Abstract
Cyclooxygenase-2 (COX2) and tumor-associated macrophages (TAMs) are associated with invasion, angiogenesis, and poor prognosis in many human cancers. However, the role of TAMs in human gastric cancer (GC) remains elusive. In the present study, we first measured COX2 expression and TAM infiltration in human GC tissues using double immunohistochemical staining. Then, we indirectly cocultured M2-polarized macrophages derived from human THP-1 cells with GC cells as an in vitro model. Transwell assays, siRNA transfection, treatment with a COX2 inhibitor and Western blotting were used to investigate the relationship among TAMs, invasion and COX2 expression as well as the underlying molecular mechanism. Double IHC staining showed that TAMs were aggregated near GC tumor nests and had high COX2 expression; moreover, the number of TAMs that infiltrated the tumor nest was correlated with the depth of invasion, COX2 expression and poor prognosis in human GC. In an in vitro assay, after treatment with phorbol myristate acetate (PMA), the THP-1 cells differentiated into M2 macrophages and induced COX2/MMP9-dependent invasiveness in GC cells. Pretreatment of GC cells with COX2 siRNA or a COX2 inhibitor (Celecoxib) can negate these promoting effects. The results of this study and those of our previous studies indicate that coculture with M2-polarized macrophages can induce the COX2-dependent release of matrix metalloproteinase-9 (MMP9), which subsequently increases the invasiveness of GC cells. Our data may provide a basis for targeting TAMs or for polarizing TAMs through immune regulation to halt GC progression, which could soon become a nonsurgical treatment for human gastric cancer.
Keywords: TAMs, COX2, gastric cancer, invasion, poor prognosis
Introduction
Despite recent developments in surgical techniques and improvements in the efficacies of anticancer drugs, gastric cancer (GC) remains a major cause of global cancer mortality [1]. There were estimated 1.3 million incident cases of GC and 819000 (95% uncertainty interval, 795000-844000) deaths in the world wide [2]. Notably, in China, due to late diagnosis (at stage III and/or IV), which is associated with a high rate of lymph node and distant metastases, the overall 5-year survival rate of patients with GC is still low at approximately 40%, with death occurring because of cancer cell invasion and distant metastasis [3].
In solid tumors, such as GC, different types of stromal cells surround the cancer core, including activated fibroblasts (myofibroblasts), smooth muscle cells, endothelial cells and inflammatory cells, which constitute a unique form of the tumor microenvironment. Recent evidence suggests that the tumor microenvironment plays a positive role in cancer invasion and metastasis because it contains numerous growth factors, cytokines, extracellular matrix constituents, and cancer-promoting immunocytes, especially tumor-associated macrophages (TAMs) [4-6]. Macrophages have functional plasticity and can change their functional profiles in response to environmental changes. When macrophages are exposed to lipopolysaccharides (LPS) or gamma interferon (IFN-γ), they are polarized to M1 macrophages and have antitumor activities. However, when they are exposed to Th2 cytokines, such as IL-4 and IL-13, they are polarized to M2 macrophages and support tumor growth [7]. Macrophages in the tumor microenvironment are defined as TAMs and chiefly exhibit M2 characteristics [8]. Importantly, collective data have shown that the high numbers of M2 macrophages in the tumor microenvironment are associated with a worse prognosis in numerous cancer types, such as GC [9,10], pancreatic cancer [10], ovarian cancer [11], breast cancer [12] and non-small-cell lung cancer [13]. Our previous data also indicated that M2 macrophages (expressing the surface markers CD206 and CD204) could promote invasion and migration of gastric cancer cells by stimulating cancer cell expression of VEGF and MMP9 [14]. However, other potential underlying molecules involved in this promotion still need to be explored. In the present study, we used an immunohistochemistry (IHC)-based double staining method to investigate the correlations between TAMs and COX2 expression. The interaction among TAMs (M2-polarized human THP-1 macrophages), GC cell migration and invasion and COX2/MMP9 axis expression was also investigated in vitro. Finally, we hypothesize that TAMs might activate COX2 in GC cells and subsequently increase their invasiveness in a COX2/MMP9-dependent manner.
Method
Cancer tissue and patients
In all, 228 GC formalin-fixed paraffin-embedded (FFPE) samples included all types of gastric carcinomas, which were collected from the departments of Gastrointestinal Surgery and Pathology of the Zhejiang Provincial People’s Hospital from January 2009 to January 2013; none of the patients had received radiotherapy and/or chemotherapy prior to surgery. The study designs and methods were approved by the Ethics Committee of Zhejiang Provincial People’s Hospital. Upon admission, all patients or their relatives provided informed consent within the written treatment contract prior to their inclusion in the study. The samples were used for tissue section preparation and IHC double staining. All patients were followed-up for over 5 years or until December 2018. The survival time was calculated from the date of surgery to the end of the follow-up period and/or the date of death. The age of the GC patients ranged from 17 to 80 (with a median age of 59.3 years), and all cases were classified according to the World Health Organization pathological classification (2010) of tumors. The clinicopathological characteristics of the GC patients are summarized in Table 1.
Table 1.
Association among COX2 and CD204 expression and clinicopathological factors
| Clinical parameters | COX2 Expression | CD204 Expression | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Positive | Negative | t/χ2 | P | High | Low | t/χ2 | P | |
| Age (yrs) | 57.08±11.49 | 56.46±11.37 | 0.278 | 0.598 | 58.17±11.51 | 55.48±11.23 | 0.608 | 0.432 |
| Gender | 2.250 | 0.134 | 1.570 | 0.210 | ||||
| Male | 88 (67.2%) | 74 (76.3%) | 76 (67.3%) | 86 (74.8%) | ||||
| Female | 43 (32.8%) | 23 (23.7%) | 37 (32.7%) | 29 (25.2%) | ||||
| Location | 3.892 | 0.147 | 0.192 | 0.907 | ||||
| Proximal | 18 (13.7%) | 14 (14.4%) | 15 (13.3%) | 17 (14.8%) | ||||
| Middle | 78 (59.5%) | 46 (47.4%) | 61 (54.0%) | 63 (54.8%) | ||||
| Distal | 35 (26.7%) | 37 (38.1%) | 37 (32.7%) | 35 (30.4%) | ||||
| Size | 3.040 | 0.081 | 3.453 | 0.063 | ||||
| ≥5 cm | 61 (46.6%) | 34 (35.1%) | 54 (47.8%) | 41 (35.7%) | ||||
| <5 cm | 70 (53.4%) | 63 (64.9%) | 59 (52.2%) | 74 (64.3%) | ||||
| Histology type | 5.132 | 0.162 | 1.727 | 0.631 | ||||
| Papillary adenocarcinoma | 6 (4.6%) | 4 (4.1%) | 6 (5.3%) | 4 (3.5%) | ||||
| Tubular adenocarcinoma | 89 (67.9%) | 60 (61.9%) | 77 (68.1%) | 72 (62.6%) | ||||
| Mucinous adenocarcinoma | 4 (3.1%) | 10 (10.3%) | 6 (5.3%) | 8 (7.0%) | ||||
| Signet-ring cell carcinoma | 32 (24.4%) | 23 (23.7%) | 24 (21.2%) | 31 (27.0%) | ||||
| Lauren classification | 8.885 | 0.012 | 0.867 | 0.648 | ||||
| Diffuse type | 68 (51.9%) | 32 (33.0%) | 53 (46.9%) | 47 (40.9%) | ||||
| Intestinal type | 59 (45.0%) | 63 (64.9%) | 57 (50.4%) | 65 (56.5%) | ||||
| Mixed type | 4 (3.1%) | 2 (2.1%) | 3 (2.7%) | 3 (2.7%) | ||||
| Differentiation | 3.577 | 0.167 | 0.565 | 0.754 | ||||
| Well | 2 (1.5%) | 6 (6.2%) | 3 (2.7%) | 5 (4.3%) | ||||
| Moderately | 42 (32.1%) | 30 (30.9%) | 35 (31.0%) | 37 (32.2%) | ||||
| Poorly | 87 (66.4%) | 61 (62.9%) | 75 (66.4%) | 73 (63.5%) | ||||
| Invasion Depth (T Grade) | 16.31 | 0.001 | 7.875 | 0.049 | ||||
| T1 | 52 (39.7%) | 38 (39.2%) | 39 (34.5%) | 51 (44.3%) | ||||
| T2 | 13 (9.9%) | 20 (20.6%) | 16 (14.2%) | 17 (14.8%) | ||||
| T3 | 50 (38.2%) | 39 (40.2%) | 45 (39.8%) | 44 (38.3%) | ||||
| T4 | 16 (12.2%) | 0 (0.0%) | 13 (11.5%) | 3 (2.6%) | ||||
| Lymphatic Metastasis (N Grade) | 12.83 | 0.000 | 8.607 | 0.003 | ||||
| N0 | 47 (35.9%) | 58 (59.8%) | 41 (36.3%) | 64 (55.7%) | ||||
| N1 | 84 (64.1%) | 39 (40.2%) | 72 (63.7%) | 51 (44.3%) | ||||
| Distant Metastasis (M Grade) | 20.78 | 0.000 | 9.194 | 0.002 | ||||
| M0 | 103 (78.6%) | 96 (99.0%) | 91 (80.5%) | 108 (93.9%) | ||||
| M1 | 28 (21.4%) | 1 (1.0%) | 22 (19.5%) | 7 (6.1%) | ||||
| TNM Stages | 28.61 | 0.000 | 13.90 | 0.003 | ||||
| I | 41 (31.3%) | 45 (46.4%) | 33 (29.2%) | 53 (46.1%) | ||||
| II | 4 (3.1%) | 12 (12.4%) | 6 (5.3%) | 10 (8.7%) | ||||
| III | 58 (44.3%) | 39 (40.2%) | 52 (46.0%) | 45 (39.1%) | ||||
| IV | 28 (21.4%) | 1 (1.0%) | 22 (19.5%) | 7 (6.1%) | ||||
| Lymphatic invasion | 5.115 | 0.024 | 5.064 | 0.024 | ||||
| Yes | 67 (51.1%) | 35 (36.1%) | 59 (52.2%) | 43 (37.4%) | ||||
| No | 64 (48.9%) | 62 (63.9%) | 54 (47.8%) | 72 (62.6%) | ||||
| Vascular invasion | 3.909 | 0.048 | 4.476 | 0.034 | ||||
| No | 61 (46.6%) | 58 (59.8%) | 51 (45.1%) | 68 (59.1%) | ||||
| Yes | 70 (53.4%) | 39 (40.2%) | 62 (54.9%) | 47 (40.9%) | ||||
| CD204 Expression | 38.21 | 0.000 | ||||||
| High | 88 (67.2%) | 25 (25.8%) | ||||||
| Low | 43 (32.8%) | 72 (74.2%) | ||||||
All cases were classified according to the World Health Organization’s (2010) pathological classification of gastric cancer. Invasion Depth (T Grade) grade T1 includes T1a and T1b, and T4 includes T4a and T4b. Lymphatic Metastasis (N Grade) grade N3 includes N3a and N3b. TNM grade I includes Ia and Ib, TNM grade II includes IIa and IIb, and TNM grade III includes IIIa, IIIb and IIIc.
IHC double staining
For the COX2 and CD204 IHC double staining, 5 μm sections of GC tissue were used. The sections were deparaffinized, rehydrated, and subjected to antigen retrieval combined with signal detection, as previously described [14,15]. Sections were incubated with blocking buffer supplied with the Polymer Double Staining Detection Kit (Mo/HRP + Rb/AP, DS-0002, Beijing Zhongshan Jinqiao Biotechnology Co. LTD., China) for 60 min and were then simultaneously incubated with two different primary antibodies overnight at 4°C. The antibodies used were mouse monoclonal anti-CD204 (1:200 dilution, aa197-451, clone 9E5, cat no. LS-C336674, LifeSpan BioSciences, Inc. USA) and rabbit polyclonal anti-COX2 (1:500, sc-514489, Santa Cruz Biotechnology, Inc. USA). After the sections were rinsed three times in TBST for 5 min each time, the sections were incubated with HRP- and AP-labeled secondary antibodies (premixed at a 1:1 ratio) for 1 h at room temperature. After the sections were rinsed three times in TBST for 5 min each time, signal detection was performed using 3,3-diaminobenzidine (DAB) and the GBI-Long red dye reagent followed by counterstaining in hematoxylin according to the instruction manual of the Polymer Double Staining Detection Kit (Mo/HRP + Rb/AP, DS-0002, Beijing Zhongshan Jinqiao Biotechnology Co. LTD., China).
Immune signal evaluation
For each sample, the immunoreactivity levels of COX2 were estimated under a light microscope by assessing the average signal intensity (on a scale of 0-3). The proportion of cells that indicated positive staining (0, <5%; 1, 5-25%; 2, 26-50%; 3, 51-75%; and 4, 76-100%) was independently estimated by two pathologists in the absence of clinical information, as described in previous studies [15,16]. The intensity and percentage scores were subsequently multiplied to obtain a composite score; a score of 0 to 3 was defined as negative, while a score of 4 to 12 was defined as positive.
CD204 is known to be a specific marker for M2-type macrophages. The number of CD204+ macrophages was initially determined using low-power magnification (100×). Then, the CD204+ macrophage count was estimated by assessing 5 high-power (400×) fields (HPFs), which were representative intratumor areas, per sample (where the staining was the strongest). Only CD204+ cells that displayed macrophage morphology were counted. The average counts were recorded as M2 macrophage counts for each patient. Finally, we used the macrophage counts to divide the patients into two subgroups (high CD204+ and low CD204+ subgroups). These analyses were performed by two independent pathologists who were blinded to the IHC evaluations.
Induction and validation of M2 macrophages
In this study, we used the human monocytic cell line THP-1 as a macrophage induction model; the details of THP-1 cell maintenance and PMA induction were reported in our previous study [13]. THP-1 cells were treated with 320 nM of PMA for 24 h to induce the cells to acquire an adherent macrophage-like phenotype after which they were washed three times with PBS to remove the PMA. After washing, the cells were treated for 72 h with 30 ng/ml IL-13 and 2 μM rosiglitazone to obtain an M2 phenotype. After inducing an M2 phenotype, macrophages and THP-1 cells were collected for downstream experiments, such as coculture with GC cells, Transwell experiments, and M2 phenotype marker validation.
For M2 phenotype marker validation, we used SYBR® Green-based real-time PCR assays with the following primers: CD206-Fwd: 5’-CCATGGACAATGCGCGAGCG-3’, CD206-Rev: 5’-CACCTGTGGCCCAAGACACGT-3’, CD204-Fwd: 5’-AGACGTTGGGGAGATGAGGA-3’, CD204-Rev: 5’-CTTCAGGAGTTGAGCTGCCA-3’, GAPDH-Fwd: 5’-TTGCAACCGGGAAGGAAATG-3’, and GAPDH-Rev: 5’-TGGAATTTGCCATGGGTGGA-3’. Briefly, the total RNA that was isolated from cells was reverse transcribed to cDNA using a PrimeScript™ RT Master Mix (Perfect Real Time) Kit (RR036A, Takara, Japan) according to the manufacturer’s instructions. Real-time PCR was performed using a SYBR Green master mix kit, and the PCR parameters were as follows: 95°C for 4 min, followed by 40 cycles of 95°C for 10 s, 60°C for 30 s and 72°C for 30 s. After PCR, melting curve analysis was performed. The relative expression levels were compared with the expression level of glyceraldehyde phosphate dehydrogenase (GAPDH) and were calculated using the 2-ΔΔCt method.
To test the M2 macrophage cytokine secretion profile, the cytokines TNF-α (DTA00D, R&D Systems, Inc., USA) and TGF-β (DB100B, R&D Systems, Inc., USA) were measured in the cell supernatant after THP-1 induction by ELISA according to the manufacturers’ instructions.
COX2 siRNA transfection and Celecoxib treatment
GC cells were transfected with 10 nM small interfering RNA (siRNA) that specifically targets COX2 (COX2 siRNA, sc-29279, Santa Cruz Biotechnology) or nontargeting control siRNA (sc-37007, Santa Cruz Biotechnology) according to the manufacturer’s instructions.
Celecoxib is a COX2 inhibitor that potently inhibits COX2 enzymatic activity and reduces the level of inflammatory prostaglandins. GC cells were treated with 10 μM Celecoxib for 24 h to block COX2 expression and enzymatic activity before coculture with the M2 macrophages or Transwell experiments.
Coculture experiments
One million GC cells (alone, individually treated with COX2 siRNA or pretreated with Celecoxib) were seeded into the upper insert of a six-well Transwell plate (0.4 μm pore size, 3412; Millipore, Billerica, MA, USA), and two million M2 macrophages were seeded into the bottom chamber; the coculture was performed for 72 h. After coculture, the cells on the upper insert were harvested for WB, RT-PCR, and migration and invasion experiments.
Western blot
Briefly, the protein samples were subjected to SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Then, the membranes were blocked with 5% nonfat dry milk in PBS/0.05% Tween 20 and probed with anti-COX2 (1:1000, sc-514489, Santa Cruz Biotechnology, Inc., USA) and anti-GAPDH (1:1000, sc-47724, Santa Cruz Biotechnology, Inc., USA) antibodies. After incubation with the corresponding HRP-labeled secondary antibodies (1:5000), the blots were developed with enhanced chemiluminescence (ECL) reagent and exposed in a Bio-Rad ChemiDoc™ MP System.
Cell migration and invasion assays
For the migration assay, briefly, 1×105 GC cells (alone, individually treated with COX2 siRNA, or pretreated with Celecoxib) were added to the upper chamber of a Boyden chamber (3422; Millipore, Billerica, MA, USA), while M2 macrophages were added to the lower chamber. In invasion assays, 2×105 GC cells (alone, individually treated with COX2 siRNA, or pretreated with 10 μM Celecoxib) were plated in the top chamber containing a membrane precoated with Matrigel (ECM554; Millipore, Billerica, MA, USA), and M2 macrophages were added to the lower chamber. After 24 h or 48 h of incubation, the cells remaining on the top layers of the inserts were removed by a cotton swab, while cells on the lower surface of the membrane were stained with H&E and counted in three HPFs under an inverted microscope (Nikon, Japan). Data are represented as the average of three counts ± SE.
Statistical analysis
All statistical analyses were performed using SPSS 13.0 or PRISM statistical software. The CD204+ cell counts, cell migration, cell invasion, WB and PCR analysis data were expressed as the mean ± SE. Statistical differences between the number of CD204+ cells were determined by 2-tailed paired Student’s t-test, independent sample t-test or one-way ANOVA. The relationship between COX2 and CD204 expression and clinicopathological characteristics was tested using the chi-square test. Survival curves were plotted using the Kaplan-Meier method and were compared by log-rank test. The significance of various survival-related variables was assessed by a Cox regression model in a multivariate analysis. A P value less than 0.05 (P<0.05) was considered statistically significant.
Results
Correlation between COX2 and CD204 expression and clinical variables
Based on the COX2 immunoreactivity scores, 131 (57.46%) of 228 GCs were considered COX2+. The COX2+ rate in patients with lymph node metastasis (68.29% or 84/123) was greater than that in patients without lymph node metastasis (44.76% or 47/105; P<0.001). The rate of COX2 positivity in patients with distant metastasis (96.55% or 28/29) was also greater than that in patients without distant metastasis (51.76% or 103/199; P<0.001). For GC patients with stages III and IV disease, 68.25% (86/126) expressed intermediate to high levels of COX2, whereas significantly fewer GC patients with stages I and II disease expressed COX2 (44.12% or 45/102; P<0.001). Patients with vascular invasion, high infiltrating depth (T grade) and lymphatic invasion had a significantly higher expression of COX2 (P<0.05, Table 1). No other variables, such as age, sex, tumor diameter, and degree of differentiation, were correlated with COX2 expression (P>0.05, Table 1).
In this cohort of patients (n=228), the number of CD204+ macrophage intratumor hotspot fields ranged from 0 to 89 per HPF, and the mean number was 39.30±18.58. The median number of CD204+ macrophages was 36.1 per HPF. The number of CD204+ macrophages in stage IV patients was 54.49±19.8, which was much higher than the values seen in patients with stage III (38.84±19.69), stage II (36.61±18.37) and stage I (35.22±14.08, P<0.05) disease, no significant difference was observed among patients with stage III, II and I disease (P>0.05). Other clinical variables, such as T grade, lymphatic metastasis (N grade), lymphatic invasion, Lauren classification, and TNM grade, were also included in the analysis of macrophage numbers between each group (Table 2).
Table 2.
Association between CD204+ cell number and clinicopathological factors
| Clinical parameters | CD204+ cell number/HPF (Mean ± SD) | t/χ2 or F Value | P |
|---|---|---|---|
| Gender | 2.229 | 0.027 | |
| Male | 37.58±18.10 | ||
| Female | 43.57±19.19 | ||
| Location | 0.006 | 0.994 | |
| Proximal | 38.99±20.09 | ||
| Middle | 39.33±18.54 | ||
| Distal | 39.42±18.22 | ||
| Size | 2.235 | 0.026 | |
| ≥5 cm | 42.54±19.32 | ||
| <5 cm | 37.00±17.75 | ||
| Histology type | 0.475 | 0.700 | |
| Papillary adenocarcinoma | 40.80±19.14 | ||
| Tubular adenocarcinoma | 40.20±19.16 | ||
| Mucinous adenocarcinoma | 35.78±21.42 | ||
| Signet-ring cell carcinoma | 37.51±16.25 | ||
| Lauren classification | 1.581 | 0.208 | |
| Diffuse type | 41.76±19.60 | ||
| Intestinal type | 37.32±17.54 | ||
| Mixed type | 38.88±19.84 | ||
| Differentiation | 1.021 | 0.362 | |
| Well | 32.33±7.86 | ||
| Moderately | 37.88±17.96 | ||
| Poorly | 40.38±19.22 | ||
| Invasion Depth (T Grade) | 7.786 | 0.000 | |
| T1 | 36.43±14.20 | ||
| T2 | 36.03±17.84 | ||
| T3 | 39.88±20.59 | ||
| T4 | 59.11±19.20 | ||
| Lymphatic Metastasis (N Grade) | 3.013 | 0.003 | |
| N0 | 35.47±14.62 | ||
| N1 | 42.59±20.89 | ||
| Distant Metastasis (M Grade) | 4.945 | 0.000 | |
| M0 | 37.10±17.37 | ||
| M1 | 54.49±19.81 | ||
| TNM Stages | 8.790 | 0.000 | |
| I | 35.22±14.08 | ||
| II | 36.61±18.38 | ||
| III | 38.84±19.69 | ||
| IV | 54.49±19.80 | ||
| Lymphatic invasion | 2.004 | 0.047 | |
| Yes | 42.11±21.14 | ||
| No | 37.04±15.94 | ||
| Vascular invasion | 1.892 | 0.060 | |
| No | 37.07±15.54 | ||
| Yes | 41.76±21.22 | ||
| COX2 Expression | 8.483 | 0.000 | |
| Positive | 46.95±17.77 | ||
| Negative | 28.99±14.18 |
All cases were classified according to the World Health Organization’s (2010) pathological classification of gastric cancer. Invasion Depth (T Grade) grade T1 includes T1a and T1b, and T4 includes T4a and T4b. Lymphatic Metastasis (N Grade) grade N3 includes N3a and N3b. TNM grade I includes Ia and Ib, TNM grade II includes IIa and IIb, and TNM grade III includes IIIa, IIIb and IIIc.
A higher number of TAMs was correlated with increased COX2 expression in GC tissue and poor prognosis
The IHC double staining results revealed conspicuous TAM (CD204+ cells) aggregations close to COX2-positive tumor nests (Figure 1A). The number of CD204+ macrophages in COX2+ patients was significantly higher than in COX2-negative patients (46.95±17.77 vs 28.99±14.18, P<0.001, Table 2).
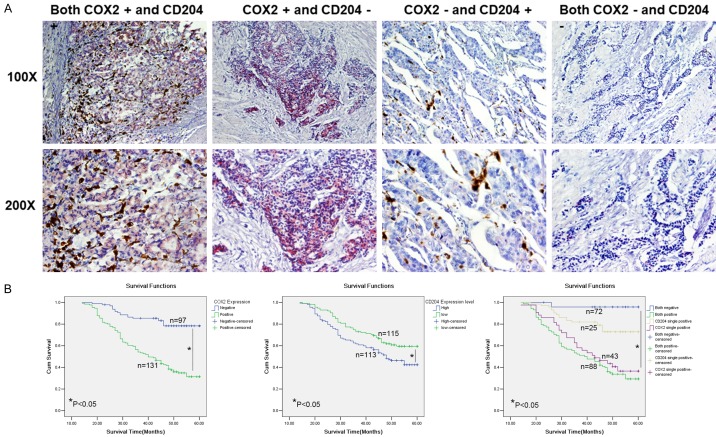
Figure 1.
COX2 and CD204 double stain (TAM infiltration) and the Kaplan-Meier survival curves of GC patients. A. Representative images of COX2 and CD204 double-positive, single COX2-positive, single CD204-positive and double-negative GC samples. High numbers of TAMs were located near COX2-expressing GC cell nests. Original magnification, ×100 and ×200. B. Kaplan-Meier survival curves of COX2 and CD204 expression in GC. The cumulative 5-year survival rate is shown.
In addition, the association between COX2 and CD204 expression level and the prognosis of GC was analyzed. In the present cohort of patients (n=228), the overall survival time was 46.68±1.04 months, and the 5-year survival time of COX2-positive patients was significantly shorter than that of COX2-negative patients (41.21±1.38 months vs 54.19±1.22 months, P<0.01). The 5-year survival rate of COX2-positive patients (31.4%) was significantly lower than that of COX2-negative patients (78.4%, P<0.05, Figure 1B). Patients with high CD204+ macrophage tumor infiltration had a poor prognosis (Figure 1B). The 5-year survival time of high CD204+ patients was 44.10±1.53 months, which was significantly shorter than that of low CD204+ patients (49.21±1.35 months, P<0.01). The 5-year survival rate of high CD204+ patients was 42.4%, which was significantly lower than that of low CD204+ patients (59.6%, P<0.05, Figure 1B). In addition, Spearman’s q-test showed a positive correlation between the levels of COX2 and CD204 (R=0.409, P<0.01). Kaplan-Meier analysis also indicated significantly worse survival in patients with both COX2 positivity and high CD204+ cell infiltration. Patients with both strong cytoplasmic COX2 intensity in cancer cells and high CD204+ cell infiltration in GC tumor nests exhibited the shortest mean survival time (40.25±1.70 months) compared with patients with single COX2-positive (43.23±2.23 month), single CD204-positive (52.77±1.53 months) or double-negative stained samples (58.58±1.38 months, P≤0.05; Figure 1B). A Cox multivariate analysis indicated that Lauren classification, invasive depth (T grade), and COX2 and CD204 expression were independent prognostic factors in this GC cohort (Table 3).
Table 3.
Multivariate analysis as determined by Cox regression analysis in 228 GC patients
| Clinicopathological Parameters | B | SE | Wald | P Value | Exp (B) | 95.0% CI for Exp (B) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower | Upper | ||||||
| Lauren classification | 0.743 | 0.235 | 9.959 | 0.002 | 2.102 | 1.325 | 3.336 |
| Invasive depth (T grade) | 0.591 | 0.204 | 8.439 | 0.004 | 1.806 | 1.212 | 2.691 |
| COX2 expression | 2.569 | 0.770 | 11.124 | 0.001 | 13.054 | 2.885 | 59.081 |
| CD204 expression | 1.083 | 0.530 | 4.175 | 0.041 | 2.952 | 1.045 | 8.340 |
M2 macrophages induced COX2 expression in GC cells
THP-1 cells are widely used as models for macrophage differentiation. When treated with PMA for 24 h, THP-1 cells quickly ceased to proliferate, at which point they attached and differentiated into macrophages (Figure 2A). The macrophages could change their functional profiles (from M1 to M2 or from M2 to M1) repeatedly, which depended on the cytokines (Th1 or Th2) to which they were exposed. CD204 and CD206 are already regarded as specific markers of M2 macrophages. In our recent study, we found that exposure of PMA-treated THP-1 macrophages to IL-13 and rosiglitazone led to a significant induction in CD204 and CD206 mRNA expression, and an M2-polarized THP-1 macrophage cytokine secretion profile (low TNF-α and high TGF-β) was seen in THP-1 macrophages. Considering these cytokine profiles and surface markers, we successfully generated an M2 response in THP-1 macrophages (Figure 2B).
Figure 2.
Induced THP-1 macrophages showed the typical functional phenotype of M2-polarized macrophages. A. Images of THP-1 cells and M2-polarized macrophages. B. THP-1 cells treated with PMA showed significant induction of CD206 and CD204 expression (both markers of M2 macrophages). An ELISA also showed that induced macrophages possessed M2-type macrophage-like secretory functions, including significantly lower levels of TNF-α and higher levels of TGF-β, than those seen with coculture with THP-1 cells (P<0.05).
We then cocultured PMA-treated and M2-polarized THP-1 macrophages with GC cells in a noncontact system. After coculture with M2-polarized macrophages, the COX2 expression level was significantly increased at both the protein and mRNA levels in GC cells (Figure 3A and 3B).
Figure 3.
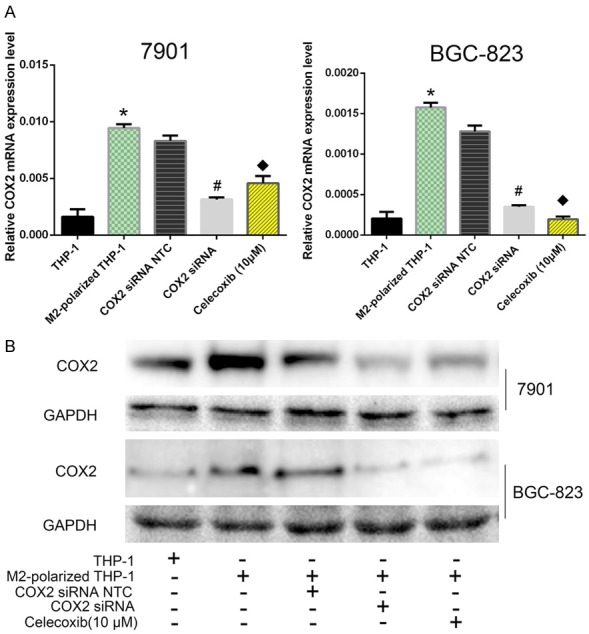
M2-polarized macrophages induced COX2 expression and COX2-dependent MMP9 expression in GC cells. A. After pretreatment with or without COX2 siRNA or Celecoxib, GC cells were cocultured with THP-1 macrophages and M2-polarized THP-1 macrophages. Real-time quantitative PCR revealed that COX2 mRNA was significantly upregulated in GC cells and that this result could be abrogated by pretreatment with COX2 siRNA or Celecoxib. Both P<0.05. *, Coculture with M2-polarized THP-1 macrophages vs coculture with THP-1 cells; #, GC cells pretreated with COX2 siRNA then cocultured with M2-polarized macrophages vs GC cells pretreated with COX2 NTC siRNA then cocultured with M2-polarized macrophages; &, GC cells pretreated with a COX2 inhibitor (Celecoxib) then cocultured with M2-polarized macrophages vs GC cells cocultured with M2-polarized macrophages. B. Western blot results of COX2 and MMP9 expression changes in GC cells after coculture with THP-1 or M2-polarized macrophages.
M2-polarized THP-1 macrophages induced COX2-dependent migration and invasiveness of GC cells
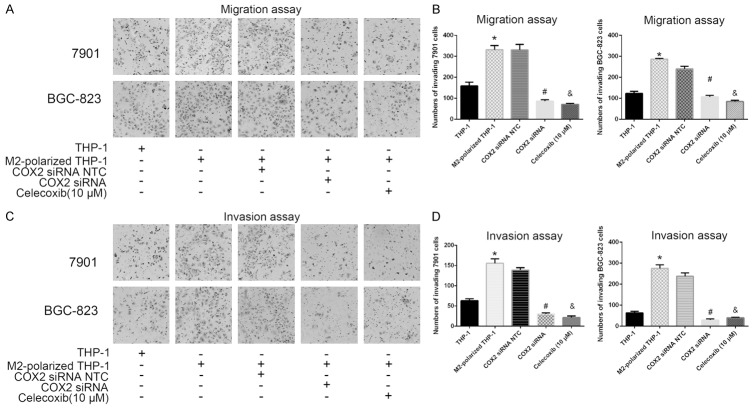
For invasion and migration assays, GC cells (in 24-well plates) were seeded into Transwell chambers (24 wells; 8 μm pore size) with or without Matrigel. After 24 or 48 h, GC cells were fixed, stained with hematoxylin, and counted to determine the number of invading cells. GC cells that were cocultured with PMA-treated M2-polarized THP-1 macrophages showed an increased number of invading cells in both the invasion and migration assays (both P<0.05; Figure 4). However, when GC cells were transiently transfected with COX2 siRNA or pretreated with Celecoxib (10 μM) for 24 h before they were cocultured with macrophages, these changes were abrogated (all P<0.05; Figure 4). These results suggest that M2 macrophages induce COX2-dependent invasion and migration in human GC cells.
Figure 4.
M2-polarized THP-1 macrophages induced COX2-dependent invasiveness of human GC. A. Transwell migration assays showed that coculture of THP-1 macrophages or M2-polarized THP-1 macrophages with GC cells led to a significant increase in the number of invading cells. These increases could be abrogated by transient transfection with COX2 siRNA or treatment with Celecoxib (10 μM). B. Data are expressed as the mean number of invading cells per field (average of 5 fields per filter) of the migration assay. C. Transwell invasion assays showed that the coculture of THP-1 macrophages or M2-polarized THP-1 macrophages with GC cells led to a significant increase in the number of invading cells. The increments could be abrogated by transient transfection with COX2 siRNA or treatment with Celecoxib (10 μM). D. Data are expressed as the mean number of invading cells per field (average of 5 fields per filter) of the invasion assay. Both P<0.05. *, Coculture with M2-polarized THP-1 macrophages vs coculture with THP-1 cells; #, GC cells pretreated with COX2 siRNA then cocultured with M2-polarized macrophages vs GC cells pretreated with COX2 NTC siRNA then cocultured with M2-polarized macrophages; &, GC cells pretreated with a COX2 inhibitor (Celecoxib) then cocultured with M2-polarized macrophages vs the GC cells cocultured with M2-polarized macrophages.
M2-polarized THP-1 macrophages promoted the migration and invasiveness of GC cells in a COX2/MMP9-dependent manner
Degradation of the extracellular matrix by matrix metalloproteinases (MMPs) is important in cancer invasion, and the same phenomenon exists in GC [17-19]. MMP9 is an important member of the MMP family and is considered a downstream molecule of the COX2 pathway, which promotes cancer progression [20]. In our previous study [14], we reported that after coculture with M2-polarized THP-1 macrophages, the expression of MMP9 in GC cells was significantly upregulated and that the number of invading cells was also significantly increased in the migration and invasion Transwell assays (data presented in our previous study) [14]. However, when MMP9 activity was blocked by specific inhibitors, the number of invading cells induced by M2-polarized THP-1 macrophages was obviously decreased (data presented in our previous study) [14]. In addition to the results of our previous study, here, we found that blocking COX2 expression in GC cells before coculture with M2-polarized THP-1 cells negated the promoting effect of MMP9 expression (Figure 3B). Taken together, these two studies suggest that M2-polarized macrophages might induce COX2-MMP9 axis expression in GC cells and subsequently promote the invasiveness of GC cells.
Discussion
Macrophages are the key cells involved in chronic inflammation, and they can be phenotypically polarized by the microenvironment to participate in specific functional programs [7]. In the tumor microenvironment, these macrophages are defined as TAMs, which exhibit M2 characteristics and promote angiogenic activity, tumor growth and metastasis [7,12,21]. TAMs demonstrate several protumor functions, including secretion of growth factors and matrix proteases, promotion of angiogenesis and suppression of adaptive immunity [22,23]. It has been reported that TAM infiltration into tumor tissue correlates significantly with tumor vascularity in gastric cancers [24]. Ishigami et al. [25] reported a direct association between the degree of TAM infiltration and the depth of tumor invasion, lymph node status and clinical stage in GC, but the potential mechanism of these correlations is still not clear. Thus, understanding the underlying mechanism of crosstalk between TAMs and cancer cells has become a hot topic in cancer research. Our current IHC results indicate that high CD204+ (M2 characteristic) macrophage infiltration in GC tumor tissue is associated with aggressive stage and poor prognosis. At the same time, coculture with M2-polarized THP-1 macrophages (CD204+) could promote the invasive ability of GC cells in vitro. These data suggest that M2 macrophages play a promoting role in the progression of gastric cancer. However, the underlying mechanism remains unclear.
Many studies have demonstrated that COX2 is one of the enzymes involved in cancer inflammation and that it catalyzes the conversion of arachidonic acid to prostaglandins, including prostaglandin E2 (PGE2), which in turn can enhance the metastatic phenotype of tumors [26]. The overexpression of COX2 in solid malignancies, including colon [27], prostate [28], breast [29], pancreas [30], non-small-cell lung [31], and bladder [32] cancers, has been reported to be significantly related to tumor invasion and metastasis and patient survival. Numerous studies have indicated that COX2 expression may contribute to an increase in cyclooxygenase activity and the synthesis of proteinoids, which have been associated with carcinogenesis and colon tumor progression [33]. Overexpression of COX2 protein was shown to be significantly associated with lymph node metastasis and depth of invasion and was reported to be an important prognostic parameter in gastric cancer [34]. However, the relationship between COX2 and TAMs has not yet been reported in GC. Based on the double staining IHC assay, we found that CD204+ macrophages aggregated near COX2-expressing cancer nests in human GC and that high M2-macrophage (CD204+) infiltration was positively associated with high COX2 expression in GC nests. Both COX2 expression and high CD204+ macrophage infiltration are correlated with the depth of invasion in human GC, and similar spatial adjacencies have been reported in prostate cancer and hepatocellular carcinoma [35,36]. In addition, we also found that patients with both high CD204+ macrophage infiltration and high COX2 expression in tumors had the poorest prognosis compared with patients with single-positive or double-negative tumors. Moreover, a Cox multivariate analysis indicated that COX2 and CD204 expression levels were independent prognostic factors in this GC cohort. Thus, we hypothesize that CD204+ TAMs (M2-polarized) might activate COX2 in GC cells and subsequently increase their invasive ability. To verify this hypothesis, we used human THP-1 cells as a model to generate an in vitro M2-polarized macrophage model and then indirectly cocultured these macrophages with GC cells in a Transwell system. As expected, M2-polarized macrophages increased GC cell invasion and induced COX2 expression. Furthermore, this promoting effect could be abrogated by blocking COX2 expression through pretreatment of GC cells with COX2 siRNA or Celecoxib. Controlled remodeling of the extracellular matrix (ECM) is essential for the growth, invasion, and metastasis of malignant tumors. Matrix metalloproteinases (MMPs) are a family of secreted, zinc-dependent endopeptidases collectively capable of degrading ECM components, and a considerable amount of evidence has shown that they play an important role in different steps of malignant tumor growth [37,38]. MMP9, an important gelatinase in the MMP family, is thought to contribute to the pathogenesis and progression of many cancer types. MMP9 has also been reported as one of the downstream target molecules of the COX2 gene, and our previous study also verified that after coculture with M2-macrophages, the expression of MMP9 was increased; we also showed that blocking MMP9 expression decreased the invasion-promoting effect of M2 macrophages [14]. This implies that M2 macrophages can induce gelatinase activity in GC cells. Based on our previous study, here, we further discovered that pre-inhibition of COX2 expression in GC cells could block the promoting effect of macrophages on MMP9 expression. Taken together, these findings suggest that GC cells are responsible for the increased invasiveness induced by macrophages in a COX2/MMP9-dependent manner.
Conclusion
In summary, we found that TAMs increased the invasiveness of human GC. M2-polarized macrophages could induce GC cells to release MMP9 to support tumor cell invasion in a COX2-dependent manner. The targeting of TAMs as a therapeutic approach has been investigated and discussed as a new strategy for cancer therapy [39-41]. Targeting TAMs or polarizing TAMs by immune modulation may soon be a nonsurgical method used to treat human GC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81502090), the Science and Technology Plan of Zhejiang Province (2017F30045, 2017C33130), the Zhejiang Provincial Natural Science Foundation (LY14H160039, LY18H160043, Y18H030007, Y18H160037, 2013RCA002) and the Medicine and Health Research Foundation of Zhejiang Province (2013KYB022 and 2020357780).
Disclosure of conflict of interest
None.
References
- 1.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strong VE, Wu AW, Selby LV, Gonen M, Hsu M, Song KY, Park CH, Coit DG, Ji JF, Brennan MF. Differences in gastric cancer survival between the U.S. and China. J Surg Oncol. 2015;112:31–37. doi: 10.1002/jso.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantano F, Berti P, Guida FM, Perrone G, Vincenzi B, Amato MM, Righi D, Dell’aquila E, Graziano F, Catalano V, Caricato M, Rizzo S, Muda AO, Russo A, Tonini G, Santini D. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. J Cell Mol Med. 2013;17:1415–1421. doi: 10.1111/jcmm.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, Di W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 2013;332:3–10. doi: 10.1016/j.canlet.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Jackute J, Zemaitis M, Pranys D, Sitkauskiene B, Miliauskas S, Vaitkiene S, Sakalauskas R. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018;19:3. doi: 10.1186/s12865-018-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma YY, He XJ, Wang HJ, Xia YJ, Wang SL, Ye ZY, Tao HQ. Interaction of coagulation factors and tumor-associated macrophages mediates migration and invasion of gastric cancer. Cancer Sci. 2011;102:336–342. doi: 10.1111/j.1349-7006.2010.01795.x. [DOI] [PubMed] [Google Scholar]
- 15.He XJ, Tao HQ, Hu ZM, Ma YY, Xu J, Wang HJ, Xia YJ, Li L, Fei BY, Li YQ, Chen JZ. Expression of galectin-1 in carcinoma-associated fibroblasts promotes gastric cancer cell invasion through upregulation of integrin beta1. Cancer Sci. 2014;105:1402–1410. doi: 10.1111/cas.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He XJ, Jiang XT, Ma YY, Xia YJ, Wang HJ, Guan TP, Shao QS, Tao HQ. REG4 contributes to the invasiveness of pancreatic cancer by upregulating MMP-7 and MMP-9. Cancer Sci. 2012;103:2082–2091. doi: 10.1111/cas.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukaszewicz-Zajac M, Mroczko B, Szmitkowski M. Gastric cancer-the role of matrix metalloproteinases in tumor progression. Clin Chim Acta. 2011;412:1725–1730. doi: 10.1016/j.cca.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Shay G, Lynch CC, Fingleton B. Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44-46:200–206. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bu X, Zhao C, Dai X. Involvement of COX-2/PGE(2) pathway in the upregulation of MMP-9 expression in pancreatic cancer. Gastroenterol Res Pract. 2011;2011:214269. doi: 10.1155/2011/214269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inflammation and cancer. New York: Springer; 2014. [Google Scholar]
- 23.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015;18:740–750. doi: 10.1007/s10120-014-0422-7. [DOI] [PubMed] [Google Scholar]
- 25.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, Matsumoto M, Miyazono F, Hokita S, Aikou T. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23:4079–4083. [PubMed] [Google Scholar]
- 26.Davila-Gonzalez D, Chang JC, Billiar TR. NO and COX2: dual targeting for aggressive cancers. Proc Natl Acad Sci U S A. 2017;114:13591–13593. doi: 10.1073/pnas.1717440114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu H, Han T, Zhuo M, Wu LL, Yuan C, Wu L, Lei W, Jiao F, Wang LW. Elevated COX-2 expression promotes angiogenesis through EGFR/p38-MAPK/Sp1-dependent signalling in pancreatic cancer. Sci Rep. 2017;7:470. doi: 10.1038/s41598-017-00288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattsson JS, Bergman B, Grinberg M, Edlund K, Marincevic M, Jirstrom K, Ponten F, Hengstler JG, Rahnenfuhrer J, Karlsson MG, Karlsson C, Helenius G, Botling J, Micke P, Gulyas M. Prognostic impact of COX-2 in non-small cell lung cancer: a comprehensive compartment-specific evaluation of tumor and stromal cell expression. Cancer Lett. 2015;356:837–845. doi: 10.1016/j.canlet.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 32.Wulfing C, Eltze E, von Struensee D, Wulfing P, Hertle L, Piechota H. Cyclooxygenase-2 expression in bladder cancer: correlation with poor outcome after chemotherapy. Eur Urol. 2004;45:46–52. doi: 10.1016/j.eururo.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004;23:63–75. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- 34.Thiel A, Mrena J, Ristimaki A. Cyclooxygenase-2 and gastric cancer. Cancer Metastasis Rev. 2011;30:387–395. doi: 10.1007/s10555-011-9312-1. [DOI] [PubMed] [Google Scholar]
- 35.Cervello M, Foderaa D, Florena AM, Soresi M, Tripodo C, D’Alessandro N, Montalto G. Correlation between expression of cyclooxygenase-2 and the presence of inflammatory cells in human primary hepatocellular carcinoma: possible role in tumor promotion and angiogenesis. World J Gastroenterol. 2005;11:4638–4643. doi: 10.3748/wjg.v11.i30.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Bergh A, Damber JE. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res. 2005;11:3250–3256. doi: 10.1158/1078-0432.CCR-04-2405. [DOI] [PubMed] [Google Scholar]
- 37.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 38.Kessenbrock K, Wang CY, Werb Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biol. 2015;44-46:184–190. doi: 10.1016/j.matbio.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mills CD, Lenz LL, Harris RA. A breakthrough: macrophage-directed cancer immunotherapy. Cancer Res. 2016;76:513–516. doi: 10.1158/0008-5472.CAN-15-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]