Abstract
Intervertebral disc degeneration (IDD) induces serious back, neck and radicular pain. Recently, moxibustion has been suggested as an effective treatment for IDD. Thus, our study aims to investigate the molecular mechanism of moxibustion in IDD. A rat model of IDD was established by moxibustion treatment. Nucleus pulposus (NP) cells isolated from IDD rats or IDD rats treated with moxibustion were transfected with plasmids harboring overexpressed hypoxia-inducible factor-1 alpha (HIF-1α) to understand the role of treatment on cell autophagy and apoptosis. To investigate the mechanism of moxibustion in IDD, aggrecan, cyclo-oxygenase 2 (COX-2), HIF-1α and vascular endothelial growth factor (VEGF) expression in NP cells was measured. The expression of aggrecan and COX-2 was elevated by moxibustion treatment. Moxibustion induced autophagy and suppressed apoptosis of NP cells from IDD rats. Compared with IDD rats, the expression of light chain 3 (LC3) II/I, Beclin-1, B-cell lymphoma-2 (Bcl-2) and HIF-1α was regulated significantly after moxibustion treatment, while the expression of cleaved-caspase-3, Bcl-2 associated protein X and VEGF was downregulated. In general, moxibustion may be beneficial to IDD by enhancing autophagy and reducing apoptosis of NP cells via the HIF-1α/VEGF pathway.
Keywords: Moxibustion, intervertebral disc degeneration, nucleus pulposus cells, apoptosis, autophagy
Introduction
Intervertebral disc degeneration (IDD) is a chronic age-related process in the intervertebral disc characterized by a decrease in the protein polysaccharide and water contents in the nucleus pulposus (NP), leading to the inability of the disc to resist compression loads. The intervertebral disc is a basic component of the spine, which is connected to the adjacent vertebral space that transfers and absorbs mechanical loads on the spine [1]. IDD is caused by complex and multifactorial processes due to mechanical stress, cell aging, and nutritional deficiencies [2,3]. Therefore, IDD is one of the main causes of motor deficiency and low back pain [4,5]. Because the molecular mechanisms of IDD remain unclear in current clinical therapies, the clinical treatment mainly aims to relieve the symptom instead of targeting IDD directly. IDD is globally prevalent and therefore a huge economic burden to our society [6], highlighting the urgency of developing novel treatment modalities against IDD.
Moxibustion, a traditional Chinese medical intervention, is a direct or indirect application of Artemisia argyi at acupoints or other specific parts to prevent diseases [7]. Moxibustion has been used for treating IDD with good outcomes. Recently, a study has noted that warm needle moxibustion stimulates the sensation of meridian-collateral transmission, so it exerts a better curative effect on IDD at lumbar intervertebral discs [8]. Moreover, moxibustion has protective effects on myocardial ischemia-reperfusion injury by decreasing the expression of hypoxia-inducible factor-1 alpha (HIF-1α), B-cell lymphoma-2 (Bcl-2), and caspase-3 in rat myocardial cells [9]. The beneficial effects of moxibustion may be achieved by alleviating hypoxia-mediated apoptosis [10]. The NP is an avascular tissue that takes nutrition diffused from the endplates; therefore, cells in the NP are physiologically hypoxic [11,12]. Changes in oxygen levels can activate or suppress various homeostatic genes for survival and accommodation of cells as part of the cellular adaptation. HIF-1, one of the key genes in this cellular adaption process, can be activated in hypoxic conditions and acclimatize tissue functions to reduce oxygen levels by mediating transcription factors and enzymes [13]. Therefore, we determined whether HIF-1α was associated with a beneficial effect of moxibustion on IDD.
Materials and methods
Ethical statement
Animal use and experimental procedures were approved by the Institutional Experimental Animal Ethics Committee of Dongying People’s Hospital (No. 201707003). Experiments were performed in compliance with the Guidelines on the Humane Treatment of Laboratory Animals established by the Ministry of Science and Technology of the People’s Republic of China (Policy No. 2006 398).
Model establishment of IDD in rat caudal spine
IDD was induced by needle puncture in the caudal spine of Sprague-Dawley (SD) rats, as described previously [14]. In brief, 42 healthy male SD rats (weighing 280-320 g) were divided into the sham group (n = 14) or IDD group (n = 28) after being acclimated for one week. The IDD model was established by the central puncture method. After pentobarbital anesthesia (30 mg/kg, intraperitoneal injection), a portable X-ray machine was used to measure the intervertebral disc (IVD) level between the 6th and 7th caudal vertebra (Co6-7 level) and center of the IVD. A 21-gauge needle was inserted into the center of the NP through the annulus fibrosus at the Co6-7 level with rotation of 180° in 5 s. The rats in each group were intraperitoneally injected with 200,000 U/kg penicillin sodium salt (800,000 U/branch, Huabei Pharmaceutical Co., Ltd., Wuhan, China) once a day for 3 consecutive days to prevent infection. Tissues from the intervertebral disc in the IDD and sham groups were stained with hematoxylin and eosin (HE), Safranin O-fast green and Sirius red after 8 weeks of standard diet feeding in single cages at 23-25°C.
Animal grouping and administration
The IDD group was further divided into 2 groups: no treatment group (n = 14) or IDD + moxibustion group (n = 14, rats received moxibustion with moxa sticks for 20 min once a day for 10 days as 1 course of treatment and continued for 3 courses). The sham group (n = 14) received a skin incision and then wound closure. After treatment, all rats were euthanized by CO2 inhalation. The intervertebral disc tissues were collected and stored for subsequent experiments.
HE staining
After fixation with 10% neutral formaldehyde solution (pH 7.0) for 24 h, the rats’ intervertebral disc tissues were processed with standard gradient alcohol dehydration, xylene clearing, wax immersion and paraffin embedding. Tissues were sliced continuously (5 μm) and placed at 80°C for 1 h. After cooling down, the tissues were dehydrated by standard gradient alcohol and cleared with xylene. Next, the tissues were stained with hematoxylin (H8070-5 g, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 4 min, differentiated by alcohol hydrochloride for 10 s, blued by ammonia for 10 min, and then stained with eosin (PT001, Shanghai Bogu Biological Technology Co., Ltd., Shanghai, China) for 2 min, followed by gradient alcohol dehydration, clearing and mounting. The pathological changes were observed under an optical microscope (DMM-300D, Shanghai Caikang Optical Instrument Co., Ltd., Shanghai, China).
Immunohistochemistry
The tissue slices were blocked with normal goat serum blocking solution (C-0005, Shanghai Haoran Biotechnology Co., Ltd., Shanghai, China) for 20 min, followed by the addition of primary anti-rabbit polyclonal antibodies for aggrecan (ab26861, 1:200), cyclo-oxygenase 2 (COX-2) (ab15191, 1:500), HIF-1α (ab51608, 1:100) and vascular endothelial growth factor (VEGF) (ab2349, 1:100) overnight at 4°C. Then, slices were treated with goat anti-rabbit immunoglobulin G (ab6721, 1:1000) at 37°C for 20 min and horseradish peroxidase-labeled streptavidin (0343-10000U, Yimo Biotechnology Co., Ltd., Beijing, China) at 37°C for 20 min. Antibodies were all from Abcam Inc. (Cambridge, MA, USA). Then, slices were developed by diaminobenzidine (DAB) (ST033, Guangzhou Whiga Technology Co., Ltd., Guangzhou, China), stained with hematoxylin (PT001, Shanghai Bogu Biological Technology Co., Ltd., Shanghai, China) and returned to blue in color by 1% ammonia, dehydrated, cleared and mounted. Images were obtained under a microscope.
Isolation and identification of NP cells
The isolation and identification of NP cells were performed according to previous studies [15-17]. Briefly, the intervertebral disc tissues were sliced (1 mm), detached with 0.1% collagenase type II at 37°C for 4 h, resuspended and seeded in 6-cm culture dishes in 5% CO2 at 37°C in Dulbecco’s modified Eagle’s medium (DMEM)-F12 containing 20% fetal bovine serum (FBS). The medium was replaced every 48 h with DMEM-F12 medium containing 10% FBS. The cell morphology was identified under a high power inverted microscope. After approximately 15 days, the cells were detached with trypsin and passaged (1:4). The expression of HIF-1α, HIF-1β, collagen I, collagen II and matrix metalloproteinase-2 (MMP-2) in NP cells was identified by immunocytochemical staining [18-20]. The cells were fixed in 4% polyformaldehyde, treated with 0.5% Triton X-100 for 15 min and incubated with 3% H2O2 for 15 min at room temperature. Then, 0.01 mol/L sodium citrate was applied for repair at 95°C for 15 min. The cells were blocked with 5% goat serum at 37°C for 30 min and incubated with primary antibodies (Abcam Inc., Cambridge, MA, USA) to HIF-1α (ab51608), HIF-1β (ab2771), collagen I (ab90395), collagen II (ab185430) and MMP-2 (ab37150) at 4°C overnight. Then, biotin-labeled secondary antibody was added and incubated with cells at room temperature for 30 min, followed by color visualization using DAB and counterstaining by hematoxylin. Microscopic observation was performed after cells were sealed with neutral gum.
Cell treatment
NP cells were isolated from IDD rats that received 30-min moxibustion treatment or not before isolation. NP cells were detached with trypsin, counted, seeded in 6-well plates, diluted with DMEM, and transfected with blank plasmid or HIF-1α overexpression plasmid for 24 h using Lipofectamine 2000 (Invitrogen, NY, CA, USA). Next, the cells were mixed, rested and incubated in 5% CO2 at 37°C. Cells were embedded with a mixture of Epon8l2 and acetone (3:1) and sliced using an ultramicrotome (Leica, Wetzler, Germany), and ultrastructural changes in the cells were observed under an H-600IV transmission electron microscope (Hitachi, Japan).
Terminal deoxynucleotidyl transferase (TdT)-mediated 2’-deoxyuridine 5’-triphosphate (dUTP) nick end labeling (TUNEL) staining
Forty-eight hours after transfection, cells were fixed with acetone for 15 min and incubated for 5 min in osmotic solution containing 0.2% Triton X-100 and 0.1% sodium citrate. The TUNEL reaction mixture (11684817910, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was added for 30 min in a wet box at 37°C, followed by the addition of the TUNEL-peroxidase converter (AP005, Shanghai 7sea Biotechnology Co., Ltd., Shanghai, China) and incubation in a wet box at 37°C for 30 min. Next, cells were incubated with DAB solution for 5-10 min, stained with hematoxylin (PT001, Shanghai Bogu Biological Technology Co., Ltd., Shanghai, China), differentiated by hydrochloric acid alcohol, returned to blue in color by tap water, dehydrated by alcohol, cleared by xylene, mounted, and analyzed under a fluorescence microscope.
RNA isolation and quantitation
A TRIzol kit (Invitrogen, New York, CA, USA) was used to extract total RNA based on the manufacturer’s instructions. RNA was then reverse transcribed into cDNA using a PrimeScriptTM reverse transcription (RT) reagent kit with a gDNA Eraser kit (TaKaRa Bio Inc., Shiga, Japan). Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was conducted in an ABI7500 PCR system (Applied Biosystems) using the SYBR Premix Ex TaqTM kit (TaKaRa, Japan). β-actin served as an internal reference. Primers were designed and synthesized by TaKaRa Biotechnology Co., Ltd., (Liaoning, China) (Table 1). The fold changes were calculated by relative quantification (2-ΔΔCt method) [21].
Table 1.
Primer sequences for RT-qPCR
| Genes | Primer sequences |
|---|---|
| HIF-1α | F: 5’-TCAAGTCAGCAACGTGGAAG-3’ |
| R: 5’-TATCGAGGCTGTGTCGACTG-3’ | |
| VEGF | F: 5’-CAATGATGAAGCCCTGGAGT-3’ |
| R: 5’-TTTCTTGCGCTTTCGTTTTT-3’ | |
| β-actin | F: 5’-CCGTGAAAAGATGGACCCAGAT-3’ |
| R: 5’-GGACAGTGAGGCCAGGATAGA-3’ |
Note: RT-qPCR, reverse transcription quantatitive polymerase chain reaction; HIF-1α, hypoxia-inducible factor-1 alpha; VEGF, vascular endothelial growth factor; F, forward; R, reverse.
Western blot analysis
Cells were lysed, and total protein was extracted and quantified with a bicinchoninic acid kit (Thermo Fisher Scientific, Waltham, MA, USA). Next, a total of 30 μg of protein was separated by polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Amersham, Piscataway, NJ, USA). The membranes were blocked in 5% skim milk for 1 h and then incubated with primary antibodies: VEGF (NB100-664, 1:1000, Bio-Techne Corporation, Minneapolis, MN, USA), HIF-1α (ab113642, 1:2000, rat antibody), light chain protein 3B (LC3B) (ab51520, 1:1000, rabbit antibody), Beclin-1 (ab207612, 1:2000, rabbit antibody), cleaved-caspase-3 (ab2302, 1:1000, rabbit antibody), Bcl-2 (ab32124, 1:1000, rabbit antibody), Bcl-2 associated protein X (Bax) (ab32503, 1:5000, rabbit antibody), and GAPDH (ab8245, 1:1000, rat antibody). The next day, the membranes were washed 3 times with Tris-buffered saline Tween 20 and incubated with secondary goat anti-rabbit (ab6721, 1:2000) or goat anti-rat (ab6785, 1:10,000) antibodies accordingly at 37°C for 1 h. All antibodies except for VEGF were purchased from Abcam Inc. (Cambridge, MA, USA). Enhanced chemiluminescence reaction solution (GK342011, Gene Tech Co., Ltd., Shanghai, China) was used for development and observation. Relative protein levels were identified using Image Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
SPSS 21.0 (IBM Corp. Armonk, NY, USA) was used for data analysis and statistics. Normal distribution was tested for all data. Data are expressed as the mean ± standard deviation, with equality of variances tested. Comparisons among multiple groups were analyzed using one-way analysis of variance (ANOVA), with Tukey’s post hoc test used for pairwise comparison between two groups. A value of P < 0.05 was considered statistically significant.
Results
Establishment of the IDD model
As shown in the HE staining in Figure 1, the lumbar intervertebral space was wide, the arrangement of fibrous rings was regular, and the number of NP cells and interstitial cells did not decrease significantly in the sham-operated rats. In contrast, IDD rats had more hyperplastic cartilage lacunae in the inferior lamina of the endplate, suggesting that the IDD animal model was successfully established.
Figure 1.
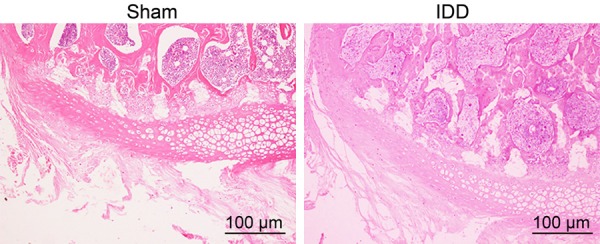
Representative images showing the lumbar intervertebral space after HE staining in sham-operated and IDD rats (× 100).
Moxibustion treatment alleviates IDD
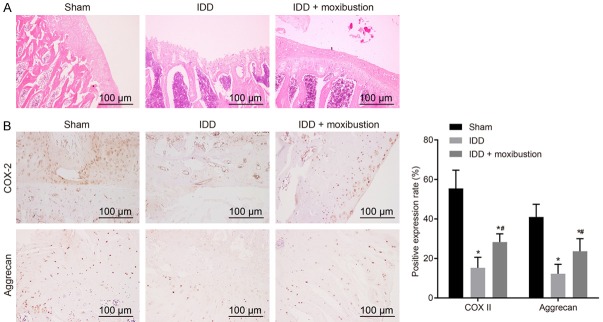
HE staining was then used to investigate the role of moxibustion in IDD. After treatment with moxibustion, the fibrous rings were arranged in an orderly fashion in IDD rats, with increment in the NP cells and extracellular matrix (Figure 2A). Furthermore, the expression of aggrecan and COX-2 in intervertebral disc tissues was also determined by immunohistochemistry (Figure 2B). The results showed that the expression of aggrecan and COX-2 significantly decreased in IDD rats when compared with that in the sham-operated rats, while that of IDD rats treated with moxibustion increased significantly (P < 0.05). These results showed that moxibustion treatment was beneficial to IDD in rats.
Figure 2.
Moxibustion treatment relieves degeneration of intervertebral discs. A. Micrographs showing histopathological changes in intervertebral discs after HE staining in IDD rats after moxibustion treatment (scale bar = 100 µm). B. Micrographs showing immunohistochemistry of aggrecan and COX-2 in intervertebral discs of IDD rats after moxibustion treatment. *P < 0.05 vs. sham-operated rats; #P < 0.05 vs. IDD rats. The measurement data were expressed as the mean ± standard error. Comparisons among multiple groups were analyzed using one-way ANOVA. Three parallel experiments were repeated; n = 14.
Identification of NP cells from rats
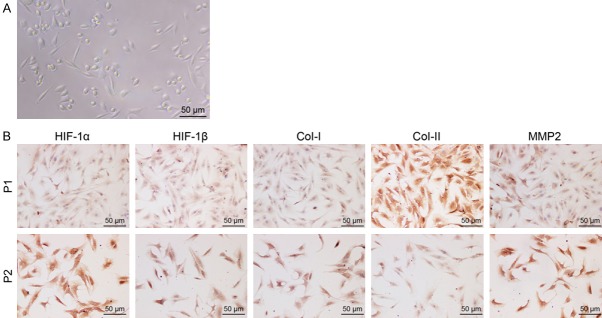
The morphology of isolated rat NP cells was observed by microscope. NP cells were spindle-shaped or angular in shape under high-power microscope, with long cytoplasmic protuberances (Figure 3A). The results of immunocytochemical staining revealed that NP cells expressed HIF-1α, HIF-1β, collagen I, collagen II and MMP-2 and that the expression of HIF-1α, HIF-1β, collagen I and MMP-2 increased while collagen II decreased with cell passages (Figure 3B). These findings confirmed the successful isolation of NP cells from rats.
Figure 3.
Micrographs identifying rat NP cells (× 200; scale bar = 50 µm). A. The rat NP cells observed under an inverted microscope. B. The expression of HIF-1α, HIF-1β, collagen I, collagen II and MMP-2 identified by immunocytochemical staining.
Moxibustion induces autophagy and suppresses apoptosis of rat NP cells
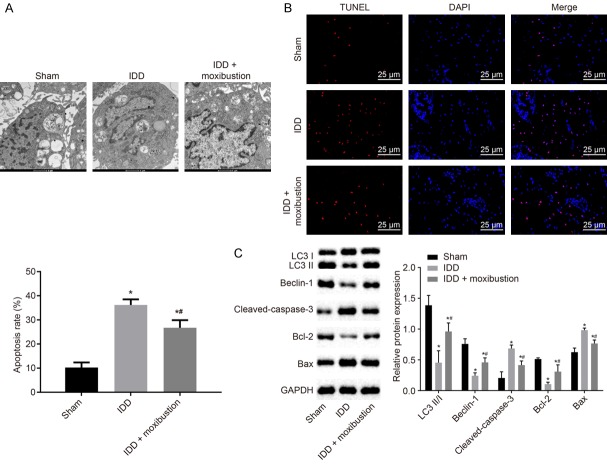
Autophagy levels were determined by transmission electron microscope in isolated NP cells. Autophages or autophagic vesicles, which were presented as bilayer membrane vesicles containing fragments of damaged organs or polymeric proteins, were detected in the cytoplasm of NP cells. IDD rats had lower levels of autophagy than the levels in sham-operated rats (Figure 4A). TUNEL staining further revealed that the IDD rats had increased apoptosis of NP cells, while that was reduced by moxibustion treatment in IDD rats (Figure 4B).
Figure 4.
Treatment with moxibustion restrains apoptosis and induces autophagy in NP cells. A. Micrographs showing autophagy of NP cells observed under transmission electron microscope. B. Apoptosis of NP cells detected by TUNEL staining (scale bar = 25 µm). C. The protein expression of autophagy and apoptosis-related genes LC3 II/I, Beclin-1, cleaved-caspase-3, Bcl-2 and Bax normalized to GAPDH in NP cells measured using western blot analysis. *P < 0.05 vs. sham-operated rats; #P < 0.05 vs. IDD rats. The measurement data were expressed as the mean ± standard error. Comparisons among multiple groups were analyzed using one-way ANOVA (Tukey’s post hoc test). Three parallel experiments were repeated.
To further validate the above results, the expression of autophagy and apoptosis-related genes was identified using western blot analysis (Figure 4C). Compared with the NP cells from sham-operated rats, the expression of LC3 II/I, Beclin-1 and Bcl-2 was downregulated in the NP cells from IDD rats (P < 0.05). However, cleaved-caspase-3 and Bax were upregulated in the NP cells from IDD rats when compared with expression in the NP cells from sham-operated rats (P < 0.05). After treatment with moxibustion, the expression of LC3 II/I, Beclin-1 and Bcl-2 increased significantly in IDD rats, and cleaved-caspase-3 and Bax expression was reduced in NP cells (P < 0.05). Thus, moxibustion may improve the degeneration of the intervertebral disc by inducing autophagy and reducing apoptosis of NP cells.
Moxibustion elevates the expression of HIF-1α but reduces that of VEGF in NP cells of rats with IDD
Previous studies have shown that HIF-1α is involved in IDD [16,17]. To further verify this, immunohistochemistry was used to assess the expression of HIF-1α and VEGF in intervertebral disc tissues. HIF-1α was poorly expressed while VEGF was highly expressed in the NP cells from IDD rats when compared with expression in the NP cells from sham-operated rats (P < 0.05, Figure 5A). After treatment with moxibustion, HIF-1α expression increased and VEGF expression decreased in the NP cells from the IDD rats. Further, western blot analysis confirmed the findings above (Figure 5B). These results indicated that moxibustion increased HIF-1α expression and reduced VEGF expression in the NP cells from rats with IDD.
Figure 5.
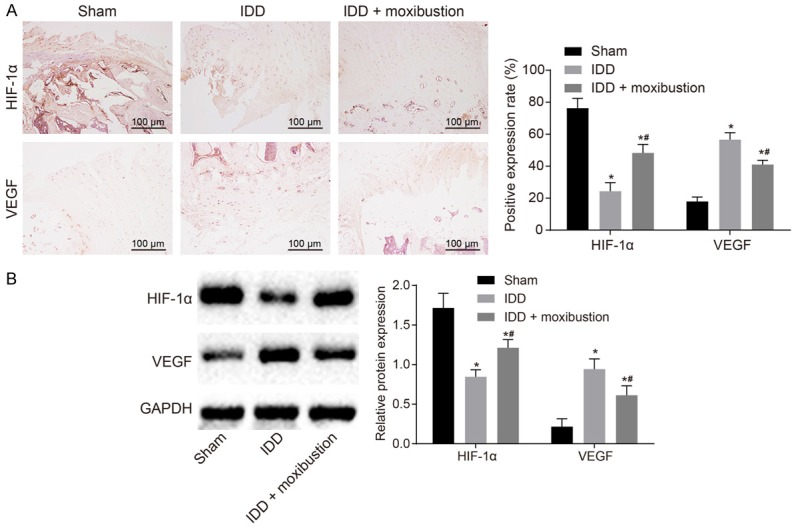
Treatment with moxibustion results in increased HIF-1α expression and reduced VEGF expression in rats with IDD. A. Expression of HIF-1α and VEGF in intervertebral disc tissues was identified by immunohistochemistry (scale bar = 100 µm). B. Western blot analysis was used to detect the expression of HIF-1α and VEGF normalized to GAPDH in NP cells. *P < 0.05 vs. sham-operated rats; #P < 0.05 vs. IDD rats. The measurement data were expressed as the mean ± standard error. Comparisons among multiple groups were analyzed using one-way ANOVA (Tukey’s post hoc test). Three parallel experiments were repeated; n = 14.
Moxibustion affects autophagy and apoptosis of NP cells in IDD rats through the HIF-1α/VEGF pathway
Moxibustion affected the expression of HIF-1α and VEGF. Here, we explored whether moxibustion could function through the HIF-1α/VEGF pathway. The expression of HIF-1α and VEGF was measured using RT-qPCR (Figure 6A) and western blot analysis (Figure 6B) in NP cells after moxibustion treatment or HIF-1α overexpression. In contrast to IDD rat NP cells transfected with blank plasmids, HIF-1α expression increased but VEGF expression declined significantly in IDD rat NP cells transfected with overexpressed HIF-1α or NP cells from IDD rats treated with moxibustion alone or in combination (P < 0.05). The combination of HIF-1α overexpression and moxibustion treatment exhibited the most significant changes (P < 0.01).
Figure 6.
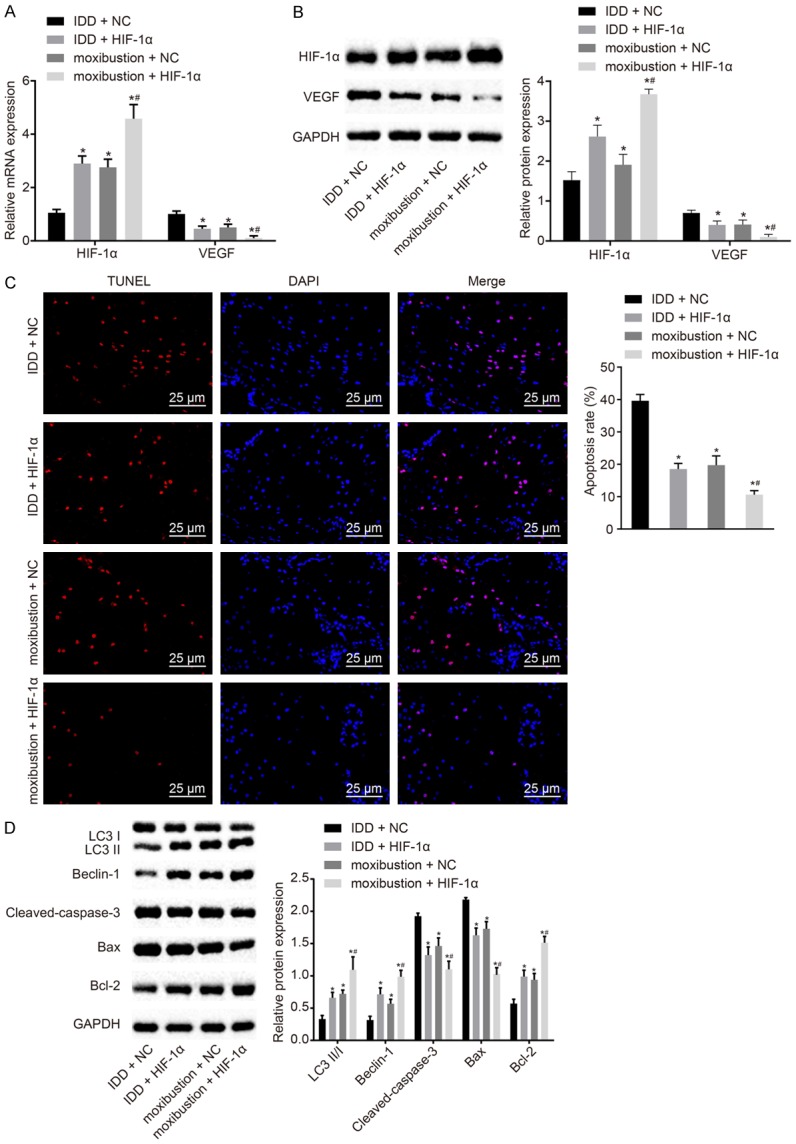
Treatment with moxibustion and HIF-1α overexpression restrains apoptosis and induces autophagy in NP cells. A. Expression of HIF-1α and VEGF in NP cells determined by RT-qPCR. B. Expression of HIF-1α and VEGF normalized to GAPDH in NP cells determined by western blot analysis. C. Apoptosis in NP cells assessed using TUNEL staining (scale bar = 25 µm). D. Expression of the autophagy and apoptosis-related genes LC3II/I, Beclin-1, cleaved-caspase-3, Bcl-2 and Bax normalized to GAPDH measured by western blot analysis. *P < 0.05 vs. IDD rat bearing NP cells transfected with blank plasmid; #P < 0.05 vs. IDD rat bearing NP cells transfected with blank plasmid. The measurement data were expressed as the mean ± standard error. Comparisons among multiple groups were analyzed using one-way ANOVA (Tukey’s post hoc test). Three parallel experiments were performed.
TUNEL staining (Figure 6C) was used for the detection of cell apoptosis. After treatment with moxibustion or HIF-1α overexpression alone or together, apoptosis in NP cells decreased (P < 0.05), with the most significant changes mediated by treatment with overexpressed HIF-1α and moxibustion together (P < 0.01). To further validate the above results, the expression of autophagy- and apoptosis-related genes was assessed (Figure 6D). It was found that the expression of LC3 II/I, Beclin-1 and Bcl-2 was enhanced after administration of moxibustion or HIF-1α overexpression, while cleaved-caspase-3 and Bax expression was diminished (P < 0.05). The combination of moxibustion treatment and HIF-1α overexpression contributed to the most effective changes (P < 0.01). Thus, apoptosis was reduced and autophagy was promoted after moxibustion and HIF-1α overexpression, therefore improving IDD.
Discussion
IDD causes spinal motion segment instability, stenosis, and deformity, resulting in low back pain and motor deficiency [4,5]. IDD is common around the globe and is therefore a huge economic burden to our society [6]. Despite this, the molecular mechanisms of IDD are not fully elucidated. The current study explored the in vivo effects of moxibustion on the occurrence and progression of IDD by establishing an IDD model in rats. Moreover, the findings from the present study suggest that moxibustion improves IDD, and moxibustion combined with HIF-1α overexpression may have more beneficial effects on IDD than moxibustion alone.
Based on the theory of traditional Chinese medicine, a possible reason for how moxibustion works is that heat regulates the functions of meridians and viscera by stimulating acupoints, thus increasing the circulation of qi and alleviating qi stagnation [21]. However, moxibustion is not risk free, as it may cause allergies, burns and infections [8]. The current study focused on the effects of moxibustion on IDD regarding the involvement of the HIF-1α/VEGF pathway. We found that moxibustion improves IDD by inducing autophagy and inhibiting apoptosis of NP cells in IDD. Apoptosis and autophagy are two main programmed cell death patterns [22], which are closely linked, and autophagy can either suppress or delay apoptosis [23]. In the present study, the expression of LC3 II/I, Beclin-1 and Bcl-2 increased and the expression of cleaved-caspase-3 and Bax was reduced. In addition to their importance as biological phenomena, defective apoptotic processes or excessive autophagy is also involved in a wide range of diseases, including IDD, and the increase in apoptosis or excessive autophagy of intervertebral disc cells is related to the pathogenesis of IDD [24].
Moxibustion increased the expression of LC3 II/I, Beclin-1 and Bcl-2, which are related to autophagy. Autophagy involves a multistep process, which is highly regulated by the autophagy-related gene LC3. Cytoplasmic tubule-associated protein LC3-I is lipid-modified and transformed into LC3-II and thus capable of translocating through the autophage membrane, making the transformation from LC3-I to LC3-II and the accumulation of LC3 a marker of autophagy. Beclin-1 is a Bcl-2 homology 3 member of the Bcl-2 gene family that can drive autophagy in mammalian cells [25]. Apoptosis can be induced by variant upstream stimuli, including caspase-3, which can be activated by cytochrome C released from mitochondria that is enhanced by Bax or suppressed by Bcl-2 [26,27]. Consistent with our findings, 3-methyladenine has been reported to promote autophagy in annulus fibrosus cells under serum deprivation in vitro, with increased Beclin-1 and LC3 II/I and Bax/Bcl-2, which are associated with mitochondrial function [28]. Thus, moxibustion may be beneficial to IDD by enhancing the autophagy of NP cells in IDD.
Subsequently, it was found that moxibustion induced HIF-1α expression and activated the HIF-1α/VEGF pathway to affect IDD. A combination treatment of moxibustion and HIF-1α overexpression exerted better effects at reducing apoptosis and enhancing autophagy. HIF-1α is implicated in the degeneration of human intervertebral discs when the expression of HIF-1α is correlated with the progression of the condition [29]. Additionally, it has been shown that HIF-1α is expressed in NP cells in rat intervertebral discs [30]. HIF-1α is implicated in hypoxia-induced autophagy [31], which decreases apoptosis and increases autophagy by removing damaged organelles in metabolic stress conditions such as hypoxia [32]. Recent findings have suggested that modification of VEGF in mesenchymal stem cells exerts angiogenetic and paracrine effects [33]. Thus, we may conclude that overexpressed HIF-1α exerted beneficial effects on IDD. These findings underscore that the role of moxibustion in IDD may be related to apoptosis and autography via the HIF-1α/VEGF pathway.
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper. This study was supported by the Shandong Provincial Traditional Chinese Medicine Science and Technology Development Plan Project (No. 2015-404).
Disclosure of conflict of interest
None.
Abbreviations
- IDD
intervertebral disc degeneration
- COX-2
cyclo-oxygenase 2
- NP
nucleus pulposus
- HIF-1α
hypoxia-inducible factor-1 alpha
- VEGF
vascular endothelial growth factor
- SD
Sprague-Dawley
- IVD
intervertebral disc
- AF
annulus fibrosus
- HE
hematoxylin and eosin
- DAB
diaminobenzidine
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- PAGE
polyacrylamide gel electrophoresis
- PVDF
polyvinylidene fluoride
- ECL
enhanced chemiluminescence
References
- 1.Wang F, Cai F, Shi R, Wang XH, Wu XT. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage. 2016;24:398–408. doi: 10.1016/j.joca.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016;2016:5952165. doi: 10.1155/2016/5952165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng KK, Berven SH, Hu SS, Lotz JC. Intervertebral discs from spinal nondeformity and deformity patients have different mechanical and matrix properties. Spine J. 2014;14:522–530. doi: 10.1016/j.spinee.2013.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vadalà G, Russo F, Ambrosio L, Loppini M, Denaro V. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016;8:185–201. doi: 10.4252/wjsc.v8.i5.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F, Shi R, Cai F, Wang YT, Wu XT. Stem cell approaches to intervertebral disc regeneration: obstacles from the disc microenvironment. Stem Cells Dev. 2015;24:2479–2495. doi: 10.1089/scd.2015.0158. [DOI] [PubMed] [Google Scholar]
- 6.Molinos M, Almeida CR, Caldeira J, Cunha C, Gonçalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12:20150429. doi: 10.1098/rsif.2014.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao XY, Chong CY, Zhang SP, Cheng KW, Zhu B. Temperature and safety profiles of needle-warming techniques in acupuncture and moxibustion. Evid Based Complement Alternat Med. 2012;2012:168393. doi: 10.1155/2012/168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JE, Lee SS, Lee MS, Choi SM, Ernst E. Adverse events of moxibustion: a systematic review. Complement Ther Med. 2010;18:215–223. doi: 10.1016/j.ctim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Liu WW, Huang J, Liu M, Tan CF, Chang XR, Yan J. Effect of different length of moxibustion preconditioning on the expression of HIF-1α, Bcl-2 and caspase-3 in myocardial cells of MIRI rats. Acta Chinese Medicine and Pharmacology. 2017 [Google Scholar]
- 10.Zhang P, Yao Q, Lu L, Li Y, Chen PJ, Duan C. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep. 2014;6:1110–1121. doi: 10.1016/j.celrep.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Arkesteijn IT, Smolders LA, Spillekom S, Riemers FM, Potier E, Meij BP, Ito K, Tryfonidou MA. Effect of coculturing canine notochordal, nucleus pulposus and mesenchymal stromal cells for intervertebral disc regeneration. Arthritis Res Ther. 2015;17:60. doi: 10.1186/s13075-015-0569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin WP, Wang XJ, Wang CR, Zhang LQ, Li N, Wang FS, Lin JH. Polymorphism in the hypoxia-inducible factor 1alpha gene may confer susceptibility to LDD in Chinese cohort. PLoS One. 2013;8:e73158. doi: 10.1371/journal.pone.0073158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu WJ, Zhang XK, Zheng XF, Yang YH, Jiang SD, Jiang LS. SHH-dependent knockout of HIF-1 alpha accelerates the degenerative process in mouse intervertebral disc. Int J Immunopathol Pharmacol. 2013;26:601–609. doi: 10.1177/039463201302600304. [DOI] [PubMed] [Google Scholar]
- 14.Jiang LB, Cao L, Ma YQ, Chen Q, Liang Y, Yuan FL, Li XL, Dong J, Chen N. TIGAR mediates the inhibitory role of hypoxia on ROS production and apoptosis in rat nucleus pulposus cells. Osteoarthritis Cartilage. 2018;26:138–148. doi: 10.1016/j.joca.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto A, Kannari M, Tsuchida Y, Sasaki S, Saito C, Matsuta T, Maeda T, Akiyama M, Nakamura T, Sakaguchi M, Nameki N, Gonzalez FJ, Inoue Y. An HNF4alpha-microRNA-194/192 signaling axis maintains hepatic cell function. J Biol Chem. 2017;292:10574–10585. doi: 10.1074/jbc.M117.785592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan X, Chen Y, Ji Z. Expressions and significance of HIF-1alpha and AQP-3 in intervertebral disc degeneration tissues of cervical spondylotic myelopathy. Minerva Med. 2017;108:593–595. doi: 10.23736/S0026-4806.17.05345-9. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Li C, Meng X, Bai Y, Qi J, Wang J, Zhou Q, Zhang W, Zhang X. Hypoxia-inducible factor-lalpha mediates aggrecan and collagen Pi expression via NOTCH1 signaling in nucleus pulposus cells during intervertebral disc degeneration. Biochem Biophys Res Commun. 2017;488:554–561. doi: 10.1016/j.bbrc.2017.05.086. [DOI] [PubMed] [Google Scholar]
- 18.Li XC, Wang MS, Liu W, Zhong CF, Deng GB, Luo SJ, Huang CM. Co-culturing nucleus pulposus mesenchymal stem cells with notochordal cell-rich nucleus pulposus explants attenuates tumor necrosis factor-alpha-induced senescence. Stem Cell Res Ther. 2018;9:171. doi: 10.1186/s13287-018-0919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JT, Cheung KM, Leung VY. Systematic study of cell isolation from bovine nucleus pulposus: improving cell yield and experiment reliability. J Orthop Res. 2015;33:1743–1755. doi: 10.1002/jor.22942. [DOI] [PubMed] [Google Scholar]
- 20.Ma X, Lin Y, Yang K, Yue B, Xiang H, Chen B. Effect of lentivirus-mediated survivin transfection on the morphology and apoptosis of nucleus pulposus cells derived from degenerative human disc in vitro. Int J Mol Med. 2015;36:186–194. doi: 10.3892/ijmm.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong X, Liu W, Yang X, Feng B, Wang J. Moxibustion for essential hypertension. Complement Ther Med. 2014;22:187–195. doi: 10.1016/j.ctim.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Booth LA, Tavallai S, Hamed HA, Cruickshanks N, Dent P. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell Signal. 2014;26:549–555. doi: 10.1016/j.cellsig.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiorini C, Menegazzi M, Padroni C, Dando I, Dalla Pozza E, Gregorelli A, Costanzo C, Palmieri M, Donadelli M. Autophagy induced by p53-reactivating molecules protects pancreatic cancer cells from apoptosis. Apoptosis. 2013;18:337–346. doi: 10.1007/s10495-012-0790-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F, Zhao X, Shen H, Zhang C. Cell death in intervertebral disc degeneration. Apoptosis. 2013;18:777–785. doi: 10.1007/s10495-013-0839-1. [DOI] [PubMed] [Google Scholar]
- 25.Kong CG, Park JB, Kim MS, Park EY. High glucose accelerates autophagy in adult rat intervertebral disc cells. Asian Spine J. 2014;8:543–548. doi: 10.4184/asj.2014.8.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, Cho K, Sheng M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 28.Ni BB, Li B, Yang YH, Chen JW, Chen K, Jiang SD, Jiang LS. The effect of transforming growth factor beta1 on the crosstalk between autophagy and apoptosis in the annulus fibrosus cells under serum deprivation. Cytokine. 2014;70:87–96. doi: 10.1016/j.cyto.2014.07.249. [DOI] [PubMed] [Google Scholar]
- 29.Wu WP, Jiang JM, Qu DB, Wei QZ, Jiang H. Expression of hypoxia-inducible factor-1 alpha and matrix metalloproteinase-2 in degenerative lumbar intervertebral disc. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:1152–1155. [PubMed] [Google Scholar]
- 30.Richardson SM, Knowles R, Tyler J, Mobasheri A, Hoyland JA. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate human intervertebral disc. Histochem Cell Biol. 2008;129:503–511. doi: 10.1007/s00418-007-0372-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ni X, Ou C, Guo J, Liu B, Zhang J, Wu Z, Li H, Chen M. Lentiviral vector-mediated co-overexpression of VEGF and Bcl-2 improves mesenchymal stem cell survival and enhances paracrine effects in vitro. Int J Mol Med. 2017;40:418–426. doi: 10.3892/ijmm.2017.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]