Abstract
Background: Pomegranate ellagic polyphenols (PEP) has been used as a good medicine in many cultures throughout history. However, the mechanism of PEP regulated insulin resistance on gestational diabetes mellitus (GDM) rats is unclear. The main purpose of the present study was to explore the efficacy and mechanisms of PEP regulated in GDM rats. Materials and methods: Then, ELISA assay indicated that the levels of serum RBP4, Hcy, GA and FFA were lower in PEP groups than GDM groups in a dose-dependent manner. TUNEL staining showed that PEP improved the pathological changes and inhibited the cell apoptosis in the pancreatic and placenta tissues, respectively. Results: We found that PEP improved the weight of pregnant rats and fetal rats and the level of blood glucose, blood biochemical index, insulin resistance in GDM rats. Results from H&E and immunohistochemical analysis found that PEP decreased the expressions of APN and Chemerin. Further, PEP decreased the levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and C-reactive protein (CRP), and increased the levels of 11β-hydroxy steroid dehydrogenase type 2 (11β-HSD2) and PPARα-TRB3-AKT2-p-FOXO1-GLUT2 signal related to insulin sensitivity in a dose-dependent manner. Conclusions: In conclusion, we have demonstrated that PEP may be a candidate drug for the treatment of GDM and guide the clinical therapy.
Keywords: Pomegranate ellagic polyphenols, insulin resistance, gestational diabetes mellitus, PPARα, 11β-HSD2, GLUT2
Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first detected during pregnancy, which affects 1-18% of all pregnancies depending on the diagnostic tests employed and the population studied [1-3]. The morbidity of GDM is increasing, and it has become a global public health problem [4]. Currently, diet and drug are the therapeutic methods most used to control blood glucose concentrations during pregnancy. The effect of drug treatment is obvious and rapid, so it is very important to find an effective and safe drug for the treatment of GDM.
The pathogenesis of GDM has not been fully elucidated. Insulin resistance (IR) is a physiologic metabolic change that is regulated during pregnancy to maintain glucose levels, including glucose transport, glycogen synthesis, and anti-lipolysis, which is often linked to cardiovascular disease and type II diabetes [5-7]. Therefore, IR is an important feature of GDM. The peroxisome proliferator-activated receptor (PPAR) is crucial transcription factors that regulate insulin responsiveness and glucose metabolism in insulin target tissues [8]. The PPAR family of ligand-activated transcription factors includes three PPAR isoforms (α, β/δ, and γ), which have prominent roles in insulin signaling pathways [9]. PPARα is known to suppress inflammation and to preserve insulin sensitivity [10] and the PPAR-α agonist fenofibrate increased insulin sensitivity and decreased glucose improved the insulin sensitivity [11]. FOXO1, as a suppressive transcription factor, attenuates PPARγ activity in adipocytes via binding and repressing the PPARγ promoter [12]. Further, Antioxidants, with suppressive effects on JNK activity, would lead to FOXO1 activity suppression via AKT activation with subsequent augmentation of PPARγ-associated lipogenesis and attenuation of blood glucose level [13,14]. However, the molecular mechanism of PPAR signal pathway in insulin resistance processes is not wholly clear.
Pomegranate has been used as good medicine in many cultures throughout history [15]. Pomegranate extract contains the virtue of its abundant and diverse polyphenols, including ellagic acid, ellagitannins and other flavonoids (quercetin, kaempferol, and luteolin glycosides) [16]. Recent a studies have shown that polyphenols can prevented and treated cancer and cardiovascular and inflammatory diseases [17]. Additionally, recent research has focused on its potential use in treatments of diabetes and cardiovascular diseases [18,19]. Pomegranate polyphenolics reduce inflammation and ulceration in dextran sodium sulfate (DSS)-induced colitis in Sprague-Dawley rats and in lipopolysaccharide (LPS)-treated CCD-18Co colon-myofibroblastic cells [20]. Pomegranate flowers extracts had a protective effect on serum lipid profile and activities of antioxidant status in STZ-induced diabetic rats, which showed the use of PEP as a dietary supplement to protect against chronic atherogenic and impaired glucose metabolic disorders [21]. Similarly, another study showed that the administration of pomegranate seeds oil (PSO) resulted in decreased body weight, less body fat mass in fat diet induced obese mice. These results indeed explained that punicic acid metabolism played a significant role and hence dietary supplementation of PSO improved peripheral insulin sensitivity in the high fat diet induced obese mice and reduced obesity [22]. Additionally, PEP was proven to enhance cholesterol metabolism in L-02 human hepatic cells via the PPARγ-ABCA1/CYP7A1 pathway [23]. To date, the mechanism of PEP reducing insulin resistance in GDM rats has been still unclear.
Our study aims to assess the effect of PEP on the body weight, IR, the pathological changes and cell apoptosis in GMD rats. Further, we investigate the molecular mechanism of PEP in insulin resistance in GDM mice.
Methods
Materials and reagents
Pomegranate ellagic polyphenols was purchased from Dalian Meilun Bioogical Technology Co., Ltd, with a mass fraction of 99%.
Animals and grouping
Wistar rats (female = 30, male = 15) weighing 180 to 250 g were procured form Beijing Vital River Laboratory Animal Technology Co., Ltd., and were raised in a controlled environmental condition (25 ± 1°C, 50 ± 10% humidity, and a 12 h day/12 h night cycle) with standard diet and water ad libitum. After a week, rats in GDM group were fed with High Fathigh Sucrose Diet (HFSD) from Week 10 onwards. Next day, the female rats with a mucus plug or sperm observed under microscopy were regarded pregnant for 0 day and then 30 pregnant rats were subcutaneously injected with 2% streptozotocin (STZ) at a dosage of 35 mg/kg to induce GDM model. GDM rats with a blood glucose concentration ≥ 13.85 mmol/L were randomly divided into the GDM group (n = 5). Four days after STZ injection, the rats in PEP groups were treated with 50, 150, 300 mg/(kg/d) of PEP by gavage for 14 days (n = 5/per group), while rats in the GDM group and normal pregnancy group were treated with the equal volume of normal saline. Treated with metformin hydrochloride (200 mg/kg·d) was regarded as the positive control group (n = 5). Last day of the treatment, all rats were weighed and fasted overnight prior to collecting maternal blood from the heart by cardiac puncture. After blood samples were collected, rats were sacrificed by cervical dislocation after pentobarbital anesthetized. Cesarean section was then performed for recording the weights of the fetuses and placentas. The placenta and pancreatic tissues samples were stored at -80°C until assayed.
All animal experiments have been approved by the animal ethics committee of Huai’an First People’s Hospital Affiliated to Nanjing Medical University.
Measurement of glucose level
The level of fasting blood-glucose (FBG) was measured using a blood glucose monitoring system method.
Measurement of insulin level
Fasting blood insulin (FINS) level was assayed using Mercodia Rat Insulin ELISA kit (Sweden). The optical densities of the samples were read at 450 nm. The concentration of FINS was obtained by computerized data reduction of the absorbance for the calibrators, except for calibrator 0, versus the concentration using cubic spline regression. FINS were assessed using an insulin radioimmunoassay kit [24].
Biochemical measurements
After blood samples were centrifuged, the serum were collected for the detection of total cholesterol (TC), triglyceride (TG) and high-density lipoprotein (HDL) levels using an automatic biochemical analyzer [ILab Chemistry Analyzer 300 PLUS (Instrumentation Laboratory, USA)] according to the procedures of the kits [25]. The insulin resistance index (HOMA-IRI) = (FBG * FINS)/22.5.
Enzyme-linked immunosorbent assay (ELISA)
Enzyme-linked immunosorbent assay (ELISA) kits were used to determine the levels of RBP4, Hcy, GA and FFA (Beyotime Biotechnology, Shanghai, China) according to the corresponding manufacturer’s instructions.
Western blotting assay
Total protein was isolated from placenta and pancreatic tissues using RIPA buffer. Protein concentrations were determined using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL), and 20 ug protein loaded and separated on 12% SDS gels and transferred to polyvinylidene fluoride (PVDF) membrane. Following transfer, membranes were blocked with 10% skimmed milk and incubated with the appropriate primary antibodies at 4°C overnight. The antibodies 11β-HSD2 (1:500, ab115696), PPARα (1:200, ab3484), TRB3 (1:1000, ab73547) and GLUT2 (1:100, ab54460) were obtained from Abcam; PI3K (1:1000, #17366), AKT (1:1000, #9271), CRP (1:1000, #14316), TNF-α (1:1000, #11948), IL-6 (1:1000, #12912), AKT2 (1:1000, #5239), p-FOXO1 (1:1000, #9461) and GAPDH (1:1000, #2118) were purchased from Cell Signaling Technology Inc.. Detection was by peroxidase-conjugated secondary antibodies. Chemiluminescent film was applied for assessment of protein expression with Image J software.
Quantitative real-time PCR assay
Total RNA was isolated from placenta and pancreatic tissues using TRIzol reagent according to the manufacturer instructions. Real-time PCR assay was performed to detect the relative mRNA levels of 11β-HSD2, CRP, TNF-α, IL-6, PPARα, TRB3, AKT2, p-FOXO1, GLUT2 in pancreatic tissues. The gene expressions of the detected genes were normalized to an endogenous reference GAPDH. The relative gene expression level was calculated by using the comparative Ct Method, and those relative to the calibrator were given by the formula 2-ΔΔCt.
Hematoxylin and eosin (H&E) staining
The placenta and pancreatic tissues were fixed with 10% buffered formalin at room temperature for 48 h, dehydrated, and embedded in paraffin. The sections were stained with hematoxylin and eosin. Blinded analysis of liver tissues was performed using a light microscope. A board-certified pathologist examined placenta and pancreatic tissues pathological alterations.
TUNEL assay
The placenta and pancreatic tissues cell apoptosis was analyzed using a TUNEL assay kit following the manufacturer’s instructions. In brief, cells were fixed, permeabilized, and incubated with fluorescein isothiocyanate (FITC)-labeled dUTP and terminal deoxynucleotide transferase for 1 h at 37°C. Nuclei counterstaining was performed using DAPI. Stained cells were observed under a fluorescence microscope and the percentage of TUNEL-positive cells was determined.
Immunohistochemistry assay
For immunohistochemistry analysis, the pancreatic tissues were fixed in 10% buffered formalin for 48 h and then put into paraffin for slices in a microtome. Following the tissue slices were incubated with primary antibodies (APN and Chemerin) at 4°C overnight. Sections were then incubated with diluted streptavidin-peroxidase HRP at room temperature with a staining kit. Sections were stained with hematoxylin for 5 min and mounted before observed under a phase-contrast microscope.
Statistical analysis
Data were analyzed using SPSS13.0 statistical software and present as the mean ± standard deviation (SD). Student’s t test was performed to statistically compare between groups and one-way analysis of variance was performed for multiple group analysis. P < 0.05 was considered as the statistical significance.
Results
Effect of PEP on the body weight, weight of fetal rats, blood glucose, FINS of GDM rats
The weight of pregnant rats and fetal rats were shown in Figure 1. The data indicated that the weight of pregnant rats and fetal rats in GDM group explored lower than control group (Figure 1A, P < 0.01, and Figure 1B, P < 0.001), but the PEP groups exhibited higher than that in GDM group in a dose-dependent manner. Meanwhile, FBG level in the GDM group was higher than that in control group (Figure 1C, P < 0.001), but PEP significantly decreased the glucose level in a dose-dependent manner (Figure 1C). The change trend of FINS was consistent with that of FBG (Figure 1D). The IRI obtained from the above results is shown in the Figure 1E, the IRI in GDM group was higher than that in control group (P < 0.001), and PEP significantly decreased IRI in a dose-dependent manner. These results indicated that PEP improved the body weight loss of pregnant rats and fetal rats, and significantly increased the insulin sensitivity of GDM rats and improved the IR, thus maintaining the normal blood glucose level.
Figure 1.
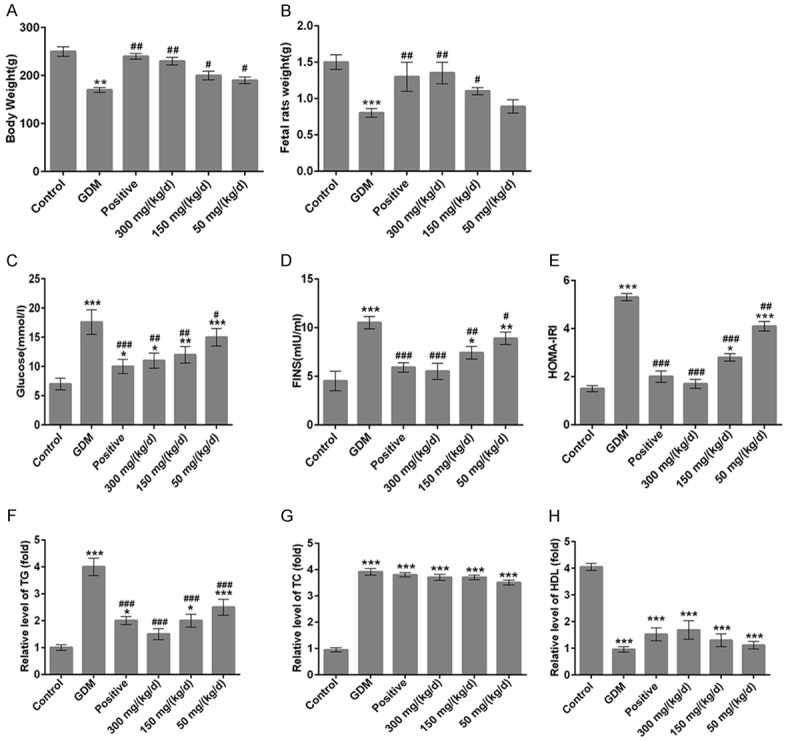
Effects of PEP on the body weight of pregnant rats and fetal rats, and the blood glucose, insulin resistance and serum biochemical indexes of GDM rats. (A) The pregnant rats weight and (B) fetal rats weight in different groups. The levels of (C) fasting plasma glucose and (D) fasting insulin in different groups. (E) Quantitative analysis of the insulin resistance index (HOMA-IRI) = (fasting blood glucose * fasting insulin)/22.5 among different groups. The levels of serum triglyceride (F), total cholesterol (G) and high-density lipoprotein (H) in different groups. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. GDM.
Effect of PEP on the biochemical criterion of GDM rats
Then, on the 14th day of pregnancy, the biochemical indexes in GDM group significantly changed. The serum TG and TC levels of GDM rats were significantly higher than that in normal pregnancy rats (Figure 1F and 1G, P < 0.001), but the serum HDL level of GDM rats was significantly lower than that in normal pregnancy rats (Figure 1H, P < 0.001). After GDM rats were administrated with doses of 50, 150 and 300 mg/kg of PEP, TG level was significantly decreased in a dose-dependent manner (Figure 1F); however, the TC and HDL levels in PEP groups showed no difference when compared with GDM group (Figure 1G and 1H). Next, we detected the levels of serum RBP4, Hcy, GA and FFA by ELISA. The levels of serum RBP4, Hcy, GA and FFA in GDM rats are all significantly higher than that in normal pregnancy rats (Figure 2A-D, P < 0.001). When GDM rats were treated with doses of 50, 150 and 300 mg/kg PEP, the levels of serum RBP4, Hcy, GA and FFA were significantly lower than GDM rats in a dose-dependent manner (Figure 2A-D). Therefore, PEP improved biochemical criterion of GDM rats.
Figure 2.
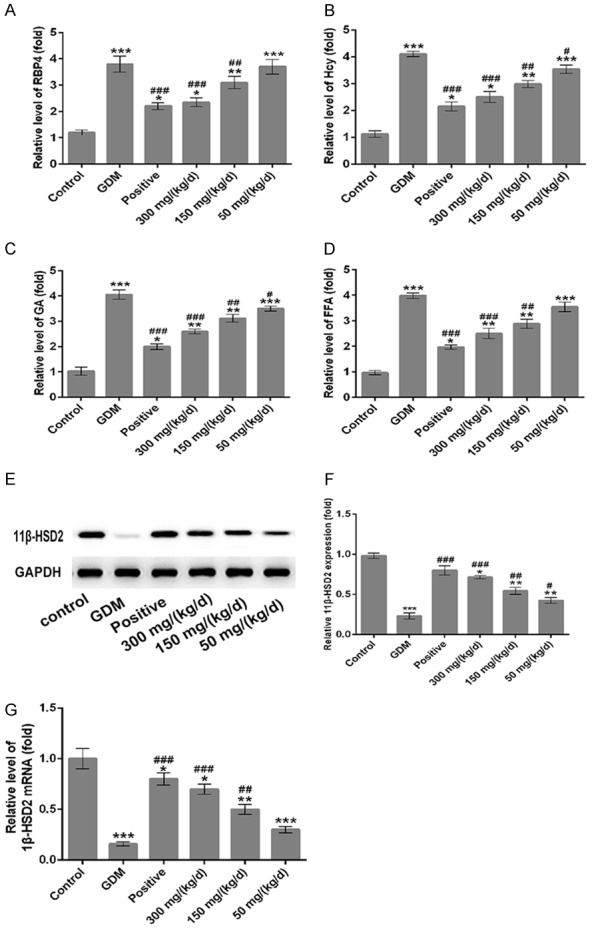
Effect of PEP on the levels of serum RBP4, Hcy, GA, FFA and 11β-HSD2 of GDM rats. The relative levels of serum RBP4 (A), Hcy (B), GA (C) and FFA (D) among different groups by an ELISA assay. (E) Western blotting was performed to detect the 11β-HSD2 expression in the placental tissue among different groups. (F) Quantitative analysis results of 11β-HSD2 expression from western blotting. (G) The mRNA levels of 11β-HSD2 was assessed by RT-PCR assay in different groups. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. GDM.
As shown in Figure 2, the protein (Figure 2E) and mRNA (Figure 2G) levels of 11β-HSD2 in the placental tissue were decreased significantly in GDM group comparing to the control group (P < 0.001), but increased in PEP groups in a dose-dependent manner when compared with the GDM group.
Effect of PEP on the pathological changes in the placenta and pancreatic tissues of GDM rats
H&E staining was conducted to observe the pathological changes of pancreatic and placenta tissues. As shown in Figure 3, the boundaries of tissue stratification were not obvious, the distribution of cells was loose and disordered, the intercellular space became larger, and the distribution of capillaries decreased in the placenta tissues of GDM rats (Figure 3A). Meanwhile, the cell is arranged disorder, nuclear pyretic of partial cell occurrence and lysis, visible inflammatory cell infiltration, capillary dilate, congestion, with haemorrhage occurrence in the pancreatic tissues of GDM rats (Figure 3B). When compared with GDM group, the cell structures and morphology of placenta and pancreatic tissues in the PEP groups gradually became more clear and PEP improved the cell arrangement in a dose-dependent manner. These results indicated that PEP improved the pathological damage of placenta and pancreatic tissues.
Figure 3.
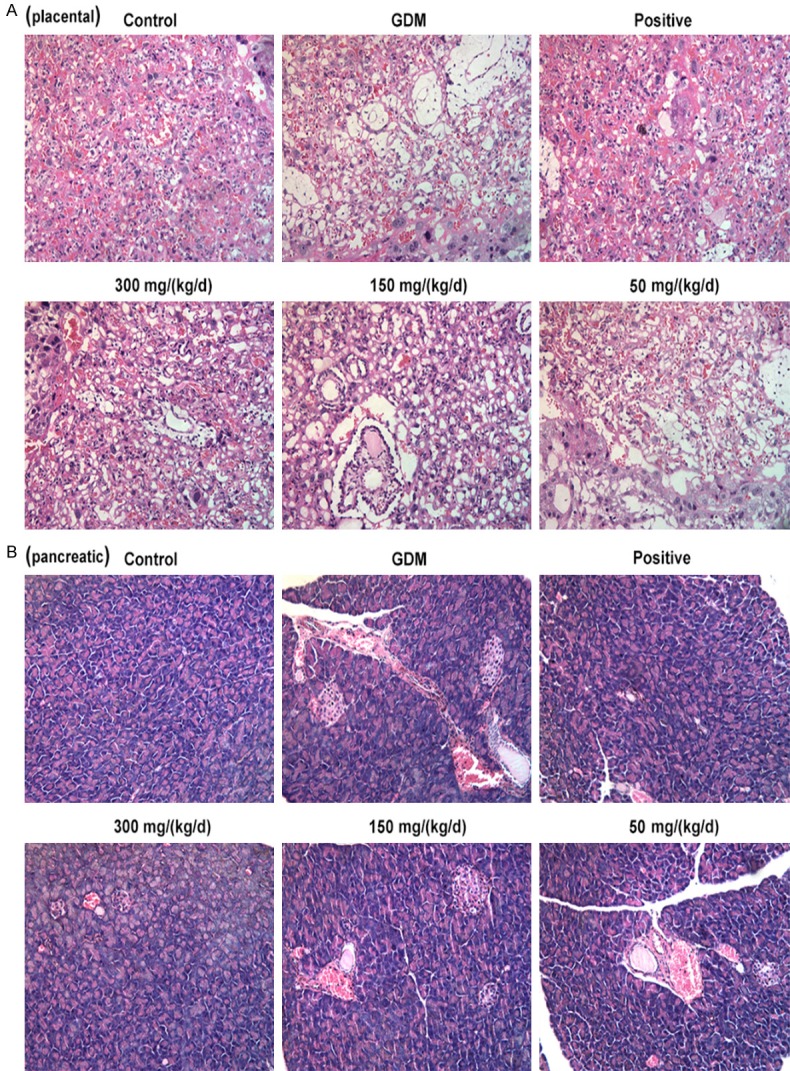
Effect of PEP on the pathological changes of pancreatic and placenta tissues of GDM rats. Representative images of H&E staining of the pathological morphology in the placenta (A) and pancreatic (B) tissue among different groups (magnification, ×200).
Effect of PEP on the cell apoptosis in the placenta and pancreatic tissue of GDM rats
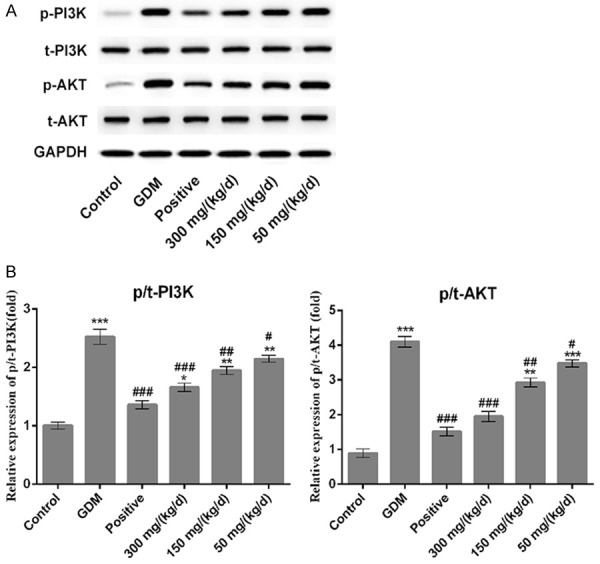
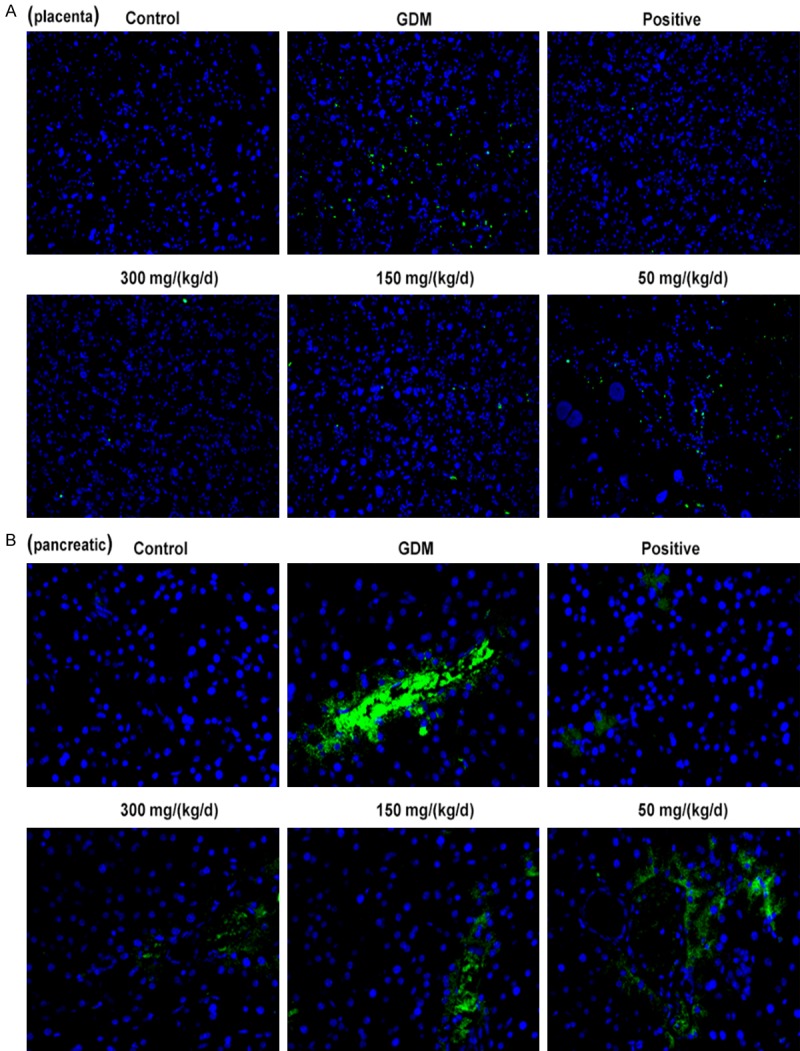
A TUNEL assay was conducted to observe the cell apoptosis of placenta and pancreatic tissues. Green indicates the apoptotic cell and more green fluorescence means more cell apoptosis in tissues. The results showed that the cell apoptosis in placenta (Figure 4A) and pancreatic (Figure 4B) tissues of GDM rats were significantly higher than that in control group. Comparatively, cell apoptosis in placenta and pancreatic tissues were decreased significantly in PEP groups in a dose-dependent manner (Figure 4). Due to the difference of apoptosis level in pancreatic tissue is obvious, western blotting assay was performed to detect the expression of apoptosis related proteins in pancreatic tissues. As shown in Figure 5, the p-PI3K and p-AKT expression in GDM group exhibited upregulated compared with control group (P < 0.001) with no difference of total (t)-PI3K and AKT. When compared with GDM group, PEP reduced p-PI3K and p-AKT expressions among PEP groups in a dose-dependent manner. Therefore, PEP could inhibit the cell apoptosis via suppressing the PI3K/AKT signal pathway.
Figure 4.
Effect of PEP on the cell apoptosis of pancreatic and placenta tissues of GDM rats. TUNEL assay was performed to value the cell apoptosis of placenta (A) and pancreatic (B) tissues in different groups (magnification, ×200). Green indicates the apoptotic cell.
Figure 5.
Effect of PEP on the PI3K/AKT expression in the pancreatic the tissue of GDM rats. (A) Western blotting was performed to detect the expression of p/t-PI3K/AKT in the placental tissue among different groups. (B) Quantitative analysis results from (A). *P < 0.05, ***P < 0.001 vs. control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. GDM. p/t-, phosphorylated/total.
Effect of PEP on the expressions of APN and Chemerin in the pancreatic tissue of GDM rats
Immunohistochemistry analysis was performed to determine the expressions of APN and Chemerin in pancreatic tissues. Results showed the GDM group exhibited upregulated ANP (Figure 6A) and Chemerin (Figure 6B) expressions compared with control group. Comparing to GDM group, PEP reduced ANP and Chemerin expressions in pancreatic tissue of GDM rats in a dose-dependent manner. Therefore, PEP downregulated ANP and Chemerin expressions, which were closely related to insulin resistance and inflammation in GDM.
Figure 6.
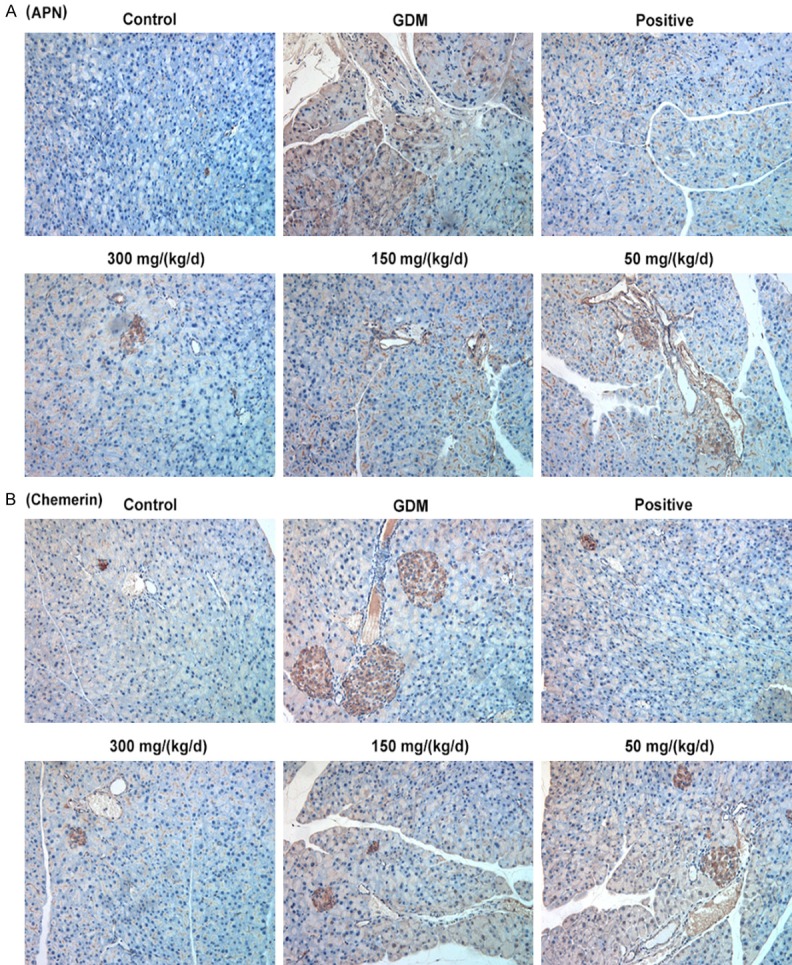
Effect of PEP on the expression of APN and Chemerin in the pancreatic tissue of GDM rats. Immunohistochemically analysis was performed to determine the expressions of APN (A) and Chemerin (B) in pancreatic tissues in different groups (magnification, ×200).
Effect of PEP on the expression of inflammation-associated factors in pancreatic tissues of GDM rats
Results showed that GDM group exhibited upregulated the mRNA and protein levels of CRP, TNF-α and IL-6 when compared with control group (Figure 7, all P < 0.001). Comparing to GDM group, PEP reduced the mRNA and protein levels of CRP, TNF-α and IL-6 in pancreatic tissues of GDM rats in a dose-dependent manner. Therefore, PEP could inhibit the expression of inflammation-associated proteins in GDM.
Figure 7.
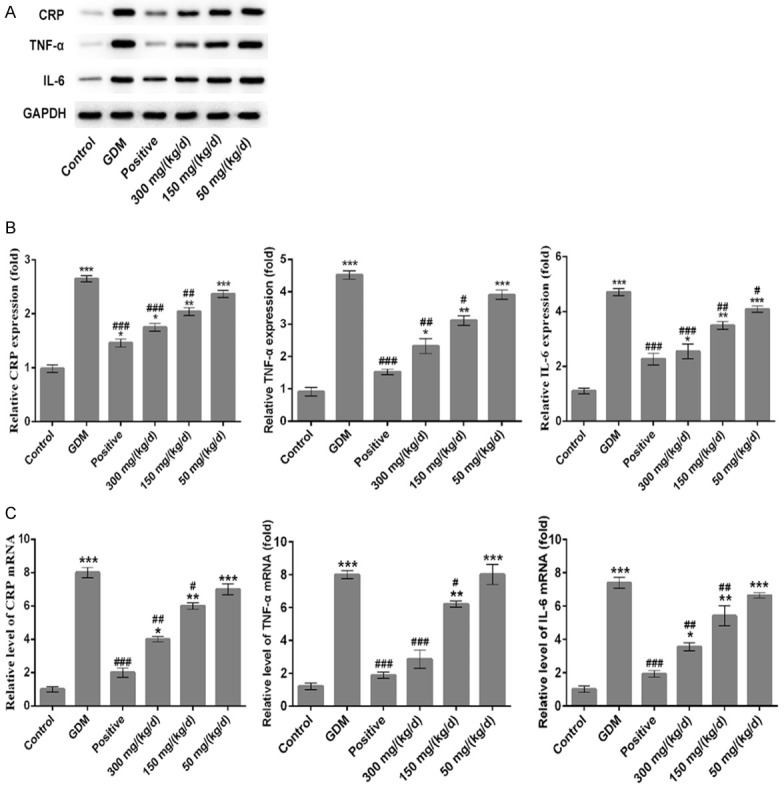
Effect of PEP on the expression of CPR, TNF-α and IL-6 in the pancreatic the tissue of GDM rats. (A) Western blotting analysis were performed to determine the protein expressions of CRP, TNF-α and IL-6 in the pancreatic tissue in different groups. (B) Quantitative analysis results from Western blotting in the (A). (C) The mRNA levels of CRP, TNF-α and IL-6 in the pancreatic tissue among different groups were assessed using qRT-PCR assay. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. GDM.
Effect of PEP on the signal pathway related to insulin resistance in pancreatic tissues of GDM rats
Results showed that GDM group exhibited downregulated the protein and mRNA levels of PPARα, TRB3, AKT2, p-FOXO1 and GLUT2 comparing to control group (Figure 8, P < 0.001). Compared with GDM group, the protein and mRNA levels of PPARα, TRB3, AKT2, p-FOXO1 and GLUT2 were increased significantly among the PEP groups in a dose-dependent manner. These results indicated that PEP could improve the insulin resistance through PPAR signal pathway.
Figure 8.
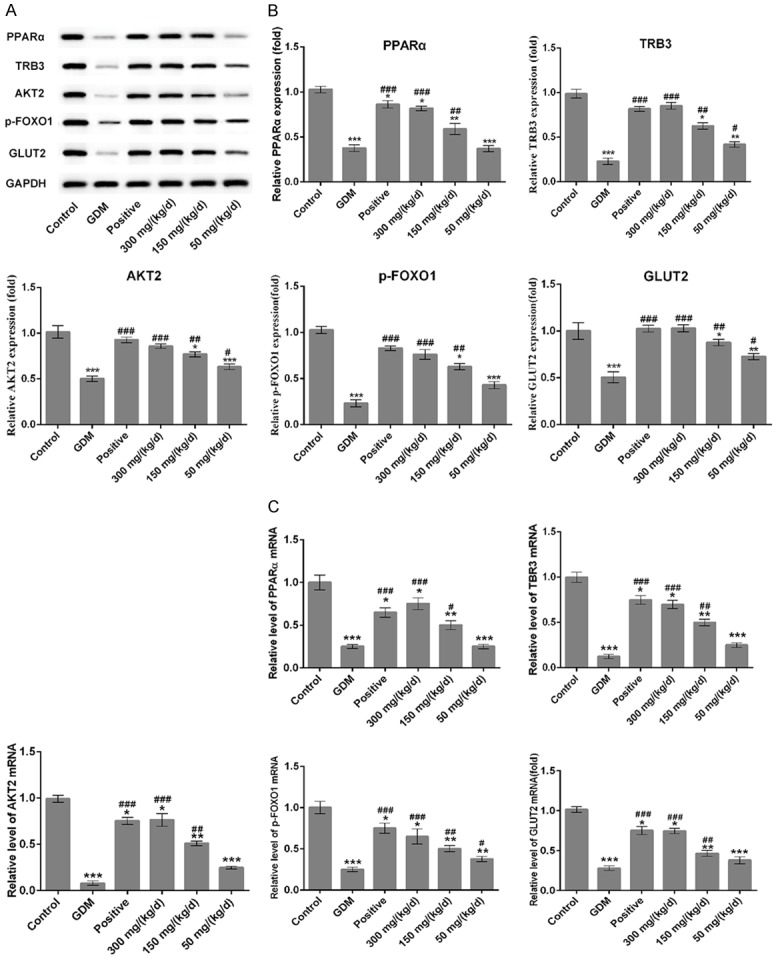
Effect of PEP on the expression of PPARα, TRB3, AKT2, p-FOXO1 and GLUT2 in the pancreatic the tissue of GDM rats. (A) Western blotting analysis were performed to detect the protein level of PPARα, TRB3, AKT2, p-FOXO1 and GLUT2 in the pancreatic tissue in different groups. (B) Quantitative analysis results from Western blotting in the (A). (C) The mRNA levels of PPARα, TRB3, AKT2, p-FOXO1 and GLUT2 in the pancreatic tissue among different groups were assessed using qRT-PCR assay. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. GDM.
Discussion
The imbalance of insulin resistance and insulin secretion capacity during pregnancy often leads to GDM [26]. Recently studies suggested that the change of glycemic parameters, lipid parameters, glucose transporters parameters, pro-inflammatory cytokines parameters, oxidative parameters and genes affecting insulin signaling were associated with in the pathogenesis of the insulin resistance of GDM [27,28]. In the present study, we found that the weight of pregnant rats and fetal rats in GDM group was lower than that in control group. Moreover, the insulin resistance of GDM rats significantly higher compared with normal pregnancy rats, and PEP elevated the weight of the pregnant and fetal rats and suppressed the IRI dose dependently. Meanwhile, PEP could reverse changes of TC, TG and HDL-C levels caused by GDM as well as the levels of serum RBP4, Hcy, GA and FFA in a dose-dependent manner. Additionally, we found that PEP improved the pathological changes of pancreatic and placenta tissues, and inhibited the cell apoptosis and the expression of p-PI3K/AKT in GDM rats. These results have suggested that PEP play important roles in GDM rats.
Pomegranate was considered an antidiabetic medicine in certain systems of traditional medicine. Pomegranate polyphenols were known as a molecular perspective the beneficial effects and traditional use of pomegranate in the prevention of metabolic-associated disorders such as obesity, diabetes and related complications [29]. In this study, the administration of PEP remarkably increased the insulin sensitivity of GDM rats and improved IR, thereby maintaining the normal blood glucose level. 11β-HSD2, an enzyme highly expressed in the placenta [30], was overexpressed in parallel with increasing cortisol levels across pregnancy and protecting the fetus from excess cortisol exposure in utero [31]. We found that 11β-HSD2 in placental tissue of GDM rats decreased significantly comparing to the normal rats, and PEP could increase 11β-HSD2 expression in a dose-dependent manner.
TNF-α, IL-6 and CRP are inflammatory cytokine that are proposed to be involved in the regulation of maternal metabolism and pathogenesis of the insulin resistance [32,33]. We found that PEP inhibited the expression of CRP, TNF-α and IL-6 in placenta tissues of GDM rats. Elevated Chemerin levels were associated with inflammation and insulin resistance [34]. APN has insulin-sensitizing and anti-inflammatory properties; however, lower levels of T-APN may be important in the pathogenesis of gestational diabetes in the Chinese population [35]. In this study, we found that PEP could downregulate the expression of ANP and Chemerin in pancreatic tissue of GDM rats in a dose-dependent manner.
Under insulin-signaling pathway, PPARα is known to suppress inflammation and to preserve insulin sensitivity [10]. TRB3 is a negative regulator of the insulin signaling, and the main role of hepatic TRB3 on AKT2 activity is to bind to AKT2 and then inhibit its phosphorylation at Thr308 and Ser473motifs, which are the critical motifs for AKT activation induced by growth factors and insulin [36]. In pancreatic β cells deacetylates FOXO1, Sirt6 increased the expression of GLUT2 to maintain the glucose-sensing ability of glucose tolerance [37]. In the present study, PEP promoted the mRNA and protein levels of PPARα, TRB3, AKT2, p-FOXO1 and GLUT2 in GDM rats. Among the molecular mechanisms involved, we emphasized that the participation of TRB3, which inhibit AKT2 activity, thereby, maintain FOXO1 activity in progress of insulin resistance.
Although our study revealed that PEP could regulate the expression of PPARα signaling pathways, which play a role in GDM, the drug target of PEP still unclear. Blocking the PPARα signaling will be conducted to explore the impact of PEP on GDM, which is also the limitations of the present study. More researches are needed to verify the exact target of PEP in the future.
Conclusion
In summary, our study provided new evidence that PEP improved insulin sensitivity through complex signal pathways. Thus, PEP has the potential to promote insulin sensitivity in GDM patients and can be used to develop new clinical drugs.
Disclosure of conflict of interest
None.
References
- 1.Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, Duncan BB, Schmidt MI. Gestational diabetes and pregnancy outcomes--a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons D. Prevention of gestational diabetes mellitus: where are we now? Diabetes Obes Metab. 2015;17:824–834. doi: 10.1111/dom.12495. [DOI] [PubMed] [Google Scholar]
- 3.Mulla WR, Henry TQ, Homko CJ. Gestational diabetes screening after HAPO: has anything changed? Curr Diab Rep. 2010;10:224–228. doi: 10.1007/s11892-010-0109-3. [DOI] [PubMed] [Google Scholar]
- 4.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103:176–185. doi: 10.1016/j.diabres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Guelfi KJ, Ong MJ, Li S, Wallman KE, Doherty DA, Fournier PA, Newnham JP, Keelan JA. Maternal circulating adipokine profile and insulin resistance in women at high risk of developing gestational diabetes mellitus. Metabolism. 2017;75:54–60. doi: 10.1016/j.metabol.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Shaat N, Ignell C, Katsarou A, Berntorp K. Glucose homeostasis, beta cell function, and insulin resistance in relation to vitamin D status after gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2017;96:821–827. doi: 10.1111/aogs.13124. [DOI] [PubMed] [Google Scholar]
- 7.Sun X, Zhang Z, Ning H, Sun H, Ji X. Sitagliptin down-regulates retinol-binding protein 4 and reduces insulin resistance in gestational diabetes mellitus: a randomized and double-blind trial. Metab Brain Dis. 2017;32:773–778. doi: 10.1007/s11011-017-9958-7. [DOI] [PubMed] [Google Scholar]
- 8.Bernal-Mizrachi C, Weng S, Feng C, Finck BN, Knutsen RH, Leone TC, Coleman T, Mecham RP, Kelly DP, Semenkovich CF. Dexamethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat Med. 2003;9:1069–1075. doi: 10.1038/nm898. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Xu CX, Krager SL, Bottum KM, Liao DF, Tischkau SA. Aryl hydrocarbon receptor deficiency enhances insulin sensitivity and reduces PPAR-alpha pathway activity in mice. Environ Health Perspect. 2011;119:1739–1744. doi: 10.1289/ehp.1103593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eder K, Ringseis R. The role of peroxisome proliferator-activated receptor alpha in transcriptional regulation of novel organic cation transporters. Eur J Pharmacol. 2010;628:1–5. doi: 10.1016/j.ejphar.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Haluzik MM, Lacinova Z, Dolinkova M, Haluzikova D, Housa D, Horinek A, Vernerova Z, Kumstyrova T, Haluzik M. Improvement of insulin sensitivity after peroxisome proliferator-activated receptor-alpha agonist treatment is accompanied by paradoxical increase of circulating resistin levels. Endocrinology. 2006;147:4517–4524. doi: 10.1210/en.2005-1624. [DOI] [PubMed] [Google Scholar]
- 12.Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, Karnieli E. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem. 2006;281:19881–19891. doi: 10.1074/jbc.M600320200. [DOI] [PubMed] [Google Scholar]
- 13.Aslian S, Yazdanparast R. Hypolipidemic activity of Dracocephalum kotschyi involves FOXO1 mediated modulation of PPARgamma expression in adipocytes. Lipids Health Dis. 2018;17:245. doi: 10.1186/s12944-018-0893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suntar IP, Akkol EK, Yilmazer D, Baykal T, Kirmizibekmez H, Alper M, Yesilada E. Investigations on the in vivo wound healing potential of Hypericum perforatum L. J Ethnopharmacol. 2010;127:468–477. doi: 10.1016/j.jep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009;27:2712–2725. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern Med Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- 19.Reddy MK, Gupta SK, Jacob MR, Khan SI, Ferreira D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007;73:461–467. doi: 10.1055/s-2007-967167. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Banerjee N, Sirven MA, Minamoto Y, Markel ME, Suchodolski JS, Talcott ST, Mertens-Talcott SU. Pomegranate polyphenolics reduce inflammation and ulceration in intestinal colitis-involvement of the miR-145/p70S6K1/HIF1alpha axis in vivo and in vitro. J Nutr Biochem. 2017;43:107–115. doi: 10.1016/j.jnutbio.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Bagri P, Ali M, Aeri V, Bhowmik M, Sultana S. Antidiabetic effect of Punica granatum flowers: effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem Toxicol. 2009;47:50–54. doi: 10.1016/j.fct.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 22.Vroegrijk IO, van Diepen JA, van den Berg S, Westbroek I, Keizer H, Gambelli L, Hontecillas R, Bassaganya-Riera J, Zondag GC, Romijn JA, Havekes LM, Voshol PJ. Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem Toxicol. 2011;49:1426–1430. doi: 10.1016/j.fct.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Lv O, Wang L, Li J, Ma Q, Zhao W. Effects of pomegranate peel polyphenols on lipid accumulation and cholesterol metabolic transformation in L-02 human hepatic cells via the PPARgamma-ABCA1/CYP7A1 pathway. Food Funct. 2016;7:4976–4983. doi: 10.1039/c6fo01261b. [DOI] [PubMed] [Google Scholar]
- 24.Kyohara M, Shirakawa J, Okuyama T, Kimura A, Togashi Y, Tajima K, Hirano H, Terauchi Y. Serum quantitative proteomic analysis reveals soluble EGFR to be a marker of insulin resistance in male mice and humans. Endocrinology. 2017;158:4152–4164. doi: 10.1210/en.2017-00339. [DOI] [PubMed] [Google Scholar]
- 25.Bellamkonda R, Rasineni K, Singareddy SR, Kasetti RB, Pasurla R, Chippada AR, Desireddy S. Antihyperglycemic and antioxidant activities of alcoholic extract of Commiphora mukul gum resin in streptozotocin induced diabetic rats. Pathophysiology. 2011;18:255–261. doi: 10.1016/j.pathophys.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Ducarme G, Desroys du Roure F, Grange J, Vital M, Le Thuaut A, Crespin-Delcourt I. Predictive factors of subsequent insulin requirement for glycemic control during pregnancy at diagnosis of gestational diabetes mellitus. Int J Gynaecol Obstet. 2019;144:265–270. doi: 10.1002/ijgo.12753. [DOI] [PubMed] [Google Scholar]
- 27.Schliefsteiner C, Peinhaupt M, Kopp S, Logl J, Lang-Olip I, Hiden U, Heinemann A, Desoye G, Wadsack C. Human placental hofbauer cells maintain an anti-inflammatory M2 phenotype despite the presence of gestational diabetes mellitus. Front Immunol. 2017;8:888. doi: 10.3389/fimmu.2017.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wedekind L, Belkacemi L. Altered cytokine network in gestational diabetes mellitus affects maternal insulin and placental-fetal development. J Diabetes Complications. 2016;30:1393–1400. doi: 10.1016/j.jdiacomp.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Les F, Arbones-Mainar JM, Valero MS, Lopez V. Pomegranate polyphenols and urolithin A inhibit alpha-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J Ethnopharmacol. 2018;220:67–74. doi: 10.1016/j.jep.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoof E, Girstl M, Frobenius W, Kirschbaum M, Repp R, Knerr I, Rascher W, Dotsch J. Course of placental 11beta-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase mRNA expression during human gestation. Eur J Endocrinol. 2001;145:187–192. doi: 10.1530/eje.0.1450187. [DOI] [PubMed] [Google Scholar]
- 32.Ryan AS. Inflammatory Markers in older women with a history of gestational diabetes and the effects of weight loss. J Diabetes Res. 2018;2018:5172091. doi: 10.1155/2018/5172091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao W, Wang X, Chen T, Xu W, Feng F, Zhao S, Wang Z, Hu Y, Xie B. Maternal lipids, BMI and IL-17/IL-35 imbalance in concurrent gestational diabetes mellitus and preeclampsia. Exp Ther Med. 2018;16:427–435. doi: 10.3892/etm.2018.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd JW, Zerfass KM, Heckstall EM, Evans KA. Diet-induced increases in chemerin are attenuated by exercise and mediate the effect of diet on insulin and HOMA-IR. Ther Adv Endocrinol Metab. 2015;6:189–198. doi: 10.1177/2042018815589088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SY, Sy V, Araki T, Babushkin N, Huang D, Tan D, Liao E, Liu G, Wan S, Poretsky L, Seto-Young D. Total adiponectin, but not inflammatory markers C-reactive protein, tumor necrosis factor-alpha, interluekin-6 and monocyte chemoattractant protein-1, correlates with increasing glucose intolerance in pregnant Chinese-Americans. J Diabetes. 2014;6:360–368. doi: 10.1111/1753-0407.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 37.Song MY, Wang J, Ka SO, Bae EJ, Park BH. Insulin secretion impairment in Sirt6 knockout pancreatic beta cells is mediated by suppression of the FoxO1-Pdx1-Glut2 pathway. Sci Rep. 2016;6:30321. doi: 10.1038/srep30321. [DOI] [PMC free article] [PubMed] [Google Scholar]


