Abstract
Background and Objectives: Diabetic nephropathy (DN) is one of the commonest microvascular complications of diabetes and has been the major cause of end-stage renal disease in many countries. It is of great clinical significance to further explore more efficacious therapeutic strategies for DN. This study aims to explore the effect of Blnc1 on renal fibrosis in diabetic nephropathy. Methods: In this study, mRNA level of Blnc1 was examined by RT-PCR. HE staining and Masson staining were adopted to detect kidney damage and renal fibrosis. The renal fibrosis was evaluated by the levels of PTEN, fibronectin, collagen I and collagen IV with immunofluorescence assay and western blot analysis. Oxidative Stress and inflammatory response were detected by ELISA assay. At the same time, western blot was performed to detect the proteins related to NRF2/HO-1 and NF-κB pathways. Results: Blnc1 has higher expression in serum of DN patients, STZ-induced DN model and HG-induced HK2 cells. Blnc1 interference significantly attenuated renal fibrosis, inflammation and oxidative stress via NRF2/HO-1 and NF-κB pathways. Conclusion: Our present study suggested that Blnc1 can affect inflammation, oxidative stress and renal fibrosis by Nrf2/HO-1 and NF-κB pathways in DN.
Keywords: LncRNA Blnc1, diabetic nephropathy, renal fibrosis
Introduction
Diabetes mellitus (DM) describes a common metabolic disorder with high blood glucose levels [1]. Persistently high blood glucose levels may lead to various complications in heart, kidneys, nerves and eyes [2]. Diabetic nephropathy (DN) is one of the commonest microvascular complications of diabetes and has been the major cause of end-stage renal disease in many countries [3]. DN is characterized by the accumulation of extracellular matrix (ECM) proteins, the activation of myofibroblasts and tubulointerstitial fibrosis (TIF) [4]. Actually, the pathogenesis of DN is extremely complicated and is the result of multiple factors synergy, such as inflammation and oxidative stress [5]. At present, there are no more effective prevention methods to control it [6].
Phosphatase and tensin homologue (PTEN) are a ubiquitously expressed phosphatase [7]. More scholars have discovered that PTEN plays an important role in mediating fibrosis development in kidney [8]. Also, mounting evidence in vivo and in vitro has indicated that PTEN activates profibrotic signaling pathways in kidney such as SMAD3, p53, and JNK to attenuate renal fibrosis [9,10]. Therefore, PTEN may be a significant way to restrain the development of TIF and DN.
Nowadays, nuclear-factor erythroid 2-related factor 2 (Nrf2) as an antioxidant transcription target has gained more and more attention [11]. In the regulation of the oxidative response, Nrf2 modulates the expressions of various antioxidant factors, such as heme oxygenase-1 (HO-1) et al [12]. It has been widely recognized that nuclear factor kappa beta (NF-κB) is an important transcription factor associated with inflammation [13]. The activation of NF-κB can promote inflammatory factors secretion including TNFα, IL-6, IL-1β [13]. A research showed that the modulation of the Nrf2/HO-1 and NF-κB pathways could affect DN due to their adjustment of oxidant and inflammatory [14]. These researches suggest that targeting Nrf2/HO-1 and NF-κB pathways might serve as a potential therapy against DN.
Long non-coding RNAs (lncRNAs) are a subset of RNAs of over 200 nucleotides that play a key role in numerous biological processes [15]. In the past decades, lncRNAs have been intensely investigated and found to regulate diabetes and fibrosis progresses [16]. Blnc1 is a conserved lncRNA that could promote thermogenic gene expression in brown adipocytes [17]. A research showed that inactivation of Blnc1 could exacerbate adipose tissue inflammation and fibrosis, insulin resistance and hepatic steatosis [18]. However, the effects and specific mechanisms of Blnc1 on inflammation, oxidative stress and renal fibrosis have not yet been fully elucidated. Therefore, the present study aims to evaluate whether Blnc1 can affect inflammation, oxidative stress and renal fibrosis by Nrf2/HO-1 and NF-κB pathways in DN. The in-depth study on the molecular mechanisms will provide new strategies and methods for the treatment of diabetic nephropathy.
Materials and methods
Patients and clinical specimens
The research protocol was approved by Shanghai Tongren Hospital. Blood were collected from DN patients (n = 30) and normal patients (n = 30) in Tongren Hospital, People’s Republic of China. Written informed consent was obtained from every patient. The serum was separated from blood by centrifugation and the supernatant serum was frozen at -80°C for analysis.
Streptomycin-induced animal model
Eight-week old SD rats were bought from Shanghai Animal Center (Shanghai, China) and were housed in animal facility at the Animal Center of Shanghai Jiaotong University School of Medicine. Streptomycin (STZ; Sigma) was dissolved in citrate buffer to a working concentration of 35 mg/ml, and purified with 0.22 µm filter. The DN model was induced with intraperitoneal administration of 35 mg/kg body weight while the negative control group were administrated with equal amount of PBS buffer. Blood glucose concentration was measured at 24 h post administration. DN mice model were defined when blood glucose level was higher than 16.7 mmol/L.
Cell culture and transfection
Human kidney-2 (HK-2) line was purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma) supplemented with 10% fetal bovine serum (FBS; Gibco, MA, USA), 100 U/ml penicillin and 100 mg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. HK-2 cells were treated with 30 mM D-glucose or mannitol for 12 h, 24 h, 48 h. Transfection with LncRNA Blnc1 siRNA was performed using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) in line with the manufacturer’s protocol.
HE staining and Masson staining
The kidney tissues from NC and DN group were fixed in 4% paraformaldehyde, and embedded in paraffin. Sections in 5 μm thickness were cut in series from the embedded tissues and stained with H&E and Masson’s trichrome. Immunohistochemical analysis was performed following its standard protocol. Five random images were chosen and analyzed with Image-Pro Plus (Medium Cybernetics, Bethesda, MD, USA).
RNA isolation and quantitative real-time PCR
TRIzol reagent (Invitrogen, CA, USA) was used to extract the total RNA from HK-2 cell line and clinical blood samples according to the manufacturer’s protocol. For the evaluation of mRNA, total cDNA was reverse-transcribed from isolated RNA using a RNA PCR kit (Takara, Japan) and quantitative real-time PCR was carried out with the SYBR premix Ex TaqII kit (Takara) in line with the manufacturer’s instructions. Reaction steps were 95°C for 30 s, 95°C for 5 s and 60°C for 34 s, for a total of 40 cycles. The relative expression level of lincRNA-Blnc1 was normalized to GAPDH as internal controls, and the 2-ΔΔCt method was adopted to calculate the relative quantities.
Protein extraction and Western blot analysis
Total protein from animals and cells were extracted with ice-cold Radio-Immunoprecipitation Assay (RIPA) lysis buffer containing protease and phosphatase inhibitors (Beyotime, Shanghai, China) in strict accordance with the manufacturer’s protocol. Equal amounts of protein per lane (16-20 mg) were loaded onto 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to different molecular weights. Proteins were transferred to polyvinylidene fluoride (PVDF) membranes. After blocked in 5% skimmed milk or BSA solution, the PVDF membranes were incubated with the primary antibodies against PTEN PTEN (1:1000, ab170941, abcam, UK), Fibronection (1:1000, ab32419, abcam, UK), Collagen I (1:1000, ab34710, abcam, UK), Collagen IV (1:1000, ab6586, abcam, UK), NRF2 (1:1000, ab137550, abcam, UK), p-NF-KB P65 (1:2000, ab86299, abcam, UK), Ikβ (1:1000, ab32518, abcam, UK), NF-KB P65 (1:1000, ab32536, abcam, UK), HO-1 (1:2000, ab13243, abcam, UK) and GAPDH (1:10000, ab181602, abcam, UK) overnight. HRP-conjugated anti-rabbit immunoglobulin G (IgG) (1:10000, ab6721, abcam, UK) and anti-mice IgG (1:10000, ab6728, abcam, UK) were used as the secondary antibodies. GAPDH was utilized as the endogenous control. Gray value of protein bands was quantified by Image J software.
Immunofluorescence assay
For the immunofluorescence assay, the fixed HK-2 cells were permeabilized with 0.1% Triton X-100 and blocked in 5% goat serum solution. Then, the coverslips were treated with primary antibodies against Collagen IV (1:100, ab6586, abcam, UK) at 4°C overnight and Cy3-labeled secondary antibody (1:1000, ab6939, abcam, UK) for 1 h at room temperature. The coverslips were sealed with glycerine after treatment with DAPI. Images were analyzed with Image-Pro Plus (Medium Cybernetics, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay (ELISA)
Medium from HK-2 cells after different treatments was collected to detect production of inflammatory factors. The contents of TNFα, IL-6, IL-1β were measured by ELISA using the appropriate ELISA kit (R&D Systems, United States) according to the manufacturer’s instructions.
Determination of ROS
The ROS level was determined using the ROS detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). After culturing under various stimulation conditions, HK-2 cells were incubated with DCFH-DA for 20 min at 37°C. Then the ROS levels were observed and recorded using a fluorescent microscope.
Statistical analysis
All statistical analysis was analyzed by SPSS 21.0 software. All the results were presented as mean ± standard deviation. Student’s t-test was applied to the analysis of two groups and one-way ANOVA was applied to the analysis of no less than three groups. A value of P < 0.05 was considered to indicate a statistically significant difference. *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Blnc1 has higher expression in serum of DN patients
In our study, qRT-PCR assay was used to detect the relative mRNA expression level of Blnc1 in normal patient serum (n = 30) and DN patient serum (n = 30). We found that compared with normal patient serum, the mRNA expression level of Blnc1 was significantly increased in DN patient serum in Figure 1.
Figure 1.
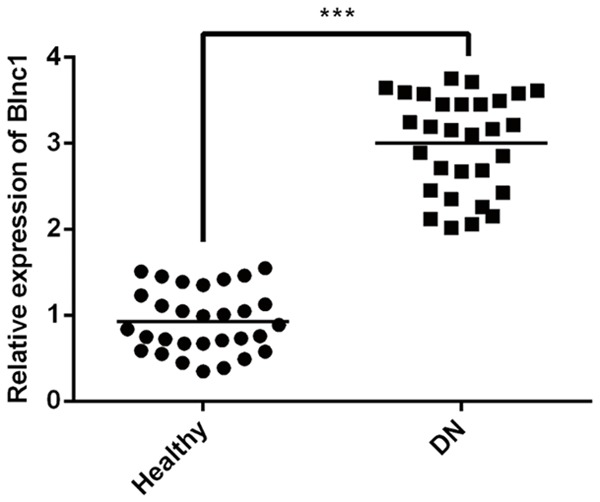
Blnc1 has higher expression in in serum of DN patients. The relative mRNA expression level of Blnc1 in normal patient serum (n = 30) and DN patient serum (n = 30) were detected by qRT-PCR. ***P < 0.001 vs. Healthy group.
Blnc1 attenuates renal dysfunction and fibrosis in STZ-induced DN model
To explore the functional role of lncRNA Blnc1, we next examined the expression level of Blnc1 and fibrosis level in normal rats and DN models. As shown in Figure 2A, these results of H&E and Masson staining suggested that kidney damage and renal fibrosis were more serious compared to the control group. At the same time, qRT-PCR assay was used to detect the relative mRNA expression level of Blnc1 in normal rats and DN models. As shown in Figure 2B, the expression of Blnc1was almost 3-fold upregulated compared to the control. These results indicated that Blnc1 attenuates renal dysfunction and fibrosis in STZ-induced DN model.
Figure 2.
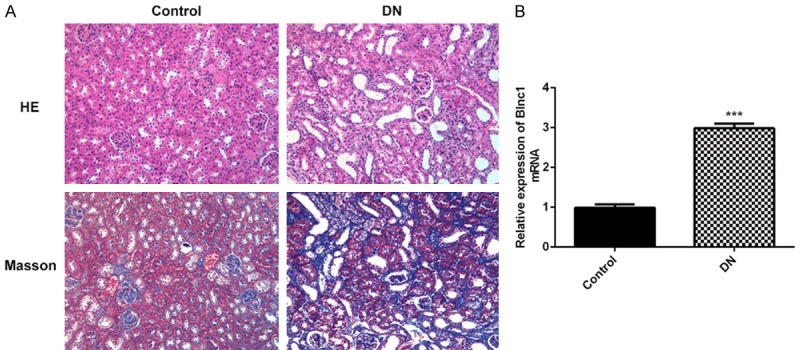
Blnc1 attenuates renal dysfunction and fibrosis in STZ-induced DN model. DN model was induced with intraperitoneal administration of STZ. A. Renal cortical tissues are collected for H&E staining and Masson staining. B. qRT-PCR assay was used to detect the relative mRNA expression level of Blnc1 in normal rats and DN models. ***P < 0.001 vs. control group.
Blnc1 expression is upregulated in HG-induced HK2 cells
We further studied the role of Blnc1 in DN by using HG-stimulated HK-2 cells. HK-2 cells were treated with normal glucose (NG, 5.5 mM D-glucose) or high glucose (HG, 30 mM D-glucose) for 12, 24, 48 h, after which levels of were examined by qRT-PCR assay. Compared with the control group, our results showed that HG could significantly increase the level of Blnc1 in a time-dependent manner in Figure 3.
Figure 3.
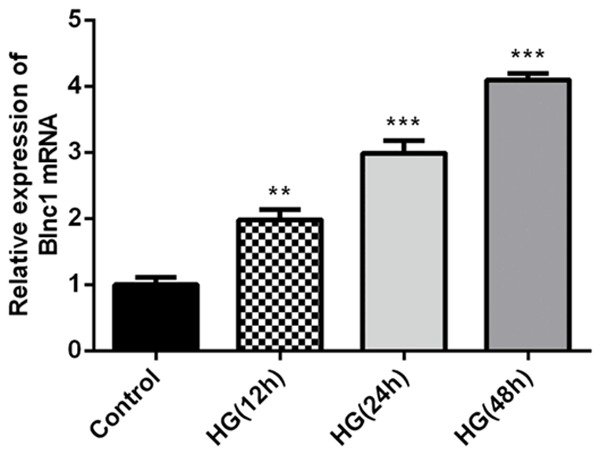
Blnc1 expression is upregulated in HG-induced HK2 cells. HK-2 cells were treated with normal glucose (NG, 5.5 mM D-glucose) or high glucose (HG, 30 mM D-glucose) for 12, 24, 48 h. Quantification of Blnc1 levels in HK-2 cells after NG or HG treatments with indicated time were detected by qRT-PCR assay. **P < 0.01, ***P < 0.001 vs. control group.
Blnc1 interference significantly inhibited renal fibrosis in HG-induced HK-2 cells
To analyze the effect of Blnc1 on renal fibrosis in DN, we inhibited the Blnc1 expression by transfecting with Blnc1 inhibitor and measured Blnc1 level by qPCR analysis in Figure 4A. Subsequently, the protein levels of PTEN, fibronectin, collagen I and collagen IV were examined by western blot in Figure 4B and 4C. Meanwhile, we confirmed the expression of collagen IV in HK-2 cells by immunofluorescence assay in Figure 4D. High glucose injury obviously increased the levels of PTEN, fibronectin, collagen I and collagen IV compared with the control group while Blnc1 interference significantly decreased the levels of PTEN, fibronectin, collagen I and collagen IV compared with the HG group. These results indicated Blnc1 interference reversed renal fibrosis in HG-induced HK-2 cells.
Figure 4.
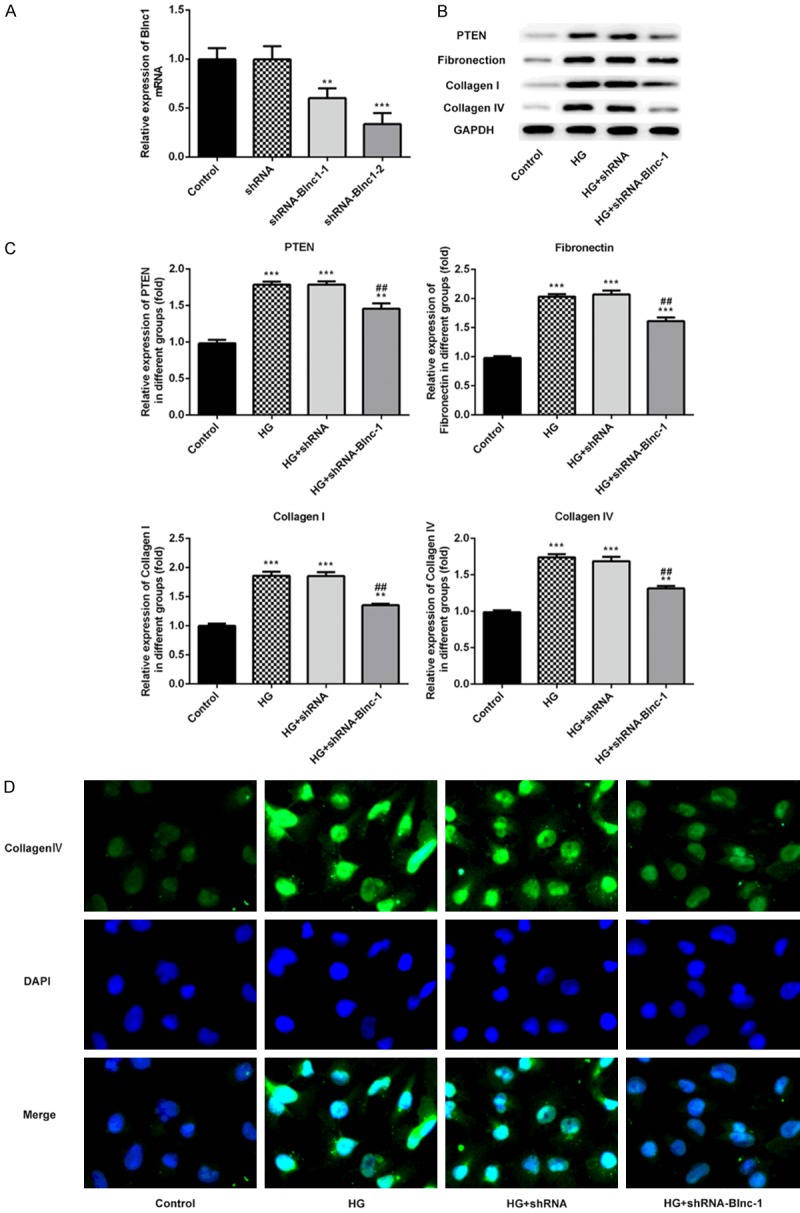
Blnc1 interference significantly inhibited renal fibrosis in HG-induced HK-2 cells. A. The mRNA level of Blnc1 was detected by qPCR assay. HK-2 cells were either untreated, or transfected with shRNA, Blnc1 inhibitor 1 or Blnc1 inhibitor 2. B and C. The protein levels of PTEN, fibronectin, collagen I, collagen IV were detected by western blot. D. The expression of collagen IV in HK-2 cells was measured by immunofluorescence assay. HK-2 cells were either untreated, or treated with D-glucose, and transfected with control or Blnc1 inhibitor. **P < 0.01, ***P < 0.001 vs. control group, ##P < 0.01 vs. HG group.
Blnc1 interference significantly inhibited inflammation factors in HG-induced HK-2 cells
To determine whether Blnc1 affected the inflammatory response in DN, we measured the levels of TNF-α, IL-6 and IL-1β by ELISA assay. Generally, the results indicated that high glucose injury obviously increased the levels of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β while Blnc1 interference could extremely decrease the expression of pro-inflammatory cytokines in Figure 5.
Figure 5.
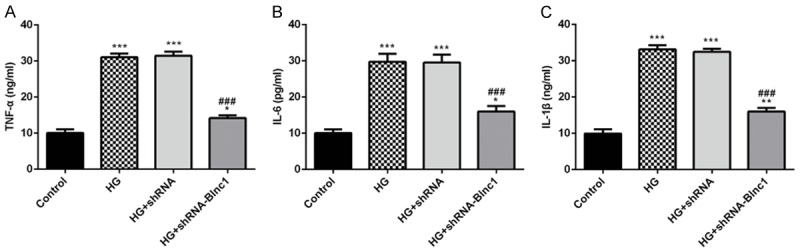
Blnc1 interference significantly inhibited inflammation factors in HG-induced HK-2 cells. The levels of TNF-α, IL-6 and IL-1β were detected with ELISA kits. HK-2 cells were either untreated, or treated with D-glucose, and transfected with control or Blnc1 inhibitor. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group, ###P < 0.001 vs. HG group.
Blnc1 interference significantly decreased the ROS level in HG-induced HK-2 cells
To analyze the effect of Blnc1 on the ROS level in HK-2 cells treated with D-glucose, we detected the ROS level using 2’, 7’-Dichlorodihydrofluorescein diacetate. We found that high glucose could significantly increase the ROS level in HK-2 cells while Blnc1 interference could inhibit the generation of ROS in Figure 6.
Figure 6.
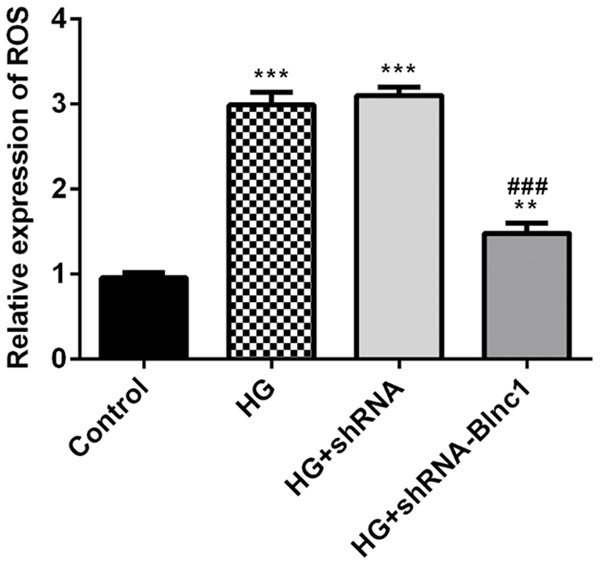
Blnc1 interference significantly decreased the ROS level in HG-induced HK-2 cells. The effect of Blnc1 on the ROS level in HK-2 cells treated with D-glucose. The ROS level was assessed with 2’, 7’-Dichlorodihydrofluorescein diacetate. HK-2 cells were either untreated, or treated with D-glucose, and transfected with control or Blnc1 inhibitor. **P < 0.01, ***P < 0.001 vs. control group, ### P < 0.001 vs. HG group.
Blnc1 interference attenuated inflammation and oxidative stress via NRF2/HO-1 and NF-κB pathways
To determine whether Blnc1 interference influenced inflammation and oxidative stress via NRF2/HO-1 and NF-κB pathways in HK-2 cells pre-incubated with D-glucose, we detected some crucial proteins related to NRF2/HO-1 and NF-κB pathways, including NRF2, HO-1, Iκβ, NF-κB P65 and p-NF-κB P65 levels. As shown in Figure 7, high glucose injury in HK-2 cells significantly downregulated NRF2, HO-1, Iκβ levels and upregulated p-NF-κB P65 level compared with the control group, which was reversed by transfecting Blnc1 inhibitor. These findings indicated that Blnc1 may regulate DN progression through NRF2/HO-1 and NF-κB pathways.
Figure 7.
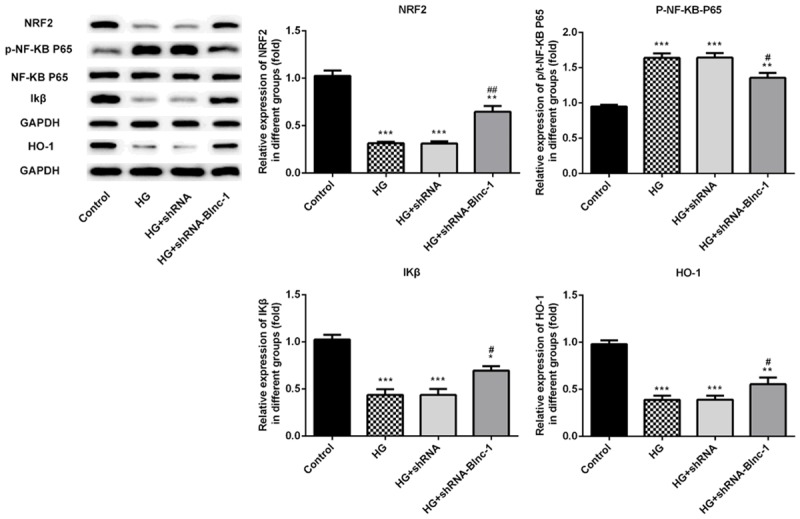
Blnc1 interference attenuated inflammation and oxidative stress via NRF2/HO-1 and NF-κB pathways. Proteins related to NRF2/HO-1 and NF-κB pathways in HK-2 cells were detected by western blot. HK-2 cells were either untreated, or treated with D-glucose, and transfected with control or Blnc1 inhibitor. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group, #P < 0.05, ##P < 0.01 vs. HG group.
Discussion
Recently, the research of lncRNAs to prevent inflammation, oxidative stress and renal fibrosis in DN is getting more and more attentions [19,20]. In our study, we revealed that the expression of Blnc1 was improved in the blood of DN patients and rats. Also, we could see tissue damage and fibrosis in the kidney of DN rat models. Besides, we proved that high glucose led to the improvement of Blnc1 in HK-2 cells. Furthermore, we evaluated that Blnc1 interference significantly reduced the fibrosis, inflammation and oxidative factors in HK-2 cells pre-incubated with high glucose. Finally, Blnc1 interference could up-regulate NRF2/HO-1 signaling pathway proteins and inhibit the activation of NF-κB pathway.
It has been well documented that the expression of many lncRNAs changed in DN and was associated with kidney fibrosis [21]. In the present study, we found that the expression of lncRNA Blnc1 increased in DN patients and rats. The damage and fibrosis were also found in DN rat kidneys. Recently, it has been demonstrated that high glucose could lead to the oxidative stress in HK-2 cells [22]. Our results revealed that HK-2 cells pre-incubated with high glucose could be found the upregulation of Blnc1. And Blnc1 interference could significantly inhibit the levels of fibrosis-related proteins, inflammatory factors and ROS induced by high glucose in HK-2 cells. It has been reported in the literature that NRF2/HO-1 and NF-κB signaling pathways had played important roles in DN [14,23]. Our research proved that pre-incubating with high glucose inhibit significantly reduced NRF2/HO-1 signaling pathway proteins and promoted the activation of NF-κB pathway in HK-2 cells, while Blnc1 interference could reverse the effects.
In conclusion, Blnc1 serves as a novel regulator of inflammation, oxidative stress and fibrosis in DN through NRF2/HO-1 and NF-κB pathways. Therefore, this study may provide new insights into the possibility of Blnc1 being a potential therapeutic target in DN.
Disclosure of conflict of interest
None.
References
- 1.Baena-Díez JM, Peñafiel J, Subirana I, Ramos R, Elosua R, Marín-Ibañez A, Guembe MJ, Rigo F, Tormo-Díaz MJ, Moreno-Iribas C, Cabré JJ, Segura A, García-Lareo M, Gómez de la Cámara A, Lapetra J, Quesada M, Marrugat J, Medrano MJ, Berjón J, Frontera G, Gavrila D, Barricarte A, Basora J, García JM, Pavone NC, Lora-Pablos D, Mayoral E, Franch J, Mata M, Castell C, Frances A, Grau M FRESCO Investigators. Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care. 2016;39:1987–1995. doi: 10.2337/dc16-0614. [DOI] [PubMed] [Google Scholar]
- 2.Packer M. Have we really demonstrated the cardiovascular safety of anti-hyperglycaemic drugs? Rethinking the concepts of macrovascular and microvascular disease in type 2 diabetes. Diabetes Obes Metab. 2018;20:1089–1095. doi: 10.1111/dom.13207. [DOI] [PubMed] [Google Scholar]
- 3.Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol. 2017;13:311–318. doi: 10.1038/nrneph.2017.31. [DOI] [PubMed] [Google Scholar]
- 4.Gao J, Wang W, Wang F, Guo C. LncRNA-NR_033515 promotes proliferation, fibrogenesis and epithelial-to-mesenchymal transition by targeting miR-743b-5p in diabetic nephropathy. Biomed Pharmacother. 2018;106:543–552. doi: 10.1016/j.biopha.2018.06.104. [DOI] [PubMed] [Google Scholar]
- 5.Wan TT, Li XF, Sun YM, Li YB, Su Y. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Biomed Pharmacother. 2015;74:145–147. doi: 10.1016/j.biopha.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Shi Y, Peng H, Shen X, Thomas S, Wang Y, Truong LD, Dryer SE, Hu Z, Xu J. Loss of PTEN promotes podocyte cytoskeletal rearrangement, aggravating diabetic nephropathy. J Pathol. 2015;236:30–40. doi: 10.1002/path.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Zang X, Ponnusamy M, Masucci MV, Tolbert E, Gong R, Zhao TC, Liu N, Bayliss G, Dworkin LD, Zhuang S. Enhancer of zeste homolog 2 inhibition attenuates renal fibrosis by maintaining smad7 and phosphatase and tensin homolog expression. J Am Soc Nephrol. 2016;27:2092–2108. doi: 10.1681/ASN.2015040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan R, Geng H, Polichnowski AJ, Singha PK, Saikumar P, McEwen DG, Griffin KA, Koesters R, Weinberg JM, Bidani AK, Kriz W, Ven-katachalam MA. PTEN loss defines a TGF-β-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Physiol Renal Physiol. 2012;302:F1210–1223. doi: 10.1152/ajprenal.00660.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samarakoon R, Helo S, Dobberfuhl AD, Khakoo NS, Falke L, Overstreet JM, Goldschmeding R, Higgins PJ. Loss of tumour suppressor PTEN expression in renal injury initiates SMAD3- and p53-dependent fibrotic responses. J Pathol. 2015;236:421–432. doi: 10.1002/path.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujiki T, Ando F, Murakami K, Isobe K, Mori T, Susa K, Nomura N, Sohara E, Rai T, Uchida S. Tolvaptan activates the Nrf2/HO-1 antioxidant pathway through PERK phosphorylation. Sci Rep. 2019;9:9245. doi: 10.1038/s41598-019-45539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Xu L, Zhou X, Gao C, Yang M, Chen G, Zhu J, Jiang L, Gan H, Gou F, Feng H, Peng J, Xu Y. High glucose induces activation of NF-κB inflammatory signaling through IκBα sumoylation in rat mesangial cells. Biochem Biophys Res Commun. 2013;438:568–574. doi: 10.1016/j.bbrc.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 14.Bao L, Li J, Zha D, Zhang L, Gao P, Yao T, Wu X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-κB pathways. Int Immunopharmacol. 2018;54:245–253. doi: 10.1016/j.intimp.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 16.Tao H, Song ZY, Ding XS, Yang JJ, Shi KH, Li J. LncRNAs and miRs as epigenetic signatures in diabetic cardiac fibrosis: new advances and perspectives. Endocrine. 2018;62:281–291. doi: 10.1007/s12020-018-1688-z. [DOI] [PubMed] [Google Scholar]
- 17.Mi L, Zhao XY, Li S, Yang G, Lin JD. Conserved function of the long noncoding RNA Blnc1 in brown adipocyte differentiation. Mol Metab. 2016;6:101–110. doi: 10.1016/j.molmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao XY, Li S, DelProposto JL, Liu T, Mi L, Porsche C, Peng X, Lumeng CN, Lin JD. The long noncoding RNA Blnc1 orchestrates homeostatic adipose tissue remodeling to preserve metabolic health. Mol Metab. 2018;14:60–70. doi: 10.1016/j.molmet.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng W, Huang S, Shen L, Tang Y, Li H, Shi Y. Long noncoding RNA NONHSAG053901 promotes diabetic nephropathy via stimulating Egr-1/TGF-β-mediated renal inflammation. J Cell Physiol. 2019;234:18492–18503. doi: 10.1002/jcp.28485. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Chen ZY, Wang Y, Liu Y, Ma JX, Li YK. Long non-coding RNA ASncmtRNA-2 is upregulated in diabetic kidneys and high glucose-treated mesangial cells. Exp Ther Med. 2017;13:581–587. doi: 10.3892/etm.2017.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Lv X, Fan Q, Wang X, Xu L, Lu X, Chen T. Analysis of circulating lncRNA expression profiles in patients with diabetes mellitus and diabetic nephropathy: differential expression profile of circulating lncRNA. Clin Nephrol. 2019;92:25–35. doi: 10.5414/CN109525. [DOI] [PubMed] [Google Scholar]
- 22.Ji L, Wang Q, Huang F, An T, Guo F, Zhao Y, Liu Y, He Y, Song Y, Qin G. FOXO1 overexpression attenuates tubulointerstitial fibrosis and apoptosis in diabetic kidneys by ameliorating oxidative injury via TXNIP-TRX. Oxid Med Cell Longev. 2019;2019:3286928. doi: 10.1155/2019/3286928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landis RC, Quimby KR, Greenidge AR. M1/M2 macrophages in diabetic nephropathy: Nrf2/HO-1 as therapeutic targets. Curr Pharm Des. 2018;24:2241–2249. doi: 10.2174/1381612824666180716163845. [DOI] [PubMed] [Google Scholar]


