Abstract
Background: Aberrant apoptosis in nucleus pulposus (NP) cells is the primary cause of intervertebral disc degeneration (IDD). In contrast, a large number of studies have confirmed that autophagy may protect NP cells from apoptosis. Sinomenine is an alkaloid monomer, which has been reported to stimulate cell autophagy. Therefore, the aim of the present study was to investigate the effects of sinomenine on IDD. Methods: The effects of sinomenine on the proliferation and apoptosis of NP cells were evaluated with the CCK-8 assay and Annexin V/PI staining, respectively. Results: The data obtained from the present study demonstrated that sinomenine could notably reverse TBHP-induced growth inhibition and apoptosis in rat NP cells. In addition, sinomenine significantly induced autophagy in rat NP cells, which was completely inhibited by 3-methyladenine (3MA). In addition, the protective effect of sinomenine against TBHP in rat NP cells was abolished following treatment with 3MA. Finally, an in vivo study further confirmed that sinomenine could ameliorate rat IDD. Conclusion: Taken together, the results of the present study indicated sinomenine could ameliorate rat IDD via induction of autophagy in vitro and in vivo. These findings suggest the therapeutic potential of sinomenine in the prevention of IDD.
Keywords: Sinomenine, nucleus pulposus cells, intervertebral disc degeneration, autophagy, apoptosis
Introduction
Intervertebral disc degeneration (IDD) is a disease characterized by cervical spondylosis, lumbar disc herniation, and degenerative scoliosis. According to statistics, the lower back pain of 40% of patients is caused by degeneration of the intervertebral disc [1]. The current treatments for such diseases include conservative anti-inflammatory analgesics, physiotherapy, surgical discectomy, replacement and spinal fusion [2]. However, these methods only cure the symptoms and not the disease; therefore, their efficacy is highest for early and middle stage disc degeneration, which does not require surgery.
The adult intervertebral disc is a completely enclosed avascular tissue composed of three highly specific structures, namely the cartilage endplate, the annulus fibrosus and the nucleus pulposus [3]. Changes in the nucleus pulposus (NP) tissue were most pronounced in IDD at the early stage of disease [4]. At present, studies have demonstrated that the loss of water and extracellular matrix in the NP, causing the living environment of NP cells to become unfavourable [5]. Therefore, aberrant apoptosis of NP cells is the principal cause of IDD [6].
Autophagy is a process whereby cells phagocytose and degrade their own cytoplasm and organelles via a lysosomal system. It serves a significant role in numerous cellular processes such as growth and development, cell self-stability, and mature differentiation [7]. A large number of studies have confirmed that autophagy protected cells by inhibiting apoptosis [8,9]. In studies investigating orthopedic degenerative diseases, autophagy has been confirmed to serve a crucial role in the development of osteoarthritis. For example, the autophagy of articular cartilage in severe osteoarthritis was significantly reduced, while the rate of apoptosis was obviously increased [10]. In addition, in a study investigating the intervertebral disc annulus of rats, autophagy protected the degeneration of the annulus by inhibiting apoptosis [11].
Sinomenine is an alkaloid monomer extracted from the Sinomenium acutum, which has anti-inflammatory and immunosuppressive effects (12). Sinomenine is widely used in the treatment of rheumatoid arthritis, systemic lupus erythematosus and other inflammatory and autoimmune diseases [12,13]. Recent studies have demonstrated that sinomenine could promote autophagy in tumor cells [14,15]. Therefore, we hypothesize that sinomenine could regulate the apoptosis of intervertebral NP cells by regulating autophagy, thereby, affecting the progression of IDD.
Therefore, the present study aimed to elucidate the biological effects of sinomenine on the apoptosis of NP cells. In addition, the therapeutic potential of sinomenine in a puncture-induced rat IDD model was further examined.
Materials and methods
Isolation and culture of rat NP cells
5 SD rats (225-250 g) purchased from the Fourth Military Medical University Model Animal Center (Xian, China) were sacrificed using CO2 at a displacement rate of 20%/L/min. The spinal column then was exposed under aseptic conditions in order to reveal the lumbar intervertebral disc, and the annulus fibrosus was isolated for separating the gelatinous nucleus pulposus. The gelatinous nucleus pulposus was placed in sterile D-hanks and washed 3 times to remove blood. The nucleus pulposus tissue was separated to a thickness of 1 mm3, and was subsequently digested with 0.1% collagenase type II (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 2 h at 37°C. The tissues were subsequently centrifuged at 800 g for 5 min, and the precipitate was incubated with 10 U/ml hyaluronidase (Sigma, St Louis, MO, USA) for an additional 2 h. NP cells were collected by centrifugation (1,000 g for 5 min) and washed three times with DMEM-F12 (Gibco; Thermo Fisher Scientific, Inc.). The cells were then cultured in DMEM-F12 with 15% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C. The National Institutes of Health guide for the care and use of laboratory animals was followed. All experimental procedures were approved by the Ethics Committee of The Zhongda Hospital Affiliated to Southeast University. The animal care and use were followed the policy of Institutional animal care and use committee (IACUC).
Sinomenine treatment
To examine the effects of sinomenine on rat NP cells, cells were pre-treated with 0.33, 0.67, 1.00, 3.33, 6.67 or 10 mM sinomenine for 24 h. The cells were then cultured with different concentrations of tert-butyl hydroperoxide (TBHP) (50, 100, 200 or 300 μM) for 24 h. For treatment with 3-methyladenine (3MA), cells were cultured with 5 mM 3MA for 1 h prior to treatment with sinomenine.
Dansylcadaverine (MDC) staining
The MDC staining kit (Beijing Solarbio, Science & Technology Co., Ltd., Beijing, China) was applied to detect cell autophagy in the present study according to the manufacture’s protocol. In brief, cells were collected and adjusted to a concentration of 1×106 cells/ml. Then, 10 μl MDC reagent was added into 90 μl cell suspension at room temperature for 30 min. The MDC staining was observed and photographed under a fluorescence microscope (Olympus Corporation, Tokyo, Japan).
LC3 immunofluorescence staining
Cells were seeded into 6-weel plates and treated with 3MA, sinomenine or TBHP. For LC3 immunofluorescence staining, cells were fixed with 4% paraformaldehyde at room temperature for 1 h and washed twice with PBS buffer. The cells were subsequently perforated with 0.5% Triton-X100 for 15 min, and 5% BSA was added into samples at room temperature for 5 min to block non-specific background staining. The cells were incubated with primary antibodies against LC3 (1:200) at 4°C overnight. Following washing with PBS, the cells were incubated with the secondary antibody and DAPI at room temperature for 1 h. Finally, LC3 immunofluorescence staining was observed and photographed under a fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Western blotting assay
Total protein was extracted with RIPA buffer. The proteins then were separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transfected onto PVDF membranes (Bio-Rad, Laboratories, Inc., Hercules, CA, USA). The membranes were blocked with 5% non-fat milk in TBST buffer for 1 h. Following blocking, the membranes were incubated with primary antibodies against Bax (Abcam, ab32503), Bcl-2 (Abcam, ab32124), active caspase 3 (Abcam, ab2302), Beclin 1 (Abcam, ab62557), ATG5 (Abcam, ab109490) and β-actin (Abcam, ab8227) at 4°C for 12 h, which was followed by incubation with the secondary antibodies. The protein expression levels were measured by an enhanced chemiluminescence detection kit (Thermo Fisher Scientific, Inc.).
In vivo rat IDD model
20 SD rats (225-250 g) were purchased from Fourth Military Medical University Model Animal Center (Xian, China) and fasted 1 day prior to surgery. The rats underwent an intraperitoneal injection with 7% chloral hydrate (0.5 g/kg) for general anesthesia (5 rats/per group). When the pain response disappeared, a right side of midline incision in rat was performed. The skin and subcutaneous tissue were subsequently cut open, and the L5/6 intervertebral disc and the endplate were exposed, and treated with the 21 G micro-puncture. A segmental puncture, parallel to the cartilage endplate needle (controlled penetration depth of 2.3 mm) was performed, and when the operation was completed, the subcutaneous fascia and skin of rat were sutured in sequence. Rats in the IDD-group were intraperitoneally injected with 0.1 ml physiological saline per 10 g body weight daily. Rats in the sinomenine-25 or 75 mg/kg groups were intraperitoneally injected with sinomenine at doses of 25 or 75 mg/kg, respectively. After 16 weeks, all rats were sacrificed using CO2 at a displacement rate of 20%/L/min.
Histopathological analysis
The samples were fixed with 4% paraformaldehyde at room temperature for 30 min, and then embedded in paraffin at a melting point of 57°C. Sections 3 μm-thick were obtained and baked at 55°C for 1 h. The samples were then stained with hematoxylin for 5 min, then added with 0.5% hydrochloric-alcohol mixture for 10 s. The samples were treated with eosin solution for 30 s, and were washed with ethanol and xylene. The morphological changes of the NP cells were observed and the annulus fibrosus were evaluated by grading scale according to the protocol outlined in previous studies [16,17]. Briefly, the cellularity and morphology of the NP and the border between the two structures were assessed. Grades ranged from 5 to 15 and all discs were divided into normal, moderately degenerated, and severely degenerated according to the histological grading scale (Table 1). The histological score of 5 was considered to be normal; 6 to 11 was moderate degeneration; and severe degeneration was 12 to 15.
Table 1.
Histologic grading scale
| Score | Morphology of the nucleus pulposus |
| 1 | > 50%: the ratio of round shape and the nucleus pulposus to the disc area |
| 2 | 25%-50%: the ratio of nucleus pulposus to the disc area; Intermediate morphology |
| 3 | < 25%: the ratio of irregular shape and the nucleus pulposus to the disc area |
| Score | Cellularity of the nucleus pulposus |
| 1 | Stellar-shaped cells distributed evenly, with a proteoglycan matrix located at the periphery |
| 2 | Partially round cells and partially stellar cells, more stellar than round |
| 3 | Mostly large and round cells, isolated by dense areas of proteoglycan matrix |
| Score | Morphology of anular fibrosus |
| 1 | Well-organized collagen lamellae with no ruptures |
| 2 | < 33%: the ratio of anulus to the ruptured fibers; Intermediate morphology |
| 3 | > 33%: the ratio of anulus to the ruptured fibers; Inward annular bulging |
| Score | Cellularity of the anular fibrosus |
| 1 | > 75%: the ratio of fibroblasts to the cells |
| 2 | Intermediate |
| 3 | < 75%: the ratio of chondrocytes to the cells |
| Score | Endplates |
| 1 | Continuous |
| 2 | Disrupted |
Statistical analysis
SPSS software (SPSS standard version 19.0; SPSS Inc.) was performed for statistical analysis. Each group were executed at least three independent experiments and all data were expressed as the mean ± standard deviation (SD). The mean value of the 3 experiments performed for each group was used. The results of different experiments were similar, since the standard deviation of each group was small. The results of the CCK-8 assay, the apoptosis assay, western blot assay, and immunofluorescence staining assay were analyzed by oneway analysis of variance (ANOVA) followed by Dunnett’s test. P values less than 0.05 was considered to be significant difference.
Results
Sinomenine reversed TBHP-induced growth inhibition in rat NP cells
To explore the effect of sinomenine on the viability of rat NP cells, the cells were treated with varying concentrations of sinomenine. The chemical structure of sinomenine is illustrated in Figure 1A. In addition, as indicated in Figure 1B, the treatment of cells with 0.33, 0.67, 1.00 or 3.33 mM sinomenine resulted in no significant changes in cell viability. However, treatment of cells with 6.67 or 10 mM sinomenine significantly decreased cell viability (P < 0.01). The rat NP cells were subsequently treated with 0, 50, 100, 200 or 300 μM TBHP. The results of the CCK-8 assay demonstrated that treatment with TBHP significantly inhibited cell viability in a dose-dependent manner (Figure 1C; P < 0.01). Due to the aforementioned results, 3.33 mM sinomenine and 100 μM TBHP were selected for use in the following studies. In addition, as presented in Figure 1D, 3.33 mM sinomenine nearly recovered cell viability to normal levels in the presence of TBHP, compared with the TBHP group. Collectively, sinomenine could reversed TBHP-induced growth inhibition in rat NP cells.
Figure 1.
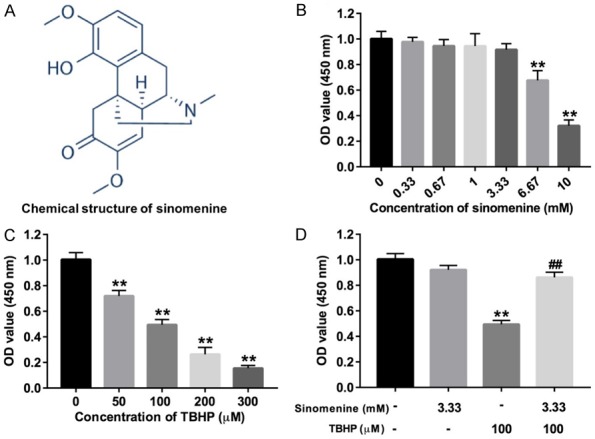
Sinomenine reversed TBHP-induced growth inhibition in rat NP cells. A. Chemical structures of sinomenine. B. CCK-8 assay was performed to evaluate the proliferation of rat NP cells treated with different concentrations of sinomenine, respectively. C. CCK-8 assay was used to evaluate the proliferation of rat NP cells treated with different concentrations of THBP, respectively. D. CCK-8 assay was performed to evaluate the proliferation of rat NP cells treated with sinomenine or/and THBP. N = 3, **P < 0.01 vs. control group. ##P < 0.01 vs. 100 μM THBP group.
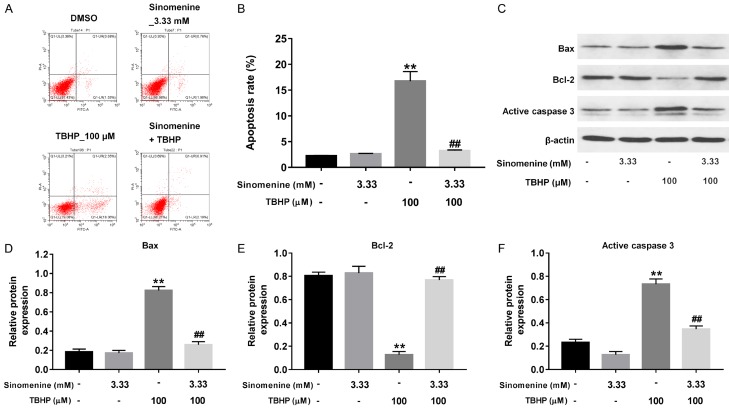
Sinomenine reversed TBHP-induced apoptosis in rat NP cells
To investigate the effects of sinomenine or/and TBHP on the apoptosis of rat NP cells, the present study performed Annexin V/PI staining. As presented in Figure 2A and 2B, treatment with 3.33 mM sinomenine alone resulted in no significant changes in cell apoptosis. However, treatment with 100 mM μM TBHP alone notably induced cell apoptosis, compared with the control cells (P < 0.01). Similar to the results of the CCK-8 assay, treatment with sinomenine significantly attenuated TBHP-induced cell apoptosis (Figure 2A and 2B, P < 0.01). Western blotting was subsequently performed to measure the expression of the apoptosis-related proteins Bax, Bcl-2 and active caspase 3. As illustrated in Figure 2C-F, the relative protein expressions of Bax and active caspase 3 were significantly up-regulated in the cells treated with 100 μM TBHP, when compared with the control cells (P < 0.01). However, compared with TBHP group, Bax and active caspase 3 protein levels were significantly decreased in cells treated with 3.33 mM sinomenine with 100 μM TBHP (Figure 2C-F, P < 0.01). As hypothesized, the protein expression of Bcl-2 was significantly decreased in cells treated with 100 μM TBHP alone (P < 0.01), which was also reversed following treatment with sinomenine (Figure 2C and 2E, P < 0.01). Collectively, the above results indicated that sinomenine could significantly reverse TBHP-induced apoptosis in rat NP cells.
Figure 2.
Sinomenine reversed TBHP-induced apoptosis in rat NP cells. A, B. PI/Annexin V assays were performed to evaluate the apoptosis rate in NP cells treated with 3.33 mM sinomenine, 100 μM THBP or 3.33 mM sinomenine + 100 μM THBP, respectively. C-F. Western blotting assay was performed to measure the protein expressions of Bax, Bcl-2 and active caspase 3 in NP cells treated with 3.33 mM sinomenine, 100 μM THBP or 3.33 mM sinomenine + 100 μM THBP, respectively. N = 3, **P < 0.01 vs. control group. ##P < 0.01 vs. 100 μM THBP group.
Sinomenine induced autophagy in rat NP cells
The present study then investigated whether sinomenine induced autophagy in rat NP cells using MDC staining. As presented in Figure 3A and 3B, treatment with 3.33 mM sinomenine significantly induced autophagy in rat NP cells, while 100 μM TBHP had no effect on it. In addition, pretreatment of 3MA notably inhibited sinomenine-induced autophagy in rat NP cells (Figure 3A and 3B, P < 0.01). The above results were validated by LC3 immunofluorescence analysis. As illustrated in Figure 3C, the fluorescence intensities of sinomenine-treated cells were significantly higher than those of the control group, while pretreatment with 3MA potently inhibited the increase of fluorescence intensities. Western blot analysis was subsequently performed to measure the expressions of the autophagy-related proteins Beclin-1 and ATG5. As indicated in Figure 3D-F, sinomenine significantly up-regulated the levels of Beclin-1 and ATG5 in cells (P < 0.01), while TBHP alone had no effect on them. As hypothesized, pretreatment of 5 mM 3MA resulted in a significant decrease of Beclin-1 and ATG5 in cells (Figure 3D-F, P < 0.01). Taken together, these results suggested that sinomenine significantly induced autophagy in rat NP cells, which was completely revered by pretreatment with 3MA.
Figure 3.

Sinomenine induced autophagy in rat NP cells, which was completely inhibited by 3MA. A, B. MDC assay was performed to measure the autophagosomes and autophagolysosomes in NP cells treated with 3.33 mM sinomenine, 100 μM THBP, 3.33 mM sinomenine + 100 μM THBP, or 3.33 mM sinomenine + 100 μM THBP + 5 mM 3MA, respectively. C. Double immunofluorescence of LC3 in NP cells. D-F. Western blotting assay was used to measure the protein levels of Beclin-1 and ATG5 in rat NP cells. N = 3, **P < 0.01 vs. control group. ##P < 0.01 vs. 3.33 mM sinomenine + 100 μM THBP.
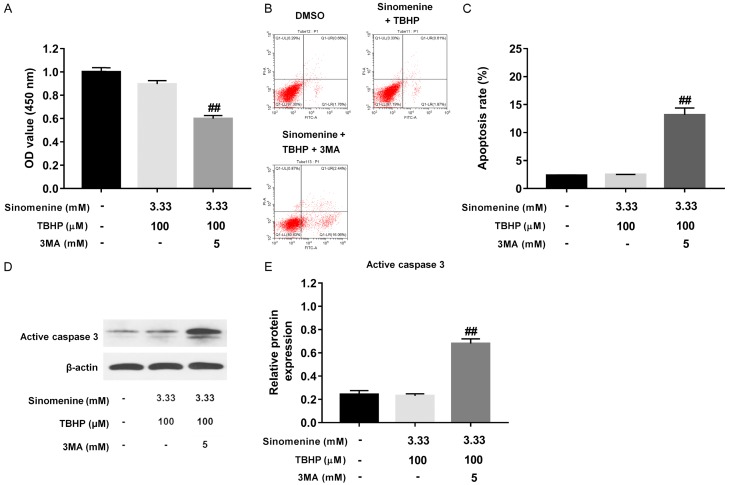
3MA abolished the protective effect of sinomenine against TBHP in rat NP cells
As presented in Figure 4A, pretreatment with 3MA resulted in a significant decrease in cell viability, compared with the cells treated with 3.33 mM sinomenine plus 100 μM TBHP (P < 0.01). The apoptosis analysis data indicated that pretreatment with 3MA significantly increased the apoptosis rate of NP cells (Figure 4B and 4C, P < 0.01) compared with the cells treated with sinomenine with TBHP. The western blotting results demonstrated that pretreatment with 3MA notably up-regulated the level of active caspase 3, compared with sinomenine plus TBHP (Figure 4D and 4E, P < 0.01). Taken together, these data indicated that 3MA could abolish the protective effect of sinomenine against TBHP in rat NP cells.
Figure 4.
3MA abolished the protective effect of sinomenine against TBHP in rat NP cells. A. CCK-8 assay was performed to evaluate the proliferation of rat NP cells treated with 3.33 mM sinomenine + 100 μM THBP or 3.33 mM sinomenine + 100 μM THBP + 5 mM 3MA, respectively. B, C. Annexin V/PI assay was performed to evaluate the apoptosis rate in NP cells treated with 3.33 mM sinomenine + 100 μM THBP or 3.33 mM sinomenine + 100 μM THBP + 5 mM 3MA, respectively. D, E. Western blotting assay was performed to measure the protein level of active caspase 3 in NP cells treated with 3.33 mM sinomenine + 100 μM THBP or 3.33 mM sinomenine + 100 μM THBP + 5 mM 3MA, respectively. N = 3, ##P < 0.01 vs. 3.33 mM sinomenine + 100 μM THBP.
Sinomenine ameliorated rat IDD in vivo
To further explore the effects of sinomenine on IDD in vivo, a rat model of IDD was established by the anulus needle puncture method. The results of HE staining indicated that the NP was fibrotic; however, the inner layer of the annulus was unable to be conclusively determined due to 16 weeks of anulus needle punctures (Figure 5A). As expected, 25 or 75 mg/kg sinomenine significantly improved IDD. Especially in the 75 mg/kg sinomenine group, the nucleus pulposus and the annulus fibrosis were clearly defined, and the structure of the annulus was close to those in the control group (Figure 5A). In addition, the histologic scores of rats in the IDD group were markedly higher than those of control group (Figure 5B, P < 0.01). As hypothesized, the histological scores were notably decreased in the rats treated with sinomenine, compared with the IDD group (Figure 5B, P < 0.05). The western blotting assay indicated the expressions of active caspase 3 in IDD group were markedly higher than those of control group (Figure 5C and 5D, P < 0.01), which was significantly inhibited following treatment with sinomenine. Furthermore, there was no difference in the Beclin 1 levels between the IDD and control groups (Figure 5C and 5E). However, the level of Beclin 1 in the sinomenine-treated groups were markedly higher than those in the IDD group (P < 0.01, Figure 5C and 5E). Both Shapiro-Wilk normality test indicated the data were normally distributed. Taken together, these results indicated that sinomenine could notably ameliorated rat IDD in vivo.
Figure 5.
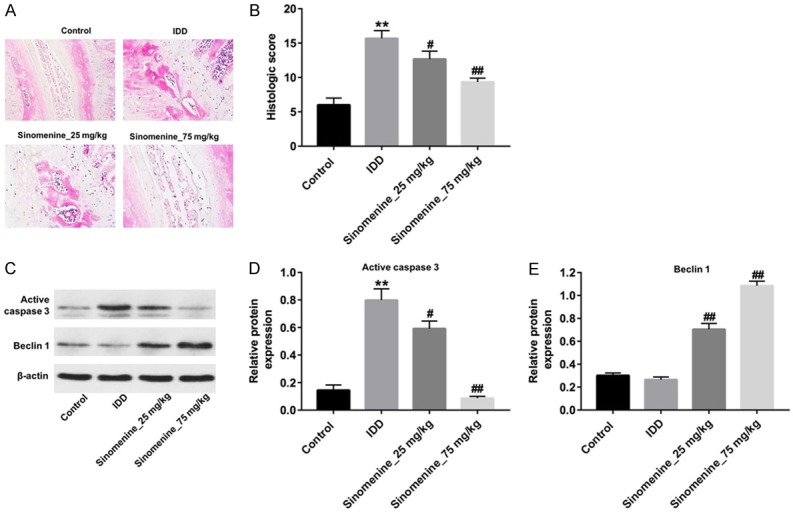
Sinomenine could ameliorated rat IDD in vivo. A. HE staining of intervertebral disc in rats treated with vehicle, 25 or 75 mg/kg group sinomenine, respectively. B. The histologic score of rats treated with vehicle, 25 or 75 mg/kg group sinomenine, respectively. C-E. Active caspase 3 and Beclin 1 in rats treated with vehicle, 25 or 75 mg/kg group were detected with Western blotting assay. N = 5, **P < 0.01 vs. vehicle group. #P < 0.05 and ##P < 0.01 vs. IDD group.
Discussion
The issue of delaying or even inhibiting the degeneration of the intervertebral disc has become the focus of an increasing amount of interest in the field of spinal surgery in recent years. Studies have confirmed that the earliest stage of degeneration of the intervertebral disc is the degeneration of the nucleus pulposus [4]. The results of the present study indicated that treatment with sinomenine inhibited oxidative stress-induced apoptosis of NP cells via promoting autophagy. In addition, the in vivo experiments confirmed that sinomenine could alleviate rat IDD.
Sinomenine is an alkaloid component extracted from the dried rhizome of Sinomenium acutum. Its pharmacological properties include anti-inflammatory and immunosuppressive effects, and it is often administered in the form of its hydrochloride [18]. Due to the increasing amount of research on sinomenine, specific novel pharmacological effects and mechanisms of action have been discovered. For example, Sun et al demonstrated that sinomenine inhibited the growth of melanoma via the enhancement of autophagy [14]. In addition, another similar study also demonstrated that sinomenine promoted apoptosis in renal carcinoma via enhancing autophagy [15]. Conversely, to the best of our knowledge, the present study demonstrated for the first time that sinomenine alleviated IDD by enhancing autophagy in NP cells in vitro and in vivo. The difference between these results may be due to the different cell types used in these investigations.
In humans, NP cells are continuously lost due to the apoptotic process. The excessive apoptosis of NP cells is considered to be an important cause of IDD [19]. It is well established that the body’s oxidative stress level and reactive oxygen species (ROS) accumulation increase with aging, which culminates in the destruction of extracellular matrix of the intervertebral disc tissue [20,21]. In present study demonstrated that sinomenine could reverse TBHP-induced apoptosis in rat NP cells. Wang et al reported that apoptosis in degenerative disc tissues is mediated by three pathways, including the death receptor pathway, the mitochondrial pathway and the endoplasmic reticulum pathway. In addition, their confirmed that these three pathways work at different stages of disc degeneration [22]. The mitochondrial pathway is primarily activated by various cellular compressive stresses and various apoptotic signals, and is a significant pathway for apoptosis during IDD [23]. Similar to these studies, the present study identified that pretreatment with sinomenine could notably decrease mitochondrial pathway markers Bax and cleaved-caspase 3, while increasing Bcl-2 in NP cells under conditions of oxidative stress.
Autophagy is an independent type II programmed cell death process and is closely associated with apoptosis [24]. The study of Jiang et al identified that SIRT1 (silent information regulation 2 homolog 1) can inhibit the apoptosis of human NP cells by up-regulating autophagy [25]. Miyazaki et al also reported that SIRT1 inhibited apoptosis in human NP cells induced by serum deprivation by promoting autophagy [26]. In addition, the study of Chen et al demonstrated that the levels of apoptosis and autophagy in rat NP cells were increased following stimulation with H2O2 (27). However, an additional study demonstrated that metformin could inhibit TBHP-induced apoptosis of rat NP cells by promoting autophagy [27]. Similarly, the results of the present study indicated that sinomenine inhibited TBHP-induced apoptosis in rat NP cells by promoting autophagy. These above findings illustrate the complex relationship between autophagy and apoptosis in intervertebral disc cells under different conditions and remains to be further investigated. Nevertheless, the limitation of current study was the lacking of mechanical testing or Magnetic Resonance Imaging (MRI), which could provide some idea that either mechanical function or hydration was protected. Thus, further investigation are needed.
In conclusion, the present study provided evidence that treatment with sinomenine exerted an anti-apoptotic effect against oxidative stress in rat NP cells by promoting autophagy, whereas the autophagy inhibitor 3MA abolished this effect. These findings suggested the therapeutic potential of sinomenine in the prevention of the IDD.
Acknowledgements
This work is supported by Six Talent Peak Projects in Jiangsu Province (No. WSW-003) and Nanjing Medical Science and Technology Development Project (No. YKK15249).
Disclosure of conflict of interest
None.
References
- 1.Colombier P, Clouet J, Hamel O, Lescaudron L, Guicheux J. The lumbar intervertebral disc: from embryonic development to degeneration. Joint Bone Spine. 2014;81:125–129. doi: 10.1016/j.jbspin.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 2.van Uden S, Silva-Correia J, Oliveira JM, Reis RL. Current strategies for treatment of intervertebral disc degeneration: substitution and regeneration possibilities. Biomater Res. 2017;21:22. doi: 10.1186/s40824-017-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maas H, Noort W, Hodges PW, van Dieen J. Effects of intervertebral disc lesion and multifidus muscle resection on the structure of the lumbar intervertebral discs and paraspinal musculature of the rat. J Biomech. 2018;70:228–234. doi: 10.1016/j.jbiomech.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, Yamamura K, Masuda K, Okano H, Ando K, Mochida J. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 6.He R, Cui M, Lin H, Zhao L, Wang J, Chen S, Shao Z. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018;199:122–130. doi: 10.1016/j.lfs.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Bishop E, Bradshaw TD. Autophagy modulation: a prudent approach in cancer treatment? Cancer Chemother Pharmacol. 2018;82:913–922. doi: 10.1007/s00280-018-3669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying L, Zhao GJ, Wu Y, Ke HL, Hong GL, Zhang H, Dong N, Wu Y, Yao YM, Lu ZQ. Mitofusin 2 promotes apoptosis of CD4(+) T cells by inhibiting autophagy in sepsis. Mediators Inflamm. 2017;2017:4926205. doi: 10.1155/2017/4926205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojha R, Ishaq M, Singh SK. Caspase-mediated crosstalk between autophagy and apoptosis: mutual adjustment or matter of dominance. J Cancer Res Ther. 2015;11:514–524. doi: 10.4103/0973-1482.163695. [DOI] [PubMed] [Google Scholar]
- 10.Ding F, Shao ZW, Xiong LM. Cell death in intervertebral disc degeneration. Apoptosis. 2013;18:777–785. doi: 10.1007/s10495-013-0839-1. [DOI] [PubMed] [Google Scholar]
- 11.Shen C, Yan J, Jiang LS, Dai LY. Autophagy in rat annulus fibrosus cells: evidence and possible implications. Arthritis Res Ther. 2011;13:R132. doi: 10.1186/ar3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Liu H, Song J, Cao L, Tang L, Qi C. Sinomenine alleviates dextran sulfate sodiuminduced colitis via the Nrf2/NQO1 signaling pathway. Mol Med Rep. 2018;18:3691–3698. doi: 10.3892/mmr.2018.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim TW, Han JM, Han YK, Chung H. Anti-inflammatory effects of sinomenium acutum extract on endotoxin-induced uveitis in lewis rats. Int J Med Sci. 2018;15:758–764. doi: 10.7150/ijms.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Z, Zheng L, Liu X, Xing W, Liu X. Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Drug Des Devel Ther. 2018;12:2413–2421. doi: 10.2147/DDDT.S155798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng F, Ma YX, Liang L, Zhang P, Feng J. The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2018;97:1269–1274. doi: 10.1016/j.biopha.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 16.Han B, Zhu K, Li FC, Xiao YX, Feng J, Shi ZL, Lin M, Wang J, Chen QX. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine (Phila Pa 1976) 2008;33:1925–1934. doi: 10.1097/BRS.0b013e31817c64a9. [DOI] [PubMed] [Google Scholar]
- 17.Mao HJ, Chen QX, Han B, Li FC, Feng J, Shi ZL, Lin M, Wang J. The effect of injection volume on disc degeneration in a rat tail model. Spine (Phila Pa 1976) 2011;36:E1062–1069. doi: 10.1097/BRS.0b013e3182027d42. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Qian X, Ji W, Lu Y, Wei G, Wang Y. Effects and safety of sinomenine in treatment of rheumatoid arthritis contrast to methotrexate: a systematic review and meta-analysis. J Tradit Chin Med. 2016;36:564–577. doi: 10.1016/s0254-6272(16)30075-9. [DOI] [PubMed] [Google Scholar]
- 19.Chen SQ, Lin JP, Zheng QK, Chen SJ, Li M, Lin XZ, Wang SZ. Protective effects of paeoniflorin against FasL-induced apoptosis of intervertebral disc annulus fibrosus cells via Fas-FasL signalling pathway. Exp Ther Med. 2015;10:2351–2355. doi: 10.3892/etm.2015.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasto LA, Robinson AR, Ngo K, Clauson CL, Dong Q, St Croix C, Sowa G, Pola E, Robbins PD, Kang J, Niedernhofer LJ, Wipf P, Vo NV. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J Orthop Res. 2013;31:1150–1157. doi: 10.1002/jor.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng C, Yang M, Lan M, Liu C, Zhang Y, Huang B, Liu H, Zhou Y. ROS: crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxid Med Cell Longev. 2017;2017:5601593. doi: 10.1155/2017/5601593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Liu H, Zheng ZM, Zhang KB, Wang TP, Sribastav SS, Liu WS, Liu T. Role of death receptor, mitochondrial and endoplasmic reticulum pathways in different stages of degenerative human lumbar disc. Apoptosis. 2011;16:990–1003. doi: 10.1007/s10495-011-0644-7. [DOI] [PubMed] [Google Scholar]
- 23.Niu CC, Lin SS, Yuan LJ, Chen LH, Wang IC, Tsai TT, Lai PL, Chen WJ. Hyperbaric oxygen treatment suppresses MAPK signaling and mitochondrial apoptotic pathway in degenerated human intervertebral disc cells. J Orthop Res. 2013;31:204–209. doi: 10.1002/jor.22209. [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK. Autophagy and apoptosis: where do they meet? Apoptosis. 2014;19:555–566. doi: 10.1007/s10495-014-0967-2. [DOI] [PubMed] [Google Scholar]
- 25.Jiang W, Zhang X, Hao J, Shen J, Fang J, Dong W, Wang D, Zhang X, Shui W, Luo Y, Lin L, Qiu Q, Liu B, Hu Z. SIRT1 protects against apoptosis by promoting autophagy in degenerative human disc nucleus pulposus cells. Sci Rep. 2014;4:7456. doi: 10.1038/srep07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazaki S, Kakutani K, Yurube T, Maeno K, Takada T, Zhang Z, Kurakawa T, Terashima Y, Ito M, Ueha T, Matsushita T, Kuroda R, Kurosaka M, Nishida K. Recombinant human SIRT1 protects against nutrient deprivation-induced mitochondrial apoptosis through autophagy induction in human intervertebral disc nucleus pulposus cells. Arthritis Res Ther. 2015;17:253. doi: 10.1186/s13075-015-0763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D, Xia D, Pan Z, Xu D, Zhou Y, Wu Y, Cai N, Tang Q, Wang C, Yan M, Zhang JJ, Zhou K, Wang Q, Feng Y, Wang X, Xu H, Zhang X, Tian N. Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo. Cell Death Dis. 2016;7:e2441. doi: 10.1038/cddis.2016.334. [DOI] [PMC free article] [PubMed] [Google Scholar]