Abstract
Traditional Chinese medicine theory indicates that Yu Jin Pulvis (YJP) could prevent liver fibrosis progression and this has been verified in liver fibrosis patients. However, the mechanism underlying the protective effects of YJP against liver fibrosis remains unclear. While different signaling pathways are involved in liver fibrosis progression, mitogen-activated protein kinase (MAPK) and phosphoinositide-3-kinase-protein kinase B/Akt (PI3K/Akt) are the most crucial. To determine whether YJP regulates these signaling pathways to prevent liver fibrosis, we used a mouse model of liver fibrosis induced by intraperitoneal injection of carbon tetrachloride (CCl4). Mice were randomly divided into normal, CCl4, YJP (300 mg/kg), CCl4+YJP (100, 200, and 300 mg/kg), and two positive control silybin (100 mg/kg) and Fuzheng Huayu (FZHY) capsule (2 g/kg) groups. The mice were gavaged daily for 6 weeks. Then liver fibrosis markers; tissue morphology; serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and proinflammatory cytokine levels; and expression of α-smooth muscle actin (α-SMA) and collagen type I (Col1) were examined to determine liver fibrosis progression. Liver injury and collagen deposition were significantly reduced in the YJP treatment group compared with the CCl4 group. Furthermore, the expression of phosphorylated-extracellular-signal-regulated kinase (p-ERK), p-jun N-terminal kinase (p-JNK), p-P38MAPK, p-PI3K and p-Akt was decreased by YJP treatment compared with CCl4 treatment. Collectively, these results demonstrate the antifibrosis effect of YJP on CCl4-induced liver fibrosis in mice, mediated through blockade of the MAPK and PI3K/Akt signaling pathways. Therefore, YJP has therapeutic potential against liver fibrosis.
Keywords: Carbon tetrachloride, Fuzheng Huayu capsule, liver fibrosis, signaling pathway, silybin, Yu Jin Pulvis
Introduction
Liver fibrosis, a wound-healing response to chronic liver and inflammatory diseases, is characterized by excessive accumulation of extracellular matrix (ECM) [1]. Under physiological condition, hepatic stellate cells (HSCs) maintain ECM homeostasis and accumulate vitamin A in the form of retinyl esters in cytoplasmic lipid droplets [2]. In response to pathological damage, quiescent HSCs lose lipid droplets, become more proliferative, and transdifferentiate into myofibroblasts, characterized by substantial up-regulation of α-smooth muscle actin (α-SMA) and collagen type I (Col1) [3]. Considerable evidence suggests that liver fibrosis is dynamic and is potentially reversible [4]. Therefore, reversing or inhibiting the proliferation of activated HSCs might be a potential strategy for inhibiting the deposition of ECM for fibrosis therapy [5].
Liver fibrosis can progress to liver cirrhosis, leading to organ failure and death [6]. Previous studies have shown that the mitogen-activated protein kinase (MAPK) and phosphoinositide-3-kinase-protein kinase B/Akt (PI3K/Akt) signaling pathways are associated with HSC proliferation and amelioration of liver fibrosis [7,8]. Inhibition of the MAPK signaling pathway down-regulates the expression of inflammatory factors, thereby improving the prognosis of inflammatory diseases [9]. The PI3K/Akt pathway is involved in regulating HSC activation, cell proliferation, and collagen synthesis, promoting the progression of hepatic fibrosis [10]. In this milieu, traditional Chinese medicine (TCM) formulation for liver fibrosis are particularly important. Fuzheng Huayu (FZHY) formula is an Food and Drug Administration (FDA) and Chinese State Food and Drug Administration (SFDA)-approved, effective, anti-fibrotic medicine [11,12]. The efficacy of TCM in the treatment of liver fibrosis has encouraged its application in the treatment of patients with liver fibrosis. Yu Jin Pulvis (YJP) is a TCM formula that has been widely used in Yanbian patients with cirrhosis, fatty liver, and chronic hepatitis. YJP can clear “liver-fire” and “gallbladder-heat”, activate blood circulation, remove stasis, clear heat, and help detoxify the body.
The pathogenesis of liver fibrosis is associated with multiple factors and links. Studies have explored the mechanism of TCM to prevent and treat liver fibrosis to develop effective antifibrosis drugs. In this study, we sought to determine whether YJP inhibits liver fibrosis progression through the MAPK and PI3K/Akt signaling pathways.
Materials and methods
Materials
YJP was prepared using the following traditional Chinese herbs: Radix Curcumae, frankincense, Herba Taraxaci, Carapax Trionycis, Excrementum pteropi, Pollen Typhae, mung bean, Panax pseudoginseng, and Commiphora in the ratio of 2:2:2:2:2:2:4:1:1, respectively. It was maintained at 4°C.
Silybin (Derick, Chengdu, China) and FZHY capsule (Shanghai Huang Hai Pharmaceutical Co., Ltd; Shanghai, China) were used as positive controls. Rabbit polyclonal anti-α-SMA (bs-10196R) and anti-Col1 (bs-0578R) antibodies were purchased from Bioss (Beijing, China). Mouse monoclonal antibodies against ERK (ab-54230), JNK (ab-179461), p-JNK (ab-124956), p-ERK (ab-5011) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, ab-8245) were purchased from Abcam (Cambridge, MA, USA). P38MAPK (cs-9212), PI3K (cs-4257), p-PI3K (cs-4228), Akt (cs-9272) were purchased from Cell Signaling Technology (Beverly, MA, USA). p-P38MAPK (sc-17852) was purchased from Santa Cruz Biotechnology Inc. (CA, USA) and p-Akt (66444-1-1g) was purchased from Proteintech (Chicago, IL, USA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit immunoglobulins were purchased from Beyotime Biotechnology (Shanghai, China).
YJP preparation
YJP (1 kg) was added to 10 L distilled water, heated at a constant temperature of 80°C to reflux for 2 h, and then filtered using 0.2 μm filter. The YJP residue was recovered, added to 7 L of distilled water, heated to reflux for 1 h, and filtered again. The residue was recovered again, added to 3 L of distilled water, heated to reflux for 1 h, and finally the filtrates were combined. After rotary evaporation, the extract was obtained, and vacuum-dried for 48 h at 80°C, to obtain a yield of 278 g YJP.
Animals and treatments
Equal numbers of male and female C57BL/6N mice (8-10-week-old, weighing 18-22 g, n = 70) were obtained from the Changchun Yisi Laboratory Animal Technology Co., Ltd. (Jilin, China) [SPF, SCXK(J)2003-0008]. All mice were acclimatized to a specific pathogen-free controlled environment, with free access to water and feed for 1 week before each experiment. Experimental procedures were approved by the Institutional Animal Care and Use Committee of Yanbian University (Resolution number, 201501022).
Mice were randomly separated into the following eight groups: normal (n = 5), CCl4 (n = 10), CCl4+YJP-100 mg/kg (n = 9), CCl4+YJP-200 mg/kg (n = 9), CCl4+YJP-300 mg/kg (n = 10), YJP-300 mg/kg alone (n = 9), silybin-100 mg/kg (n = 9), and FZHY capsule-2 g/kg (n = 9). Except for the normal and YJP alone groups, liver fibrosis was established in the other groups by injecting 10% CCl4 ip twice a week for 3 weeks, and then once a week after that for 3 weeks. YJP, silybin, and FZHY were dissolved in sepatate samples of physiological saline containing 2% (v/v) Tween 80. The three test drugs were administered by gavage once a day for 6 weeks. The normal group was administered the same volume of physiological saline. CCl4 and other treatments were administered to mice on the same day, intraperitoneally 1 h after gavage.
Sample harvest
Mice were enthanized at the end of week 6 and blood and tissue samples were collected after 6-h starvation. Serum was obtained by centrifuging the blood samples collected via the orbital sinus vein at 3000 rpm for 30 min at 4°C and stored at -80°C for later use. Mouse liver tissue samples were washed with physiological saline to remove the blood, blotted with filter paper to remove the remaining saline buffer, and weighed. A small portion of the liver was fixed with 10% formaldehyde, dehydrated, embedded in paraffin, and sectioned. Another portion of the liver was stored at -80°C until reverse transcription-polymerase chain reaction (RT-PCR) and immunoblotting were performed.
Serum biochemistry
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), IL-12, and IL-18 levels were measured by enzyme-linked immunosorbent assay (Nanjing Jiancheng Bioengineering Institute, China; Mlbio, Shanghai, China respectively).
Histopathological and immunohistochemistry examination
Paraffin blocks were cut into 4 μm-thick sections, stained with 0.1% (w/v) Sirius red and hematoxylin-eosin (H&E, Fuzhou Maixin Biotechnology Development Co. Ltd., Fuzhou, China), and observed under an Olympus light microscope. For immunohistochemistry, the mouse liver samples were embedded in the optimal cutting temperature (OCT) compound and stored at -80°C until use. Cryosections (5 μm) were fixed in acetone/methanol (1:1) and incubated with normal goat serum to reduce non-specific binding. The tissue sections were incubated with anti-CoI1 (1:200) and anti-α-SMA (1:500) overnight in a humidified chamber at 4°C. HRP-labeled secondary antibodies in the MaxVision™ HRP-Polymer Anti-Mouse/Rabbit IHC kit, were applied for 30 min at room temperature, followed by incubation at room temperature with diaminobenzidine (DAB) chromogen for color development. The tissue sections were counterstained with Mayer’s hematoxylin.
RNA extraction and RT-PCR
Total RNA was extracted from liver tissues using Eastep total RNA extraction kit (Shanghai, China) according to the manufacturer’s instructions. cDNA was prepared using 1 μg total RNA. RT-PCR was performed using the ABI Veriti® thermal cycler (Applied Biosystems, Foster City, CA, USA). The primer sequence used in the PCR are as follows: α-SMA (sense, 5’-CATCAGGGAGTAATGGTTGG-3’ and antisense, 5’-CACAATACCAGTTGTACGTC-3’); Collagen-I (sense, 5’-TGAGTCAGCAGATTGAGAAC-3’ and antisense, 5’-TACTCGAACGGGAATCCATC-3’); GAPDH (sense, 5’-CTTGTGCAGTGCCAGCC-3’ and antisense, 5’-GCCCAATACGGCCAAATCC-3’).
Western blotting
After 6-week YJP treatments, liver tissues were collected and lysed with radioimmunoassay precipitation (RIPA) buffer. Then, 50 μg extracted protein was separated using 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electroblotted onto polyvinylidene difluoride membranes. The membranes were incubated with blocking solution (5% skim milk) for 1 h at room temperature, followed by incubation with the following specific primary antibodies at the indicated dilutions: α-SMA, 1:200; Col1, 1:500; GAPDH, 1:500; ERK, 1:500; p-ERK, 1:500; JNK, 1:500; p-JNK, 1:500; P38MAPK, 1:500; p-P38MAPK, 1:500; PI3K, 1:500; p-PI3K, 1:500; Akt, 1:500; and p-Akt, 1:500. The blots were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBST), and then incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Immunoreactive proteins were visualized using the Beyo-ECL plus kit (Beyotime Institute of Biotechnology). The band intensity was quantified using Quantity One software (Bio-Rad, USA).
Statistical analyses
All values are expressed as the means ± standard deviation (SD). The results were compared using a one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests. The calculations were performed using GraphPad Prism program (Graph Pad Software, Inc., San Diego, USA). Results with P < 0.05 were considered statistically significant.
Results
YJP prevented CCl4-induced liver injury in mice
The liver fibrosis-induced mice showed lusterless hair and irritability. Interestingly, mice in the YJP treatment and positive control groups had glossy hair and a good physical condition. Furthermore, the liver surface was rough in the CCl4-induced liver fibrosis group, whereas surfaces of the YJP and two positive control group mice were smooth. This phenomenon illustrated the potential therapeutic effects of YJP (Figure 1).
Figure 1.
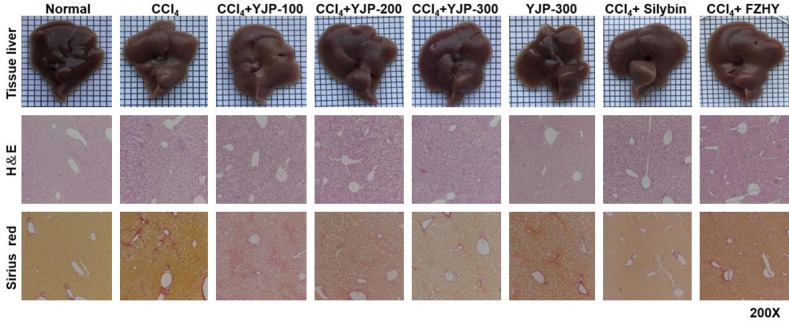
Effects of YJP on hepatic morphology. Liver appearance pictures, H&E staining and Sirius red staining. H&E and Sirius red staining were 200× magnifications. H&E staining could observe inflammatory cell infiltration, steatosis and integrity of hepatic lobule structure. The red part of Sirius red staining represented the deposition of collagen fibers in liver tissue.
ALT/AST levels increased in the CCl4 group compared with levels in the normal group. However, YJP and the two positive control groups showed effectively reduced ALT and AST levels compared with the CCl4 treatment group. There was no significant difference between the YJP and positive control groups. We determine whether YJP suppressed the inflammation in CCl4-induced liver fibrosis by examining the liver tissue levels of relevant proinflammatory cytokines using ELISA. The levels of TNF-α, IL-1β, IL-12, and IL-18 in the CCl4 group were significantly higher than those in the normal group were (Figure 2). In contrast, YJP treatment attenuated these cytokine levels. There was no significant change in the level of these mediators between the normal and YJP alone groups. H&E staining showed that CCl4 resulted in massive steatosis and inflammatory infiltration in the livers of mice compared with the liver tissues from normal mice. Administration of YJP to the CCl4-induced liver fibrosis mice ameliorated these histological changes and dose dependently prevented liver destruction (Figure 1). Liver injury in the YJP group (300 mg/kg) improved more than that in the positive control groups.
Figure 2.
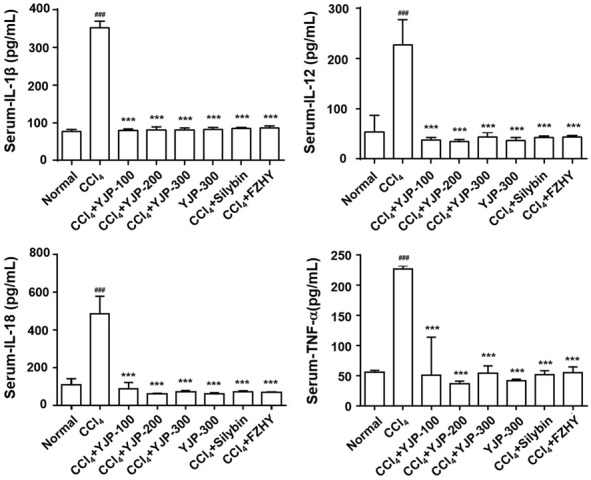
Effects of YJP on the serum IL-1β, IL-12, IL-18 and TNF-α levels. Data were presented as the mean ± SD. ###P < 0.001 versus normal group, ***P < 0.001 versus CCl4 group.
Effect of YJP on liver fibrosis in CCl4-induced mice
Sirius red staining showed significant collagen deposition surrounding the portal area and central vein in the CCl4 group mice. However, collagen deposition was decreased after YJP, silybin, and FZHY treatments (Figure 1). There was almost no change in the group administered YJP alone compared with the normal group.
Immunohistochemistry revealed no expression of α-SMA and Col1 in the normal and YJP alone groups. However, the above markers were strongly expressed in the portal area and around the central vein in the CCl4 group mice, whereas their expression levels were low in the YJP group, which showed a dose-dependent effect (Figure 3). The therapeutic effects of silybin and FZHY were not as potent as that of YJP.
Figure 3.
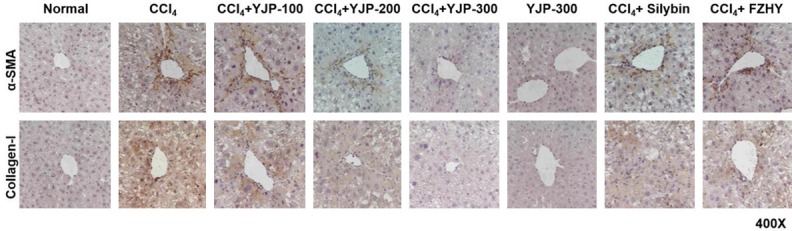
Effects of YJP inhibits hepatic fibrosis. Immunohistochemical staining of α-SMA and Col(1) (×400). The positive expression of α-SMA and Collagen-I around the portal area and central vein represented the degree of liver fibrosis in mice tissue.
In addition, the protein and mRNA expression of α-SMA and Col1 was significantly higher in the CCl4 group than it was in the normal group (P < 0.001, Figures 4A, 4B and S1). YJP at doses of 200 and 300 mg/kg significantly inhibited the protein and mRNA expression of α-SMA and Col1, compared with that of CCl4 group (P < 0.001, Figures 4A, 4B and S1). However, there was no significant difference in the protein expression of Col1 after YJP (100 mg/kg) treatment (P > 0.05, Figures 4A and S1). After silybin and FZHY treatments, the expression of the two proteins decreased significantly (P < 0.001, Figures 4A, 4B and S1). In the YJP alone group, there were no significant changes in the protein and mRNA expression levels of α-SMA and Col1 compared with that in the normal group.
Figure 4.
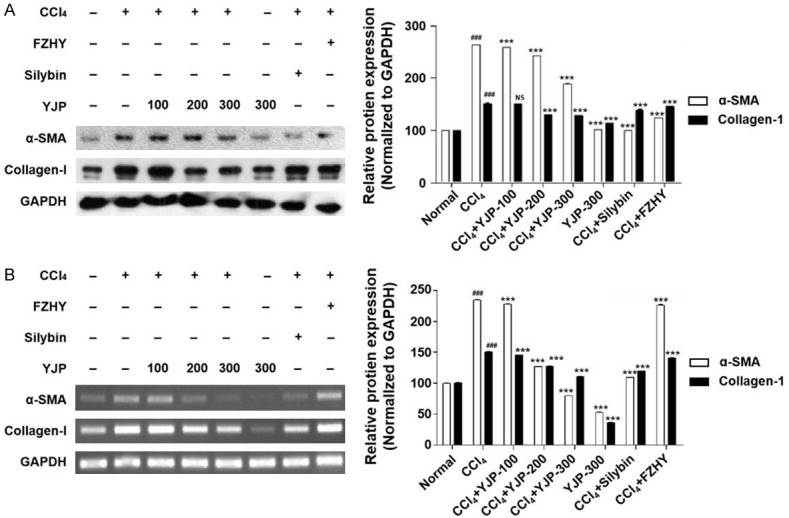
YJP treatment ameliorated hepatic fibrosis in CCl4-induced mice. A. Relative protein levels of α-SMA and Col(1) detected by Western blotting. The protein levels were normalized by GAPDH. B. Relative mRNA levels of α-SMA and Col(1) measured by reverse transcription polymerase chain reaction. The mRNA levels were normalized by GAPDH. Data were presented as the mean ± SD. ###P < 0.001 versus normal group, ***P < 0.001 versus CCl4 group.
YJP blocked the MAPK and PI3K/Akt signaling pathways to protect against CCl4-induced liver fibrosis
To confirm whether the anti-fibrotic mechanism of YJP involved regulation of the MAPK signaling pathway, we examined the phosphorylation of ERK, JNK, and P38 MAPK in the eight groups. Compared with the normal group, YJP treatment (100 and 300 mg/kg) significantly reduced the phosphorylation of ERK, JNK, and P38 MAPK following CCl4 treatment (Figures 5A, 5B, S2A and S2B). However, YJP treatment (200 mg/kg) significantly reduced the expression of p-JNK and p-P38MAPK, but not p-ERK. Interestingly, YJP treatment alone suppressed the expression of p-P38MAPK. Silybin treatment significantly inhibited the phosphorylation of the above three proteins whereas FZHY only inhibited that of JNK and P38 MAPK, but not ERK (Figures 5A, 5B, S2A and S2B). The inhibitory effect of YJP on the phosphorylation of three proteins was most significant at the high dose, which reduced p-ERK and p-P38 more potently than silybin did. In the JNK pathway, the inhibitory effect of high-dose YJP was more potent than that of FZHY, but weaker than that of silybin.
Figure 5.
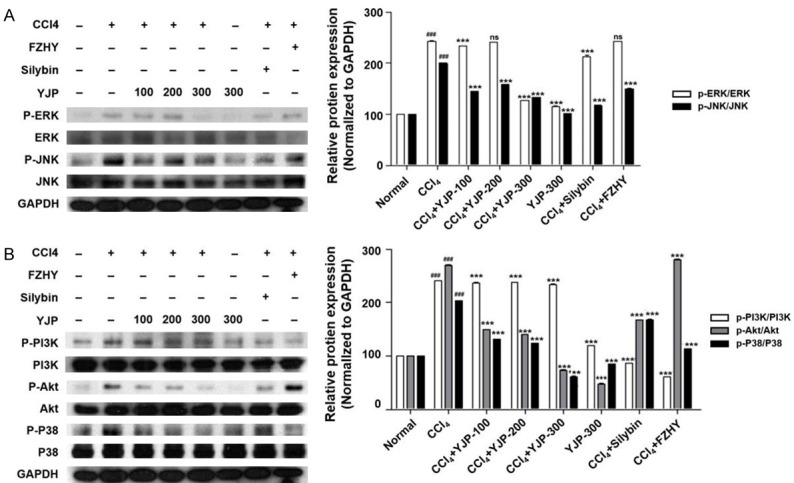
Effects of YJP on the protein expression of MAPK and PI3K/Akt signaling pathways. A. MAPK signaling pathway changed in mice liver fibrosis. Western blotting analysis of p-ERK/ERK, p-P38/P38 and p-JNK/JNK relative protein expressions. The protein expressions were normalized by GAPDH. B. PI3K/Akt signaling pathway changed in mice liver fibrosis. Western blotting analysis of p-PI3K/PI3K and p-Akt/Akt relative protein expressions. The protein expressions were normalized by GAPDH. Data were presented as the mean ± SD. ###P < 0.001 versus normal group, ***P < 0.001 versus CCl4 group.
The phosphorylation of PI3K and Akt was significantly lower in the YJP (100, 200, and 300 mg/kg) groups than it was in the CCl4 group (Figure 5B). Furthermore, YJP treatment alone also decreased the phosphorylation of Akt but not of PI3K while both silybin and FZHY inhibited the phosphorylation of both proteins. The inhibitory effect of silybin and FZHY on PI3K was more potent than that of the different doses of YJP. However, the phosphorylation of the Akt signaling pathway was more strongly inhibited by the medium dose of YJP than it was by the other two positive control treatments.
Discussion
Recently, information regarding the pathophysiology of liver fibrosis has been accumulating, which has contributed to the development of Chinese herbal medicines that could potentially inhibit and even reverse the fibrosis process. Chinese herbal medicines such as FZHY capsule and Bie-Jia-Ruan-Gan tablet, which have been approved by the People’s Republic of China State Food and Drug Administration, have been shown to have anti-fibrotic effects in patients with chronic liver diseases and in animal models [13,14]. Although YJP, a compound preparation of a secret TCM formula, has satisfactory effects in the clinical treatment of liver injury and hepatic fibrosis in patients, little is known about the mechanisms of underlying its protective effect against liver fibrosis in vivo. Thus, we used CCl4 to establish a mouse model of liver fibrosis to investigate the signaling pathway by which YJP likely inhibits liver fibrosis.
CCl4-induced liver fibrosis is a well-established animal model of chronic liver injury. The symptoms in this model are similar to those of chronic liver injury in humans [15]. CCl4-induced liver fibrosis model at 6 weeks was used to simulate liver injury in the present study. The histological and biochemical examinations of mice injected with CCl4 confirmed that liver fibrosis in the mice. Our results showed that YJP decreased the area of collagen fiber in the fibrotic liver tissue, demonstrating its anti-fibrotic effect on CCl4-induced liver fibrosis in vivo. It is noteworthy that YJP alone minimally altered the morphology of the hepatocytes.
The MAPK protein family includes ERK, JNK, and P38 MAPK [16], which recruit Ras following their activation, leading to the transcription of cell-proliferative and profibrogenic factors. Our data showed that the phosphorylation levels of ERK, P38 MAPK, and JNK decreased significantly after YJP treatment in mice. This suggests that YJP inhibited liver fibrosis by inhibiting the phosphorylation of ERK, P38 MAPK, and JNK in the MAPK pathway. These results are consistent with those of previous studies, including an investigation of the role of Toll-like receptor 5 (TLR5) in CCl4-induced liver fibrosis [7]. The results revealed that TLR5 knockout attenuated CCl4-induced liver fibrosis by inhibiting HSC activation via modulation of the NF-κB and MAPK signaling pathways [7]. JNK1 and JNK2 regulate α-SMA in HSCs during CCl4-induced fibrosis in the rat liver [17]. In addition, studies have shown that activation of PI3K can further activate the downstream kinase Akt, promote HSC proliferation, and inhibit HSC apoptosis [18]. The PI3K/Akt pathway plays an important role in the occurrence and development of liver fibrosis by regulating ECM degradation and HSC activation [19]. In the present study, YJP significantly reduced the phosphorylation of PI3K and Akt. We also observed that the protein and mRNA expression of Col1 and α-SMA in the YJP group was obviously reduced compared with that in the CCl4 group. This suggests that the improvement in CCl4-induced liver fibrosis might be associated with inhibition of the PI3K/Akt pathway, followed by inhibition of HSC activation and reduction in Col1 deposition to prevent liver fibrosis induced by CCl4.
Our present findings suggest that YJP was highly effective in inhibiting liver fibrosis and blocking inflammation and liver injury induced by CCl4 in mice. Interestingly, YJP alone had no significant effect on liver function and liver fibrosis. Moreover, YJP exhibited no obvious liver toxicity at a high dose. The effect of 300 mg/kg on liver fibrosis was most satisfactory. Further, YJP might inhibit the activation of the MAPK and PI3K/Akt signaling pathways to achieve its anti-hepatic fibrosis effect.
As mentioned, FZHY has a positive effect on liver fibrosis and silybin has recently been used to treat liver cirrhosis, hepatitis, liver fibrosis, and alcoholic liver diseases [20]. In the present study, we also found that FZHY and silybin have an antifibrosis effect, which is associated with inhibition of the MAPK and PI3K/Akt signaling pathways. Chinese herbal medicines are used widely without serious side effects in clinical practice; therefore, YJP might provide a novel alternative strategy for the treatment of liver fibrosis in the future.
Finally, we suggest that future studies should focus on determining whether the three MAPK pathways regulate each other and the relationship between MAPK and PI3K/Akt pathways following intervention with YJP. This would contribute to further clarifying the mechanism underlying the attenuation of liver fibrosis by YJP and its potential as a therapeutic intervention.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 31460294 and 81860651) and projects funded by the Education Department of Jilin (No. 2016-263) and the Yanbian University (No. 2016-5).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Sebastiani G, Gkouvatsos K, Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol. 2014;20:11033–11053. doi: 10.3748/wjg.v20.i32.11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohara M, Ohnishi S, Hosono H, Yamamoto K, Fu Q, Maehara O, Suda G, Sakamoto N. Palmitoylethanolamide ameliorates carbon tetrachloride-induced liver fibrosis in rats. Front Pharmacol. 2018;9:709. doi: 10.3389/fphar.2018.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 6.Elpek GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: an update. World J Gastroenterol. 2014;20:7260–7276. doi: 10.3748/wjg.v20.i23.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu M, Huang DD, Hung ZA, Hu XR, Zhang S. Inhibition of MAPK and NF-κB signaling pathways alleviate CCl4-induced liver fibrosis in Toll-like receptor 5 (TLR5) deficiency mice. Biochem Biophys Res Commun. 2016;471:233–239. doi: 10.1016/j.bbrc.2016.01.119. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Y, Wang J, Chen Y, Zhou K, Wen J, Wang Y, Zhou Y, Pan W, Cai W. Up-regulation of miR-200b in biliary atresia patients accelerates proliferation and migration of hepatic stellate cells by activating PI3K/Akt signaling. Cell Signal. 2014;26:925–932. doi: 10.1016/j.cellsig.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Yang YA, Chen LZ, Chen SC, Zhang J, Tang WJ. 18α-Glycyrrhetinic acid monoglucuronide as an anti-inflammatory agent through suppression of the NF-κB and MAPK signaling pathway. Medchemcomm. 2017;8:1498–1504. doi: 10.1039/c7md00210f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng R, Wang S, Wang R, Wang Y, Wu Y, Yuan Y. Antifibrotic effects of tanshinol in expermental hepatic fibrosis by targeting PI3K/AKT/mTOR/p70s6K1 signaling pathways. Discov Med. 2017;23:81–94. [PubMed] [Google Scholar]
- 11.Zhao CQ, Wu YQ, Xu LM. Curative effects of fuzheng huayu capsules on hepatic fibrosis and the functional mechanisms: a review. Zhong Xi Yi Jie He Xue Bao. 2006;4:467–472. doi: 10.3736/jcim20060505. [DOI] [PubMed] [Google Scholar]
- 12.Song YN, Sun JJ, Lu YY, Xu LM, Gao YQ, Zhang W, Wang XS, Xue DY, Zheng QS, Su SB. Therapeutic efficacy of fuzheng-huayu tablet based traditional Chinese medicine syndrome differentiation on hepatitis-B-caused cirrhosis: a multicenter double-blind randomized controlled trail. Evid Based Complement Alternat Med. 2013;2013:709305. doi: 10.1155/2013/709305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao CQ, Zhou Y, Ping J, Xu LM. Traditional Chinese medicine for treatment of liver diseases: progress, challenges and opportunities. J Integr Medb. 2014;12:401–408. doi: 10.1016/S2095-4964(14)60039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Q, Li N, Chen M, Zheng J, Qian Z, Wang X, Huang C, Li Q, Lin Q, Shi G. Fuzheng Huayu inhibits carbon tetrachloride-induced liver fibrosis in mice through activating hepatic NK cells. J Ethnopharmacol. 2013;145:175–181. doi: 10.1016/j.jep.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Risal P, Hwang PH, Yun BS, Yi HK, Cho BH, Jang KY, Jeong YJ. Hispidin analogue davallialactone attenuates carbon tetrachloride-induced hepatotoxicity in mice. J Nat Prod. 2012;75:1683–1689. doi: 10.1021/np300099a. [DOI] [PubMed] [Google Scholar]
- 16.Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway in melanoma. Curr Opin Oncol. 2008;20:183–189. doi: 10.1097/CCO.0b013e3282f5271c. [DOI] [PubMed] [Google Scholar]
- 17.Hong IH, Park SJ, Goo MJ, Lee HR, Park JK, Ki MR, Kim SH, Lee EM, Kim AY, Jeong KS. JNK1 and JNK2 regulate α-SMA in hepatic stellate cells during CCl4-induced fibrosis in the rat liver. Pathol Int. 2013;63:483–491. doi: 10.1111/pin.12094. [DOI] [PubMed] [Google Scholar]
- 18.Parsons CJ, Takashima M, Rippe RA. Molecularmechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22:S79–S84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- 19.Voloshenyuk TG, Landesman ES, Khoutorova E, Hart AD, Gardner JD. Induction of cardiac fibroblast lysyl oxidase by TGF-β1 requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine. 2011;55:90–97. doi: 10.1016/j.cyto.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Fehér J, Lengyel G. Silymarin in the treatment of chronic liver diseases: past and future. Orv Hetil. 2008;149:2413–2418. doi: 10.1556/OH.2008.28519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


