and bioincompatible PD fluid exposure
The peritoneal transport characteristics were evaluated by the two-hour D/PCr and nUF via a PET. As shown in Table 2, the two-hour D/PCr was significantly increased in uremia rats compared to that in control group rats (0.65 ± 0.13 vs 0.50 ± 0.09, P < 0.05) and was further significantly increased in uremia+PD rats (0.92 ± 0.06 vs 0.65 ± 0.13, P < 0.05). Correspondingly, the nUF was significantly reduced in uremia rats compared to that in control rats (1.17 ± 0.78 vs 4.67 ± 0.95 ml, P < 0.05) and was further significantly decreased in uremia+PD rats (-6.63 ± 3.98 vs 1.17 ± 0.78 ml, P < 0.05). However, after COMP-Ang-1 treatment, the two-hour D/Pcr significantly decreased, and the nUF significantly increased relative to those values in the uremia+PD group (Table 2).
Table 2.
Comparison of peritoneal transport characteristics among different groups
| Parameter | Control | Uremia | Uremia+PD | Ade-control | Ade-COMP-Ang-1 |
|---|---|---|---|---|---|
| 2 h D/Pcr | 0.50 ± 0.09 | 0.65 ± 0.13* | 0.92 ± 0.06*,# | 0.93 ± 0.06*,# | 0.58 ± 0.10§ |
| nUF (ml) | 4.67 ± 0.95 | 1.17 ± 0.78* | -6.63 ± 3.98*,# | -5.74 ± 3.37*,# | 1.45 ± 0.75§ |
D/Pcr: dialysate-to-plasma ratio of creatinine. nUF: net ultrafiltration. Data are expressed as the means ± SDs.
P < 0.05 versus the control group;
P < 0.05 versus the uremia group;
P < 0.05 versus the uremia+PD group.
Discussion
The integrity of the vascular endothelium in response to physical, biochemical, and immune-mediated damage is highly important in maintaining endothelial function and preventing vascular diseases [11,20-22]. In this study, we demonstrated that both uremia and PD therapy significantly induced pericyte detachment and reduced endothelial junction protein expression, concomitant with a marked increase in peritoneal vascular permeability and enhanced expression of proinflammatory adhesion molecules and cytokines, which collectively might be strongly involved in the increase in the D/Pcr and decrease in ultrafiltration induced during PD therapy. However, COMP-Ang-1 administration substantially alleviated these pathological findings and improved peritoneal transport function.
Successful PD therapy largely depends on the preservation of peritoneal membrane function. However, during long-term PD, the peritoneal membrane undergoes both structural and functional alterations [2,3]. The most common functional alteration during long-term PD is an increased PSTR, which is the major contributor to impaired ultrafiltration and ultimate discontinuation of PD treatment [5]. Studies have demonstrated that an increase in the PSTR depends not only on the increased number of peritoneal vessels but also on changes in peritoneal vessel hyperpermeability; thus, the latter is also responsible for ultrafiltration failure [3,23-25]. Pericytes are specialized cells that wrap around the endothelial cells of arteries, capillaries and venules, sharing a basement membrane with the endothelium. Pericytes differ in origin, morphology, and function depending on different organs’ vascular beds and play critical roles in various physiological contexts, including vascular remodeling and stabilization and the generation and maintenance of the blood-brain barrier (BBB) and blood-retinal barrier (BRB) [26-29]. Studies have revealed that pericyte dropout or loss is one of the hallmarks of diabetic retinopathy (DR), and it has been postulated to initiate the development of several pathological features, including abnormal leakage, edema and ischemia, provoking proliferative neovascularization in the retina [30]. In addition, vessel permeability is closely associated with continuous complexes of endothelial junctions, including both tight junctions (claudin, occludin and ZO-1) and adherens junctions (VE-cadherin) [31,32]. It has been suggested that the function of the endothelial barrier is directly correlated with the expression levels of occludin and VE-cadherin [33,34], and disruption of these proteins’ functions was sufficient to disturb the endothelial barrier function, causing an increase in vascular permeability, interstitial edema and accumulation of inflammatory cells in the heart and lung microcirculation [34,35]. In the present study, we observed that both uremia and bioincompatible PD fluid exposure significantly reduced peritoneal pericyte coverage and decreased endothelial junction protein expression, resulting in markedly increased peritoneal vascular leakage of BSA and dextran, as well as increased expression of proinflammatory adhesion molecules and cytokines, followed by increased D/Pcr and decreased ultrafiltration.
In a previous study, we demonstrated that uremic serum, high glucose (HG) levels and glucose degradation products significantly inhibited pericyte activity and proliferation in vitro [36]. It was also confirmed that HG inhibited pericyte activity and proliferation in a DR model [37]. Miller et al. [38] reported that glucose degradation products induced human retinal pericyte apoptosis in vitro. The underlying mechanisms might be related to oxidative stress, mitochondrial overproduction of ROS, accumulation of advanced glycation end products (AGEs), upregulation of protein kinase C, increased polyol pathway flux and focal aggregation of leukocytes [37-39]. The increased permeability of peritoneal vessels caused by uremia and bioincompatible PD fluid has a pathological background similar to that of those mechanisms mentioned above. During long-term PD therapy, pericytes encased within the periphery of peritoneal microvessels are continuously exposed to various uremic toxins and PD fluid containing HG levels and large amounts of glucose degradation products. A continuous effect of various uremic toxins and PD fluid stimulation is inevitably exerted on pericytes, leading to the inhibition of pericyte growth and proliferation and even to pericyte injury and detachment from preexisting vessels.
In addition, it has been suggested that downregulated occludin expression in cells exposed to HG [33] and in the retinas of diabetic rats [39] and diabetic patients [40] could lead to excessive vascular permeability. Loss of occludin at interendothelial junctions appeared to result from Raf-1-dependent activation of the MAP kinase signal transduction cascade [41,42]. VE-cadherin is another target of the signaling pathway of agents that increase vascular permeability, such as VEGF [43,44]. VEGF-R2 interacts with VE-cadherin, and together, they maintain the endothelial cell barrier [45]. When VEGF is present, it binds to VEGF-R2, initiating the activation, internalization, and degradation of VE-cadherin and disruption of AJs, which results in increased permeability and loss of endothelial cell barrier integrity [46]. Gorbunova et al. [47] also demonstrated an increase in VEGF-R2 phosphorylation and internalization of VE-cadherin in hantavirus-infected human lung endothelial cells treated with high levels of exogenous VEGF. Furthermore, Armulik et al. [48] showed markedly distributed patterns of both tight (claudin and ZO-1) and adherens (VE-cadherin) junctions in the BBB of pericyte-deficient mice, indicating a possible role of pericyte attachment in the organization of continuous endothelial junction complexes. Our results suggest that uremia- and glucose-based PD fluid-induced loss of endothelial junction protein expression might represent another pathway involved in the increased peritoneal vascular permeability and inflammation during PD therapy.
Moreover, we found that the levels of Ang-1 protein expression and Tie-2 phosphorylation were significantly decreased in uremia nondialysis rats and were further markedly reduced after exposure to glucose-based PD fluid for 4 weeks. The Ang-1/Tie-2 signaling pathway has been reported to play an important role in the reciprocal interactions between pericytes and endothelial cells. Augustin et al. [49] reported that Ang-1 could stimulate pericyte coverage and basement membrane deposition, thereby promoting proper vessel permeability. Tian et al. [50] suggested that Ang-1 overexpression prevented the dissociation of perivascular cells from the endothelium of tumor edge-associated blood vessels and induced an influx of stromal cells into tumors, which greatly enhanced pericyte coverage. However, it has also been suggested that Ang-1 could inhibit endothelial monolayer permeability through regulating endothelial junction complexes, which are also involved in preventing the leakiness of blood vessels observed in inflammatory or allergic reactions [9]. In the present study, we selected a designed Ang-1 variant, COMP-Ang-1, which has been demonstrated to be more soluble, stable and potent than naturally occurring Ang-1, to investigate whether the preservation of Ang-1/Tie-2 signaling could alleviate these injuries and improve peritoneal transport function as mentioned above. After COMP-Ang-1 administration, the phosphorylation of the Tie-2 receptor was significantly increased. Furthermore, we observed that COMP-Ang-1 treatment significantly enhanced pericyte coverage, upregulated endothelial junction protein expression, lessened the leakage of BSA and dextran from the peritoneal vasculature, and inhibited the expression of proinflammatory adhesion molecules and cytokines induced by PD therapy; these effects were accompanied by a decreased D/Pcr and increased nUF. Iivanainen et al. [51] suggested that the binding of Ang-1 to Tie-2 results in the upregulation of endothelial HB-EGF, which substantially promotes pericyte migration by binding to ErbB1 and ErbB2 expressed on endothelial cells. However, the specific molecular mechanisms by which COMP-Ang-1 exerts its effects on pericyte coverage and endothelial junction protein expression are still unclear, and further studies on this aspect are required.
Our study has several limitations that need to be acknowledged. Firstly, the peritoneal membrane injury observed in the uremic PD model has not been further assessed by varied methods. Also, there has been a lack of in vitro experiments to explore the specific molecular mechanisms underlying these pathological changes. Clearly more studies are needed to further confirm our findings.
Taken together, our results indicate that COMP-Ang-1 exerts a protective effect against damage-induced peritoneal vascular permeability and inflammation, at least in part, by enhancing pericyte coverage and endothelial junction protein expression, which subsequently significantly improves peritoneal transport function in a uremic PD model. These findings suggest the potential therapeutic benefits of COMP-Ang-1 on peritoneal injury from PD and might provide a novel, endothelium-specific therapeutic modality for peritoneal membrane failure.
Acknowledgements
This study was supported in part by grants from the National Natural Science Foundation of China (Nos. 30600290, 81370864, 81670691) and the Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20152211).
Disclosure of conflict of interest
None.
References
- 1.Nagy JA. Peritoneal membrane morphology and function. Kidney Int Suppl. 1996;56:S2–S11. [PubMed] [Google Scholar]
- 2.Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG. Meta-analysis: peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol. 2006;17:2591–2598. doi: 10.1681/ASN.2006030194. [DOI] [PubMed] [Google Scholar]
- 3.Rumpsfeld M, McDonald SP, Johnson DW. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and new Zealand peritoneal dialysis patient populations. J Am Soc Nephrol. 2006;17:271–278. doi: 10.1681/ASN.2005050566. [DOI] [PubMed] [Google Scholar]
- 4.Lambie ML, John B, Mushahar L, Huckvale C, Davies SJ. The peritoneal osmotic conductance is low well before the diagnosis of encapsulating peritoneal sclerosis is made. Kidney Int. 2010;78:611–618. doi: 10.1038/ki.2010.186. [DOI] [PubMed] [Google Scholar]
- 5.Krediet RT, Lindholm B, Rippe B. Pathophysiology of peritoneal membrane failure. Perit Dial Int. 2000;20(Suppl 4):S22–S42. [PubMed] [Google Scholar]
- 6.Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Exp Cell Res. 2013;319:1271–1280. doi: 10.1016/j.yexcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 8.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 9.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603–607. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 10.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 11.Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci U S A. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho CH, Kammerer RA, Lee HJ, Yasunaga K, Kim KT, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. Designed angiopoietin-1 variant, COMP-Ang1, protects against radiation-induced endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101:5553–5558. doi: 10.1073/pnas.0307575101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim W, Moon SO, Lee SY, Jang KY, Cho CH, Koh GY, Choi KS, Yoon KH, Sung MJ, Kim DH, Lee S, Kang KP, Park SK. COMP-angiopoietin-1 ameliorates renal fibrosis in a unilateral ureteral obstruction model. J Am Soc Nephrol. 2006;17:2474–2483. doi: 10.1681/ASN.2006020109. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Kim W, Kim DH, Moon SO, Jung YJ, Lee AS, Kang KP, Jang KY, Lee SY, Sung MJ, Koh GY, Park SK. Protective effect of COMP-angiopoietin-1 on cyclosporine-induced renal injury in mice. Nephrol Dial Transplant. 2008;23:2784–2794. doi: 10.1093/ndt/gfn168. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Kim W, Moon SO, Sung MJ, Kim DH, Kang KP, Jang KY, Lee SY, Park BH, Koh GY, Park SK. Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrol Dial Transplant. 2007;22:396–408. doi: 10.1093/ndt/gfl598. [DOI] [PubMed] [Google Scholar]
- 16.Jung YJ, Kim DH, Lee AS, Lee S, Kang KP, Lee SY, Jang KY, Sung MJ, Park SK, Kim W. Peritubular capillary preservation with COMP-angiopoietin-1 decreases ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol. 2009;297:F952–F960. doi: 10.1152/ajprenal.00064.2009. [DOI] [PubMed] [Google Scholar]
- 17.Yuan J, Fang W, Ni Z, Dai H, Lin A, Cao L, Qian J. Peritoneal morphologic changes in a peritoneal dialysis rat model correlate with angiopoietin/Tie-2. Pediatr Nephrol. 2009;24:163–170. doi: 10.1007/s00467-008-0944-5. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Yan H, Yuan J, Cao L, Lin A, Dai H, Ni Z, Qian J, Fang W. Pharmacological inhibition of heparin-binding EGF-like growth factor promotes peritoneal angiogenesis in a peritoneal dialysis rat model. Clin Exp Nephrol. 2018;22:257–265. doi: 10.1007/s10157-017-1440-7. [DOI] [PubMed] [Google Scholar]
- 19.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 20.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 21.Opal SM, van der Poll T. Endothelial barrier dysfunction in septic shock. J Intern Med. 2015;277:277–293. doi: 10.1111/joim.12331. [DOI] [PubMed] [Google Scholar]
- 22.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zareie M, de Vriese AS, Hekking LH, ter Wee PM, Schalkwijk CG, Driesprong BA, Schadee-Eestermans IL, Beelen RH, Lameire N, van den Born J. Immunopathological changes in a uraemic rat model for peritoneal dialysis. Nephrol Dial Transplant. 2005;20:1350–1361. doi: 10.1093/ndt/gfh835. [DOI] [PubMed] [Google Scholar]
- 24.Kim YL. Update on mechanisms of ultrafiltration failure. Perit Dial Int. 2009;29(Suppl 2):S123–S127. [PubMed] [Google Scholar]
- 25.Shi Y, Yan H, Yuan J, Zhang H, Huang J, Ni Z, Qian J, Fang W. Different patterns of inflammatory and angiogenic factors are associated with peritoneal small solute transport and peritoneal protein clearance in peritoneal dialysis patients. BMC Nephrol. 2018;19:119. doi: 10.1186/s12882-018-0921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenerg. 2010;2:1–14. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Korn C, Augustin HG. Mechanisms of vessel pruning and regression. Dev Cell. 2015;34:5–17. doi: 10.1016/j.devcel.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 31.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 32.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Antonetti DA, Barber AJ, Khin S, Lieth E, Tarbell JM, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn state Retina research group. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- 34.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 35.Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279:L419–L422. doi: 10.1152/ajplung.2000.279.3.L419. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Fang W, Huang C, Lin A, Dai H, Ni Z, Qian J. Effects of high glucose and methylglyoxal on proliferation and expression of angiopoietin-1 of vascular pericytes. J Shanghai Jiaotong Univ. 2009;29:496–499. [Google Scholar]
- 37.Ejaz S, Chekarova I, Ejaz A, Sohail A, Lim CW. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obes Metab. 2008;10:53–63. doi: 10.1111/j.1463-1326.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 38.Miller AG, Smith DG, Bhat M, Nagaraj RH. Glyoxalase I is critical for human retinal capillary pericyte survival under hyperglycemic conditions. J Biol Chem. 2006;281:11864–11871. doi: 10.1074/jbc.M513813200. [DOI] [PubMed] [Google Scholar]
- 39.Kim JH, Kim JH, Jun HO, Yu YS, Kim KW. Inhibition of protein kinase C delta attenuates blood-retinal barrier breakdown in diabetic retinopathy. Am J Pathol. 2010;176:1517–1524. doi: 10.2353/ajpath.2010.090398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang GE. Diabetic macular edema. Ophthalmologica. 2012;227(Suppl 1):21–29. doi: 10.1159/000337156. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Wade P, Mandell KJ, Akyildiz A, Parkos CA, Mrsny RJ, Nusrat A. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene. 2007;26:1222–1230. doi: 10.1038/sj.onc.1209902. [DOI] [PubMed] [Google Scholar]
- 42.González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 44.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 45.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, Kowalczyk AP. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J Biol Chem. 2003;278:19199–19208. doi: 10.1074/jbc.M211746200. [DOI] [PubMed] [Google Scholar]
- 47.Gorbunova E, Gavrilovskaya IN, Mackow ER. Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. J Virol. 2010;84:7405–7411. doi: 10.1128/JVI.00576-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 49.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 50.Tian S, Hayes AJ, Metheny-Barlow LJ, Li LY. Stabilization of breast cancer xenograft tumour neovasculature by angiopoietin-1. Br J Cancer. 2002;86:645–651. doi: 10.1038/sj.bjc.6600082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iivanainen E, Nelimarkka L, Elenius V, Heikkinen SM, Junttila TT, Sihombing L, Sundvall M, Maatta JA, Laine VJ, Yla-Herttuala S, Higashiyama S, Alitalo K, Elenius K. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J. 2003;17:1609–1621. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]