Abstract
Multiple sclerosis (MS), one of the autoimmune and inflammatory diseases, is a major cause of neurological disability worldwide. The existing clinical treatments are not curable, and better treatments are urgently needed. Mesenchymal stromal cells (MSCs) have shown promise for treating MS, but the favorable effects and mechanism of MSC therapy on MS are still not fully understood. In this study, we analyzed the phenotypic feature of peripheral blood mononuclear cells (PBMCs) in MS patients and found that the patients exhibited an increase in the frequency of B cells, but a markedly decrease in frequency of CD5+ and IL-10+ B cells compared to healthy controls. Infusion of MSCs exhibited a significant therapeutic effect on the experimental autoimmune encephalomyelitis (EAE) mice, infiltration of mononuclear cells and demyelination of the spinal cords were both reduced in CNS of the mice, the frequency of CD5+ IL-10+ B cells in the mice was significantly increased. Additionally, when PBMCs or B cells from MS patients were co-cultured with MSCs, the frequency of CD5+ IL-10+ B cells also increased, the proliferative and immunosuppressive capacity of CD5+ B cells were significantly enhanced while the apoptosis ratio of this cellular subset significantly decreased. Moreover, those effects could be eliminated while the indoleamine 2,3-dioxygenase (IDO) inhibitor, D/L-1MT, was added to the co-cultured cells. In summary, this study suggests that MSCs can control EAE via IDO pathway to promote the proportion and function of CD5+ IL-10+ B cells, providing a promise to treat patients with MS in the clinical setting.
Keywords: Multiple sclerosis; mesenchymal stromal cells; experimental autoimmune encephalomyelitis; indoleamine 2,3-dioxygenase; regulatory B cell
Introduction
Multiple sclerosis (MS) is an autoimmune disease that is characterized by central nervous system (CNS) inflammation, demyelination, axonal loss, and degeneration [1]. Approximately 2.5 million people suffered from MS worldwide. Current MS therapies can only relieve the inflammatory damage, but cannot recover the damage. There are more than 80% patients finally develop the progressive disability [2,3]. Thus, it is imperative to develop new drugs or therapeutic approaches that are able to overcome or even cure the disease.
The etiology of MS is still not clear. It was originally thought to be a T cell-mediated disease [4-6], but accumulating data has also demonstrated the involvement of B cells [7,8], the most convincing evidence comes from the clinical efficacy outcomes of selective B cell depletion therapies, such as rituximab, a depleting CD20 antibody in clinical trials in MS [9,10]. B cells contribute to MS pathology by producing antibodies and cytokines, and by functioning as antigen-presenting cells. In addition to these potentially pathogenic roles, recent experimental evidence indicates that IL-10 producing B cells, a specific subset of B cells with negative regulatory function (Bregs), suppress the progression of immune-mediated diseases. In experimental autoimmune encephalomyelitis (EAE) mice, the animal model for MS [11,12], studies demonstrate that numbers of endogenous or adoptively transferred Bregs directly interfere with the outcome of EAE pathogenesis [13,14]. While Bregs were showed to produce low levels of IL-10 in patients with MS [15,16], the depletion and repopulation of B cells in rituximab-treated patients, seem to reset the production of IL-10 in B cells [15]. These studies indicated that Bregs play an important role in controlling the MS, development of targeting and promoting Bregs therapies could be beneficial for the treatment of patients with MS.
Recently, there has been increasing recognition of the potential of mesenchymal stromal cells (MSC)-based therapies for the treatment of MS [17-20]. Clinical trials of MSC transplantation in MS patient appears feasible, safe, well tolerated and effective [21,22]. These exciting results implied that MSCs might be a potential therapeutic agent for MS. MSCs are multipotent progenitor cells that can be isolated from various adult tissues [23-27], in addition to their self-renewal capacity and multipotency [28], MSCs also have potent immunomodulatory effects on both innate and adaptive immune cells [29-33]. Studies in EAE have provided abundant evidence that MSCs decreased inflammatory infiltrates and demyelination [20,34,35]. Some studies suggested that MSCs could ameliorate EAE by preventing Th1 and Th17 cell population [36], or by enhancing the function of Tregs in vitro [37]. However, the immunomodulatory and neuroprotective effects of MSC therapy for MS on B cells has been less illustrated.
In this study, we demonstrated that a subset of CD5+ IL-10+ B cells was indeed decreased in PBMCs of patients with MS. Additionally, we observed that infusion of MSCs attenuated EAE through upregulation of CD5+ IL-10+ Breg cells. Moreover, the MSCs prompted upregulation of Breg cells via IDO pathway.
Materials and methods
Processing of peripheral blood cells
This study was approved by the Research Ethics Committee of the Third Affiliated Hospital at the Sun Yat-sen University and written informed consent was obtained from each participant according to the Declaration of Helsinki. Heparinized peripheral blood was obtained from MS patients and the healthy subjects. Ten patients (three men and seven women) along with age and sex matched controls enrolled in this study. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation using Ficoll-Paque PLUS media (GE Healthcare, USA) and stored in aliquots.
Cell culture
Human umbilical cord-derived MSCs (hUC-MSCs) and normal skin-derived fibroblast (NFs) were isolated and cultured as previously described [38,39]. Briefly, fresh human umbilical cords were obtained after birth, with the written consent of parents, and collected in phosphate buffered saline (PBS; Sigma, USA) containing 100 UI/ml penicillin and streptomycin (Gibco-BRL, USA) at 4°C. The cords were washed twice and cut into pieces and floated in Dulbecco’s modified Eagle’s medium with low glucose (DMEM-LG) containing 10% FBS (Gbico), 5% HS, penicillin and streptomycin at 37°C in a humidified atmosphere with 5% CO2. The medium was changed every 2 days, and non-adherent cells were removed by washing after 7 days. When well-developed colonies of fibroblast-like cells appeared after 10 days, the cultures were trypsinized and transferred (without dilution) into a new flask for further expansion. NFs were obtained from foreskin, the tissues were minced and digested in Roswell Park Memorial Institute 1640 (RPMI 1640; Invitrogen, USA) supplemented with 10% FBS, 1 mg/ml collagenase type I (Sigma) and 100 U/ml hyaluronidase (Sigma) at 37°C for 8 hours, washed twice with PBS (Sigma) and centrifuged at 450 g for 8 minutes each time. Cells were finally resuspended in RPMI 1640 supplemented with 10% FBS, 100 IU/ml penicillin, 100 mg/ml streptomycin, and then cultured at 37°C in a humidified 5% CO2 environment.
EAE induction and MSC treatment
All animal studies were approved by the Institutional Animal Care and Use Committee of the Third Hospital at the Sun Yat-Sen University (Approve Number: 160520). Female mice (C57BL/6, 18-20 g, 8-10 weeks) were randomly divided into three groups: control group, EAE model group and hUC-MSC treatment group (n = 6 per group). To induce EAE in mice, complete Freund’s adjuvants (CFA) was prepared by mixing Mycobacterium tuberculosis (Difco, USA) (2 mg/mL) with Freund’s adjuvants (Sigma). An equal amount of MOG35-55 peptide (GL Biochem, China) (2 mg/mL in ddH2O) and CFA solution were mixed to have a final concentration of 1 mg/mL before injected into each mouse. 100 µL antigen/CFA emulsion was delivered to two different sites of each hind flank, immediately after that, 400 ng pertussis toxin (Enzo life sciences) was intraperitoneally injected. Another pertussis toxin was given to the mice two days later.
For the treatment of EAE, 2×106 hUC-MSCs in 200 µL PBS or PBS alone were intravenously injected into mice via the tail vein on 12th and 22nd days after immunization of the EAE model. Disease score was monitored every day for up to 30 days as follows: 0, no sign of disease; 1, loss of tone in the tail; 2, partial hind limb paralysis; 3, complete hind limb paralysis; 4, front limb paralysis; and 5, dead or moribund [40,41].
Histology
Thirty days after cell injection, mice were sacrificed and perfused transcardially with 0.1 M PBS containing 4% paraformaldehyde (PFA, Sigma), spinal cords were dissected out and post-fixed overnight and then mounted in paraffin. The spinal cord sections were incubated in 0.1% luxol fast blue (LFB; Sigma) for 4 hours at 60°C, the excess stain will be differentiated with 0.01% Lithium carbonate (Sigma). The quantification of demyelination was performed on at least five spinal cord cross-sections per animal.
Co-culture experiment
PBMCs or B cells were isolated from untreated-MS patients or healthy person. Once MSCs or NFs reached 50% confluence, PBMCs or B cells were seeded onto MSCs or NFs monolayers at a density of 1×106 PBMCs or 1×105 B cells per 4 mL per well in 6-well plates, and MSC culture medium was replaced with RPMI 1640 supplemented with 5% FBS. To block the activity of indoleamine 2,3-dioxygenase (IDO), 0.5 mM D/L-1MT (Enzo life sciences, USA) was added after co-culture for 5 days. At day 9, B cells were separated from MSCs by spinning the cells in suspension and then washing them.
Flow cytometric analysis
The flow cytometric analysis of cell phenotypes and cell sorting were performed according to routine laboratory methods. Flow cytometric analyses or sorting were performed on LSRFortessa (BD, USA) or MoFlo Astrios EQs (Beckman, USA) flow cytometer. The antibodies used for flow cytometry were APC, FITC, PE-CF594 or PE-Cy7 conjugated anti-CD3, CD4, CD5, CD28, CTLA-4, PD1, CD19, B7-1, CD107a, TRAIL, CD8, CD56, NKG2D and IL-10, which were purchased from BD (USA), while anti-CD96, LAG-3, TIM3 and CD40 were purchased from BioLegend (USA), anti-TIGIT was brought from eBioscience (USA). The fresh isolated or cultured cells were collected, washed twice, and resuspended in 100 mL of PBS containing 0.1% BSA, and were stained and labeled with either specific antibodies or the appropriate isotype controls.
Sorted CD19+ B cells from healthy donors and MS patients were resuspended in RPMI-1640 medium. After 5 hours of stimulation with BFA (10 μg/mL; Sigma), PMA (50 ng/mL; Sigma), and ionomycin (1 μg/mL; Sigma), the cells were fixed, permeabilized, and stained for cell surface CD3, CD19, and CD5 and cytoplasmic IL-10 according to the manufacturer’s instructions. The stained cells were then analyzed by flow cytometry.
Cell proliferation and cytokine production assays
To evaluate the suppressive functions of CD5+ B cells, 5×105 CD3+ T cells were labeled in 1 mL of PBS containing 2.5 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen, USA) for 5 min at room temperature and seeded in a 96-well plate in 100 μL of RPMI 1640 medium containing 10% FBS. CD5+ B cells were purified by flow cytometry and added to CD3+ T cells at ratios of 1:1 (105 cells). Next, the CD3+ T cells were activated by the addition of 2 μg/mL of anti-CD3 and 5 μg/mL of anti-CD28 mAb beads per well for 3 days. Subsequently, T cell proliferation were measured by flow cytometry.
For cytokine production assays, 2×105 CD4+ T cells were co-cultured with CD5+ B cells and then activated as same as the above mentioned. Expression of IL-17A, Foxp3 and IFN-γ were determined by qRT-PCR.
Cell apoptosis assay
B cell apoptosis was quantified using a PI/Annexin V apoptosis detection kit according to the manufacturer’s instructions (Invitrogen). The binding of Annexin V-FITC and PI to the cells was measured by flow cytometry.
RT-PCR analyses
Using a commercial kit (Roche, Switzerland), total RNA was extracted from PBMCs in MS patients both before and after the MSC treatment. Reverse transcription was performed using the Quantitect Reverse Transcription kit (Qiagen, USA), according to the manufacturer’s protocol, followed by qPCR using the DyNAmo ColorFlash SYBR Green qPCR kit (Thermo Fisher Scientific, USA) on a LightCycler 480 Detection System (Roche). The samples were transferred to the thermal cycler, and DNA was amplified using the following thermocycling conditions: 40 cycles of denaturation at 95°C for 10 seconds, annealing at 60°C for 10 seconds, and extension at 72°C for 30 seconds. GAPDH served as an internal control. The primers used for IL-17: forward 5’-CGAGATCTCCGAGATGCC-3’ and reverse 5’-AGTTCACATGCGCCTTGA-3’, IFN-γ: forward 5’-CGAGATCTCCGAGATGCC-3’ and reverse 5’-AGTTCACATGCGCCTTGA-3’, Foxp3: forward 5’-CGAGATCTCCGAGATGCC-3’ and reverse 5’-AGTTCACATGCGCCTTGA-3’.
Statistical analysis
The results are expressed as the Means ± SEM. The statistical significance of differences between groups was determined by Student’s t-test. SPSS statistical software (version 13.0) was used for all statistical analyses. All data were analyzed using two-tailed tests unless otherwise specified, and P < 0.05 was considered statistically significant.
Results
The frequency of CD5+ and IL-10+ B cells decreases in PBMCs of MS patients
To study the relationship between immunology dysfunction and patients with MS, we collected blood samples from 10 MS patients, including 9 who were clinically diagnosed with relapsing-remitting MS (RRMS) and 1 with secondary-progressive MS (SPMS). All patients were untreated before sample collection, the clinical features of the patients were listed in Table 1. In these samples, we did not detect any striking changes in CD4+ or CD8+ T cell subsets of the MS patients compared to healthy controls (Figure 1A-C). We also examined the T cell markers related to T cell activation and tolerance, such as Tim3, PD-1, CD107a, TIGIT, TRAIT, B7-1, LAG3, and CD28 (Figure 2A and 2B), and found no significant changes as well. Nonetheless, the significant changes in natural killer (NK) cells (Figure 1D and 1E) and B cells (Figure 1F and 1G) were observed. Compared to the healthy donors, although the MS patients exhibited an increase in B cell and a decrease in NK frequency, detection of NKG2D and CTLA4 in NK showed non-significant changes (Figure 2C and 2D). Interestingly, the frequency of CD5+ and IL-10+ B cells within the B cell population markedly decreased (Figure 2E-H). Importantly, the frequency of CD5+ and IL-10+ B cells in MS patients with an EDSS score of 2 or less was significantly greater than these in MS patients with an EDSS score greater than 2 (Figure 2I and 2J). These results showed that MS progression is accompanied by an increase in B cells but a decrease in CD5+ and IL-10+ B cells in PBMCs of MS patients.
Table 1.
Demographic and baseline clinical characteristics of enrolled patients
| Characteristic | MS patients (n = 10) | Health controls (n = 10) |
|---|---|---|
| Gender (Male: Female) | 3:7 | 3:7 |
| Age (Mean ± SEM) | 34.10 ± 13.96 | 34.10 ± 13.96 |
| EDSS Score (Mean ± SEM) | 2.60 ± 1.05 | 0 |
Figure 1.
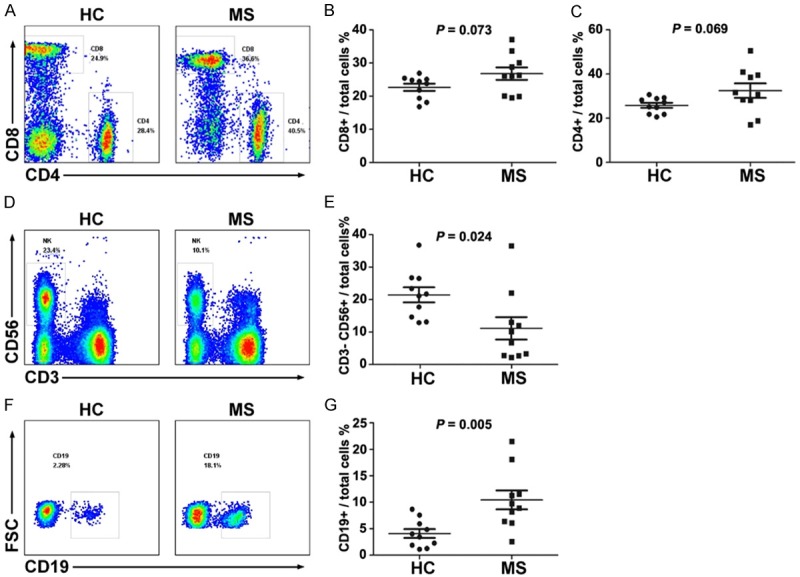
The frequency of CD19+ B cells increases in MS patients. The frequencies of CD4+ and CD8+ T cells (A-C), CD3- CD56+ NK cells (D and E), and CD19+ B cells (F and G) in the MS patients and healthy subjects were detected by FASC (n = 10). The symbols represent individual samples, the horizontal bars represent the mean, and the error bars show the SEM. P < 0.05 was considered statistically significant.
Figure 2.
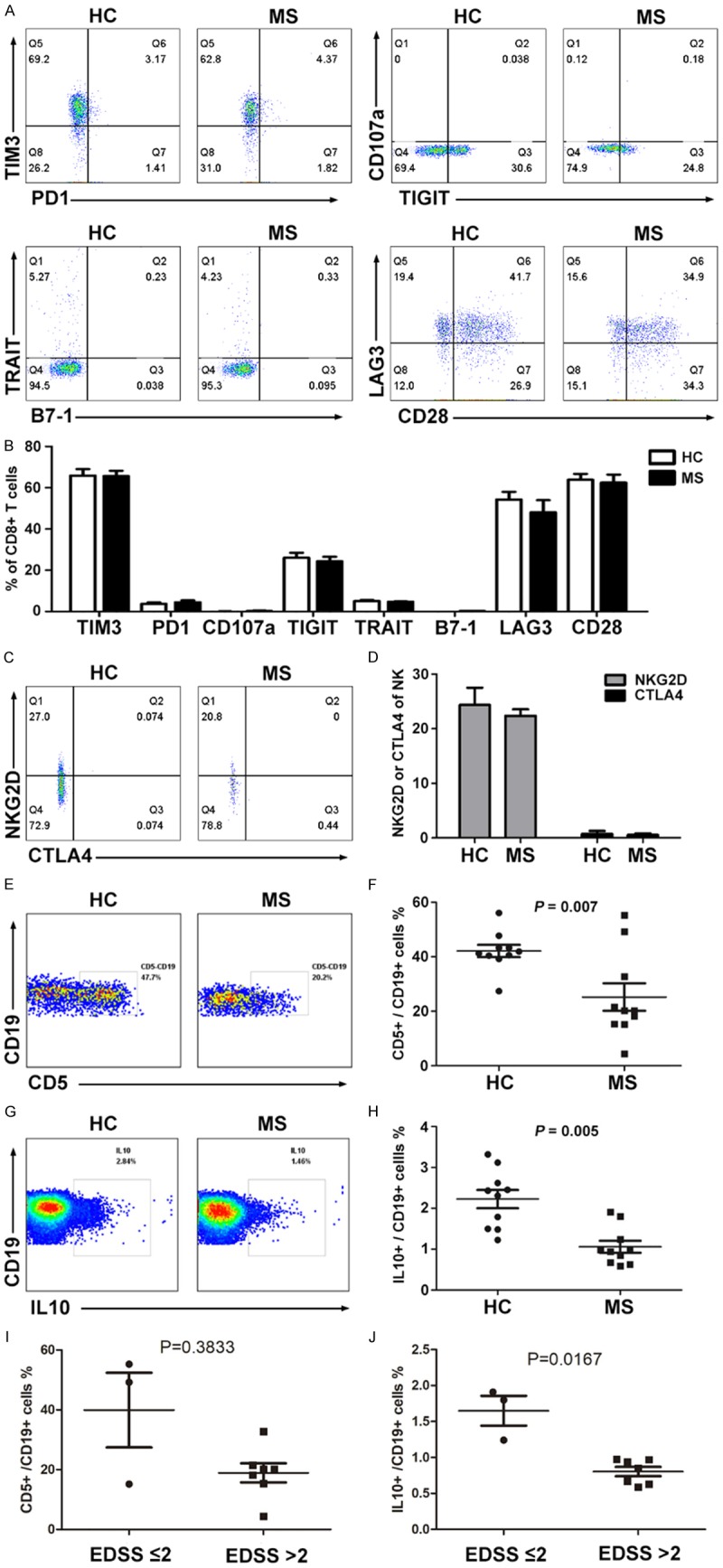
The frequency of CD5+ IL-10+ B cells decreases in MS patients. The expression of TIM3, PD1, CD107a, TIGIT, TRAIT, B7-1, LAG3 and CD28 in T cells (A and B), the NKG2D and CTLA4 expression in CD3- CD56+ NK cells (C and D), and the CD5 and IL-10 expression in CD19+ B cells (E-H) in the PBMCs of MS patients and healthy subjects were detected and analyzed by FASC (n = 10). The correlation between EDSS score and CD5+ or IL-10+ B cell ratio (I and J) in the PBMCs of MS patients. The symbols represent individual samples, the horizontal bars represent the mean, and the error bars show the SEM. P < 0.05 was considered statistically significant.
MSCs ameliorate symptoms and increase the proportion of CD5+ IL-10+ B cells in EAE mice
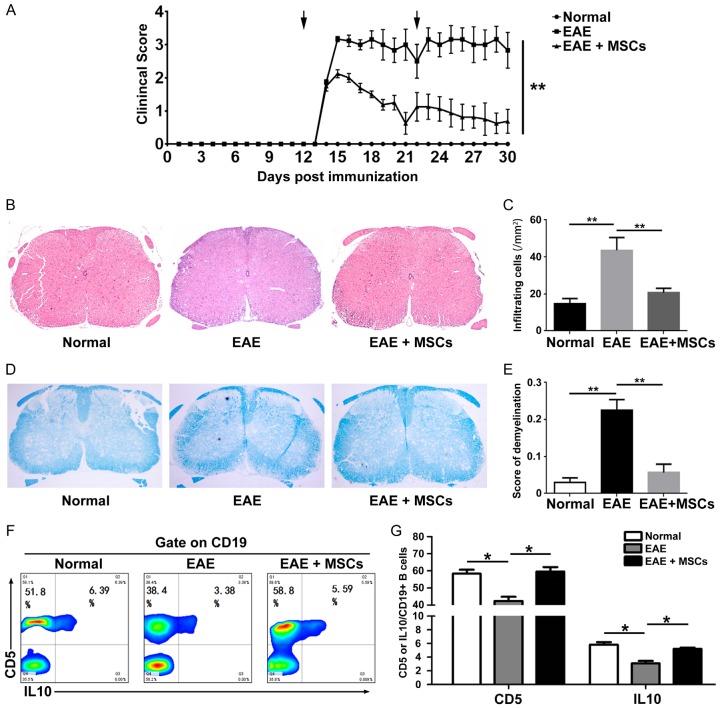
Given that the decrease of CD5+ IL-10+ B cells is possibly associated with the development and progression of MS and MSC therapy has an effect on controlling the animal model of MS, we next examined the therapeutic effects and alteration of CD5+ IL-10+ B cells in EAE mice that underwent MSC treatment. Interestingly, administration of MSCs significantly suppressed the EAE clinical course compared to PBS (Figure 3A). Histological examination showed there was less infiltration of mononuclear cells and decreased demyelination in the spinal cords of MSC-treated mice as compared with those treated with PBS (EAE vs. EAE+MSCs, number of infiltrating mononuclear cells: 23.63 vs. 43.63, P < 0.001; demyelination score: 0.23 vs. 0.06, P < 0.001) (Figure 3B-E). Moreover, we found that MSC treatment significantly increased the frequency of CD5+ IL-10+ B cells in peripheral blood of EAE mice (Figure 3F and 3G).
Figure 3.
MSCs improve MS clinical symptoms and increase the proportion of CD5+ IL-10+ B cells in vivo. After EAE in mice was induced, 2×106 hUC-MSCs were injected into mice on days 12 and 22 (arrows). Administration of MSCs with a therapeutic protocol significantly improved the neurological function score on EAE mice compared with PBS (n = 6, each group, A). Histological examination showed there was less infiltration of mononuclear cells (B and C) and decreased demyelination in the spinal cords of MSC-treated mice as compared with those treated with PBS (D and E). MSCs significantly increased the frequency of CD5+ IL-10+ B cells in EAE model (F and G). *P < 0.05, **P < 0.01.
MSCs increase the frequency of CD5+ and IL-10+ B cells in vitro
To determine the effects of MSCs on CD5+ B cells, PBMCs from four MS patients were isolated and cultured either alone or with MSCs or with NFs. After 5 days of co-culture with MSCs or culture alone, the frequency of CD5+ B cells among CD19+ B cells from the MS patients increased from 38.1 ± 1.65 to 50.2 ± 2.01 (P = 0.0035, Figure 4A and 4B), while the frequency of IL-10+ B cells among CD19+ B cells from the MS patients increased from 1.97 ± 0.24 to 4.02 ± 0.58 (P = 0.017) (Figure 4D and 4E). Moreover, after 5 days of co-culture with MSCs, the frequency of CD5+ or IL-10+ B cells in CD19+ B cells from the MS patients were both markedly increased (38.6 ± 1.79 to 53.75 ± 1.89; 1.48 ± 0.28 to 3.68 ± 0.46, P < 0.01) (Figure 4A, 4C, 4D and 4F). However, the effects of NFs on CD5+ or IL-10+ B cells were not observed. To further prove the effects of MSCs on CD5+ or IL-10+ B cells, PBMCs from the healthy person were isolated and cultured either alone or with MSCs. After 5 days of co-culture with MSCs or culture alone, the frequency of CD5+ B cells among CD19+ B cells from the healthy person increased from 38.2 ± 3.54 to 49.2 ± 1.64 (P = 0.0212, Figure S1A and S1B), while the frequency of IL-10+ B cells among CD19+ B cells from the healthy person increased from 2.13 ± 0.11 to 5.02 ± 0.14 (P = 0.0005, Figure S1C and S1D).
Figure 4.
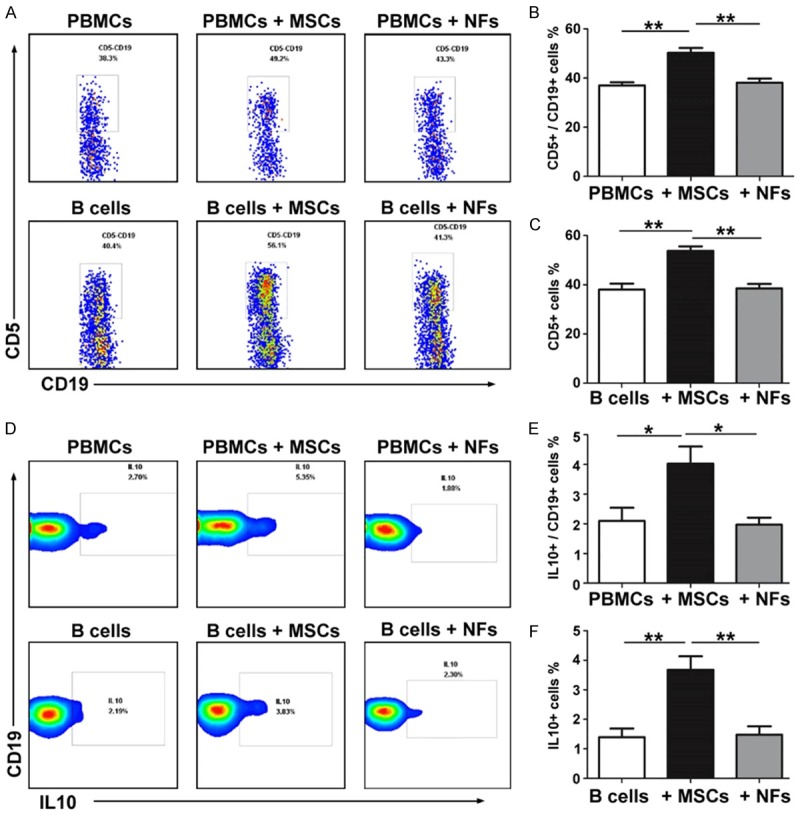
MSCs increase the frequency of CD5+/IL-10+ B cells in vitro. The CD5 expression of CD19+ B cells (A-C) in PBMCs or B cells alone and PBMCs or B cells co-culture with MSCs or NFs were detected by FASC. The IL-10 expression of CD19+ B cells (D-F) in PBMCs or B cells alone and PBMCs or B cells co-culture with MSCs or NFs were detected by FASC. The symbols represent individual samples, the horizontal bars represent the mean, and the error bars show the SEM. Significant differences are indicated as follows: *P < 0.05, **P < 0.01.
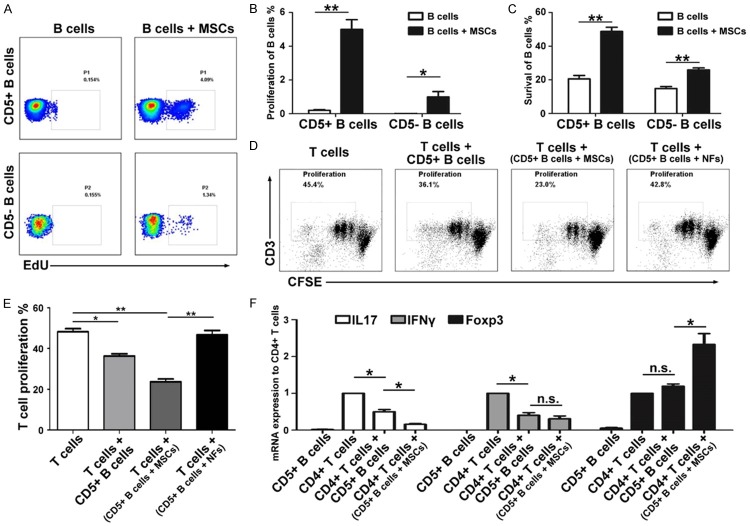
MSCs promote the proliferation, survival and function of CD5+ B cells
We next assessed the effect of MSCs on CD5+ B cell proliferation. CD5+ B cells from the MS patients exhibited a much greater proliferative capacity in the presence of MSCs than in the absence of MSCs (5 ± 1.12 vs. 0.2 ± 0.08, P < 0.001); CD5- B cells also presented the same tendency, but the efficiency was much lower than that of the CD5+ B cells co-cultured with MSCs (1 ± 0.64 vs. 0.01 ± 0.008, P = 0.022) (Figure 5A and 5B). Moreover, we analyzed the effects of MSCs on the survival of CD5+ B cells and found that the survival rate of CD5+ B cells from the MS patients after 96 hours was much lower in culture alone than in the presence of MSCs; MSCs also increased the survival rate of CD5- B cells, but the efficiency was relatively low (Figure 5C). To further prove the effects of MSCs on CD5+ B cell proliferation and survival, PBMCs from the healthy person were isolated and cultured either alone or with MSCs. CD5+ B cells from the healthy person exhibited a much greater proliferative capacity in the presence of MSCs than in the absence of MSCs (4.2 ± 0.25 vs. 1.8 ± 0.23, P = 0.01) (Figure S1E and S1F). The survival rate of CD5+ B cells from the healthy person after 96 hours was much lower in culture alone than in the presence of MSCs (Figure S1G and S1H).
Figure 5.
MSCs influence the proliferation, survival and function of CD5+ B cells. In the presence of MSCs, the proliferation (A and B) and survival rate (C) of CD5+ B cells were much higher than those of CD5- B cells in the MS patients and also much higher than those in the absence of MSCs. MSC-activated CD5+ B cells from MS patients markedly inhibit the proliferation of CD3+ T cells compared with the NF-activated or inactivated CD5+ B cells (D and E). MSC-activated CD5+ B cells from patients markedly inhibit the IL-17 production by T cells, and markedly promoted the Foxp3 expression of T cells compared with the NF-activated or inactivated CD5+ B cells (F). The symbols represent individual samples, the horizontal bars represent the mean, and the error bars show the SEM. Significant differences are indicated as follows: *P < 0.05, **P < 0.01.
In addition, we analyzed the effect of MSCs on the function of CD5+ B cells. As shown in Figure 5D-F, CD5+ B cells derived from the MS patients displayed a significant immunosuppressive capacity on T cells by inhibiting their proliferation, as well as the production of IL-17 (0.5 ± 0.12, P < 0.001) and IFN-γ (0.4 ± 0.15, P < 0.001). In contrast, after co-culturing with MSCs, CD5+ B cells showed a much more robust function of inhibiting T cell proliferation and IL-17 production (0.16 ± 0.04, P = 0.0017), and of producing a significantly higher level of Foxp3 (2.33 ± 0.58, P = 0.009). However, we did not observe the effect of NFs on the function of CD5+ B cells (Figure 5E).
MSCs act via IDO pathway to modulate the proliferation and function of CD5+ B cells
Our previous study found that MSCs can suppress human T cell-mediated diseases by secreting IDO [27]. Thus, to gain further insight into the mechanism of the MSC-mediated proliferation, survival and function of CD5+ B cells, we tested IDO mediators using specific inhibitors for IDO, D/L-1MT. We found that D/L-1MT could significantly reverse the MSC-mediated effects on CD5+ B cell proliferation (2.29 ± 0.15 vs. 5.06 ± 0.58, P = 0.0037) (Figure 6A and 6B), survival (28.03 ± 1.97 vs. 48.3 ± 2.05, P < 0.001) (Figure 6C and 6D). The suppressive function of CD5+ B cells on proliferation of CD3+ T cells (40.85 ± 2.49 vs. 23.83 ± 1.99, P = 0.0017) (Figure 6E and 6F), and also on production of IL-17 (0.47 ± 0.08 vs. 0.16 ± 0.04, P = 0.0044) and Foxp3 (1.08 ± 0.03 vs. 2.08 ± 0.38, P = 0.011) in CD4+ T cells (Fiugre 6G) were both reduced.
Figure 6.
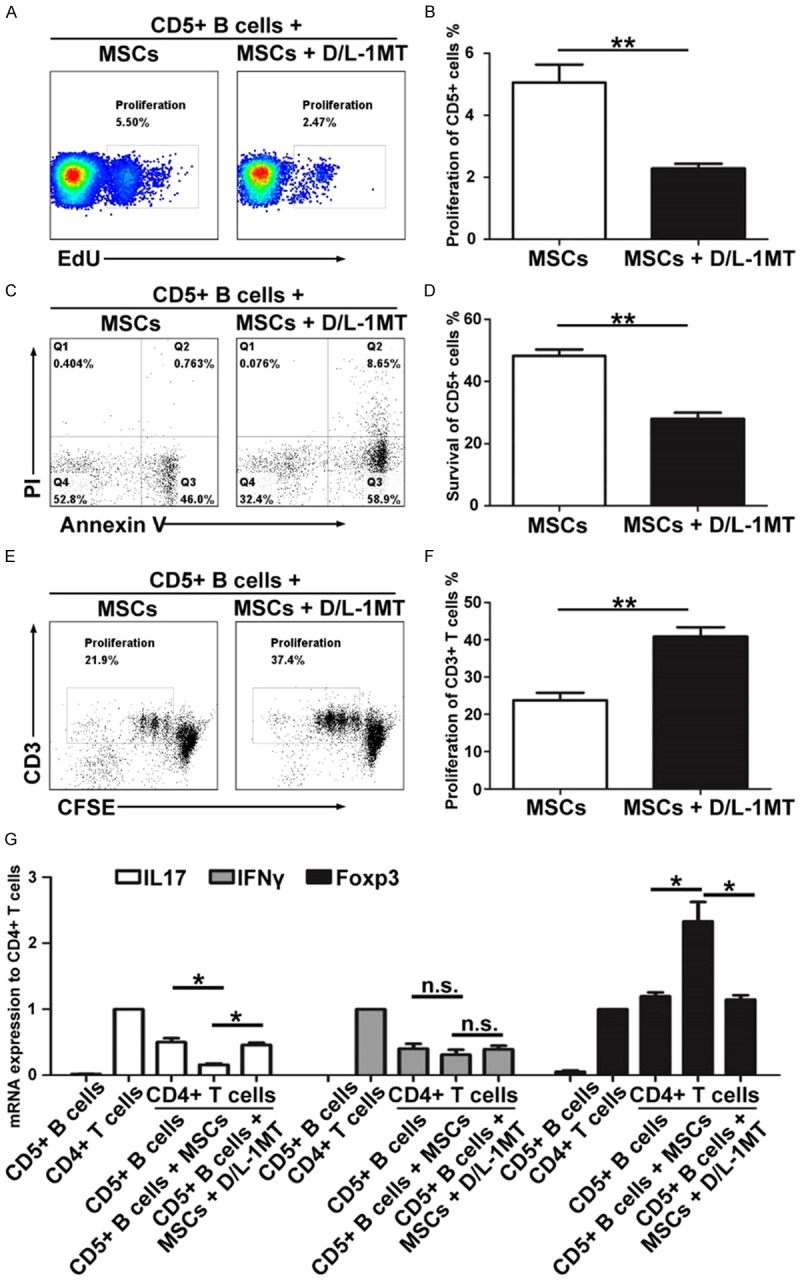
MSCs influence the proliferation, survival and function of CD5+ B cells via the IDO pathway in vitro. D/L-1MT partially reversed the MSC-mediated effects on CD5+ B cell proliferation (A and B), survival (C and D), and function (E-G). The symbols represent individual samples, the horizontal bars represent the mean, and the error bars show the SEM. Significant differences are indicated as follows: *P < 0.05, **P < 0.01.
Discussion
The pathophysiology of MS involves several components, the role of CD4+ T cells has long been considered to be central to MS pathophysiology [42-45]; increasing evidence also suggests additional immune cellularity, including B cells and NK cells, dendritic cells, and macrophages, play roles in this complex disorder [46]. However, the role of these immune cell subsets in the process of MS pathology has been both understudied and controversial. It is likely that different stages and different treatments have interfered with the explanation of these results.
In this study, we analyzed the phenotype distribution changes of PBMCs in 10 newly diagnosed patients with MS. Compared with the healthy subjects, we observed an increasing trend but not statistically significant in the T cell subsets in PBMCs of the MS patients (Figure 1A-C). MS had been viewed as a CD4+ T cell-mediated autoimmune disease, but studies found that the frequency of CD8+ T cells was greater than that of CD4+ T cells in inflamed plaques, CSF and blood of some MS patients [5]. While there also were the reports of reduced frequency and function of CD4+ CD25+ Foxp3+ Tregs in MS patients [47,48], our results demonstrated there is no significant difference on T cell subpopulations on newly diagnosed patients with MS. Thus, the immunologic mechanism of MS is complex and multifactorial. Future studies on different stages, dynamic change with increased sample sizes are warranted.
In MS patients, significant change in B cells was observed in their PBMCs (Figure 1A-C), this finding in accordance with previously reported results [49]. We also found a decrease in the frequency of NK cells (Figure 1D and 1E), but the functional activity of NK cells seemed no significantly different between MS patients and healthy control (Figure 2C and 2D). Previous studies have revealed that activity of NK cells dramatically changed in different stages of MS [50].
The MS patients exhibited an increase in B cell frequency in PBMCs (Figure 1F and 1G), which indicates that MS patient’s humoral immune system was activated, causing the corresponding immune damage, leading to the development of MS disease. But the frequency of CD5+ B cells within the B cell population of MS markedly decreased, B cells from MS patients were also shown to have a significantly diminished capacity to express IL-10 (Figure 2E-H), this alternation was recently observed by Piancone et al [51]. Importantly, the frequency of CD5+ and IL-10+ B cells in MS patients with an EDSS score of 2 or less is significantly higher than these in MS patients with an EDSS score greater than 2 (Figure 2I and 2J), which indicates that MS progression is negatively correlated with the ratio of regulatory B cells. In EAE model, studies have found that specifically IL-10 producing B cells (Bregs) predominantly control the disease initiation [13,14], these B cell subsets are necessary for EAE recovery [52], lack of these cells causes chronic inflammation [16]. Although other molecular markers including FSC, Foxp3, CD1d, CD24hiCD38hi, CD27+, Granzyme, PD1, and IL35 have been suggested to identify Breg cells, CD5+ IL-10+ are mostly applied molecular markers and they may somehow overlay with these molecules. In addition, Tregs are considered as an important regulator for immune response [53-55], nonetheless, Bregs were also able to alleviate the inflammation by a direct effect or an indirect effect that promotes Tregs differentiation and maintenance, and then reduces the differentiation of Th1 and Th17 cells [56]. These reports and our results indicate that the frequency and function of IL-10 producing B cells were both impaired in MS and EAE.
MSCs have been demonstrated in preclinical and clinical therapies for a variety of inflammatory and autoimmune diseases [57,58]. In a recent clinical trial report, in comparison with placebo treatment, the frequency of CD19+ IL-10+ B cells was found an increase in the blood when MS patients were treated with MSCs [59], even if the percentage of this B cell subset respect to the total B population in MSC-treated patients was decreased. However, the mechanism underlying this observation remains to be elucidated. To verify the effect of MSCs on B cells, we established an MSC-treated EAE model. Administration of MSCs with a therapeutic protocol significantly decreased the infiltration of mononuclear cells and demyelination in the spinal cords compared with those treated with PBS (Figure 3A-D), and MSCs significantly increased the frequency of CD5+ IL-10+ B cells in EAE model (Figure 3E and 3F). These results indicate that MSCs may improve the symptoms of MS patients by increasing Bregs.
We next analyzed the impact of MSCs on the phenotype and function of B cells from MS patients in vitro. Our results showed that co-culturing with MSCs increased the frequency of CD5+ IL-10+ B cells (Figure 4), enhanced the immunosuppressive capacity of CD5+ B cells (Figure 5D-F). In order to clarify how MSCs modulate the proportion of CD5+ B cells, we analyzed the effects of MSCs on CD5+ B cell proliferation and apoptosis. We found that MSCs not only enhanced proliferative capacity but also inhibited apoptosis in CD5+ B cells. These results indicate that MSCs are able to increase the proportion of Bregs by promoting proliferation but inhibiting apoptosis of CD5+ B cells of MS patients.
Several studies had demonstrated that MSCs were capable of producing a wide range of immunomodulatory factors, such as IDO, prostaglandin E2 (PGE-2), nitric oxide (NO) and TGF-β when they stimulated by an adequate pro-inflammatory [60]. In chronic graft-versus-host disease (cGVHD), MSC infusion was found to increase the number of IL-10 producing B cells in an IDO pathway dependent manner [61]. So we investigated potential mechanisms by which MSCs might regulate CD5+ B cells. Our results showed that D/L-1MT, the specific IDO inhibitor, could partially reverse the MSC-mediated effects on proliferation and survival of CD5+ B cells of MS patients, and the immunosuppressive function of CD5+ B cells which enhanced by MSCs was also blocked by D/L-1MT. These results suggest that MSCs may promote proliferation, survival, and function of CD5+ B cells via IDO pathway.
In summary, this study suggests that MSC treatment likely improves symptoms in EAE model and MS patients by increasing CD5+ IL-10+ B cells. In MS patients, we found that CD5+ IL-10+ B cells markedly decreased, although the sample size still needs to be enlarged to confirm this observation. For the first time, we found that MSCs could increase the frequency and function of CD5+ B cells by promoting proliferation and survival of CD5+ B cells from MS patient via IDO pathway. Our findings provide new clues for illustrating the therapeutic mechanisms of MSC-based treatments for patients with MS.
Acknowledgements
This study was supported by the National Key R&D Program of China (2017YFA0105800), the National Natural Science Foundation of China (No. 81671611), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2016ZT06S252), the Natural Science Foundation of Guangdong Province (No. 2018A030313259), the Key Scientific & Technological Projects of Guangdong Province (No. 2017B030314027). The authors thank Dr. Zezhi Li and Dr. Lu Han for EAE model support.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Mc Guire C, Beyaert R, van Loo G. Death receptor signalling in central nervous system inflammation and demyelination. Trends Neurosci. 2011;34:619–628. doi: 10.1016/j.tins.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 3.Rice CM, Kemp K, Wilkins A, Scolding NJ. Cell therapy for multiple sclerosis: an evolving concept with implications for other neurodegenerative diseases. Lancet. 2013;382:1204–1213. doi: 10.1016/S0140-6736(13)61810-3. [DOI] [PubMed] [Google Scholar]
- 4.Friese MA, Fugger L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain. 2005;128:1747–1763. doi: 10.1093/brain/awh578. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Xia Z, Lan Q, Wang J, Su W, Han YP, Fan H, Liu Z, Stohl W, Zheng SG. BAFF promotes Th17 cells and aggravates experimental autoimmune encephalomyelitis. PLoS One. 2011;6:e23629. doi: 10.1371/journal.pone.0023629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol. 2008;7:852–858. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- 8.Disanto G, Morahan JM, Barnett MH, Giovannoni G, Ramagopalan SV. The evidence for a role of B cells in multiple sclerosis. Neurology. 2012;78:823–832. doi: 10.1212/WNL.0b013e318249f6f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH HERMES Trial Group. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 10.Milo R. Therapeutic strategies targeting B-cells in multiple sclerosis. Autoimmun Rev. 2016;15:714–718. doi: 10.1016/j.autrev.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 12.Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2011;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 16.Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW, Hupperts R, Damoiseaux J. Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naive/memory Breg ratio during a relapse but not in remission. J Neuroimmunol. 2011;239:80–86. doi: 10.1016/j.jneuroim.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JA, Imrey PB, Planchon SM, Bermel RA, Fisher E, Fox RJ, Bar-Or A, Sharp SL, Skaramagas TT, Jagodnik P, Karafa M, Morrison S, Reese Koc J, Gerson SL, Lazarus HM. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult Scler. 2018;24:501–511. doi: 10.1177/1352458517703802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng M, Liu Y, Wang W, Wei C, Liu F, Du Z, Xie Y, Tang W, Hou Z, Li Q. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am J Transl Res. 2018;10:212–223. [PMC free article] [PubMed] [Google Scholar]
- 19.Uccelli A, Laroni A, Brundin L, Clanet M, Fernandez O, Nabavi SM, Muraro PA, Oliveri RS, Radue EW, Sellner J, Soelberg Sorensen P, Sormani MP, Wuerfel JT, Battaglia MA, Freedman MS MESEMS study group. ME-senchymal StEm cells for multiple sclerosis (MESEMS): a randomized, double blind, cross-over phase I/II clinical trial with autologous mesenchymal stem cells for the therapy of multiple sclerosis. Trials. 2019;20:263. doi: 10.1186/s13063-019-3346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahraian MA, Mohyeddin Bonab M, Baghbanian SM, Owji M, Naser Moghadasi A. Therapeutic use of intrathecal mesenchymal stem cells in patients with multiple sclerosis: a pilot study with booster injection. Immunol Invest. 2019;48:160–168. doi: 10.1080/08820139.2018.1504301. [DOI] [PubMed] [Google Scholar]
- 21.Harris VK, Vyshkina T, Sadiq SA. Clinical safety of intrathecal administration of mesenchymal stromal cell-derived neural progenitors in multiple sclerosis. Cytotherapy. 2016;18:1476–1482. doi: 10.1016/j.jcyt.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Riordan NH, Morales I, Fernandez G, Allen N, Fearnot NE, Leckrone ME, Markovich DJ, Mansfield D, Avila D, Patel AN, Kesari S, Paz Rodriguez J. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J Transl Med. 2018;16:57. doi: 10.1186/s12967-018-1433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, Zheng SG. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65:1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su W, Wan Q, Huang J, Han L, Chen X, Chen G, Olsen N, Zheng SG, Liang D. Culture medium from TNF-alpha-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol. 2015;136:423–432. e428. doi: 10.1016/j.jaci.2014.12.1926. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Zhou L, Dang J, Zhang X, Wang J, Chen Y, Liang J, Li D, Ma J, Yuan J, Chen W, Zadeh HH, Olsen N, Zheng SG. Human gingiva-derived mesenchymal stem cells ameliorate streptozoticin-induced T1DM in mice via Suppression of T effector cells and up-regulating treg subsets. Sci Rep. 2017;7:15249. doi: 10.1038/s41598-017-14979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang F, Chen M, Chen W, Gu J, Yuan J, Xue Y, Dang J, Su W, Wang J, Zadeh HH, He X, Rong L, Olsen N, Zheng SG. Human gingiva-derived mesenchymal stem cells inhibit xeno-graft-versus-host disease via CD39-CD73-adenosine and IDO signals. Front Immunol. 2017;8:68. doi: 10.3389/fimmu.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barzilay R, Melamed E, Offen D. Introducing transcription factors to multipotent mesenchymal stem cells: making transdifferentiation possible. Stem Cells. 2009;27:2509–2515. doi: 10.1002/stem.172. [DOI] [PubMed] [Google Scholar]
- 29.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 30.Dazzi F, Lopes L, Weng L. Mesenchymal stromal cells: a key player in ‘innate tolerance’? Immunology. 2012;137:206–213. doi: 10.1111/j.1365-2567.2012.03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapoor S, Patel SA, Kartan S, Axelrod D, Capitle E, Rameshwar P. Tolerance-like mediated suppression by mesenchymal stem cells in patients with dust mite allergy-induced asthma. J Allergy Clin Immunol. 2012;129:1094–1101. doi: 10.1016/j.jaci.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Huang F, Li W, Dang JL, Yuan J, Wang J, Zeng DL, Sun CX, Liu YY, Ao Q, Tan H, Su W, Qian X, Olsen N, Zheng SG. Human gingiva-derived mesenchymal stem cells modulate monocytes/macrophages and alleviate atherosclerosis. Front Immunol. 2018;9:878. doi: 10.3389/fimmu.2018.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo Y, Wu W, Gu J, Zhang X, Dang J, Wang J, Zheng Y, Huang F, Yuan J, Xue Y, Fu Q, Kandalam U, Colello J, Zheng SG. Human gingival tissue-derived MSC suppress osteoclastogenesis and bone erosion via CD39-adenosine signal pathway in autoimmune arthritis. EBioMedicine. 2019;43:620–631. doi: 10.1016/j.ebiom.2019.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al Jumah MA, Abumaree MH. The immunomodulatory and neuroprotective effects of mesenchymal stem cells (MSCs) in experimental autoimmune encephalomyelitis (EAE): a model of multiple sclerosis (MS) Int J Mol Sci. 2012;13:9298–9331. doi: 10.3390/ijms13079298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morando S, Vigo T, Esposito M, Casazza S, Novi G, Principato MC, Furlan R, Uccelli A. The therapeutic effect of mesenchymal stem cell transplantation in experimental autoimmune encephalomyelitis is mediated by peripheral and central mechanisms. Stem Cell Res Ther. 2012;3:3. doi: 10.1186/scrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E, Yuan S, Young YK, Boivin MN, Forner K, Basik M, Galipeau J. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Sun J, Wang F, Li Y, Bi J, Qu T. Umbilical cord-derived mesenchymal stem cells reversed the suppressive deficiency of T regulatory cells from peripheral blood of patients with multiple sclerosis in a co-culture - a preliminary study. Oncotarget. 2016;7:72537–72545. doi: 10.18632/oncotarget.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng Y, Yi S, Wang G, Cheng J, Zhang Y, Chen W, Tai Y, Chen S, Chen G, Liu W, Zhang Q, Yang Y. Umbilical cord-derived mesenchymal stem cells instruct dendritic cells to acquire tolerogenic phenotypes through the IL-6-mediated upregulation of SOCS1. Stem Cells Dev. 2014;23:2080–2092. doi: 10.1089/scd.2013.0559. [DOI] [PubMed] [Google Scholar]
- 39.Jia CC, Wang TT, Liu W, Fu BS, Hua X, Wang GY, Li TJ, Li X, Wu XY, Tai Y, Zhou J, Chen GH, Zhang Q. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS One. 2013;8:e63243. doi: 10.1371/journal.pone.0063243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 41.Gao Y, Tang J, Chen W, Li Q, Nie J, Lin F, Wu Q, Chen Z, Gao Z, Fan H, Tsun A, Shen J, Chen G, Liu Z, Lou Z, Olsen NJ, Zheng SG, Li B. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc Natl Acad Sci U S A. 2015;112:E3246–3254. doi: 10.1073/pnas.1421463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Probert L, Eugster HP, Akassoglou K, Bauer J, Frei K, Lassmann H, Fontana A. TNFR1 signalling is critical for the development of demyelination and the limitation of T-cell responses during immune-mediated CNS disease. Brain. 2000;123:2005–2019. doi: 10.1093/brain/123.10.2005. [DOI] [PubMed] [Google Scholar]
- 43.Stoll G, Jander S, Schroeter M. Cytokines in CNS disorders: neurotoxicity versus neuroprotection. J Neural Transm Suppl. 2000;59:81–89. doi: 10.1007/978-3-7091-6781-6_11. [DOI] [PubMed] [Google Scholar]
- 44.Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol. 2011;74:1–13. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- 45.Jiang HR, Milovanovic M, Allan D, Niedbala W, Besnard AG, Fukada SY, Alves-Filho JC, Togbe D, Goodyear CS, Linington C, Xu D, Lukic ML, Liew FY. IL-33 attenuates EAE by suppressing IL-17 and IFN-gamma production and inducing alternatively activated macrophages. Eur J Immunol. 2012;42:1804–1814. doi: 10.1002/eji.201141947. [DOI] [PubMed] [Google Scholar]
- 46.Miljkovic D, Spasojevic I. Multiple sclerosis: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2013;19:2286–2334. doi: 10.1089/ars.2012.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, Medaer R, Hupperts R, Stinissen P. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 49.Habib J, Deng J, Lava N, Tyor W, Galipeau J. Blood B cell and regulatory subset content in multiple sclerosis patients. J Mult Scler (Foster City) 2015;2 doi: 10.4172/2376-0389.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chanvillard C, Jacolik RF, Infante-Duarte C, Nayak RC. The role of natural killer cells in multiple sclerosis and their therapeutic implications. Front Immunol. 2013;4:63. doi: 10.3389/fimmu.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piancone F, Saresella M, Marventano I, La Rosa F, Zoppis M, Agostini S, Longhi R, Caputo D, Mendozzi L, Rovaris M, Clerici M. B lymphocytes in multiple sclerosis: bregs and BTLA/CD272 expressing-CD19+ lymphocytes modulate disease severity. Sci Rep. 2016;6:29699. doi: 10.1038/srep29699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 53.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 54.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 55.Liu ZM, Wang KP, Ma J, Guo Zheng S. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell Mol Immunol. 2015;12:553–557. doi: 10.1038/cmi.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5:173ra123. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 57.Ben-Ami E, Berrih-Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev. 2011;10:410–415. doi: 10.1016/j.autrev.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Francois M, Galipeau J. New insights on translational development of mesenchymal stromal cells for suppressor therapy. J Cell Physiol. 2012;227:3535–3538. doi: 10.1002/jcp.24081. [DOI] [PubMed] [Google Scholar]
- 59.Llufriu S, Sepulveda M, Blanco Y, Marin P, Moreno B, Berenguer J, Gabilondo I, Martinez-Heras E, Sola-Valls N, Arnaiz JA, Andreu EJ, Fernandez B, Bullich S, Sanchez-Dalmau B, Graus F, Villoslada P, Saiz A. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS One. 2014;9:e113936. doi: 10.1371/journal.pone.0113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 61.Peng Y, Chen X, Liu Q, Zhang X, Huang K, Liu L, Li H, Zhou M, Huang F, Fan Z, Sun J, Liu Q, Ke M, Li X, Zhang Q, Xiang AP. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29:636–646. doi: 10.1038/leu.2014.225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.