Abstract
The down-regulation of long non-coding RNA (lncRNA) MEG3 has been observed in various cancers; nonetheless, underlying mechanisms are still unclear. The current research work aims at exploring the roles of MEG3 in the pathogenesis of CRC and the associated mechanism. We observed that MEG3 was significantly down-regulated in both CRC tumor tissue and cell lines; also, the transient over-expression of MEG3 in CRC cell line SW480 and LoVo inhibited the proliferation and the migration and clone formation capability of cells; on the other hand, the knockdown of MEG3 has revealed opposite effects. Eventually, we figured it out that target miR-376 directly targeted both MEG3 and PRDK1 in SW480 and LoVo cells. To conclude, as our findings proved, MEG3 is likely to act as a tumor suppressor in the pathogenesis of CRC by means of the regulation of the miR-376/PRDK1 signal axis, suggesting that MEG3 has the potential to become a novel therapeutic target for the treatment of CRC.
Keywords: MEG3, proliferation, migration, colorectal cancer cells
Introduction
Colorectal cancer (CRC) is counted among the most common gastrointestinal cancers (incidence number > 900,000/year), besides being one the leading causes of cancer-related motility across the globe [1,2]. The pathogenesis of CRC remains unclear. On the basis of the findings of previous reports, CRC is likely to derive from a series of abnormal genetic and molecular alterations in colon epithelial cells, accordingly causing the uncontrolled proliferation of cancer cells [3,4]. Current methodologies put to use for treating CRC include surgery, chemotherapy, and radiotheapy or surgery; however, for the robust metastasis activity of the disease, none of the current anti-CRC methodologies have attained the desired therapeutic efficacy, and the long-term prognosis of patients with CRC remains poor as well [5-7]. In this manner, it is urgently required to figure out the novel diagnostic biomarkers as well as novel therapeutic targets for the early diagnosis and treatment of CRC.
Long non-coding RNAs (LncRNAs) are a group of non-coding RNA having the length of more than 200 nucleotides, and some of the LncRNAs are capable of reaching kilobases in length [8,9]. Like other types of non-coding RNAs i.e. microRNAs and Circular RNAs, the regulatory roles of LncRNA were on the post-transcriptional level. Recently, with the comprehensive investigation of LncRNAs from varying aspects, it has now been extensively accepted that LncRNAs have involvement in the process of multiple biological process, which includes the proliferation, migration and apoptosis of cells, in addition to angiogenesis, embryo development and tumorigenesis [10-12]. In a recent LncRNA microarray-based bioinformatic analysis, the researchers compared the LncRNAs expression profile in the CRC tumor tissues and adjacent tissues. They observed that a novel lncRNA, MEG3, was significantly down-regulated in CRC [13,14]. Interestingly, the increased expression of MEG3 in the tissue samples of patients with CRC is likely to be an indication of the better prognosis of patients. Maternally expressed gene3 (MEG3), located at chromosome 14q32, is the first identified tumor suppressive lncRNA. Furthermore, the decreased expression of MEG3 has been observed in various cancers that include not just lung cancer [15], but also hepatocellular cancer [16], bladder cancer [17], prostate cancer [18], gastric cancer [19], and breast cancer [20]. Also, in some cancers like neuroblastoma [21] and renal cell carcinoma [22], MEG3 expression was observed as lost. Nonetheless, the underlying mechanism of how MEG3 was involved in the pathogenesis of CRC still requires further investigation.
MiRNAs are also a group of non-coding RNAs, which are 22-28 oligonucleotide-long, besides regulating the gene expression by base pairing with the 3’untranslated region (3’-UTR) of target genes at the post-transcriptional level, accordingly being involved in a number of cell physiological mechanisms [23].
In the present research work, we have carried out both clinical and in vitro cellular analysis, aimed at investigating the roles of MEG3 in CRC. Firstly, we compared the expression of MEG3 in the CRC tumor tissues and adjacent tissues, and the correlation between the levels of MEG3 and the clinical characteristics of the patients was analysed as well. Besides, the impact of MEG3 on the proliferation and migration of the CRC cancer cells and the associated mechanism involving the regulation of miRs were also examined. Our findings proved that MEG3 is tumor suppressor in CRC, together with providing a potential novel therapeutic target for the treatment of CRC.
Methods
Patients and clinical tissue samples
A number of 25 of CRC tissue samples and adjacent tissue samples were obtained from CRC patients in the First Affiliated Hospital of Wenzhou Medical University between 2017 and 2019. All patients were diagnosed as CRC pathologically, and patients have the history of preoperative radio and/or chemotherapies were excluded from this study. The tissue samples were quick frozen in liquid nitrogen after surgery and stored in -80°C. The informed consent was obtained from each patient. This study was approved by the ethical committee of First Affiliated Hospital of Wenzhou Medical University.
Cell culture
Human CRC cell lines DLD-1, HT-29, SW480, SW620 and LoVo were purchased from Shanghai Institutes for Biological Sciences (Shanghai, China) and the normal colonic mucosa cell line FHC was purchased from INCELL (San Antonio, TX, USA). Cells were maintained in RPMI-1640 medium (Invitrogen, USA) supplied with 10% of FBS (fetal bovine serum, Invitrogen, Carlsbad, CA, USA) at 37°C in an incubator (with 5% CO2 humidified).
Transfection and treatment
MEG3 siRNA and MEG3 over-expression plasmid were synthesized by Shanghai GenePharma Co., Ltd (Shanghai, China). SW480 or LoVo cells were transfected with MEG3 siRNA or MEG3 overexpression plasmid by lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. Transfection efficiency was determined by RT-qPCR.
Reverse transcript PCR and quantitative real-time PCR
Total RNAs were extracted from cells or clinical tissue samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The total RNAs weren then reversed transcribed into cDNAs by PrimeScript RT Master Mix (Takara, Dalian, China). Next, quantitative real-time PCR (RT-qPCR) was performed to detect the expression levels of MEG3 using the SYBR premix Ex Taq (Takara, Dalian, China) on the ABI Biosystems. The relative expression level of MEG3 was normalized by 2-ΔΔCt method, and GAPDH has been applied for normalization. The real time PCR reactions were performed with the following thermo profiles: 95°C for 30 seconds, followed by 40 cycles of 95°C for 5 seconds and 60°C for 30 seconds. The primers were synthesized by Sangon Biotech Co., Ltd (Shanghai, China).
Cell proliferation analysis
The effect of MEG3 on cell proliferation were determined by Cell Counting Kit-8 (CCK-8) kit (Beyotime, Shanghai, China) 48 hours after transfection according to the manufacturer’s instructions. Briefly, SW480 or LoVo cells were washed with PBS (pH 7.4), trypsinized and seeded onto 96-well plates. Then 10 μl of the CCK-8 solution was added to each well, and the plate was incubated at 37°C for 12 to 48 hours. At each time point, the viability of the cells in each well was evaluated through detecting the absorbance at 450 nm using a microplate reader.
Flow cytometry assay
At 72 h after transfection, SW480 and LoVo cells of different treatment were collected, re-suspended in 500 μl × binding buffer, and stained with 2.5 μl propidium iodide (PI). The cell cycle of the cells was then determined with FACSCalibur system (BD Biosciences, San Jose, CA).
Transwell assay
Transwell assay was performed using transwell chambers (Corning Inc., Corning, USA). SW480 or LoVo cells were seeded onto the upper of the chamber of the transwell with the density of 5 × 104 cells/well and placed on 24-well plates. After 24 h incubation at 37°C, cells invaded into the membrane of the lower chamber were fixed in methanol, stained with crystal violet and photographed by a microscope.
Western blot
The total proteins were isolated from the cells using protease inhibitor cocktail. Protein concentration was examined by BCA protein assay kit (Beyotime, Shanghai, China). Then appropriate amount of proteins were loaded onto 10% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) gels, and when the process of gel electrophoresis was accomplished, the proteins were then transferred onto polyvinylidene fluoride (PVDF) membranes, and blocked in 5% non-fat dry milk in Tris-buffered saline (pH 7.4) containing 0.05% Tween 20. Then membranes were than incubated with the primary antibodies at 4°C overnight. GAPDH was served as the control. In the following day, the membranes were incubated with HRP and secondary antibodies, washed and incubated with chemiluminescent reagent BeyoECL Plus (Beyotime, Shanghai, China). The signals were detected and photographed by Tanon 6100 Chemiluminescent Imaging System (Tanon, Shanghai, China).
Luciferase activity assay
The wild-type or mutant sequence at the predicted 3’UTR region of MEG3 were synthesized and cloned into the pGL3 Luciferase Reporter Vectors (Promega, CA, USA) at the KpnI and BamHI sites. colorectal cancer cells were co-transfected with miR-376 mimics or mimic control along with pGL3 vectors containing the wild type or mutant 3’UTR region of MEG3. TRL-SV40 plasmid (Promega, CA, USA) was also transfected as a internal control. The cells were harvested and subsequently measured the luciferase activity using the Dual-Luciferase Assay (Promega, WI, USA) at 48 h after transfection.
Immunohistochemical staining (IHC)
The tumor tissues were fixed in 4% paraformaldehyde for 24 h, dehydrated in a graded alcohol series and embedded in paraffin followed by cutting into 5 μm sections. The sections were deparaffinized, rehydrated with a graded alcohol series and then incubated in 96°C 0.01 mol/l sodium citrate buffer for the antigen retrieval. After incubation in 5% H2O2, was applied for quenching endogenous peroxidase activity. The sections were blocked with 10% non-immune goat serum to reduce non-specific binding. The sections were incubated with primary antibody overnight at 4°C. Immunostaining was performed using streptavidin-peroxidase and diaminobenzidine (DAB) according to the manufacturer’s instructions (Beyotime, Shanghai, China). The sections were mounted with gummi after staining the nucleus with hematoxylin.
Xenograft model
A total number of 3 × 106 SW480 cells were harvested and resuspended in 100 μL DMED medium. The nude mice were injected with cells infected with MEG3 overexpressed or deleted SW480 cells along with their vector control respectively at the posterior flank. The length (L) and width (W) of tumor were measured with calipers every 3 days. After 28 days, the tumors were excised out from the sacrificed mice and weighed.
Colony-formation assay
The cells treated with indicated conditions and then seeded in 12-well plates (100/well). After incubated for 2 weeks, crystal violet (0.05%, Beyotime, Shanghai, China) was used to stain the colonies. Colonies containing more than 50 cells were counted.
Statistical analysis
All data are presented as the means ± standard deviation (SD). The statistical analyses were performed using one-way ANOVA and Student’s test. The data were analyzed using GraphPad Prism 5.0 and P<0.05 was considered to be statistically significant. All experiments were performed independently triplicates.
Results
Down-regulation of MEG3 in colorectal cancer tissues and cell lines
QPCR was used to evaluate the expression levels of MEG3 in colorectal cancer tissues and the adjacent normal tissues as well as colorectal cancer cells and normal colon epithelial cell line FHC. We observed that MEG3 was down-regulated notably in the colorectal cancer tissues and cell lines in comparison with the normal tissues and FHC cell (Figure 1A-C). The survival curve analysis results showed that there is a correlation between MEG3 expression and the patient survival (Figure 1D).
Figure 1.

MEG3 was down-regulated in CRC and is correlated with the survival time. A, B. Expression of MEG3 in CRC tissue specimens and adjacent normal tissues were determined by a qRT-PCR assay (n=15), the level of MEG3 was down-regulated in CRC tissues. C. Expression of MEG3 in CRC cells and normal colonic mucosa cells were determined by a qRT-PCR assay, the level of MEG3 was down-regulated in CRC cell lines. D. The overall survival in the patients with high MEG3 (n=68) was significantly shorter than that in the patients with low MEG3 (n=97) as determined by Kaplan-Meier analyses. *P<0.05 verse control or FHC group.
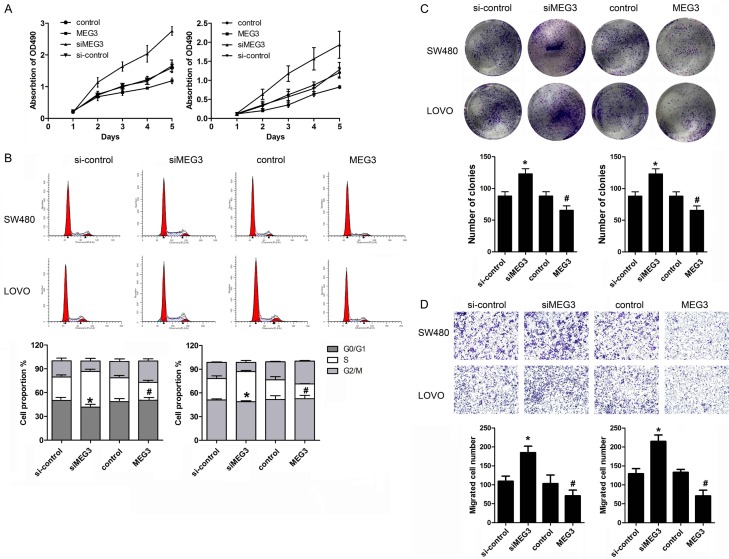
MEG3 inhibited the proliferation, migration and induced G0/G1 arrest of colorectal cancer cells
To determine the function of MEG3 in colorectal cancer, we carried out loss and gain of function study. We observed that MEG3 overexpression was capable of inhibiting the proliferation, migration and colony formation ability of colorectal cancer cells. In addition, the proportion of cells in the S phase was decreased by MEG3 overexpression compared to the vector control group. In contrast, we found that knock down of MEG3 by transfection of siRNA of MEG3 significantly promoted the proliferation, migration and colony formation ability of colorectal cancer cell. Meantime, MEG3 knock down notably induced G0/G1 cell cycle arrest in CRC cells (Figure 2A-D).
Figure 2.
MEG3 inhibited tumor growth of colorectal cancer cells. A. SW480 and LOVO cells were used in the function study. After transfection of overexpressing and knock down vetor of MEG3, CCK-8 assay was carried out to detect the proliferation of cells. B. PI staining and flow cytometry was used to detect the cell cycle of CRC cells. C. Colony formation assay was performed to evaluate the clone-forming capability of CRC cells. D. Transwell assay was performed to detect the migration of CRC cells. *P<0.05 verse control group. #P<0.05 verse si-control group.
MEG3 inhibited tumor growth of colorectal cancer
In order to elucidate the role of MEG3 in the regulation of colorectal cancer, xenograft nude mice model was established though subcutaneously injecting genetically modified SW480 and LoVo cells. As expected, compared with control group, MEG3 overexpression significantly inhibited the tumor growth of colorectal cancer and knock down of MEG3 promoted the tumor growth as shown in the tumor growth curve (Figure 3A-C). QPCR was performed to evaluate the MEG3 expression level in the tumor tissues indicating that we successfully established the MEG3 over and low-expressed CRC cells (Figure 3D). IHC assay was used to detect the expression of ki67 which is a marker of the cell proliferation. We found that ki67 was significantly up-regulated in the MEG3 treatment group and down-regulated in si-MEG3 treatment group (Figure 3E).
Figure 3.

MEG3 inhibited tumor growth of colorectal cancer. Stable cell line overexpressing and knocking dwon MEG3 was used to establishe xenograft model. A. Representative images of nude mice and tumors from implanted mice. B, C. The tumor volume and weight were measured in different groups, MEG3 overexpressing inhibited while knock down of MEG3 promoted the growth of colorectal cancer. D. Expression level of MEG3 in tumor tissues were evaluated by qPCR indicating that the overexpressing and knock down vector are effective. E. IHC assay was used to detect the expression of ki67 which is a marker of the cell proliferation. *P<0.05 verse control or FHC group.
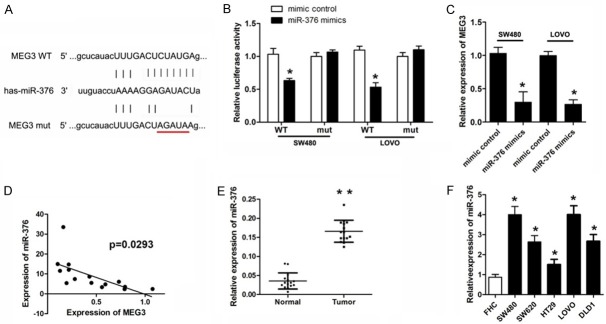
MiR-376 was upregulated in colorectal cancer and directly target MEG3
LncRNA was able to sponge miRNAs to regulate the gene expression and cell process. Thus, we predicted the potential miR of MEG3 using bioinformatics analysis. Figure 4A showed the target region between MEG3 and miR-376. To verify whether miR-376 directly target MEG3 in colorectal cancer cell, luciferase activity assays was performed. The results revealed that miR-376 notably down-regulated the luciferase activity in SW480 and LoVo cells co-transfected with pGL3-3’UTR of MEG3 but not the pGL3-3’UTR-mut (Figure 4B). QPCR analyses further confirmed that miR-376 reduced the RNA expression of MEG3 (Figure 4C). MiR-376 level in colorectal cancer tissue and cells was detected subsequently. The results indicated a significant elevated expression of miR-376 in both colorectal cancer tissue and cells (Figure 4E, 4F). Person analysis revealed a negative correlation between MEG3 and miR-376 (Figure 4D).
Figure 4.
EMG3 is the direct target of miR-376. (A) Prediction of binding sites for EMG3 and miR-376 by Targetscan. (B) CRC cells were transfected luciferase reporter vector with wild or mutant type of targeting region along with miR-376 mimic or control. Luciferase activity assay was used to determine whether miR-376 target EMG3 (C) qPCR was used to detect the MEG3 level in different groups. (D) The correlation analysis was performed between EMG3 and miIR-376 expression levels in clinical specimen. (E, F) QPCR was used to evaluate the expression level of MEG3 in CRC tissues and cell lines. *P<0.05 verse control group.
MiR-376 reversed the effect of MEG3 in CRC cells
In order to further confirm the relation between miR-376 and MEG3. We carried out rescue experiment. Cell proliferation, migration, cell cycle and clone formation ability were investigated. The results indicated that miR-376 overexpression significantly reversed the effect of EMG3 on inhibiting the growth of CRC cells (Figure 5A-D).
Figure 5.
MiR-376 overexpression reversed the effect of MEG3 in CRC cells. CRC cells were transfected with MEG3 overexpressing vector or vector control along with miR-376 mimic or mimic control. A. CCK-8 assay was carried out to detect the proliferation of CRC cells. B. Colony formation assay was performed to evaluate the clone-forming capability of CRC cells. C. PI staining and flow cytometry was used to detect the cell cycle of CRC cells. D. Transwell assay was performed to detect the migration of CRC cells. *P<0.05 verse vector group. #P<0.05 verse MEG3 + mimic control group.
MiR-376 directly targets PRKD1 in CRC cells
To further elucidate the molecular mechanism underlying the effect of MEG3 in CRC cells. We focused on the downstream target gene of miR-376. Interestingly, we found that PRKD1 was a potential target of miR-376 (Figure 6A). Luciferase activity assay and qPCR detection confirmed out prediction (Figure 6B, 6C). We next evaluate the level of PRKD1 in the CRC tissues and cell lines. The results come from starbase showed that PRDK1 was downregulated in CRC tissues (Figure 6D) QPCR and IHC results indicated that PRKD1 was notably down-regulated in CRC tissues and cell lines (Figure 6E-G). The person analysis indicated a negative correlation between miR-376 and PRKD1, in addition, a positive correlation between MEG3 and PRDK1 (Figure 6H, 6I).
Figure 6.
MiR-376 directly targets PRKD1 in CRC cells. (A) Prediction of binding sites for EMG3 and miR-376 by Targetscan. (B) CRC cells were transfected luciferase reporter vector with wild or mutant type of targeting region of PRKD1 along with miR-376 mimic or control. Luciferase activity assay was used to determine whether miR-376 target EMG3. (C) Expression data of PRKD1 in CRC and adjacent normal tissues obtained from Starbase. (D) qPCR was used to detect the MEG3 level in different groups. (E) QPCR and (F) IHC was used to evaluate the expression level of MEG3 in CRC tissues. (G) QPCR was used to evaluate the expression level of MEG3 in CRC cell lines. The correlation analysis was performed between (H) PRKD1 and miIR-376 along with (I) miR-376 and MEG3 expression levels. *P<0.05 verse mimic control group. #P<0.05 verse control group.
Discussion
MEG3 has been investigated in different kinds of cancers that include CRC as well. In the present research work, we confirmed that the level of MEG3 was modified in both CRC tissue and cell lines in comparison with the adjacent normal tissues and cells, which is in accordance with the previous studies. Previous reports highlighted that MEG3 was capable of not just promoting apoptosis but also enhancing apoptosis of CRC cells induced by oxaliplatin. Additionally, MEG3 also inhibited the proliferation and migration of CRC cell. We firstly demonstrated that MEG3 induced the G0/G1 cell cycle arrest of CRC, accordingly further clarifying its function.
MEG3 is located in the chromosome 14 DLK1-MEG3 imprinting region, which contains multiple imprinted genes along with a number of miRNAs [24,25]. This characteristic makes MEG3 a key lncRNA that regulates different types of genes. For instance, MEG3 participates in the regulation of the critical signal pathways in cancer including p53 pathway. It is fully known that TP53 activation had the potential of inducing the cell cycle arrest and apoptosis, leading to the slow growth of the CRC cells. Previous research works illustrated that MEG3 promoted expression level of TP53 in colorectal cancer and osteosarcoma cancer cell lines [26,27]. As in CRC, MEG3 has been observed as involved in the regulation of TGF-β pathway [28] as well as Clustering protein [29].
As indicated earlier, there exist a number of miRNAs and binding cites in the intron region of MEG3. The molecular mechanism of lncRNAs are quite intricate. LncRNAs was capable of directly interacting with the DNAs, RNAs and even proteins for the change of their conformation, stability or expression levels. Among these, ceRNA mechanism has been most extensively investigated as a molecular sponge for microRNAs by means of their binding sites. MEG3 has been demonstrated as the sponge for miR-494, miR-212, and miR-181. We discovered an innovative target miR that is miR-376. A few research works have illustrated the anti-cancer role of miR-376 in breast cancer and ovarian cancer [30,31]. Nonetheless, it has not been extensively investigated, in particular, in CRC. We figured it out that MEG3 could significantly inhibit the expression of miR-376. Luciferase activity assay and rescue experiments confirmed our speculation as well.
Accumulative evidence has shed light on the fact that lncRNAs participate in the regulation of their downstream genes by playing the role of ceRNA [32]. Previous investigations have revealed that the Protein Kinase D1 (PKD1) had the potential of promoting both proliferation and oestrogen independence in breast cancer cells [33,34]. PKD1 is a serine/threonine kinase belonging to the PKD family [35] that can be activated by not just growth factors, but also mitogenic neuropeptides, and oxidative stress, regulating various biological mechanisms [36,37]. We discovered that PRKD1 constituted a direct target gene of miR-376. Together with that, there exists a positive correlation between MEG3 expression and PRKD1.
In the current research work, we figured it out that MEG3 inhibited the proliferation and migration of CRC. Further mechanism research firstly suggested that MEG3 upregulated PRKD1 by means of acting as a ceRNA of miR-376 in CRC. It provides a better understanding of the role of MEG3, besides being likely to contribute to the diagnosis and therapy strategies in CRC. With the development of epigenetics research, different mechanisms like hypermethylation, coupled with the post translational degradation via miRNAs, are likely to cause the inhibited expression or deletion of MEG3. Hypermethylation in the MEG3 promoter and IG-DMR regions is likely to be of crucial significance for lowering the MEG3 level. The regulation of MEG3 in CRC has not been elucidated, and it is under our arrangement as well.
Acknowledgements
The research was supported by Natural Science Foundation of Zhejiang province (LY17H160056).
Disclosure of conflict of interest
None.
References
- 1.Fillon M. Study aims to improve colorectal cancer screening rates. CA Cancer J Clin. 2019;69:161–163. doi: 10.3322/caac.21472. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, He X, Fuchs CS, Ogino S, Willett WC, Chan AT, Giovannucci EL, Cao Y. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5:37–44. doi: 10.1001/jamaoncol.2018.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, Lee JK, Zhao WK, Udaltsova N, Ghai NR, Lee AT, Quesenberry CP, Fireman BH, Doubeni CA. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155:1383–1391. e5. doi: 10.1053/j.gastro.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu GF, Xu YW, Li J, Niu HL, Ma WX, Xu J, Zhou PR, Liu X, Ye DL, Liu XR, Yan T, Zhai WK, Xu ZJ, Liu C, Wang L, Wang H, Luo JM, Liu L, Li XQ, Guo S, Jiang HP, Shen P, Lin HK, Yu DH, Ding YQ, Zhang QL. Mir20a/106a-WTX axis regulates RhoGDIa/CDC42 signaling and colon cancer progression. Nat Commun. 2019;10:112. doi: 10.1038/s41467-018-07998-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Lu Y, Kweon SS, Tanikawa C, Jia WH, Xiang YB, Cai Q, Zeng C, Schmit SL, Shin A, Matsuo K, Jee SH, Kim DH, Kim J, Wen W, Shi J, Guo X, Li B, Wang N, Zhang B, Li X, Shin MH, Li HL, Ren Z, Oh JH, Oze I, Ahn YO, Jung KJ, Conti DV, Schumacher FR, Rennert G, Jenkins MA, Campbell PT, Hoffmeister M, Casey G, Gruber SB, Gao J, Gao YT, Pan ZZ, Kamatani Y, Zeng YX, Shu XO, Long J, Matsuda K, Zheng W. Large-scale genome-wide association study of East Asians identifies loci associated with risk for colorectal cancer. Gastroenterology. 2018;156:1455–1466. doi: 10.1053/j.gastro.2018.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siravegna G, Lazzari L, Crisafulli G, Sartore-Bianchi A, Mussolin B, Cassingena A, Martino C, Lanman RB, Nagy RJ, Fairclough S, Rospo G, Corti G, Bartolini A, Arcella P, Montone M, Lodi F, Lorenzato A, Vanzati A, Valtorta E, Cappello G, Bertotti A, Lonardi S, Zagonel V, Leone F, Russo M, Balsamo A, Truini M, Di Nicolantonio F, Amatu A, Bonazzina E, Ghezzi S, Regge D, Vanzulli A, Trusolino L, Siena S, Marsoni S, Bardelli A. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell. 2018;34:148–162. e7. doi: 10.1016/j.ccell.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Wang FW, Cao CH, Han K, Zhao YX, Cai MY, Xiang ZC, Zhang JX, Chen JW, Zhong LP, Huang Y, Zhou SF, Jin XH, Guan XY, Xu RH, Xie D. APC-activated long noncoding RNA inhibits colorectal carcinoma pathogenesis through reduction of exosome production. J Clin Invest. 2019;129:727–743. doi: 10.1172/JCI122478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan Y, Xiao X, He Z, Luo Y, Wu C, Li L, Song X. Long noncoding RNA OCC-1 suppresses cell growth through destabilizing HuR protein in colorectal cancer. Nucleic Acids Res. 2018;46:5809–5821. doi: 10.1093/nar/gky214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham JM, Meltzer SJ. Long noncoding RNAs in the pathogenesis of Barrett’s esophagus and esophageal carcinoma. Gastroenterology. 2017;153:27–34. doi: 10.1053/j.gastro.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin C, Yang L. Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol. 2018;28:287–301. doi: 10.1016/j.tcb.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Invest. 2016;126:2775–82. doi: 10.1172/JCI84421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong X, Wang J, Li T, Xu YP, Li SY. Down regulation of lncRNA MEG3 promotes colorectal adenocarcinoma cell proliferation and inhibits the apoptosis by up-regulating TGF-beta1 and its downstream sphingosine kinase 1. Eur Rev Med Pharmacol Sci. 2018;22:8265–8272. doi: 10.26355/eurrev_201812_16522. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Li H, Zhang L, Yang D. Overexpression of MEG3 sensitizes colorectal cancer cells to oxaliplatin through regulation of miR-141/PDCD4 axis. Biomed Pharmacother. 2018;106:1607–1615. doi: 10.1016/j.biopha.2018.07.131. [DOI] [PubMed] [Google Scholar]
- 15.Wu JL, Meng FM, Li HJ. High expression of lncRNA MEG3 participates in non-small cell lung cancer by regulating microRNA-7-5p. Eur Rev Med Pharmacol Sci. 2018;22:5938–5945. doi: 10.26355/eurrev_201809_15923. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Cao FL, Qu LL, Wang ZM, Liu XY. MEG3 promotes liver cancer by activating PI3K/AKT pathway through regulating AP1G1. Eur Rev Med Pharmacol Sci. 2019;23:1459–1467. doi: 10.26355/eurrev_201902_17103. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Liao X, Jin H, Xie F, Zheng F, Li J, Zhou C, Jiang G, Wu XR, Huang C. MEG3, as a competing endogenous RNA, binds with miR-27a to promote PHLPP2 protein translation and impairs bladder cancer invasion. Mol Ther Nucleic Acids. 2019;16:51–62. doi: 10.1016/j.omtn.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu M, Huang Y, Chen T, Wang W, Yang S, Ye Z, Xi X. LncRNA MEG3 inhibits the progression of prostate cancer by modulating miR-9-5p/QKI-5 axis. J Cell Mol Med. 2019;23:29–38. doi: 10.1111/jcmm.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaedi H, Mozaffari MAN, Salehi Z, Ghasemi H, Zadian SS, Alipoor S, Hadianpour S, Alipoor B. Co-expression profiling of plasma miRNAs and long noncoding RNAs in gastric cancer patients. Gene. 2019;687:135–142. doi: 10.1016/j.gene.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Wu J, Jing H, Huang G, Sun Z, Xu S. Long noncoding RNA MEG3 inhibits breast cancer growth via upregulating endoplasmic reticulum stress and activating NF-kappaB and p53. J Cell Biochem. 2019;120:6789–6797. doi: 10.1002/jcb.27982. [DOI] [PubMed] [Google Scholar]
- 21.Tang W, Dong K, Li K, Dong R, Zheng S. MEG3, HCN3 and linc01105 influence the proliferation and apoptosis of neuroblastoma cells via the HIF-1alpha and p53 pathways. Sci Rep. 2016;6:36268. doi: 10.1038/srep36268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He H, Dai J, Zhuo R, Zhao J, Wang H, Sun F, Zhu Y, Xu D. Study on the mechanism behind lncRNA MEG3 affecting clear cell renal cell carcinoma by regulating miR-7/RASL11B signaling. J Cell Physiol. 2018;233:9503–9515. doi: 10.1002/jcp.26849. [DOI] [PubMed] [Google Scholar]
- 23.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–65. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyoshi N, Wagatsuma H, Wakana S, Shiroishi T, Nomura M, Aisaka K, Kohda T, Surani MA, Kaneko-Ishino T, Ishino F. Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes Cells. 2000;5:211–20. doi: 10.1046/j.1365-2443.2000.00320.x. [DOI] [PubMed] [Google Scholar]
- 25.Da RS, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008;24:306–16. doi: 10.1016/j.tig.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu WQ, Xie WP, Hou YY. Long non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces apoptosis by affecting p53 expression. BMC Cancer. 2013;13:461. doi: 10.1186/1471-2407-13-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin R, Chen Z, Ding Y, Hao J, Hu J, Guo F. Long non-coding RNA MEG3 inhibits the proliferation of cervical carcinoma cells through the induction of cell cycle arrest and apoptosis. Neoplasma. 2013;60:486–92. doi: 10.4149/neo_2013_063. [DOI] [PubMed] [Google Scholar]
- 28.Dong X, Wang J, Li T, Xu YP, Li SY. Down regulation of lncRNA MEG3 promotes colorectal adenocarcinoma cell proliferation and inhibits the apoptosis by up-regulating TGF-beta1 and its downstream sphingosine kinase 1. Eur Rev Med Pharmacol Sci. 2018;22:8265–8272. doi: 10.26355/eurrev_201812_16522. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Chen P, Gao Y, Ta N, Zhang Y, Cai J, Zhao Y, Liu S, Zheng J. MEG3 activated by vitamin D inhibits colorectal cancer cells proliferation and migration via regulating clusterin. EBioMedicine. 2018;30:148–157. doi: 10.1016/j.ebiom.2018.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An N, Luo X, Zhang M, Yu R. MicroRNA-376b promotes breast cancer metastasis by targeting Hoxd10 directly. Exp Ther Med. 2017;13:79–84. doi: 10.3892/etm.2016.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Wei QM, Zhang XW, Sheng Q, Yan XT. MiR-376a promotion of proliferation and metastases in ovarian cancer: potential role as a biomarker. Life Sci. 2017;173:62–67. doi: 10.1016/j.lfs.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Zhang N, Meng X, Mei L, Zhao C, Chen W. LncRNA DLX6-AS1 promotes tumor proliferation and metastasis in osteosarcoma through modulating miR-641/HOXA9 signaling pathway. J Cell Biochem. 2019 doi: 10.1002/jcb.28426. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Karam M, Bieche I, Legay C, Vacher S, Auclair C, Ricort JM. Protein kinase D1 regulates ERalpha-positive breast cancer cell growth response to 17beta-estradiol and contributes to poor prognosis in patients. J Cell Mol Med. 2014;18:2536–52. doi: 10.1111/jcmm.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karam M, Legay C, Auclair C, Ricort JM. Protein kinase D1 stimulates proliferation and enhances tumorigenesis of MCF-7 human breast cancer cells through a MEK/ERK-dependent signaling pathway. Exp Cell Res. 2012;318:558–69. doi: 10.1016/j.yexcr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–8. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 36.Rozengurt E. Protein kinase D signaling: multiple biological functions in health and disease. Physiology (Bethesda) 2011;26:23–33. doi: 10.1152/physiol.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merzoug-Larabi M, Spasojevic C, Eymard M, Hugonin C, Auclair C, Karam M. Protein kinase C inhibitor Go6976 but not Go6983 induces the reversion of E- to N-cadherin switch and metastatic phenotype in melanoma: identification of the role of protein kinase D1. BMC Cancer. 2017;17:12. doi: 10.1186/s12885-016-3007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]