Abstract
Many traditional Chinese medicine tonifying prescriptions for kidney and spleen have been proved to play various roles in osteoporosis. This study aimed to explore whether Anti-Osteoporosis Decoction (AOD) and Yougui Pill (YGP) have potential therapeutic effects on osteoporosis in ovariectomy-induced rat model. The osteoporosis rat model was established with female Wistar rats by the way of ovariectomy. The chosen rats were randomly divided into five groups (control group, model group, sham group, model + AOD group, and model + YGP group). H&E staining was used to detect the bone histological pathology changes. The bone mineral density (BMD) was assessed with dual-energy X-ray absorptiometry. In addition, western blotting assay was applied to explore the expressions of BMP2, Runx2, Collagen I and Opn. Next, we examined the expression of collagen I by immunohistochemistry staining. Finally, the levels of Alkaline phosphatase (ALP), procollagen type I N propeptide (PINP) and β-C-terminal telopeptide of type I collagen (β-CTX) were detected. The results revealed that the OVX osteoporosis model was successfully established. AOD and YGP treatment effectively inhibited osteoporosis and reduced the broken trabecular bones. BMD was increased in AOD and YGP treatment. In addition, AOD and YGP treatment groups significantly increased the ALP levels. Furthermore, AOD and YGP significantly increased the expressions of BMP2, Runx2, Collagen I and Opn, while reduced the levels of PINP and β-CTX in serum compared to OVX model group. In conclusion, AOD and YGP exert regulatory effects on osteoporosis in ovariectomized rats. They may be potential candidates for the therapy and cure of human osteoporosis.
Keywords: Anti-Osteoporosis Decoction, Yougui Pill, osteoporosis, ovariectomy, bone metabolism, BMD
Introduction
Osteoporosis is a common disease characterized by low bone mass, microarchitectural deterioration in bone tissue, susceptibility to fracture and reduce of bone strength [1,2]. The morbidity and mortality of osteoporotic have been raising in China [3]. It has been estimated that osteoporosis contributes to 90% of hip and spine fractures in women 65 to 84 years of age [4,5]. The prevention of osteoporosis-associated fractures should include fall prevention, calcium supplementation and life-style advice, as well as pharmacological therapy using agents with proven anti-fracture efficacy [6,7]. However, the long-term administration of these drugs may cause severe side effects. Therefore, there has been an interest in developing approaches to prevent osteoporotic.
Traditional Chinese medicine (TCM) have been regarded as promising alternative therapy for osteoporotic [8]. “Kidney dominates bone” is one of the most important theories in TCM [9]. The primary pathogenesis of osteoporosis is insufficiency of kidney. According to this theory, Yougui pill (YGP), a classic TCM prescription, has been reported in Jingyue quanshu and used to treat bone-related diseases including osteoporosis for hundreds of years because of its definite treatment effects [10,11]. Recent studies suggested that YGP enhanced the activation of TGF-β/Smad pathway so that it can restrain cartilage degradation [12]. Anti-Osteoporosis Decoction (AOD), a new Chinese medicine prescription that is based on Yougui pill and contains several tonifying Chinese herbs such as Placenta, donkey-hide gelatin, and semen coicis. Although AOD and YGP are admitted in curative effect of osteoporosis, the underlying mechanism and the target for the drug is still unclear.
Several biochemical markers of bone formation and bone turnover have the potential to provide early feedback to patients during osteoporosis treatments, including bone-specific alkaline phosphatase (bone ALP), C-terminal telopeptide of type I collagen (CTX-I) and N-terminal pro-peptide of type I procollagen (PINP) [13-16]. For treatment of osteoporosis, high bone turnover may cause elevated ALP in postmenopausal women [17]. Bone morphogenetic protein 2 (BMP2), a growth factor that belongs to superfamily of TNF-proteins, is actively involved in bone tissue metabolism [18]. BMP2, Runx2, Collagen I and Opn play vital role in osteoblast differentiation and bone formation [19]. Overexpression of circRunx2 prevented osteoporosis by promoting the expression of osteogenic differentiation-related proteins such as Runx2 and Opn [20].
In this study, we explored the therapeutic effects of AOD and YGP on histological pathology changes, the bone mineral density, bone metabolism proteins, and bone turnover biomarks in the OVX osteoporosis rats.
Materials and methods
AOD preparation
Radix rehmanniae praeparata 0.252 g, Yam 0.126 g, dodder 0.126 g, Cornel 0.090 g, lycium chinensis 0.090 g, Eucommia ulmoides 0.126 g, Radix Angelicae sinensis 0.090 g, cinnamon 0.126 g, processed Radix Aconiti Lateralis 0.126 g, Placenta 0.126 g, donkey-hide gelatin 0.126 g, Radix Codonopsis 0.200 g, rhizoma atractylodis macrocephalae 0.126 g, semen coicis 0.126 g, fructus amomi 0.090 g (these drugs are granules), Baked tortoise shell 0.128 g, and Antlers plastic 0.126 g. All drugs were purchased from Pharmacy of traditional Chinese medicine, Nantong hospital of traditional Chinese medicine, Break, melt by heat, and mix the above-mentioned granules. Finally, 20 ml distilled water was added to mixture the granules. AOD was suspended in water and administered orally to each rat with a volume of 0.875 ml/100 g body weight.
YGP preparation
Radix rehmanniae praeparata 0.252 g, Yam 0.126 g, dodder 0.126 g, Cornel 0.090 g, lycium chinensis 0.090 g, Eucommia ulmoides 0.126 g, Radix Angelicae sinensis 0.090 g, cinnamon 0.126 g, processed Radix Aconiti Lateralis 0.126 g (these drugs are granules), and Antlers plastic 0.126 g. All drugs were purchased from Pharmacy of traditional Chinese medicine, Nantong hospital of traditional Chinese medicine, Break, melt by heat, and mix the above-mentioned granules. Finally, 20 ml water was added to mixture the granules. YGP was suspended in water and administered orally to each rat with a volume of 0.875 ml/100 g body weight.
Experimental design: establishment of osteoporosis model
Four-month-old female Wistar rats (250-300 g) were purchased from the Laboratory Animal Center of China. The animals were housed under controlled conditions including a room temperature of 22±1°C with 12: 12-hour light-dark cycle. The animals were fed with standard food pellets and water ad libitum. Bilateral ovaries of rats were extirpated to establish Osteoporosis model [21,22]. The animals were divided into five groups (n=12): control group (treated with 0.9% NaCl), model group (treated with 0.9% NaCl), sham group (treated with 0.9% NaCl), model + AOD group (treated with AOD), and model + YGP group (treated with YGP), and were administered orally with 0.9% NaCl, AOD or YGP (equivalent to the administration of 10 g/kg body weight of the crude drug) once daily for 12 week. Body weights were taken at weekly until the final day of administration.
Hematoxylin and eosin (H&E) staining
Briefly, the left femur bones were collected, fixed in 10% neutral formaldehyde for 72 h, removed and decalcified in 10% ethylenediamine tetraacetic acid solution (pH 7.4) at 4°C for 4 weeks. Then, paraffin was used to embed the fixed samples. Following cut into 4 μm slice, samples were stained with H&E staining and observed with a light microscope.
Measurement of bone mineral density
Total bone mineral density (T-BMD) was measured by using dual-energy X-ray absorptiometry (DXA) with Hologic DXA equipment (Hologic Discovery W 81507). Results were obtained as grams of mineral content per square centimeter of bone area (g/cm2). The scanner was calibrated daily by inhouse certified technician.
Measurement of ALP
ALP from serum converts phenyl phosphate to inorganic phosphate and phenol at pH 10. Phenol so formed in alkaline medium with 4-aminoantipyrine in the presence of oxidizing agent potassium ferricyanide and forms an orange colored complex, which was measured colorimetrically using an autoanalyzer [23,24]. In vitro determination of ALP was carried out by using commercially available kits (Roche Diagnostics, Mannheim, Germany) analyzed by an automated multi-item analyzer (TMS-1024, Tokyo Boeki, Japan).
Western blotting assay
Total protein was isolated from peritoneal tissues using RIPA buffer. Protein concentrations were determined with a Pierce BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL), and 10 ug protein loaded and separated on 12% SDS gels and transferred to polyinylidene difluoride membrane (Millipore, Bedford, MA). Following transfer, membranes were blocked with 10% skimmed milk and incubated with the appropriate primary antibodies against collagen I (1:1000, ab34710), BMP2 (1:1000, ab14933), Runx2 (1 µg/ml, ab23981), Opn (1:1000, ab8448) and GAPDH (1:2500, ab9485) at 4°C overnight, followed by peroxidase-conjugated secondary antibodies for 1 h at 37°C. Chemiluminescent film was applied for assessment of protein expression with Image J software.
Immunohistochemistry staining
The fresh bone tissue samples were collected and fixed in formalin overnight. The tissue was then put into paraffin and cut into 5 μm slides. The samples were prepared for blocking and incubating with collagen I antibody (Abcam, 1:100, ab34710) at 4°C overnight after washing three times. PBS was used as the negative control of the staining. The slides were then incubated with diluted streptavidin-peroxidase HRP for 20 minutes at room temperature, followed by staining with hematoxylin for 5 min and analyzed with Image-Pro Plus 6.0 (Media Cybernetics, Inc.).
Determination of serum PINP and β-CTX levels
Blood from the rat tail vein was collected in a blood collection tube with EDTA-Na2 (1 mg/ml) for anticoagulation. The levels of PINP and β-CTX were determined via ELISA according to the manufacturer’s instructions. Five replicates were established for each group and results were from triplicate experiments.
Statistical analysis
All the statistics analyses were performed using SPSS 18.0 software (v.18; SPSS, Inc., Chicago, IL, USA). All data are expressed as the mean ± standard deviation. Differences between two groups were analyzed using the Student’s t-test (two-tailed). Multiple comparisons were analyzed using one-way analysis of variance (ANOVA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of AOD and YGP on the bone histological pathology changes in the OVX osteoporosis rats
To evaluate the effects of AOD and YGP on the bone histological pathology changes in the OVX osteoporosis model, we observed femoral cortical and the microarchitecture of trabecular bone by using H&E staining. As shown in Figure 1, the OVX model group revealed the feature of osteoporosis and the increased breakage of trabecular bone. OVX led to significant reductions of both cortical thickness and trabecular bone area in the OVX model group when compared to the sham group. The bones in model + AOD and model + YGP groups have no obvious difference when compared with the sham group. OVX rats administrated with AOD and YGP experienced an obvious increase in trabecular bone area compared with OVX model group rats. Thus, these results demonstrated that AOD and YGP treatment inhibited effectively OVX-induced osteoporosis in vivo.
Figure 1.
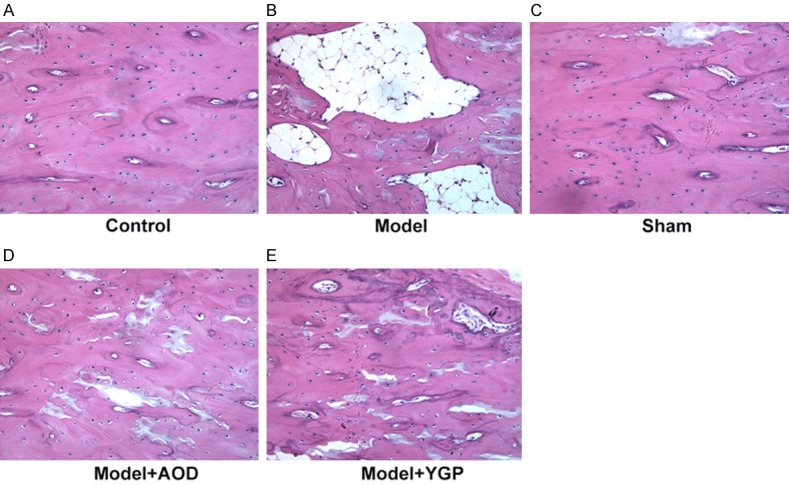
Histological analysis on the morphometry of left femur bones. (A) Control group. (B) Model group. These rats show decreases in cortical thickness and trabecular bone area. (C) Sham group. (D) Model + AOD group and (E) model + YGP group show increases cortical thickness and trabecular bone area. Original magnification, ×200.
Effects of AOD and YGP on the bone mineral density in the OVX osteoporosis rats
Total bone mineral density (T-BMD) was measured by using dual-energy X-ray absorptiometry (DXA) with Hologic DXA equipment. Compared with the sham group, the BMD of the samples in the OVX rats was decreased while the BMD was elevated after treatment with AOD and YGP for 12 weeks. In addition, the BMD in AOD group has no significant difference compared with the YGP group (Figure 2). The results indicated that AOD and YGP alleviate ovariectomy-induced osteoporosis and ameliorate the BMD in vivo.
Figure 2.
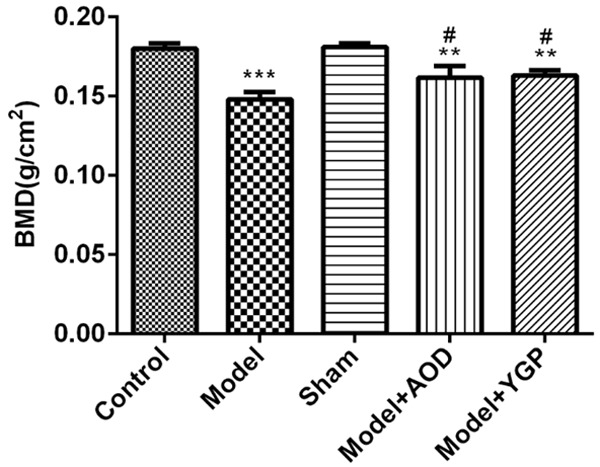
Effects of 12-week treatment with AOD and YGP on bone mineral density (BMD) of the left femur in OVX rats with dual-energy X-ray absorptiometry. Each bar represents the mean ± SD calculated from three independent experiments. **P<0.01, ***P<0.001 versus sham control. #P<0.05 versus model control.
Effects of AOD and YGP on the alkaline phosphatase (ALP) in the OVX osteoporosis rats
We then detected the level of bone formation biomarker ALP to detect the effects of AOD and YGP on bone metabolism in OVX osteoporosis rats. The results revealed that the ALP level in the OVX model group significantly lower compared with the sham group, and the AOD and YGP treatment groups markedly increased the ALP level compared to OVX model group (Figure 3). These results suggested that AOD and YGP treatment effectively ameliorated biochemical parameters in OVX osteoporosis rats.
Figure 3.
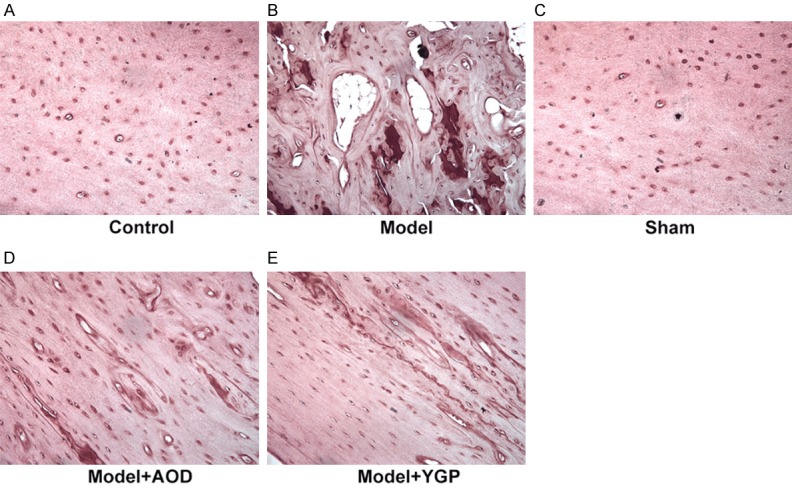
Effects of AOD and YGP on alkaline phosphatase (ALP) in the OVX osteoporosis rats. (A) Control group. (B) Model group show decreases ALP level in the OVX osteoporosis rats. (C) Sham group. (D) Model + AOD group and (E) model + YGP group show increases ALP level in the OVX osteoporosis rats. Original magnification, ×200.
Effects of AOD and YGP on the level of proteins related to bone metabolism in the OVX osteoporosis rats
To further explore the effects of AOD and YGP on bone metabolism of OVX osteoporosis rats, the level of proteins related to bone metabolism were detected. Result of western blotting assay showed the decreased levels of BMP2, Runx2, Collagen I and Opn in the OVX model group when compared with the sham group, and the AOD and YGP treatment groups significantly increased the level of BMP2, Runx2, Collagen I and Opn compared to OVX model group. Nevertheless, when compared the AOD and YGP groups, we found no distinct difference (Figure 4A). Next, we examined the expression of collagen I by immunohistochemistry staining. As indicated in Figure 4B, the results showed that expression of collagen I was significantly reduced in OVX model group, and AOD and YGP treatment increased the expression of collagen I compared to OVX model group. These results suggested that AOD and YGP treatment effectively improved the level of bone metabolism-related proteins in OVX osteoporosis rats.
Figure 4.
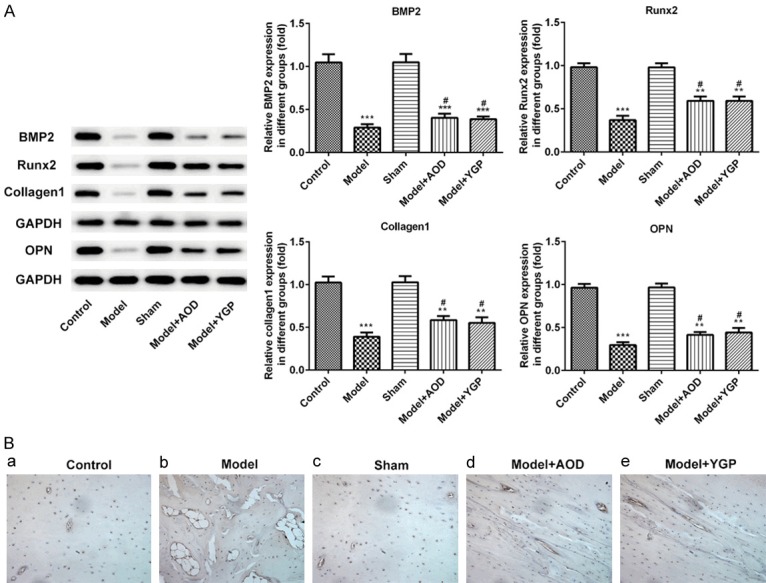
Effects of AOD and YGP on protein levels related to bone metabolism in the OVX osteoporosis rats. A. Western blotting was used to detect the expression of BMP2, Runx2, Collagen I and Opn in the OVX model treated with AOD or YGP. Each bar represents the mean ± SD calculated from three independent experiments. **P<0.01, ***P<0.001 versus sham control. #P<0.05 versus model control. B. Immunohistochemistry staining of Collagen I in the OVX osteoporosis rats treated with AOD or YGP. Original magnification, ×200.
Effects of AOD and YGP on the expression of PINP and β-CTX in the OVX osteoporosis rats
PINP and CTX-I in blood to be the reference markers of bone turnover for the fracture risk prediction and monitoring of osteoporosis treatment [16]. ELISA results showed that the level of serum PINP and β-CTX in the OVX model group was significantly enhanced compared with the sham group, and the AOD groups significantly reduced the level of serum PINP and β-CTX compared to OVX model group, as well as the YGP treatment (Figure 5). Thus, these results suggested that AOD and YGP treatment effectively ameliorated OVX-induced osteoporosis in rats.
Figure 5.
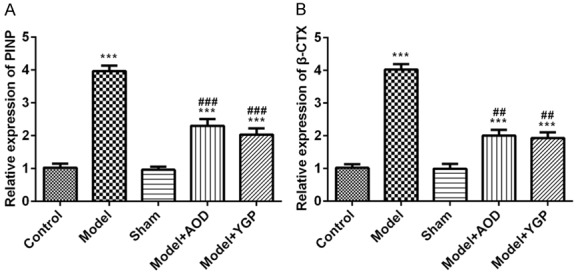
Effects of AOD and YGP on the levels protein PINP and β-CTX in the OVX osteoporosis rats. Each bar represents the mean ± SD calculated from three independent experiments. ***P<0.001 versus sham control. ##P<0.01, ###P<0.001 versus model control.
Discussion
Chinese herbal medicines have been commonly used in China, and have long been used as an alternative therapy in Western Society [25]. In the present study, we investigated the impact of AOD and YGP on the bone histological pathology changes in OVX osteoporosis model. OVX rats treated with AOD and YGP showed a significant increase in trabecular bone area, in contrast to the OVX model group rats. Further, AOD and YGP ameliorated osteoporosis after ovariectomy and bone mineral density. These results support the hypothesis of effects of AOD and YGP on articular cartilage protection and disease modifying in OVX osteoporosis. Although the precise mechanisms are not explicit, significant increases in the level of proteins involved in bone metabolism may be involved in the process.
BMP2, Runx2, Collagen I and osteopontin (Opn) are involved in bone tissue metabolism and played a role in osteoblast differentiation and bone formation [19,26-28]. BMP2 takes part in the process of osteogenesis by showing the osteoinduction potential and regulating growth of cartilage plate [29]. Runx2 is expressed in osteoblasts, periosteocytes and perichondrocytes after partum, and deletion of Runx2 leads to absence of ossification and skeletons that only consist of chondrocytes and cartilage [30]. Yoon et al. found that remifentanil increased osteoblast differentiation by upregulation of Runx2 expression in C2C12 cells [31]. In this study, the AOD and YGP treatment significantly increased the expression of BMP2, Runx2, Collagen I and Opn compared to OVX model group. These results suggested that AOD and YGP treatment effectively increased the level of proteins related to bone metabolism in OVX osteoporosis rats.
PINP and β-CTX as biochemical markers of bone formation and bone turnover during treatment for osteoporosis [32,33]. In this study, we found that AOD and YGP treatment groups significantly reduced the level of serum PINP and β-CTX compared to OVX model group. These results suggested that AOD and YGP treatment effectively ameliorated OVX-induced osteoporosis in rats. In addition, ALP is a commonly used marker for bone formation and is regarded as the most important marker of osteoblast differentiation [34]. For postmenopausal women, elevated ALP is mainly caused by high bone turnover [35]. We then detected bone formation biomarkers (ALP) to analyze the effects of AOD and YGP on bone metabolism in model rats. AOD and YGP treatment groups significantly increased the ALP levels compared to OVX model group. Thus, these results suggested that AOD and YGP treatment effectively ameliorated OVX-induced osteoporosis in rats.
In conclusion, our results demonstrated that AOD and YGP improved the OVX-induced osteoporosis. AOD and YGP increased the bone mineral density in OVX model. AOD and YGP effectively protected the bone formation and bone turnover of osteoporosis, indicating its potential therapeutic for the control of osteoporosis.
Acknowledgements
This work was supported by Science and Technology Funding Projects of Nantong Science and Technology Bureau (MS32017015).
Disclosure of conflict of interest
None.
References
- 1.Riggs BL, Melton LJ 3rd. Involutional osteoporosis. N Engl J Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 2.Lecart MP, Reginster JY. Current options for the management of postmenopausal osteoporosis. Expert Opin Pharmacother. 2011;12:2533–2552. doi: 10.1517/14656566.2011.618123. [DOI] [PubMed] [Google Scholar]
- 3.Ye Q, Ma XQ, Hu CL, Lin B, Xu LS, Zheng CJ, Qin LP. Antiosteoporotic activity and constituents of Podocarpium podocarpum. Phytomedicine. 2015;22:94–102. doi: 10.1016/j.phymed.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2005;353:595–603. doi: 10.1056/NEJMcp043801. [DOI] [PubMed] [Google Scholar]
- 5.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.Curtis EM, Moon RJ, Dennison EM, Harvey NC, Cooper C. Recent advances in the pathogenesis and treatment of osteoporosis. Clin Med (Lond) 2016;16:360–364. doi: 10.7861/clinmedicine.16-4-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Liu H, Shi W, Liu H, Yang J, Xu D, Huang H, Wu L. Insights into the action mechanisms of traditional Chinese medicine in osteoarthritis. Evid Based Complement Alternat Med. 2017;2017:5190986. doi: 10.1155/2017/5190986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WH, Liu XX, Tong PJ, Zhan HS Orthopaedic Professional Committee, Chinese Association of Research and Advancement of Chinese Traditional Medicine, China; Joint Professional Committee, Branch of Orthopaedic of Chinese Association of Integrative Medicine, China. Diagnosis and management of knee osteoarthritis: Chinese medicine expert consensus (2015) Chin J Integr Med. 2016;22:150–153. doi: 10.1007/s11655-015-2432-7. [DOI] [PubMed] [Google Scholar]
- 10.Shu B, Shi Q, Wang YJ. Shen (kidney)-tonifying principle for primary osteoporosis: to treat both the disease and the Chinese medicine syndrome. Chin J Integr Med. 2015;21:656–661. doi: 10.1007/s11655-015-2306-z. [DOI] [PubMed] [Google Scholar]
- 11.Pan M, Li M. Progressive studies on effects of traditional Chinese medicines on differentiation of human bone mesenchymal stem cells. Zhongguo Zhong Yao Za Zhi. 2010;35:1892–1895. doi: 10.4268/cjcmm20101428. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Wang PE, Ying J, Jin X, Luo C, Xu T, Xu S, Dong R, Xiao L, Tong P, Jin H. Yougui Pills attenuate cartilage degeneration via activation of TGF-beta/Smad signaling in chondrocyte of osteoarthritic mouse model. Front Pharmacol. 2017;8:611. doi: 10.3389/fphar.2017.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA IOF-IFCC Bone Marker Standards Working Group. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 14.Civitelli R, Armamento-Villareal R, Napoli N. Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporos Int. 2009;20:843–851. doi: 10.1007/s00198-009-0838-9. [DOI] [PubMed] [Google Scholar]
- 15.Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET National Bone Health Alliance Bone Turnover Marker Project. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 2017;28:2541–2556. doi: 10.1007/s00198-017-4082-4. [DOI] [PubMed] [Google Scholar]
- 16.Krege JH, Lane NE, Harris JM, Miller PD. PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos Int. 2014;25:2159–2171. doi: 10.1007/s00198-014-2646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukaiyama K, Kamimura M, Uchiyama S, Ikegami S, Nakamura Y, Kato H. Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin Exp Res. 2015;27:413–418. doi: 10.1007/s40520-014-0296-x. [DOI] [PubMed] [Google Scholar]
- 18.Riley EH, Lane JM, Urist MR, Lyons KM, Lieberman JR. Bone morphogenetic protein-2: biology and applications. Clin Orthop Relat Res. 1996:39–46. [PubMed] [Google Scholar]
- 19.Li H, Xie H, Liu W, Hu R, Huang B, Tan YF, Xu K, Sheng ZF, Zhou HD, Wu XP, Luo XH. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest. 2009;119:3666–3677. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin Q, Wang J, Fu Q, Gu S, Rui Y. CircRUNX2 through has-miR-203 regulates RUNX2 to prevent osteoporosis. J Cell Mol Med. 2018;22:6112–6121. doi: 10.1111/jcmm.13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jee WS, Yao W. Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact. 2001;1:193–207. [PubMed] [Google Scholar]
- 22.Komori T. Animal models for osteoporosis. Eur J Pharmacol. 2015;759:287–294. doi: 10.1016/j.ejphar.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Ho HY, Wu JB, Lin WC. Flemingia macrophylla extract ameliorates experimental osteoporosis in ovariectomized rats. Evid Based Complement Alternat Med. 2011;2011:752302. doi: 10.1093/ecam/nep179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, He B, Zhang X, Yang R, Li S, Song B, Zhang Y, Yun Y, Yan H, Chen P, Shen Z. Osteoprotective effect of geraniin against ovariectomy-induced bone loss in rats. Bioorg Med Chem Lett. 2015;25:673–679. doi: 10.1016/j.bmcl.2014.11.081. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZQ, Li JL, Sun YL, Yao M, Gao J, Yang Z, Shi Q, Cui XJ, Wang YJ. Chinese herbal medicine for osteoporosis: a systematic review of randomized controlled trails. Evid Based Complement Alternat Med. 2013;2013:356260. doi: 10.1155/2013/356260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu X, Gui Y, Xu Y, Li D, Wang L. DHEA promotes osteoblast differentiation by regulating the expression of osteoblast-related genes and Foxp3(+) regulatory T cells. Biosci Trends. 2015;9:307–314. doi: 10.5582/bst.2015.01073. [DOI] [PubMed] [Google Scholar]
- 27.Liu K, Jing Y, Zhang W, Fu X, Zhao H, Zhou X, Tao Y, Yang H, Zhang Y, Zen K, Zhang C, Li D, Shi Q. Silencing miR-106b accelerates osteogenesis of mesenchymal stem cells and rescues against glucocorticoid-induced osteoporosis by targeting BMP2. Bone. 2017;97:130–138. doi: 10.1016/j.bone.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Komori T. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20071694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolski H, Bogacz A, Bartkowiak-Wieczorek J, Greber A, Pienkowski W, Drews K, Klejewski A, Seremak-Mrozikiewicz A. Polymorphism of bone morphogenetic protein (BMP2) and osteoporosis etiology. Ginekol Pol. 2015;86:203–209. doi: 10.17772/gp/2006. [DOI] [PubMed] [Google Scholar]
- 30.Komori T. Roles of Runx2 in skeletal development. Adv Exp Med Biol. 2017;962:83–93. doi: 10.1007/978-981-10-3233-2_6. [DOI] [PubMed] [Google Scholar]
- 31.Yoon JY, Kim TS, Ahn JH, Yoon JU, Kim HJ, Kim EJ. Remifentanil promotes osteoblastogenesis by upregulating Runx2/osterix expression in preosteoblastic C2C12 cells. J Dent Anesth Pain Med. 2019;19:91–99. doi: 10.17245/jdapm.2019.19.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Q, Ouyang X. Biochemical-markers for the diagnosis of bone metastasis: a clinical review. Cancer Epidemiol. 2012;36:94–98. doi: 10.1016/j.canep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 33.de la Piedra C, Castro-Errecaborde NA, Traba ML, Mendez-Davila C, Garcia-Moreno C, Rodriguez de Acuna L, Rodriguez-Molina J. Bone remodeling markers in the detection of bone metastases in prostate cancer. Clin Chim Acta. 2003;331:45–53. doi: 10.1016/s0009-8981(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 34.Iba K, Takada J, Yamashita T. The serum level of bone-specific alkaline phosphatase activity is associated with aortic calcification in osteoporosis patients. J Bone Miner Metab. 2004;22:594–596. doi: 10.1007/s00774-004-0528-9. [DOI] [PubMed] [Google Scholar]
- 35.Saha MK, Agrawal P, Saha SG, Vishwanathan V, Pathak V, Saiprasad SV, Dhariwal P, Dave M. Evaluation of correlation between salivary calcium, alkaline phosphatase and osteoporosis - a prospective, comparative and observational study. J Clin Diagn Res. 2017;11:ZC63–ZC66. doi: 10.7860/JCDR/2017/24960.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]


