Abstract
Alopecia areata is characterized by the loss of hair on the scalp and elsewhere on the body. It affects approximately 2% of the general population. It is believed to be an autoimmune disease. However, its pathogenesis remains incompletely understood. Recent studies have revealed a substantial link between vitamin D and alopecia areata. But the underlying mechanism still yet to be deciphered. This article reviews the current literature and discusses the possible roles of vitamin D in the pathogenesis of alopecia areata in the context of (1) loss of immune privilege in hair follicle, (2) autoreactive effector T cells and mast cells, (3) nature killer group 2 member d-positive cytotoxic T cells, (4) Janus kinase/signal transducers and activators of transcriptional signaling pathway, (5) regulatory T cells, (6) immune checkpoints, and (7) oxidative stress, which are believed to play important roles in autoimmunity in AA. This paper provides new insights into research directions to elucidate the exact mechanisms of vitamin D in the pathogenesis. Calcipotriol, a vitamin D analog, has been reported to be topically used in treating alopecia areata with promising results. Combination therapy of vitamin D analogs with corticosteroids might also be used in treating alopecia areata.
Keywords: Alopecia areata, vitamin D, autoimmune, calcipotriol
Introduction
Alopecia areata (AA) is a common autoimmune skin disease characterized by loss of the hair on the scalp and elsewhere on the body, affecting approximately 2% of the general population at some point during their lifetime. AA may cause anxiety on patients and increases the risks of developing psychological and psychiatric complications [1]. The hair loss in AA is believed to result from an autoimmune-mediated hair follicle (HF) destruction consequent to a loss of immune privilege (IP) in the HF. Autoreactive effector T cells and mast cells, CD8-positive nature killer group 2 member D (NKG2D)-positive cytotoxic T cells (CD8+NKG2D+ cytotoxic T cells), Janus kinase/signal transducers and activators of transcriptional signaling (JAK/STAT) pathways, regulatory T cells (Tregs) and immune checkpoints and oxidative stress (OS) are involved in AA [1,2]. However, the pathogenesis of AA remains incompletely understood and AA remains incurable.
Vitamin D has been associated with various autoimmune diseases. Recently, vitamin D deficiency has been reported in AA [3]. Moreover, topical calcipotriol has been reported to be used successfully in treating AA [4-6]. These reports attracted the attention on the relationship between vitamin D and AA. The aim of this review is to summarize the current knowledge on the possible roles of vitamin D in the pathogenesis of AA and to discuss the potential implication of vitamin D in the treatment of AA.
Vitamin D sources, metabolism and functions
In the human body there are two major forms of vitamin D. More than 90% is vitamin D3 (cholecalciferol), which is converted from 7-dehydrocholesterol by ultraviolet light B (UVB) exposure in the skin. About 10% is vitamin D2 (ergocalciferol), which comes from dietary sources. Both are biologically inactive [7]. In the liver, vitamin D is metabolically converted into 25-hydroxyvitamin D (25(OH)D) which is biologically inactive at physiological concentration. 25(OH)D has been considered as one of the most reliable indicator of Vitamin D levels in humans [8]. In the kidney, 25(OH)D is further converted to the biologically active metabolites 1,25(OH)2D3 (calcitriol) by 25(OH) 1α-hydroxy-D [9]. The 1α-hydroxy enzyme is also widely expressed in non-kidney cells including immune cells and is able to convert the inactive 25(OH)D into the active 1,25(OH)2D in either an autocrine or paracrine manner [9].
Vitamin D functions by binding to the vitamin D receptor (VDR), a member of nuclear hormone receptors which is widely expressed in the kidney, immune cells, osteocytes and other types of cells. VDR activated by vitamin D forms a heterodimeric complex with retinoid X receptor. This complex is recruited to the vitamin D response elements in the target genes and interacts with additional co-regulators, influencing the expression of many genes. As such, vitamin D possesses multiple functions and target organs [7,10].
At present, the function of vitamin D can be divided into two categories. The classical function involves mineral balance and skeletal maintenance. The non-classical function involves regulation of cellular proliferation, differentiation, apoptosis, innate and adaptive immunity, anti-oxidation and others. The role of vitamin D in many conditions such as cancer, diabetes, hypertension, cardiovascular, and autoimmune and dermatological diseases is being extensively studied [7,10].
Vitamin D status in AA
A number of studies demonstrated significantly lower levels of vitamin D in the patients with AA than the control group [11-17]. Several studies showed significantly higher prevalence of vitamin D insufficiency in patients with AA than the control group [12,13,18,19]. But there were two reports of inconsistent results. A Turkish study found that AA patients had a deficiency of 25(OH)D, but there was no statistically significant difference in the serum vitamin D levels between AA patients and healthy controls. The authors said that this might be due to the universal tendency toward lower values of 25(OH)D in their geographical area, and they noted that the blood samples were collected only once during the late fall and winter months [20]. In a study involving 55,929 women in the Nurses’ Health Study, 133 cases of AA were identified over a follow-up of 12 years. The association between estimated vitamin D status and self-reported incident AA was prospectively evaluated. No significant association between a predictive score of serum 25(OH)D levels and risk of incident AA was found [21]. Importantly, two systemic reviews and meta-analyses published in 2018 did demonstrate that, patients with AA have a higher prevalence of vitamin D deficiency and lower vitamin D levels than the control group [3,22] (Table 1). Moreover, several studies revealed that serum vitamin D levels significantly and inversely correlate with the severity of AA [12,16,17,19,23].
Table 1.
Vitamin D status in AA
| Study | Patient Number | Study Design | Results | Country |
|---|---|---|---|---|
| Yilmaz et al, 2012 [11] | 42 | Case control | Lower serum vitamin D. | Turkey |
| Aksu Cerman et al, 2014 [12] | 86 | Cross-sectional | Lower serum vitamin D. | Turkey |
| Higher vitamin D deficiency prevalence. | ||||
| Mahamid et al, 2014 [13] | 23 | Perspective | Lower serum vitamin D. | Israel |
| Higher vitamin D deficiency prevalence. | ||||
| Bakry et al, 2016 [14] | 60 | Case control | Lower serum vitamin D. | Egypt |
| Ghafoor et al, 2017 [15] | 30 | Case control | Lower serum vitamin D. | Pakistan |
| Bhat et al, 2017 [16] | 50 | Cross-sectional | Lower serum vitamin D. | India |
| Gade et al, 2018 [17] | 45 | Cross-sectional | Lower serum vitamin D. | India |
| d’Ovidio et al, 2018 [18] | 156 | Case control | Higher vitamin D deficiency prevalence. | Italy |
| Daroach et al, 2018 [19] | 30 | Perspective study | Lower serum vitamin D. | India |
| Higher vitamin D deficiency prevalence. | ||||
| Erpolat et al, 2017 [20] | 41 | Case control | Serum vitamin D: no difference from control. 93.8% patients had vitamin D deficiency. | Turkey |
| Thompson et al, 2016 [21] | 133 | Perspective study | No association between serum vitamin D and risk of AA. | USA (women) |
| Tsai et al, 2018 [3] | Systematic review & meta-analysis | AA patients have higher vitamin D deficiency prevalence and lower serum vitamin D than controls. | ||
| Lee et al, 2018 [22] | Systematic review & meta-analysis | AA patients have higher vitamin D deficiency prevalence and lower serum Vitamin D than controls. |
Studies also showed that serum [24] and tissue [19,24] VDR levels were lower in AA. One study found a negative correlation of tissue VDR and extent of AA [24].
Taken together, the above data show a substantial link between the levels of vitamin D and AA, suggesting an important role of vitamin D in the pathogenesis of the disease. However, the mechanism underlying this relationship still has to be deciphered.
Possible role of vitamin D in pathogenesis of AA
Hair loss in AA is caused by the destruction of HF cycle. However, the role of vitamin D in in HF cycling is not clear. Although a study showed in mice that in calbindin-D9k knockout pups, a maternal vitamin D-deficient/low-calcium diet leads to transient noncicatricial alopecia, suggesting a role for calcium and possibly vitamin D in postnatal HF cycling [25], studies published in the literature have not demonstrated a role of vitamin D in HF cycling. On the other hand, it is now fully accepted that VDR has important function in hair cycling. However, this function of VDR appears to be vitamin D-independent [26]. VDR may function as a selective suppressor/de-repressor of gene expression in the absence of 1,25(OH)2D3 [26]. Wnt/β-catenin signaling is a key player in inducing the onset of anagen and maintaining the cycling transition during the initiation and regeneration of HFs [27]. Reduction of VDR expression in AA may be related to decreased hair cycle-related signals-Wnt/β-catenin signals [28]. The decreased expression of VDR in AA is believed to be involved in the disruption of HF cycling in AA.
The disruption of HF cycling in AA is caused by an autoimmune response [2], and there is mounting evidence that vitamin D has an impact on the pathophysiological mechanisms of autoimmunity [29]. Therefore vitamin D may affect the HF cycling through its impact on autoimmunity in AA pathogenesis. In this article, we review the possible roles of vitamin D in the pathogenesis of AA focusing on several key elements of autoimmune mechanism in AA: (1) Loss of IP in HF, (2) Autoreactive effector T cells and mast cells, (3) CD8+NKG2D+ cytotoxic T cells, (4) JAK/STAT pathway, (5) Tregs, (6) Immune checkpoints and (7) OS.
Loss of IP in HF
The anagen HF is normally an immune privileged (IP) site. The loss of the IP of the anagen hair bulb is a key element in AA pathogenesis. One of the most crucial mechanisms of IP collapse is an increase in major histocompatibility complex (MHC) I and II molecules [2]. IFN-γ is prominently expressed in lesional skin from patients with AA and is believed to contribute to the collapse of IP through increased follicular expression of MHC class I and II molecules [2,30] (Figure 1).
Figure 1.
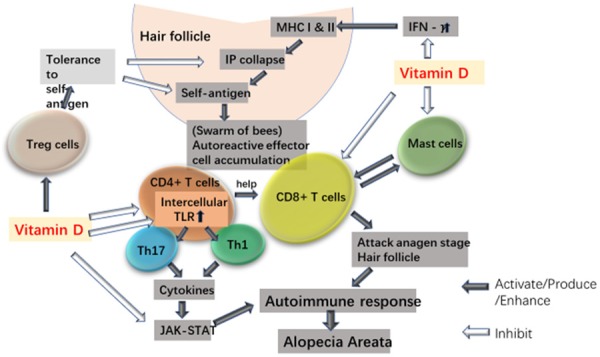
Possible effects of vitamin D on immune cells in AA. HF IP collapse is a key element in AA immunopathogenesis. IFN-γ contributes to IP collapse by increasing HF MHC class I and II molecules. Vitamin D may contribute to maintain the IP of HF by decreasing the production of IFN-γ. HF IP collapse with exposure of autoantigens leads to an accumulation of autoreactive effector T cells (“swarm of bees”) in which CD8+ T cells attack the anagen stage HF with help of CD4+ T cell (Th1 and Th17). Vitamin D can inhibit proliferation and cytokine production of CD8+ T cell and CD4+ T cell (Th1 and Th17). Besides, vitamin D may inactivate T cells by down-regulating abnormally up-regulated intracellular TLRs in AA. Vitamin D may also contribute to prevent HF IP collapse and decrease CD8+ T cell activation in AA by inhibiting MCs and interfering cross-talk between MCs and CD8+ T cells. Cytokines involved in the pathogenesis of AA are dependent on JAK-STAT pathway. Inhibition of JAK/STAT pathway by vitamin D may in turn block the effects of key cytokines involved in the immunopathogenesis of AA. Treg cells maintain peripheral tolerance to prevent IP collapse and inhibit auto-reactive effector T cells. Vitamin D may contribute to control the overactive self-effector T cells in AA by enhancing the inhibitory function of Treg cells.
Studies revealed that vitamin D3 significantly inhibits the production of IFN-γ by human peripheral blood mononuclear cells (PBMCs) activated in vitro by phytohemagglutinin [31] and decreases the secretion of IFN-γ by human CD4+ T cells [32,33]. Kokic et al [34] showed that in patients with inactive SLE, lower levels of vitamin D are corelated with higher levels of IFN-γ. In vitamin D deficient patients, IFN-γ was 150% higher compared with patients without vitamin D deficiency. Mrad et al [35] investigated the immunologic effects of vitamin D replacement in relapsing remitting multiple sclerosis (RRMS) patients. The patients categorized as vitamin D deficient were treated with high dose vitamin D (10,000 IU orally daily for 3 months), while those with normal vitamin D maintained their usual medical care. They observed a decreased IFN-γ secretion by CD4+ T cells in vitamin D deficient group but not in the sufficient group, and a negative correlation between baseline serum vitamin D and IFN-γ production. These findings suggest that normally vitamin D might contribute to maintain the IP of HF by modulating the production of IFN-γ. Contrarily, insufficiency of vitamin D might lead to over-secretion of IFN-γ and play a role in the collapse of IP of the anagen hair bulb (Figure 1).
Autoreactive effector T cells and mast cells (MCs)
The collapse of HF IP with exposure of autoantigens leads to an accumulation of autoreactive effector T cells in and around the lesional hair bulb, which is histologically referred to as “swarm of bees”. Substantial progress in immunology research supports that AA is an autoimmune reaction with a CD8+ T cell attack on the anagen stage HF with CD4+ T cell help as its underlying mechanism [36]. In addition, recent research has proposed that AA is also associated with Th17 cells [37,38] (Figure 1).
Earlier studies have demonstrated that 1,25(OH)2D inhibits the proliferation of human CD8 and CD4 T cells [39]. More recently, Sheikh et al [33] found that stimulation of human CD4+ T cell in antigen presenting cells (APCs) free condition in vitro with VitD3, suppresses proliferation capacity and diminishes the pro-inflammatory cytokines including, IFN-γ, IL-17, and IL-22 in CD4+ T cells. Schardey et al [40] found that incubation of peripheral and intestinal T cells isolated from inflammatory bowel disease (IBD) patients with 1,25(OH)2D resulted in strongly reduced frequencies of CD4+ and CD8+ T cells producing IFN-γ, IL-17, IL-22, IL-9 and TNF. In in vivo studies, Penna-Martinez et al [41] found that high dose vitamin D treatment significantly downregulates both late-activated CD4+ and CD8+ T cells in patients with Addison’s disease. Tom et al [42] found that 1α,25-dihydroxyvitamin D3 decreases IFN-γ-expressing CD8+ T cells in patients with IBD (Figure 1).
A number of studies have shown that vitamin D reduces differentiation of Th17 cells and secretion of pro-inflammatory cytokines by these cells. In CD4+ T cells from normal human [32] and asthmatic patients [43], vitamin D3 reduced the differentiation of Th17 cells and their secretion of pro-inflammatory cytokines. In peripheral blood monocuclear cells from patients with SLE [44] and early rheumatoid arthritis [45], vitamin D3 significantly decreased the proportion of Th17 cells. In vivo, supplementation with Vit D3 in Hashimoto’s thyroiditis patients for 3 months caused a significant decrease in Th17/Tr1 ratio [46] (Figure 1).
The regulation of vitamin D on the effector T cells including CD8+ T cells, CD4+ T cells and Th17 cells may play a role in the pathogenesis of AA by contributing to attenuate or prevent the autoimmune response that drives the disease (Figure 1).
Toll like receptors (TLRs) expressed in T cells have been suggested to act as co-stimulatory molecules inducing T-cell activation [47]. Alzolibani et al [48] reported that in PBMCs of AA patients, intracellular TLRs (TLRs 3, 7, 8 and 9) are significantly up-regulated associated with significantly higher expression of IL-2, TNF-α, and IL-17A gene expression, suggesting abnormal activation of Th1 and Th17 cells. Yazdanpanah et al [49] reported that culturing PBMCs with vitamin D3 significantly down-regulated the expression of TLR3, TLR7 and TLR9 in SLE patients in comparison with healthy controls. Vitamin D might also down-regulate the intercellular TLRs in AA patients, and consequently restore the abnormal regulation of Th-1 and Th-17 cell activation (Figure 1).
MCs are crucial immunomodulatory cells implicated in the regulation of T cell-dependent immunity. Recently, positive correlations were found existing between MCs and CD8(+) T cells in AA [50]. Bertolini et al [51] reported that the number, degranulation and proliferation of perifollicular MCs are increased in human AA lesions compared to healthy or non-lesional control skin. Moreover, these MCs switch from an immuno-inhibitory to a pro-inflammatory phenotype. Lesional AA HFs also displayed significantly more physical MC/CD8+ T cell contacts than healthy or non-lesional human control skin. MC may present autoantigens to CD8+ T cells and/or co-stimulatory signals. The cross-talk between MCs and CD8+ T cells may contribute to triggering HF IP collapse in AA. Vitamin D appears to have inhibitory actions on MCs. Vitamin D inhibits IL-33 action, and IL-33 acts synergistically with the neuropeptide substance P to stimulate skin MCs [52]. Yip et al [53] reported that the vitamin D3 metabolites, 1α,25(OH)2D3 and 25OHD3 can attenuate the generation of pro-inflammatory signals from IgE-activated mouse and human mast cells. Liu et al [54] tested the stability of MC lines in the presence or absence of Vitamin D3. The results demonstrated that vitamin D is required to maintain the stability of mast cells. The deficiency of vitamin D results in mast cell activation. Taken together, vitamin D might contribute to prevent HF IP collase and decrease CD8+ T cell activation in AA by inhibiting MCs and interfering cross-talk between MCs and CD8+ T cells (Figure 1).
CD8+NKG2D+ T cells
Studies support a role for CD8+NKG2D+ cytotoxic T cells in AA [2]. Dai et al [55] determined that CD8+NKG2D+ T cells are the dominant immune effectors infiltrating the HF in both humans and mice with AA. NKG2D is an activating receptor with MHC class I related chain (MIC)A and MICB as its activating ligands. Ito et al [56] reported that human AA skin abounds in strong, widespread, extra- and intrafollicular MICA immunoreactivity. Besides, it was shown that CXCR3+ CD8+ T cells markedly infiltrate in and around HFs from AA patients and it was demonstrated that CXCR3 plays a key role in skin CD8+NKG2D+ T cell trafficking in AA mice. Ligands of CXCR3, including CXCL9, CXCL10 and CXCL11, are dramatically upregulated in human and mouse with AA [55]. Taken together, findings from AA in human and in mouse suggest that upregulated MICA and CXCL10 in HP may activates and recruits CD8+NKG2D+ T cells, infiltrating the HP and contributing to the autoimmune response in AA (Figure 2).
Figure 2.
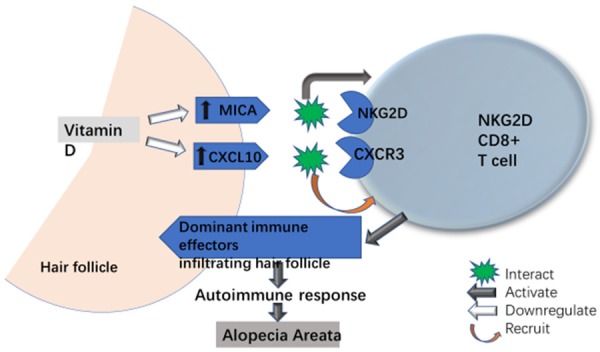
Possible effects of vitamin D on CD8+NKG2D+ T cells in AA. CD8+NKG2D+ T cells are the dominant immune effectors in AA. These cells express CXCR3 that plays a key role in skin CD8+NKG2D+ T cell trafficking in AA. NKG2D activating ligand MICA and CXCR3 ligand CXCL-10 are upregulated in AA. Vitamin D can downregulate MICA and CXCL-10, contributing to prevent the skin NKG2D+CD8+ T cell activation and trafficking in AA.
It was reported that vitamin D significantly downregulates the mRNA expression of MICA and MICB in human hepatic stellate cells [57]. Calcitriol reduced the production of CXCL-10 by SLE myeloid angiogenic cells [58] and IL-1β-stimulated human periodontal ligament cells [59]. Komolmit et al [60] reported that vitamin D suppressed expression of CXCL10 from monocytes in vitro. Moreover, a double-blind, placebo-controlled trial revealed that in the chronic hepatitis C patients with vitamin D levels less than 30 ng/mL treated with vitamin D supplements for 6 weeks, the mean 25(OH)D level in vitamin D group was significantly increased and normalized, and the serum CXCL10 levels were decreased significantly as compare to the placebo group. Wu et al [61] reported that in mouse model, vitamin D treatment before infection of Plasmodium berghei ANKA reduced the mRNA expression levels of CXCR3, CXCL9 and CXCL10 in the brains of infected mice. Since vitamin D can down-regulate the NKG2D activating ligands MICA/B and Ligands of CXCR3 that plays a key role in skin CD8+NKG2D+ T cell trafficking, vitamin D might contribute to prevent the skin NKG2D+CD8+ T cell activation and trafficking in AA (Figure 2).
JAK/STAT pathway
Cytokines involved in the pathogenesis of AA, most notably interferon (IFN)-γ and interleukin (IL)-15, are dependent on the Janus kinase and signal transducers and activators of transcription (JAK-STAT) pathway [62]. It is assumed that CD8+NKG2D+ T cells mediate AA in part through JAK signaling [63]. Skin biopsy specimens from patients with AA displayed a strong JAK3 expression [64]. JAK activation was also shown by the presence of phosphorylated STAT proteins in AA HF, but not in normal HF [65] (Figure 1).
Studies have shown an inhibitory effect of vitamin D on the JAK/STAT pathway. Employing a mouse model of type 2 diabetes mellitus, Zhang et al [66] observed that 25VD3-enhanced protein tyrosine phosphatase non-receptor type 2 expression decreased the expression of the JAK1/STAT3 signaling proteins in the gingival epithelium. Zeitelhofer et al [67] showed in a functional genomics analysis of vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis that JAK/STAT signaling pathway genes are down-regulated upon vitamin D supplementation. Inhibition of JAK/STAT pathway by vitamin D may in turn block the effects of key cytokines involved in the pathogenesis of AA (Figure 1).
Treg cells
Treg cells maintain peripheral tolerance to limit autoimmune pathology by inhibiting auto-reactive effector T cells. It has recently been hypothesized that HF IP may have been established as a mechanism to promote the de novo induction of peripheral tolerance [30]. Peripheral tolerance is believed to organize the IP which is disrupted in AA. Speiser et al [68] reported that Treg cell frequency is significantly lower in AA skin specimens when compared to other cutaneous diseases. A genome-wide association study demonstrated that a number of identified risk loci for AA are shared with other forms of autoimmunity, in particular genes critical to the function of Tregs [69]. Han et al [70] reported that circulating Treg levels in patients with AA and counts of Foxp3+ lymphocytes surrounding HFs of AA patients were significantly lower than those of controls. The deficiency in Tregs may result in breakdown of immune tolerance and may enhance T-cell-mediated autoimmunity. This may facilitate the occurrence of AA (Figure 1).
The impact of vitamin D on Treg cells has been studied. Fawaz et al [32] investigated the in vitro effect of 1,25(OH)2D3 and 25(OH)D3 on the differentiation and cytokine production of primary CD4+ T cells from normal donors. Both vitamin D forms induced an expansion of CD25hi cells and upregulated their expression of CTLA-4 and Foxp3 regulatory markers. Another in vitro studies showed that 1,25(OH)2D3 induces the differentiation of human Treg T cells [71]. The maternal blood and cord blood Treg cell populations were found to be significantly lower in 25(OH)D3 deficient pregnant women compared to sufficient pregnant women [72]. Mattozzi et al [73] found in psoriatic patients an association of serum levels of vitamin D with Treg population (P<0.001), suggesting that low levels of vitamin-D may decrease the number of circulatory Treg. In female BALB/c mice, dietary vitamin D3 increased the percentage of (CD3+CD4+CD25+Foxp3+) cells in the skin-draining lymph nodes (SDLN). The suppressive activity of cells in the SDLN, mesenteric lymph nodes, spleen, and blood was upregulated by vitamin D3 [74]. A double-blind, placebo controlled study in healthy individuals showed that vitamin D3 supplementation leads to significantly increased numbers of peripheral Tregs in vivo [75] (Figure 1).
These data suggest that vitamin D may contribute to control the overactive self-effector T cells in the pathogenesis of AA by enhancing the inhibitory function of Treg cells (Figure 1).
Immune checkpoints
Immune checkpoints are negative regulators of immune responses to avoid immune injury. They are thought to actively participate in the prevention of autoimmunity and tumor immune evasion [76]. In recent years, inhibitors of immune checkpoints, including the cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) and the programmed cell death protein (PD-1) receptors and its ligand (PD-L1), are being explored for a growing number of solid and hematological malignancies. Their mechanism of action involves the inhibition of negative regulators of immune activation, which results in many patients developing immune-related adverse events. Alopecia (areata and universalis) is a known side-effect of PD-1 receptor inhibitors and anti-CTLA-4 agents with a prevalence of 1.0-2.0% [77] (Figure 3A). Therefore the possible role of immune checkpoints in the pathogenesis of AA has attracted attention.
Figure 3.
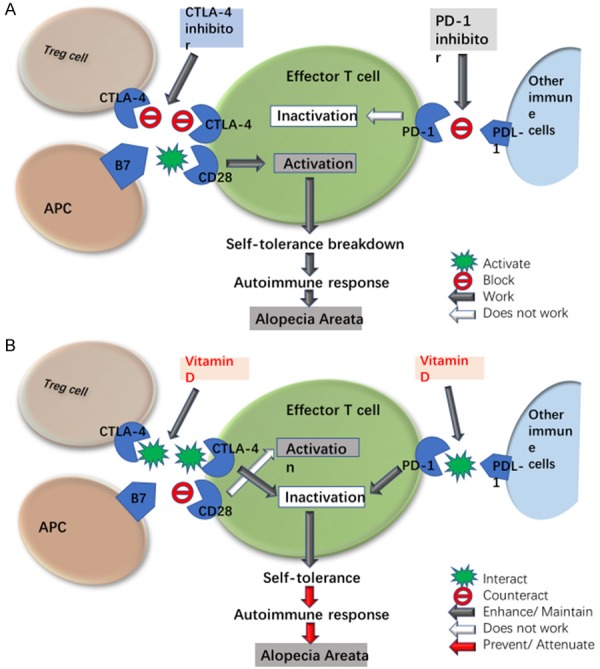
Possible effects of vitamin D on CTLA-4 and PD-1 in AA. A. Effect of CTLA-4 and PD-1 inhibitors. CTLA-4 is induced in effector T cells upon activation and is constitutively expressed by Treg cells. CTLA-4 preferentially binds to B7 co-stimulatory molecules on APCs, depriving effector T cells of the CD28 co-stimulatory signal to impair the activation of effector T cells. PD-1 expresses in T cells and various immune cells. PD-L1, a ligand of PD-1, can be found on several kinds of immune cells. Binding of PD-1 to PD-L1 generates a strong inhibitory signal, down-regulating T cell activity. CTLA-4 and PD-1 are critical in maintenance of tolerance to self-antigens to prevent autoimmunity. CTLA-4 inhibitor blocks the binding of CTLA-4 to B7 and PD-1 inhibitor blocks the binding of PD-1 to PDL-1 respectively, enhancing T cell activation. Activation of effector T cells leads to self-tolerance breakdown and autoimmune response as immune-related adverse events including AA. B. Effect of vitamin D. Vitamin D enhances CTLA-4, depriving effector T cells of the CD28 co-stimulatory signal to impair the activation of effector T cells. Vitamin D enhances PD-1/PDL-1 to impair the activation of effector T cells. Inactivation of effector T cells maintains self-tolerance, preventing autoimmune response in AA.
CTLA-4 is an inhibitory receptor induced in effector T cells upon activation and constitutively expressed by Tregs. CTLA-4 preferentially binds to B7 co-stimulatory molecules on APCs, depriving effector T cells of the CD28 co-stimulatory signal and impairs the activation of effector T cells [76]. Consequently, CTLA-4 is critical in maintenance of tolerance to self-antigens to prevent autoimmunity. Reduced expression or function of CTLA-4 can lead to autoimmune T cell clonal proliferation and contribute to the pathogenesis of autoimmune diseases, including AA (Figure 3A). Genetic studies including a genome-wide association study have confirmed CTLA-4 as a proven susceptibility gene for AA [78-80].
Jeffery et al [81] showed a striking ability of 1,25(OH)2D3 to enhance CTLA-4. Kickler et al [82] found that in CD4+ T cells isolated from healthy donors, addition of calcitriol promoted CTLA-4 expression in activated T cells. A similar increase in CTLA-4 was detected in multiple sclerosis T cells. Sheikh et al [33] studied the effect of VitD3 on human CD4+ T cell proliferation in APC free condition in vitro. They found that the frequency of CTLA4 expression on CD4+ T cells showed significant enhancement in the cultures with VitD3 compared with the cultures with no VitD3. Van Belle et al [83] showed that treatment of 1,25(OH)2D3 increases the expression of CTLA-4 in type 1 diabetes patient’s T cells. By increasing the expression of CTLA-4, vitamin D may contribute to maintaining peripheral immune tolerance. This may play a protective role in AA (Figure 3B).
PD-1 is an immune-inhibitory receptor expressed in T cells, B cells, natural killer (NK) cells and antigen presenting cells. PD-L1 and PD-L2 are two known ligands of PD-1. PD-L1 can be found on several immune cells and in various tissues. Once PD-1 binds to PD-L1, it generates a strong inhibitory signal, resulting in down-regulation of pro-inflammatory T-cell activity [76]. As such, PD-1/PDL-1 plays a key role for the maintenance of self-tolerance. Of note, patients receiving PD-1 inhibitors are at risk for increased AA. PD-1 inhibitors block the immune system’s typical regulatory measure, including T cell inactivation and likely facilitates the T-cell mediated attack on the hair bulb that leads to AA [84] (Figure 3B).
Sheikh et al [33] observed a significant increase of PD-1 and PD-L1 on CD4+ T cells isolated from normal human when VitD3 was added to the cell cultures in APC free condition in vitro. Dimitrov et al [85] found that 1,25D treatment increases expression of PD-L1 in human epithelial cells. In co-culture experiments with primary human T cells, epithelial cells pretreated with 1,25D suppress activation of CD4+ and CD8+ cells and inhibit inflammatory cytokine production in a manner that is abrogated by anti-PD-L1 blocking antibody. Bak et al [86] reported that high dose vitamin D3 supplementation significantly increases the intestinal expression of the inhibitory ligand PD-L1 in healthy subjects, suggesting that high vitamin D levels may have a beneficial effect in the of a balanced immune response in the intestine. Bendix et al [87] investigated the PD-1 expression in T cells from Crohn’s disease patients who had received oral vitamin D or placebo. They found that vitamin D treatment significantly increased the PD-1 expression upon TCR stimulation in activated T cells, while the expression remained unchanged in patients who received placebo. Vanherwegen et al [88] found that human monocyte-derived dendritic cells treated with 1,25(OH)2D3 displayed a stable tolerogenic phenotype with increased inhibitory molecules including PD-L1. Taken together, down-regulation of PD-1 facilitates the breakdown of self-tolerance that results in the autoimmune response in AA. The effect of vitamin D to up-regulate PD-1/PDL-1 may restore the immune tolerance and contribute to prevent the autoimmune response in AA (Figure 3B).
OS
OS and damage to SOD may play a part in the induction of alopecia areata [1,89].
OS is a common feature in autoimmune. It occurs as a result of inadequate antioxidant defense or overproduction of reactive oxygen species (ROS). Lipid peroxidation represents the hallmark of OS. Lipid peroxides and their breaking-down products such as malondialdehyde (MDA) can affect the normal function of most mammalian cells [90]. A common method used to assess the changes in MDA production is thiobarbituric acid reactive substances (TBARS) assay [91]. The damaging effect of ROS is counteracted by the action of antioxidants. Enzymatic antioxidant response is carried out by superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) related enzymes such as glutathione peroxidase (GPx) [92].
Studies have revealed an association between OS and AA. Bakry et al [93] showed that the serum total oxidant capacity (TOC), MDA, and oxidative stress index (OSI) values are significantly higher in AA patients than in the control group. The serum total antioxidant capacity (TAC) value is significantly lower in AA cases than in controls. Significantly higher MDA, TOC, and OSI values and lower TAC values are found in severe AA than in mild or moderate AA. Two recent review articles documented that OS plays a part in the induction of AA [1,89]. A study on organ cultured HFs showed that ROS exposure can induce hair growth retardation and catagen induction [94]. By this way, OS may contribute to the hair cycle dysfunction in AA.
Currently, Vitamin D has been shown as an antioxidant [95]. Randomized, double-blind, placebo-controlled clinical trials performed in patients with major depressive disorder [96], patients with non-alcoholic fatty liver disease [97], patients in maintenance methadone treatment [98] and patients with diabetic foot ulcer [99] demonstrated that vitamin D intake resulted in significantly increases in TAC [96,98] and GSH [96,98] levels and significant decrease in MDA level [97,99] compared with the placebo. In a systematic review and meta-analysis of randomized controlled trials including 33 studies, it was found that vitamin D supplementation significantly reduced serum MDA levels while significantly increased TAC and total GSH levels in diabetic patients [100]. In a systematic review and meta-analysis including 7 randomized controlled trials, vitamin D supplementation in women with polycystic ovary syndrome significantly decreased MDA and significantly increased TAC levels [101]. In a systematic review and meta-analysis published in 2019 including 17 trials in patients with different diseases, it was found that vitamin D supplementation significantly increased serum levels of TAC and GSH, and significantly decreased MDA concentration compared to placebo [102]. These data indicate that vitamin D can restore the oxidant-antioxidant balance in multiple clinical settings. Thus it can be inferred that vitamin D may also contribute to attenuate the OS in AA.
One of the important genes activated by vitamin D is Nrf2 [103]. Nrf2 encodes the transcription factor, nuclear factor (erythroid-derived 2)-like 2 (Nrf2), which maintains redox homeostasis and is defined in the HF. The activation of Nrf2 can maintain hair growth during OS [104]. 1,25(OH)2D3 prevents leptin-induced OS in human endothelial cells by activating the Nrf2 system [105]. More recently, the study of Chen et al [106] provided a model that suggests that 1,25(OH)2D3 deficiency results in increasing oxidative stress through inhibiting transcription of Nrf2 and enhancing DNA damage. Their results indicate that 1,25(OH)2D3 exerts an antioxidant role in large part by transcriptional regulation of Nrf2 mediated through the VDR. These data suggest that vitamin D may contribute to restore the hair growth cycle via activation of Nrf2.
To sum up, data published in literature suggest that vitamin D, due to its diverse biological effects, plays important roles in the complex pathogenesis of AA. Understanding the possible roles of vitamin D in AA may provide new insights into research direction to elucidate the exact mechanisms of vitamin D in the pathogenesis of AA.
Vitamin D for treatment of AA
Since vitamin D plays a role in the pathogenesis of AA, it may be considered as a treatment for the disease. The beneficial effects of 1,25(OH)2D3 supplementation have been observed in experimental autoimmune models, but the systemic use of vitamin D in the treatment of human autoimmune diseases is still under investigation [107]. In the experimental autoimmune models, animals are mostly supplemented with a high dose of 1,25(OH)2D3, but in humans, this strategy may lead to hypercalcemia [107]. In clinical application of active vitamin D, the supraphysiological doses needed to modulate immune responses may elicit concomitant calcemic side effects. To overcome this limitation, hypocalcemic analogs of active vitamin D with similar immunoregulatory activity are being exploited. Calcipotriol, a vitamin D3 analogue, which is at least 100 times less calcemic than calcitriol [108], has been topically used in treating psoriasis with beneficial effects. Early in 1991, Berth-Jones and Hutchinson [109] reported a group of 20 patients with alopecia totalis or universalis studied by placebo-controlled double-blind design. Each subject applied ointment containing 50 pg/g calcipotriol to one side of the scalp and matching vehicle to the other. There was no evidence of a response to calcipotriol in this group of subjects with very severe alopecia. However, recent studies reported encouraging results. In 2012, Kim et al [4] reported a 7-year-old boy with reduced VDR expression, whose AA did not respond to various treatments, including topical and intralesional corticosteroids. Recovery of whom was observed by topical application of calcipotriol. In 2015, Çerman et al [5] treated 48 mild-to-moderate AA patients with calcipotriol cream. At week 12, the total response was achieved in 69.2% of patients. The mean Severity of Alopecia Tool (SALT) score of patients at week 12 was significantly lower than that at baseline. Complete regrowth rate (hair regrowth = 100%) was 27.1%. The authors concluded that calcipotriol may serve as a safe and effective treatment option in mild-to-moderate patchy AA. More recently, Narang et al [6] conducted a prospective study, in which 22 patients with AA were treated with calcipotriol lotion 0.005% twice daily for 3 months. After 12 weeks of treatment, hair regrowth was observed in 13 (59.1%) patients. Mean period for onset of disease stabilization and hair regrowth was 4 weeks and 4.21±2.13 weeks, respectively. Among these 13 patients, SALT50 and SALT100 was observed in 6 (46.2%) and 2 (9%) patients, respectively. Response to treatment was significantly better in patients with lower vitamin D levels. The authors concluded that topical calcipotriol can be an alternative treatment in AA. Taken together, trials of topical calcipotriol are promising. Further studies, especially larger placebo-controlled double-blind trials are required to confirm these findings. Besides, potential efficacy of systemic vitamin D or its analogs in the treatment of AA still should be investigated in the future.
Combination therapy may have complementary effects on the underlying pathophysiology of a disease, resulting in increased therapeutic response and attenuates side effects associated with its individual components. Vitamin D has been used as combination therapies in treating diseases. Topical corticosteroids/vitamin D analogs combination therapy has been successfully used in treating psoriasis. Recent studies showed that corticosteroids/vitamin D analog combination therapy is more effective than the respective monotherapies in inducing immunomodulation and normalizing keratinocytes [110]. Vitiligo and AA are both autoimmune diseases, and striking similarities in immune-pathogenesis have been identified [111]. Topical calcipotriol in combination with corticosteroids has also been reported to effectively treat vitiligo [112]. The calcipotriol/corticoteroid combined therapy appeared to give a significantly faster onset and better stability of re-pigmentation along with lesser side-effects than single agent treatments [113]. Therefore, we suppose that combination therapy of vitamin D analogs and corticosteroids might also be beneficial in treating AA.
Conclusion
Studies have shown a substantial link between the levels of vitamin D and AA, even though the underlying mechanism is not well known. By reviewing relevant literature data, we put forward the possible roles of vitamin D in AA pathogenesis as follows: (1) contribute to maintain the IP of HF by decreasing the production of IFN-γ; (2) attenuate or prevent the autoimmune response that drives AA by regulating the autoreactive effector T cells and mast cells; also by down-regulating CXCL-10 and the intercellular TLRs; (3) contribute to prevent the skin NKG2D+CD8+ T cell activation and trafficking in AA by down-regulating both NKG2D-activating and CXCR3-activating ligands; (4) inhibit JAK/STAT pathway and in turn block the effects of key cytokines in the pathogenesis of AA; (5) control the overactive self-effector T cells by enhancing the inhibitory function of Treg T cells; (6) by increasing the expression of CTLA-4 and PD-1; and (7) contribute to restoring the hair growth cycle by attenuating the OS in AA. This provides new insights into research direction to further elucidate the mechanisms of vitamin D in the pathogenesis of AA. As a link between vitamin D and AA pathogenesis has been establish, vitamin D should be considered for the treatment of AA. However, systemic supplement of vitamin D in the treatment of human autoimmune diseases is still under investigation. Calcipotriol, a vitamin D analog, has been used topically in treating AA with promising results. In the future, vitamin D analogs and corticosteroids combination treatment might provide potential benefit in the treatment of AA.
Disclosure of conflict of interest
None.
References
- 1.Pratt CH, King LE Jr, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. doi: 10.1038/nrdp.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, Shapiro J. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1–12. doi: 10.1016/j.jaad.2017.04.1141. [DOI] [PubMed] [Google Scholar]
- 3.Tsai TY, Huang YC. Vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;78:207–209. doi: 10.1016/j.jaad.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Lee JW, Kim IS, Choi SY, Lim YY, Kim HM, Kim BJ, Kim MN. Successful treatment of alopecia areata with topical calcipotriol. Ann Dermatol. 2012;24:341–344. doi: 10.5021/ad.2012.24.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Çerman AA, Solak SS, Altunay İ, Küçükünal NA. Topical calcipotriol therapy for mild-to-moderate alopecia areata: a retrospective study. J Drugs Dermatol. 2015;14:616–620. [PubMed] [Google Scholar]
- 6.Narang T, Daroach M, Kumaran MS. Efficacy and safety of topical calcipotriol in management of alopecia areata: a pilot study. Dermatol Ther. 2017;30 doi: 10.1111/dth.12464. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Chen W, Li D, Yin X, Zhang X, Olsen N, Zheng SG. Vitamin D and chronic diseases. Aging Dis. 2017;8:346–353. doi: 10.14336/AD.2016.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D. The role of vitamin D in thyroid diseases. Int J Mol Sci. 2017;18:E1949. doi: 10.3390/ijms18091949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umar M, Sastry KS, Chouchane AI. Role of vitamin D beyond the skeletal function: a review of the molecular and clinical studies. Int J Mol Sci. 2018;19:E1618. doi: 10.3390/ijms19061618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yilmaz N, Serarslan G, Gokce C. Vitamin D concentrations are decreased in patients with alopecia areata. Vitam Miner. 2012;1:105–109. [Google Scholar]
- 12.Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299–1304. doi: 10.1111/bjd.12980. [DOI] [PubMed] [Google Scholar]
- 13.Mahamid M, Abu-Elhija O, Samamra M, Mahamid A, Nseir W. Association between vitamin D levels and alopecia areata. Isr Med Assoc J. 2014;16:367–370. [PubMed] [Google Scholar]
- 14.Bakry OA, El Farargy SM, El Shafiee MK, Soliman A. Serum Vitamin D in patients with alopecia areata. Indian Dermatol Online J. 2016;7:371–377. doi: 10.4103/2229-5178.190504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghafoor R, Anwar MI. Vitamin D deficiency in alopecia areata. J Coll Physicians Surg Pak. 2017;27:200–202. [PubMed] [Google Scholar]
- 16.Bhat YJ, Latif I, Malik R, Hassan I, Sheikh G, Lone KS, Majeed S, Sajad P. Vitamin D level in alopecia areata. Indian J Dermatol. 2017;62:407–410. doi: 10.4103/ijd.IJD_677_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gade VKV, Mony A, Munisamy M, Chandrashekar L, Rajappa M. An investigation of vitamin D status in alopecia areata. Clin Exp Med. 2018;18:577–584. doi: 10.1007/s10238-018-0511-8. [DOI] [PubMed] [Google Scholar]
- 18.d’Ovidio R, Vessio M, d’Ovidio FD. Reduced level of 25-hydroxyvitamin D in chronic/relapsing Alopecia Areata. Dermatoendocrinol. 2013;5:271–273. doi: 10.4161/derm.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daroach M, Narang T, Saikia UN, Sachdeva N, Sendhil Kumaran M. Correlation of vitamin D and vitamin D receptor expression in patients with alopecia areata: a clinical paradigm. Int J Dermatol. 2018;57:217–222. doi: 10.1111/ijd.13851. [DOI] [PubMed] [Google Scholar]
- 20.Erpolat S, Sarifakioglu E, Ayyildiz A. 25-hydroxyvitamin D status in patients with alopecia areata. Postepy Dermatol Alergol. 2017;34:248–252. doi: 10.5114/ada.2017.67847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson JM, Li T, Park MK, Qureshi AA, Cho E. Estimated serum vitamin D status, vitamin D intake, and risk of incident alopecia areata among US women. Arch Dermatol Res. 2016;308:671–676. doi: 10.1007/s00403-016-1687-y. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Kim BJ, Lee CH, Lee WS. Increased prevalence of vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:1214–1221. doi: 10.1111/jdv.14987. [DOI] [PubMed] [Google Scholar]
- 23.Unal M, Gonulalan G. Serum vitamin D level is related to disease severity in pediatric alopecia areata. J Cosmet Dermatol. 2018;17:101–104. doi: 10.1111/jocd.12352. [DOI] [PubMed] [Google Scholar]
- 24.Fawzi MM, Mahmoud SB, Ahmed SF, Shaker OG. Assessment of vitamin D receptors in alopecia areata and androgenetic alopecia. J Cosmet Dermatol. 2016;15:318–323. doi: 10.1111/jocd.12224. [DOI] [PubMed] [Google Scholar]
- 25.Mady LJ, Ajibade DV, Hsaio C, Teichert A, Fong C, Wang Y, Christakos S, Bikle DD. The transient role for calcium and vitamin D during the developmental hair follicle cycle. J Invest Dermatol. 2016;136:1337–1345. doi: 10.1016/j.jid.2016.02.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SM, Pike JW. The vitamin D receptor functions as a transcription regulator in the absence of 1,25-dihydroxyvitamin D(3) J Steroid Biochem Mol Biol. 2016;164:265–270. doi: 10.1016/j.jsbmb.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Nan W, Wang S, Zhang T, Si H, Yang F, Li G. Epidermal growth factor promotes proliferation and migration of follicular outer root sheath cells via Wnt/β-catenin signaling. Cell Physiol Biochem. 2016;39:360–370. doi: 10.1159/000445630. [DOI] [PubMed] [Google Scholar]
- 28.Gerkowicz A, Chyl-Surdacka K, Krasowska D, Chodorowska G. The role of vitamin D in non-scarring alopecia. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18122653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen Y, Daich J, Soliman I, Brathwaite E, Shoenfeld Y. Vitamin D and autoimmunity. Scand J Rheumatol. 2016;45:439–447. doi: 10.3109/03009742.2016.1151072. [DOI] [PubMed] [Google Scholar]
- 30.Paus R, Bulfone-Paus S, Bertolini M. Hair follicle immune privilege revisited: the key to alopecia areata management. J Investig Dermatol Symp Proc. 2018;19:S12–S17. doi: 10.1016/j.jisp.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Ragab D, Soliman D, Samaha D, Yassin A. Vitamin D status and its modulatory effect on interferon gamma and interleukin-10 production by peripheral blood mononuclear cells in culture. Cytokine. 2016;85:5–10. doi: 10.1016/j.cyto.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Fawaz L, Mrad MF, Kazan JM, Sayegh S, Akika R, Khoury SJ. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol. 2016;166-167:59–71. doi: 10.1016/j.clim.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Sheikh V, Kasapoglu P, Zamani A, Basiri Z, Tahamoli-Roudsari A, Alahgholi-Hajibehzad M. Vitamin D3 inhibits the proliferation of T helper cells, downregulate CD4(+) T cell cytokines and upregulate inhibitory markers. Hum Immunol. 2018;79:439–445. doi: 10.1016/j.humimm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Kokic V, Martinovic Kaliterna D, Radic M, Tandara L, Perkovic D. Association between vitamin D, oestradiol and interferon-gamma in female patients with inactive systemic lupus erythematosus: a cross-sectional study. J Int Med Res. 2018;46:1162–1171. doi: 10.1177/0300060517734686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mrad MF, El Ayoubi NK, Esmerian MO, Kazan JM, Khoury SJ. Effect of vitamin D replacement on immunological biomarkers in patients with multiple sclerosis. Clin Immunol. 2017;181:9–15. doi: 10.1016/j.clim.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Guo H, Cheng Y, Shapiro J, McElwee K. The role of lymphocytes in the development and treatment of alopecia areata. Expert Rev Clin Immunol. 2015;11:1335–1351. doi: 10.1586/1744666X.2015.1085306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanemura A, Oiso N, Nakano M, Itoi S, Kawada A, Katayama I. Alopecia areata: infiltration of Th17 cells in the dermis, particularly around hair follicles. Dermatology. 2013;226:333–336. doi: 10.1159/000350933. [DOI] [PubMed] [Google Scholar]
- 38.Hong JW, Lee CY, Ha SM, Choi SH, Kim TH, Song KH, Kim KH. The contributory roles of Th17 lymphocyte and cytotoxic T lymphocyte at the hair bulge region as well as the hair bulb area in the chronic alopecia areata patients. Ann Dermatol. 2017;29:156–166. doi: 10.5021/ad.2017.29.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schardey J, Globig AM, Janssen C, Hofmann M, Manegold P, Thimme R, Hasselblatt P. Vitamin D inhibits pro-inflammatory T cell function in patients with inflammatory bowel disease. J Crohns Colitis. 2019 doi: 10.1093/ecco-jcc/jjz090. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Penna-Martinez M, Filmann N, Bogdanou D, Shoghi F, Huenecke S, Schubert R, Herrmann E, Koehl U, Husebye ES, Badenhoop K. High-dose vitamin D in Addison’s disease regulates T-cells and monocytes: a pilot trial. Nutrition. 2018;49:66–73. doi: 10.1016/j.nut.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Tom MR, Li J, Ueno A, Fort Gasia M, Chan R, Hung DY, Chenoo S, Iacucci M, Jijon HB, Kaplan GG, Beck PL, Panaccione R, Barkema HW, Buret AG, Yajnik V, Ghosh S. Novel CD8+ T-cell subsets demonstrating plasticity in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:1596–1608. doi: 10.1097/MIB.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 43.Hamzaoui A, Berraïes A, Hamdi B, Kaabachi W, Ammar J, Hamzaoui K. Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology. 2014;219:873–879. doi: 10.1016/j.imbio.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Reihani H, Rastin M, Mahmoudi M, Ghoryani M, Abdollahi N, Tabasi NS, Zamani Taghizadeh Rabe S, Sahebari M. Influence of 1 alpha, 25-dihydroxyvitamin D3 on T helper 17 cells and related cytokines in systemic lupus erythematosus. Iran J Immunol. 2015;12:82–93. [PubMed] [Google Scholar]
- 45.Wen HY, Luo J, Li XF, Wei DD, Liu Y. 1,25-Dihydroxyvitamin D(3) modulates T cell differentiation and impacts on the production of cytokines from Chinese Han patients with early rheumatoid arthritis. Immunol Res. 2019;67:48–57. doi: 10.1007/s12026-018-9033-4. [DOI] [PubMed] [Google Scholar]
- 46.Nodehi M, Ajami A, Izad M, Asgarian Omran H, Chahardoli R, Amouzegar A, Yekaninejad S, Hemmatabadi M, Azizi F, Esfahanian F, Mansouri F, Mazaheri Nezhad Fard R, Saboor-Yaraghi AA. Effects of vitamin D supplements on frequency of CD4(+) T-cell subsets in women with Hashimoto’s thyroiditis: a double-blind placebo-controlled study. Eur J Clin Nutr. 2019;73:1236–1243. doi: 10.1038/s41430-019-0395-z. [DOI] [PubMed] [Google Scholar]
- 47.Jin B, Sun T, Yu XH, Yang YX, Yeo AE. The effects of TLR activation on T-cell development and differentiation. Clin Dev Immunol. 2012;2012:836485. doi: 10.1155/2012/836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alzolibani AA, Rasheed Z, Bin Saif G, Al-Dhubaibi MS, Al Robaee AA. Altered expression of intracellular Toll-like receptors in peripheral blood mononuclear cells from patients with alopecia areata. BBA Clin. 2016;5:134–142. doi: 10.1016/j.bbacli.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yazdanpanah E, Mahmoudi M, Sahebari M, Rezaieyazdi Z, Esmaeili SA, Tabasi N, Jaberi S, Sahebkar A, Rastin M. Vitamin D3 alters the expression of Toll-like receptors in peripheral blood mononuclear cells of patients with systemic lupus erythematosus. J Cell Biochem. 2017;118:4831–4835. doi: 10.1002/jcb.26155. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Zhao Y, Ye Y, Li S, Qi S, Yang Y, Cao H, Yang J, Zhang X. Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia areata. Arch Dermatol Res. 2015;307:319–331. doi: 10.1007/s00403-015-1539-1. [DOI] [PubMed] [Google Scholar]
- 51.Bertolini M, Zilio F, Rossi A, Kleditzsch P, Emelianov VE, Gilhar A, Keren A, Meyer KC, Wang E, Funk W, McElwee K, Paus R. Abnormal interactions between perifollicular mast cells and CD8+ T-cells may contribute to the pathogenesis of alopecia areata. PLoS One. 2014;9:e94260. doi: 10.1371/journal.pone.0094260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theoharides TC. Vitamin D and atopy. Clin Ther. 2017;39:880–883. doi: 10.1016/j.clinthera.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Yip KH, Kolesnikoff N, Yu C, Hauschild N, Taing H, Biggs L, Goltzman D, Gregory PA, Anderson PH, Samuel MS, Galli SJ, Lopez AF, Grimbaldeston MA. Mechanisms of vitamin D3 metabolite repression of IgE-dependent mast cell activation. J Allergy Clin Immunol. 2014;133:1356–64. 1364.e1–14. doi: 10.1016/j.jaci.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu ZQ, Li XX, Qiu SQ, Yu Y, Li MG, Yang LT, Li LJ, Wang S, Zheng PY, Liu ZG, Yang PC. Vitamin D contributes to mast cell stabilization. Allergy. 2017;72:1184–1192. doi: 10.1111/all.13110. [DOI] [PubMed] [Google Scholar]
- 55.Dai Z, Xing L, Cerise J, Wang EH, Jabbari A, de Jong A, Petukhova L, Christiano AM, Clynes R. CXCR3 blockade inhibits T cell migration into the skin and prevents development of alopecia areata. J Immunol. 2016;197:1089–1099. doi: 10.4049/jimmunol.1501798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546. doi: 10.1155/2013/348546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seydel S, Beilfuss A, Kahraman A, Aksoy K, Gerken G, Akkiz H, Canbay A. Vitamin D ameliorates stress ligand expression elicited by free fatty acids in the hepatic stellate cell line LX-2. Turk J Gastroenterol. 2011;22:400–407. doi: 10.4318/tjg.2011.0254. [DOI] [PubMed] [Google Scholar]
- 58.Reynolds JA, Haque S, Williamson K, Ray DW, Alexander MY, Bruce IN. Vitamin D improves endothelial dysfunction and restores myeloid angiogenic cell function via reduced CXCL-10 expression in systemic lupus erythematosus. Sci Rep. 2016;6:22341. doi: 10.1038/srep22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hosokawa Y, Hosokawa I, Shindo S, Ozaki K, Matsuo T. Calcitriol suppressed inflammatory reactions in IL-1β-stimulated human periodontal ligament cells. Inflammation. 2015;38:2252–2258. doi: 10.1007/s10753-015-0209-y. [DOI] [PubMed] [Google Scholar]
- 60.Komolmit P, Charoensuk K, Thanapirom K, Suksawatamnuay S, Thaimai P, Chirathaworn C, Poovorawan Y. Correction of vitamin D deficiency facilitated suppression of IP-10 and DPP IV levels in patients with chronic hepatitis C: a randomised double-blinded, placebo-control trial. PLoS One. 2017;12:e0174608. doi: 10.1371/journal.pone.0174608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu B, Du Y, Feng Y, Wang Q, Pang W, Qi Z, Wang J, Yang D, Liu Y, Cao Y. Oral administration of vitamin D and importance in prevention of cerebral malaria. Int Immunopharmacol. 2018;64:356–363. doi: 10.1016/j.intimp.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 62.Crowley EL, Fine SC, Katipunan KK, Gooderham MJ. The use of janus kinase inhibitors in alopecia areata: a review of the literature. J Cutan Med Surg. 2019;23:289–297. doi: 10.1177/1203475418824079. [DOI] [PubMed] [Google Scholar]
- 63.Blume-Peytavi U, Vogt A. Translational positioning of janus kinase (JAK) inhibitors in alopecia areata. EBioMedicine. 2015;2:282–283. doi: 10.1016/j.ebiom.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alves de Medeiros AK, Speeckaert R, Desmet E, Van Gele M, De Schepper S, Lambert J. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One. 2016;11:e0164080. doi: 10.1371/journal.pone.0164080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, de Jong A, Harel S, DeStefano GM, Rothman L, Singh P, Petukhova L, Mackay-Wiggan J, Christiano AM, Clynes R. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang P, Zhang W, Zhang D, Wang M, Aprecio R, Ji N, Mohamed O, Li Y, Ding Y, Wang Q. 25-Hydroxyvitamin D(3) -enhanced PTPN2 positively regulates periodontal inflammation through the JAK/STAT pathway in human oral keratinocytes and a mouse model of type 2 diabetes mellitus. J Periodontal Res. 2018;53:467–477. doi: 10.1111/jre.12535. [DOI] [PubMed] [Google Scholar]
- 67.Zeitelhofer M, Adzemovic MZ, Gomez-Cabrero D, Bergman P, Hochmeister S, N’diaye M, Paulson A, Ruhrmann S, Almgren M, Tegnér JN, Ekström TJ, Guerreiro-Cacais AO, Jagodic M. Functional genomics analysis of vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2017;114:E1678–E1687. doi: 10.1073/pnas.1615783114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Speiser JJ, Mondo D, Mehta V, Marcial SA, Kini A, Hutchens KA. Regulatory T-cells in alopecia areata. J Cutan Pathol. 2019;46:653–658. doi: 10.1111/cup.13479. [DOI] [PubMed] [Google Scholar]
- 69.Jabbari A, Petukhova L, Cabral RM, Clynes R, Christiano AM. Genetic basis of alopecia areata: a roadmap for translational research. Dermatol Clin. 2013;31:109–117. doi: 10.1016/j.det.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han YM, Sheng YY, Xu F, Qi SS, Liu XJ, Hu RM, Miao Y, Huang GQ, Yang QP. Imbalance of T-helper 17 and regulatory T cells in patients with alopecia areata. J Dermatol. 2015;42:981–988. doi: 10.1111/1346-8138.12978. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Q, Qin S, Zhang J, Zhon L, Pen Z, Xing T. 1,25(OH)(2)D(3) induces regulatory T cell differentiation by influencing the VDR/PLC-γ1/TGF-β1/pathway. Mol Immunol. 2017;91:156–164. doi: 10.1016/j.molimm.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Vijayendra Chary A, Hemalatha R, Seshacharyulu M, Vasudeva Murali M, Jayaprakash D, Dinesh Kumar B. Vitamin D deficiency in pregnant women impairs regulatory T cell function. J Steroid Biochem Mol Biol. 2015;147:48–55. doi: 10.1016/j.jsbmb.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 73.Mattozzi C, Paolino G, Salvi M, Macaluso L, Luci C, Morrone S, Calvieri S, Richetta AG. Peripheral blood regulatory T cell measurements correlate with serum vitamin D level in patients with psoriasis. Eur Rev Med Pharmacol Sci. 2016;20:1675–1679. [PubMed] [Google Scholar]
- 74.Gorman S, Geldenhuys S, Judge M, Weeden CE, Waithman J, Hart PH. Dietary vitamin D increases percentages and function of regulatory T cells in the skin-draining lymph nodes and suppresses dermal inflammation. J Immunol Res. 2016;2016:1426503. doi: 10.1155/2016/1426503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prietl B, Treiber G, Mader JK, Hoeller E, Wolf M, Pilz S, Graninger WB, Obermayer-Pietsch BM, Pieber TR. High-dose cholecalciferol supplementation significantly increases peripheral CD4+ Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur J Nutr. 2014;53:751–759. doi: 10.1007/s00394-013-0579-6. [DOI] [PubMed] [Google Scholar]
- 76.Miko E, Meggyes M, Doba K, Barakonyi A, Szereday L. Immune checkpoint molecules in reproductive immunology. Front Immunol. 2019;10:846. doi: 10.3389/fimmu.2019.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zarbo A, Belum VR, Sibaud V, Oudard S, Postow MA, Hsieh JJ, Motzer RJ, Busam KJ, Lacouture ME. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649–1652. doi: 10.1111/bjd.15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, Kim H, Singh P, Lee A, Chen WV, Meyer KC, Paus R, Jahoda CA, Amos CI, Gregersen PK, Christiano AM. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–117. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.John KK, Brockschmidt FF, Redler S, Herold C, Hanneken S, Eigelshoven S, Giehl KA, De Weert J, Lutz G, Kruse R, Wolff H, Blaumeiser B, Böhm M, Becker T, Nöthen MM, Betz RC. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169–1172. doi: 10.1038/jid.2010.427. [DOI] [PubMed] [Google Scholar]
- 80.Megiorni F, Mora B, Maxia C, Gerardi M, Pizzuti A, Rossi A. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in Alopecia Areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305:665–670. doi: 10.1007/s00403-013-1348-3. [DOI] [PubMed] [Google Scholar]
- 81.Jeffery LE, Qureshi OS, Gardner D, Hou TZ, Briggs Z, Soskic B, Baker J, Raza K, Sansom DM. Vitamin D antagonises the suppressive effect of inflammatory cytokines on CTLA-4 expression and regulatory function. PLoS One. 2015;10:e0131539. doi: 10.1371/journal.pone.0131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kickler K, Ni Choileain S, Williams A, Richards A, Astier AL. Calcitriol modulates the CD46 pathway in T cells. PLoS One. 2012;7:e48486. doi: 10.1371/journal.pone.0048486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Belle TL, Vanherwegen AS, Feyaerts D, De Clercq P, Verstuyf A, Korf H, Gysemans C, Mathieu C. 1,25-Dihydroxyvitamin D3 and its analog TX527 promote a stable regulatory T cell phenotype in T cells from type 1 diabetes patients. PLoS One. 2014;9:e109194. doi: 10.1371/journal.pone.0109194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guidry J, Brown M, Medina T. PD-1 inhibitor induced alopecia areata. Dermatol Online J. 2018;24 [PubMed] [Google Scholar]
- 85.Dimitrov V, Bouttier M, Boukhaled G, Salehi-Tabar R, Avramescu RG, Memari B, Hasaj B, Lukacs GL, Krawczyk CM, White JH. Hormonal vitamin D up-regulate tissue-specific PD-L1 and PD-L2 surface glycoprotein expression in humans but not mice. J Biol Chem. 2017;292:20657–20668. doi: 10.1074/jbc.M117.793885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bak NF, Bendix M, Hald S, Reinert L, Magnusson MK, Agnholt J. High-dose vitamin D(3) supplementation decreases the number of colonic CD103(+) dendritic cells in healthy subjects. Eur J Nutr. 2018;57:2607–2619. doi: 10.1007/s00394-017-1531-y. [DOI] [PubMed] [Google Scholar]
- 87.Bendix M, Greisen S, Dige A, Hvas CL, Bak N, Jørgensen SP, Dahlerup JF, Deleuran B, Agnholt J. Vitamin D increases programmed death receptor-1 expression in Crohn’s disease. Oncotarget. 2017;8:24177–24186. doi: 10.18632/oncotarget.15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vanherwegen AS, Cook DP, Ferreira GB, Gysemans C, Mathieu C. Vitamin D-modulated dendritic cells delay lethal graft-versus-host disease through induction of regulatory T cells. J Steroid Biochem Mol Biol. 2019;188:103–110. doi: 10.1016/j.jsbmb.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 89.Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: a multifactorial autoimmune condition. J Autoimmun. 2019;98:74–85. doi: 10.1016/j.jaut.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Prie BE, Voiculescu VM, Ionescu-Bozdog OB, Petrutescu B, Iosif L, Gaman LE, Clatici VG, Stoian I, Giurcaneanu C. Oxidative stress and alopecia areata. J Med Life. 2015;8:43–46. [PMC free article] [PubMed] [Google Scholar]
- 91.Siddique YH, Ara G, Afzal M. Estimation of lipid peroxidation induced by hydrogen peroxide in cultured human lymphocytes. Dose Response. 2012;10:1–10. doi: 10.2203/dose-response.10-002.Siddique. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quiñonez-Flores CM, González-Chávez SA, Del Río Nájera D, Pacheco-Tena C. Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. Biomed Res Int. 2016;2016:6097417. doi: 10.1155/2016/6097417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bakry OA, Elshazly RM, Shoeib MA, Gooda A. Oxidative stress in alopecia areata: a case-control study. Am J Clin Dermatol. 2014;15:57–64. doi: 10.1007/s40257-013-0036-6. [DOI] [PubMed] [Google Scholar]
- 94.Haslam IS, Jadkauskaite L, Szabó IL, Staege S, Hesebeck-Brinckmann J, Jenkins G, Bhogal RK, Lim FL, Farjo N, Farjo B, Bíró T, Schäfer M, Paus R. Oxidative damage control in a human (mini-) organ: Nrf2 activation protects against oxidative stress-induced hair growth inhibition. J Invest Dermatol. 2017;137:295–304. doi: 10.1016/j.jid.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 95.Afshari L, Amani R, Soltani F, Haghighizadeh MH, Afsharmanesh MR. The relation between serum Vitamin D levels and body antioxidant status in ischemic stroke patients: a case-control study. Adv Biomed Res. 2015;4:213. doi: 10.4103/2277-9175.166150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sepehrmanesh Z, Kolahdooz F, Abedi F, Mazroii N, Assarian A, Asemi Z, Esmaillzadeh A. Vitamin D supplementation affects the beck depression inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: a randomized, controlled clinical trial. J Nutr. 2016;146:243–248. doi: 10.3945/jn.115.218883. [DOI] [PubMed] [Google Scholar]
- 97.Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47:70–80. doi: 10.1007/s12020-014-0336-5. [DOI] [PubMed] [Google Scholar]
- 98.Ghaderi A, Banafshe HR, Motmaen M, Rasouli-Azad M, Bahmani F, Asemi Z. Clinical trial of the effects of vitamin D supplementation on psychological symptoms and metabolic profiles in maintenance methadone treatment patients. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:84–89. doi: 10.1016/j.pnpbp.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 99.Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, Asemi Z. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complications. 2017;31:766–772. doi: 10.1016/j.jdiacomp.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 100.Mansournia MA, Ostadmohammadi V, Doosti-Irani A, Ghayour-Mobarhan M, Ferns G, Akbari H, Ghaderi A, Talari HR, Asemi Z. The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2018;50:429–440. doi: 10.1055/a-0630-1303. [DOI] [PubMed] [Google Scholar]
- 101.Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, Heydari ST, Kavari SH, Mirhosseini N, Mafi A, Dastorani M, Asemi Z. The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2018;50:271–279. doi: 10.1055/s-0044-101355. [DOI] [PubMed] [Google Scholar]
- 102.Sepidarkish M, Farsi F, Akbari-Fakhrabadi M, Namazi N, Almasi-Hashiani A, Maleki Hagiagha A, Heshmati J. The effect of vitamin D supplementation on oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Pharmacol Res. 2019;139:141–152. doi: 10.1016/j.phrs.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 103.Berridge MJ. Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150434. doi: 10.1098/rstb.2015.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jadkauskaite L, Coulombe PA, Schäfer M, Dinkova-Kostova AT, Paus R, Haslam IS. Oxidative stress management in the hair follicle: could targeting NRF2 counter age-related hair disorders and beyond? Bioessays. 2017;39 doi: 10.1002/bies.201700029. [DOI] [PubMed] [Google Scholar]
- 105.Teixeira TM, da Costa DC, Resende AC, Soulage CO, Bezerra FF, Daleprane JB. Activation of Nrf2-antioxidant signaling by 1,25-dihydroxycholecalciferol prevents leptin-induced oxidative stress and inflammation in human endothelial cells. J Nutr. 2017;147:506–513. doi: 10.3945/jn.116.239475. [DOI] [PubMed] [Google Scholar]
- 106.Chen L, Yang R, Qiao W, Zhang W, Chen J, Mao L, Goltzman D, Miao D. 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell. 2019;18:e12951. doi: 10.1111/acel.12951. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol. 2017;7:697. doi: 10.3389/fimmu.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dubertret L, Wallach D, Souteyrand P, Perussel M, Kalis B, Meynadier J, Chevrant-Breton J, Beylot C, Bazex JA, Jurgensen HJ. Efficacy and safety of calcipotriol (MC 903) ointment in psoriasis vulgaris. A randomized, double-blind, right/left comparative, vehicle-controlled study. J Am Acad Dermatol. 1992;27:983–988. doi: 10.1016/0190-9622(92)70299-u. [DOI] [PubMed] [Google Scholar]
- 109.Berth-Jones J, Hutchinson PE. Alopecia totalis does not respond to the vitamin-D analogue calcipotriol. J Dermatol Treat. 1991;1:293–294. [Google Scholar]
- 110.Segaert S, Shear NH, Chiricozzi A, Thaçi D, Carrascosa JM, Young H, Descamps V. Optimizing anti-inflammatory and immunomodulatory effects of corticosteroid and vitamin D analogue fixed-dose combination therapy. Dermatol Ther (Heidelb) 2017;7:265–279. doi: 10.1007/s13555-017-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rork JF, Rashighi M, Harris JE. Understanding autoimmunity of vitiligo and alopecia areata. Curr Opin Pediatr. 2016;28:463–469. doi: 10.1097/MOP.0000000000000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ibrahim ZA, Hassan GF, Elgendy HY, Al-Shenawy HA. Evaluation of the efficacy of transdermal drug delivery of calcipotriol plus betamethasone versus tacrolimus in the treatment of vitiligo. J Cosmet Dermatol. 2019;18:581–588. doi: 10.1111/jocd.12704. [DOI] [PubMed] [Google Scholar]
- 113.Kumaran MS, Kaur I, Kumar B. Effect of topical calcipotriol, betamethasone dipropionate and their combination in the treatment of localized vitiligo. J Eur Acad Dermatol Venereol. 2006;20:269–273. doi: 10.1111/j.1468-3083.2006.01420.x. [DOI] [PubMed] [Google Scholar]


