Abstract
Background: Nobiletin (NOB), isolated from Citrus nobilis, has been reported to inhibit cerebral ischemia/reperfusion (I/R) induced cell apoptosis in the brain. The mechanisms and the protective ability of NOB on cerebral I/R rats are unclear. Methods: A middle cerebral artery occlusion (MCAO) rat model was established and treated with different doses of NOB. The neurological deficits, brain water content and brain index were explored after reperfusion, and TTC staining was applied to assess the infarct area. The production of reactive oxygen species (ROS) related enzymes in the ischemic cortex samples from each group was measured. TUNEL staining was performed to evaluate neuronal cell apoptosis in brain tissues. The expression of cell apoptosis related proteins, p-p38 and MAPKAP-2 and the levels of inflammatory factors were examined by western blotting assay and ELISA. Results: NOB treatment notably improved the neurological deficits, brain water content and brain index in an MCAO model, accompanied by decreased infarct area in the brain tissue. Apoptosis induced by cerebral I/R was also decreased by NOB administration via upregulating Bcl-2 and downregulating Bax and caspase3. The levels of pro-inflammatory mediators TNF-α, IL-6 were reduced and anti-inflammatory cytokine IL-10 was increased by NOB treatment in MCAO rats. Further, we found that the expression of p-p38 and MAPKAP-2 was reduced by NOB treatment in MCAO rats. Conclusion: The present results suggest that NOB serves a protective role in I/R-induced cerebral-neuron injury. The mechanisms underlying these effects may be associated with the MAPK signaling pathway.
Keywords: Nobiletin, middle cerebral artery occlusion, ischemic-reperfusion injury, apoptosis, p38, MAPKAP-2
Introduction
Cerebral ischemia, also known as stroke, is an acute disease with severe complications and high mortality caused by insufficient blood supply to the brain [1,2]. Stroke is the leading cause of adult disability and the fifth account of death worldwide [3]. Neuroprotection and reperfusion are the major therapeutic strategies for the treatment of ischemic stroke [4,5]. However, reperfusion may aggravate the injury initially caused by ischemia [6]. During I/R process, excessive production of ROS by the mitochondria suppressed the endogenous anti-oxidative defense system, caused neuronal apoptosis and interfered with energy metabolism [7,8]. Studies had showed that I/R injury could affect apoptosis, oxidative stress, mitochondrial homeostasis, and energy metabolism [9,10]. The pathogenesis of cerebral I/R injury including neuronal survival and apoptosis, ROS production followed by oxidative stress and inflammation [11-13]. I/R injury could aggravate cerebral damage through a series of inflammatory cascades [14]. Thus, it will bring significant societal and health impacts by preventing cerebral ischemia-reperfusion injury.
NOB is a ubiquitous polyphenolic ingredient and bioflavonoid extracted from Citrus nobilis Lour, C. aurantium L and C. reticulata Blanco (Figure 1). It has been emphasized that NOB exerts pleiotropic biological activities, including the anti-agglutination, anti-thrombosis, anti-inflammation, anti-tumor and cardiovascular-protections [15-18]. Yang G, et al. found that NOB reduced the inflammation of the proximal aorta in Sprague-Dawley rats in vivo and trimethylamine oxide-induced vascular inflammation via inhibiting the NF-κB/MAPK pathways in vitro [19]. Recently, it has been reported that protective effects of NOB against I/R injury after liver transplantation are mainly manifested as the reduction of inflammation [20]. It had been demonstrated that NOB improved brain ischemia-induced learning and memory deficits and protected against cerebral ischemia/reperfusion injury in many animal studies [21-23]. Previous study revealed that the anti-apoptotic effect was considered as a neuroprotective mechanism of NOB in rats with transient MCAO [22]. The protective roles of NOB for I/R injury had raised attention. Several researches had shown NOB owned the anticancer effects through the p38 mitogen activated protein kinase (MAPK) pathway [24-26]. However, the mechanism of the neuroprotective effect of NOB on cerebral I/R injury remains to be characterized.
Figure 1.

The chemical structure of nobiletin.
Therefore, the aim of the present study is to detect the influences of NOB on focal cerebral I/R injury using through a MCAO-reperfusion model, and investigate the potential mechanisms underlying its protective effects.
Methods
Experimental animals and MCAO model
Adult male Wistar rats (body weight, 270-330 g) were maintained under standard conditions (25±1°C, with 12 h light/12 h dark cycle of housing, food and water available), which were obtained from Laboratory animal center of Wuxi People’s Hospital. All animal experiment protocols were approved by the Institutional Animal Care Committee of Wuxi People’s Hospital, and were performed in strict consistence with its guidelines.
After a week of adjustable feeding, the rats were subjected to MCAO surgery as the previous described [27,28]. Briefly, following anesthetization with 3% isoflurane, rats were placed in a prone position and treated with left MCAO. During surgery, body temperature was maintained at 36.5-37.0°C using a heating pad on the surgical table. The incision region was disinfected with povidone-iodine solution. The external carotid artery (ECA) was separated and ligatured. A nylon suture with a blunted coated tip (0.35 mm diameter) was drawn into the ECA and then into internal carotid artery (ICA) until resistance was felt. The middle cerebral artery was occluded by the suture 18 mm distal from the carotid bifurcation. Ischemia reperfusion injury was executed by removing the filament after 2 h of occlusion. After infiltrated with 1% lidocaine, the neck wound was closed with sutures. Following disinfection of the surgical region, the rats were returned to cages with food and water available. The sham operated rats were subjected to the same procedures except the insertion of filament. The major limitation associated with this model is subarachnoid hemorrhage due to vessel rupturing and hyperthermia.
Treatment and grouping
Different dosages of NOB were administrated intravenously at the start of reperfusion to rats undergoing MCAO. The same volumes of saline were administered in the same manner to rats of the sham and MCAO groups, and the same volumes of nimodipine were administered in the same manner to rats of positive group. Then, the rats were randomly divided into 6 groups (each group containing 10 rats): Sham group, sham operated control; MCAO group, rats undergoing MCAO surgery and saline was i.p. injected at the start of reperfusion; NOB groups, 5, 10 and 20 mg/kg of NOB was i.p. injected at the start of reperfusion to rats undergoing MCAO; Positive group, nimodipine (20 mg/kg) was i.p. injected at the start of reperfusion to rats undergoing MCAO.
Assessment of neurological deficit scores
After 24 h of reperfusion, neurologic deficit scores was performed blindly 24 h after reperfusion, according to Bederson method [29]. The scoring system was as follows: Grade 0, no neurological deficits; Grade 1, unable to entirely extend contralateral forelimb; Grade 2, falling to the contralateral side; Grade 3, rat circle to the right side and listlessness. The score was performed by an observer blinded to group assignment.
Assessment of brain water content and brain index
After neurological deficit scores and body weight, rats were sacrificed by cervical dislocation. The brains were then rapidly removed and weighed to obtain the wet weight. After 24 h of drying in an oven at 120°C until the weight was invariable, the brains were weighed again to obtain the dry weight. Brain water content and brain index can reflect cerebral edema to some extent. Brain water content was calculated as follows: (wet weight-dried weight)/wet weight × 100%. Brain index was calculated as follows: (wet weight/rat weight) × 100%.
Assessment of cerebral infarction area
Rats were sacrificed by cervical dislocation and the brains were rapidly stored at 20°C for 20 min. The uniform of 2 mm-thick coronal slices were cut with the aid of a brain slicer matrix, and six coronal slices were obtained and then stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma, USA) at 37°C for 15 min in the dark. Pale unstained sections were considered to be indicative of infarct regions, whereas red-stained sections were indicative of normal tissue. Then he coronal sections were fixed in 4% paraformaldehyde and filter paper absorb the liquid for photography. The infarct area ratio (%) was then measured by utilizing Image J (version 1.8.0), computer based program. The infarcted regions and the areas of both hemispheres were calculated for each brain slice. The average infarct volume (%) was expressed as: [left hemisphere area-(right hemisphere area-infarct area)]*2.
Measurement of SOD, MDA and LDH and inflammatory mediators
Rats were sacrificed by cervical dislocation at 24 h after reperfusion and the brains were removed. infarction size were homogenized on ice in PBS buffer to prepare a 10% homogenate. The homogenate samples were transferred to sterile tubes and centrifuged at 10000 rmp at 4°C. Then the supernatant was collected for the determination of levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and interleukin-10 (IL-10). Meanwhile, the content of superoxide dismutase (SOD), malo-ndialdehyde (MDA) and lactate dehydrogenase (LDH) were measured by ELISA kits method according to the manufacturer’s instructions (Sigma, USA).
Terminal transferase-mediated dUTP nick-end labeling (TUNEL) staining
TUNEL staining was performed using a situ TUNEL apoptosis detection kit (#G002-1, Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) according to the manufacturer’s instructions. Briefly, paraffin-embedded sections were deparaffinized and rehydrated, and then dealed with proteinase K (10 μg/ml) at 37°C for 30 minutes. The sections were washed in PBS for 3 times and then incubated in the 50 μl TUNEL reaction buffer including the enzyme at 37°C for 30 minutes away from light. Finally, the samples were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI) (Sigma) to visualize cell nuclei and photographed using a fluorescence microscope (Leica, Biosystems, Germany). Count positive and total cells in three fields at 200 magnification by blinded technicians.
Protein extraction and Western blot analysis
Total protein was isolated from each group brain tissue using RIPA buffer. Protein concentrations were determined with a Pierce BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). Equal amount of protein (30 μg per sample) were electrophoresed using 8%-12% SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membrane (Beyotime Institute of Biotechnology). Following transfer, membranes were blocked with 10% skimmed milk at 37°C for 2 hours. And then, incubate the membranes using the listed primary antibody: rabbit anti-Cleaved-caspase3 (ab49822, 1:500, Abcam), rabbit anti-caspase3 (ab13847, 1:500, Abcam), rabbit anti-Bax (ab53154, 1:500, Abcam), rabbit anti-Bcl-2 (ab59348, 1:1000, Abcam), rabbit anti-MAPKAP-2 (#3042, 1:1000, Cell Signaling Technology, Inc.), rabbit anti-p38 (ab170099, 1:1000, Abcam), rabbit anti-p-p38 (ab47363, 1:1000, Abcam) and rabbit anti-GAPDH (ab37168, 1:1000, Abcam) at 4°C overnight. Then, the membranes were incubated with goat anti rabbit secondary antibody HRP-conjugated IgG (ab97051, 1:5000, Abcam). The protein bands were detected using an enhanced chemiluminescence detection kit (Beyotime Institute of Biotechnology) using a BioRad imaging system according to the manufacturer’s introduction.
Statistical analysis
All data were expressed as the mean ± standard deviation and analyzed by student’s t-tests and one-way ANOVA followed by Dunnett test. All the statistics analyses were performed using SPSS software (v.18; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.0 software. P<0.05 was considered to indicate a statistically significant difference.
Results
NOB improved nerve function deficit of MCAO rats
Neurological deficit scores were evaluated after 24 h of reperfusion according to the Bederson method. No neurological deficit observed in sham rats, whereas MCAO rats suffered from I/R injury, showed all the characteristics of neuron damage and had relatively high neurological deficit scores (2.51±0.64; Table 1). The positive nimodipine drug (20 mg/kg) treatment showed markedly reduced the neurological deficit scores (1.11±0.84, Table 1). In addition, the results also displayed that NOB treatment (5, 10 and 20 mg/kg) reduced the neurological deficit scores of MCAO rats, presenting dose dependence with the scores 2.32±0.39, 1.95±0.57 and 0.98±0.25, respectively (Table 1). These results indicated NOB treatment could effectively improve nerve function deficit in MCAO rats.
Table 1.
Effects of nobiletin on neurological deficit scores of MCAO rats 24 h after reperfusion
| Group | Dosage/(mg·kg-1) | Neurological deficit scores (0~3) |
|---|---|---|
| Sham | - | 0.00±0.00 |
| MCAO | - | 2.51±0.64*** |
| Nimodipine (20 mg/kg) | 20 | 1.11±0.84### |
| NOB (20 mg/kg) | 20 | 0.98±0.25### |
| NOB (10 mg/kg) | 10 | 1.95±0.57## |
| NOB (5 mg/kg) | 5 | 2.32±0.39 |
Data are represented as mean ± S.D. of 10 rats of each group (n = 10).
P<0.001 vs. Sham.
P<0.01 vs. MCAO;
P<0.001 vs. MCAO.
NOB, nobiletin.
NOB reduced brain water content and cerebral index in MCAO rats
The effects of NOB on brain water content and cerebral index have been measure and calculated (Table 2). The results showed that the brain water content (85.08±0.61) and cerebral index (0.75±0.03) were significantly increased in the MCAO group compared with the sham group (82.44±0.49 and 0.60±0.01). The positive nimodipine drug (20 mg/kg) treatment remarkably reduced the brain water content (82.88±0.41) and cerebral index (0.61±0.02) of MCAO rats. Additionally, NOB treatment improved the brain water content and cerebral index of MCAO rats in a dose-dependent manner, and 10 mg/kg and 20 mg/kg NOB significantly decreased the brain water content (83.33±0.35 and 83.08±0.48) and brain index (0.64±0.02 and 0.62±0.01) of MCAO rats, but 5 mg/kg NOB treatment had no significant effect. These results indicated that NOB reduced brain water content and brain index in MCAO rats.
Table 2.
Effects of nobiletin on brain water content and cerebral index of MCAO rats 24 h after reperfusion
| Group | Dosage/(mg·kg-1) | Cerebral index/% | Brain water content/% |
|---|---|---|---|
| Sham | - | 0.60±0.01 | 82.44±0.49 |
| MCAO | - | 0.75±0.03** | 85.08±0.61*** |
| Nimodipine (20 mg/kg) | 20 | 0.61±0.02## | 82.88±0.41### |
| NOB (20 mg/kg) | 20 | 0.62±0.01## | 83.08±0.48### |
| NOB (10 mg/kg) | 10 | 0.64±0.02# | 83.33±0.35## |
| NOB (5 mg/kg) | 5 | 0.69±0.02 | 83.51±0.81 |
Data are represented as mean ± S.D. of 10 rats of each group (n = 10).
P<0.01 vs. Sham;
P<0.001 vs. Sham.
P<0.05 vs. MCAO;
P<0.01 vs. MCAO;
P<0.001 vs. MCAO.
NOB, nobiletin.
NOB reduced cerebral infarction area in brain tissue of MCAO rats
Infarct area of brain tissues from MCAO rats were measured 24 h after I/R injury by TTC staining. As shown in Figure 2, no infarct area was observed in sham rats, whereas the infarct area of MCAO rats reached about 35% the whole brain (Figure 2B, P<0.001). The positive nimodipine drug (20 mg/kg) treatment remarkably reduced the cerebral infarction area of MCAO rats (Figure 2B, P<0.001). Similarly, NOB (20 mg/kg) significantly decreased cerebral infarction area of MCAO rats (Figure 2B, P<0.001). The cerebral infarction area when treatment of 5 and 10 mg/kg NOB tended to be smaller than that in MCAO group (Figure 2B, P<0.05). Representative sections of each group are presented in Figure 2A. These results indicated that NOB effectively improved cerebral infarction area in MCAO rats.
Figure 2.
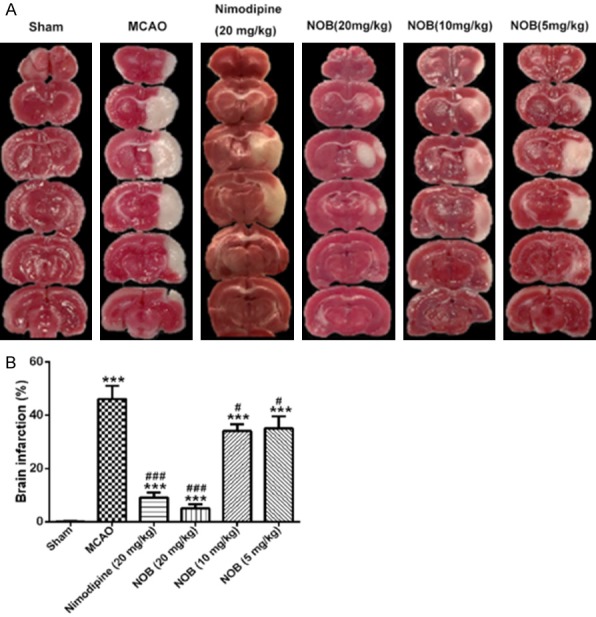
Effects of nobiletin on brain infarction. Brain coronal sections were stained with TTC, which distinguishes between ischemic and non-ischemic areas. A. Representative coronal sections of each group. B. Infarct volume of each group. Data are represented as mean ± S.D. of 10 rats of each group (n = 10). ***P<0.001 vs. Sham; ###P<0.001 vs. MCAO. NOB, nobiletin.
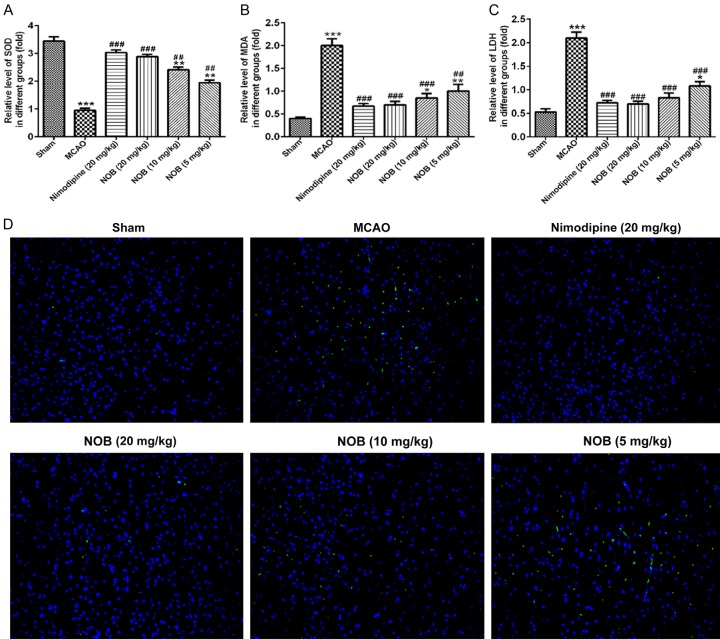
NOB suppressed production of reactive oxygen species (ROS) and ROS-induced cell apoptosis in brain tissue of MCAO rats
As shown in Figure 3, the results showed that the level of SOD was significantly decreased (Figure 3A, P<0.001), and MDA (Figure 3B, P<0.001) and LDH (Figure 3C, P<0.001) were increased in the brain tissue after I/R injury. Compared with the MCAO group, treatment with nimodipine drug (20 mg/kg) and different dosages of NOB improved oxidative damage. Briefly, the level of SOD significantly increased, and MDA and LDH levels observed reduced among the positive group and NOB groups when compared with the MCAO group. Moreover, the high dose group of NOB (20 mg/kg) and the positive nimodipine drug (20 mg/kg) had no significant. These results indicated that NOB reduced production of ROS in MCAO rats.
Figure 3.
Effects of nobiletin on oxidant enzymes activities in brain tissues. A-C. The SOD, MDA and LDH levels of each group. D. TUNEL staining with representative photos showed apoptotic cells (scale bar = 40 μm). Images are representative of experiments performed three times. MCAO causes significant cell apoptosis; while treatment with NOB attenuated apoptotic damage. *P<0.05, **P<0.01, ***P<0.001 vs. Sham; ##P<0.01, ###P<0.001 vs. MCAO. NOB, nobiletin.
Meanwhile, cell apoptosis in brain tissue was measured by TUNEL staining. Compared with sham group, a significant number of TUNEL-positive cells was observed in the infarcted area of the cortex region of MCAO rats (Figure 3D) after I/R injury. The infarcted area was accompanied with fragmented apoptotic bodies, a phenomenon that could be significantly decreased by NOB and nimodipine drug treatment with dose dependent effect. These results showed the neuroprotective role of NOB on cell apoptosis in MCAO rats.
Effects of NOB on apoptosis related proteins in brain tissue
Our results showed that Bcl2 protein level was significantly decreased in MCAO group as compared to sham group (Figure 4A, P<0.001), while the expression changes could be reversed by NOB treatment in brain tissue in a dose-dependent manner. Meanwhile, the expression of Bax and cleaved-caspase-3 were markedly increased in MCAO group, while the expression changes could be reversed by NOB treatment with dose-dependence (Figure 4B and 4C, P<0.001). Furthermore, the level of Bcl2 was increased and the levels of Bax, cleaved-caspase3 were notably decreased after nimodipine treatment compared with MCAO group. There was no significant change in caspase3 expression in each group. Moreover, NOB (20 mg/kg) group and the positive nimodipine drug (20 mg/kg) group had no significant. These results indicated that NOB inhibited the cell apoptosis in MCAO animals.
Figure 4.
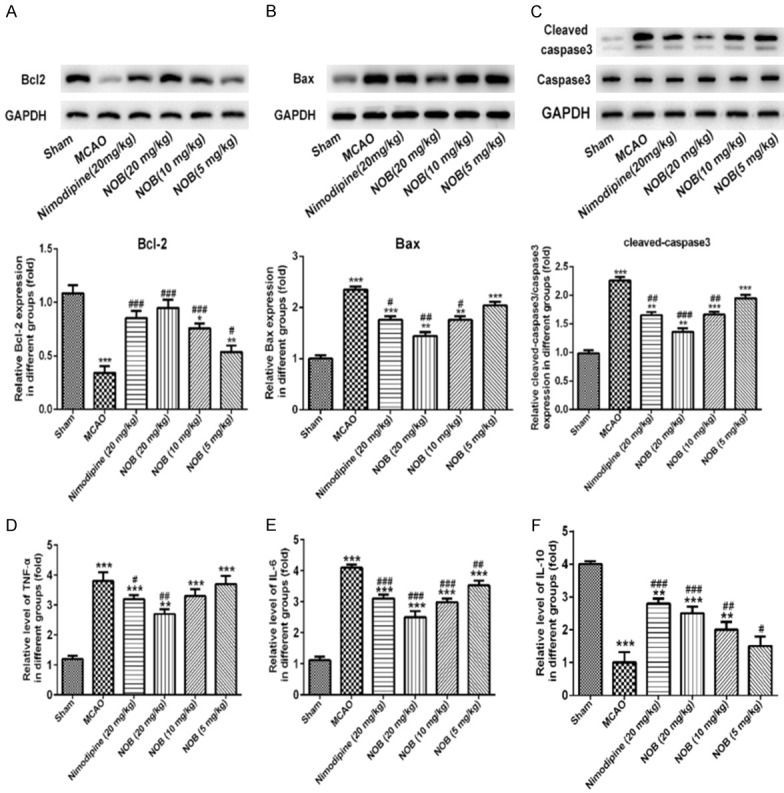
Effects of nobiletin on the expression of Bcl2, Bax, cleaved-caspase3 and caspase3, and inflammatory cytokines in brain tissue. Western blot analysis of Bcl2 (A), Bax (B), cleaved-caspase3 and caspase3 (C) from each group (n = 10 rats per group). ELISA analysis of TNF-α (D), IL-6 (E) and IL-10 (F) levels of each group. Data are represented as mean ± S.D. of 10 rats of each group. *P<0.05, **P<0.01, ***P<0.001 vs. Sham; #P<0.05, ##P<0.01, ###P<0.001 vs. MCAO. NOB, nobiletin.
Effects of NOB on inflammatory factors in brain tissue
As shown in Figure 4, high levels of pro-inflammatory mediators TNF-α, IL-6 (Figure 4D and 4E, P<0.001) and low level of the anti-inflammatory cytokine IL-10 (Figure 4F, P<0.001) were identified in MCAO group compared with sham group. These results confirmed that inflammatory damage occurred in ischemic brain injury. Treatment with NOB (5, 10 and 20 mg/kg) or positive nimodipine drug (20 mg/kg) reduced TNF-α and IL-6 levels and increased IL-10 level. These results indicated that NOB decreased the inflammatory factors in MCAO rats.
Effects of NOB on p38, p-p38 and MAPKAP-2 protein expression
As shown in Figure 5, the expression of p-p38 (Figure 5A, P<0.001) and MAPKAP-2 (Figure 5B, P<0.001) were significantly increased in MCAO group compared with sham group. Furthermore, the protein expression of p-p38 and MAPKAP-2 was reduced after treatment with nimodipine (20 mg/kg) or NOB group as the concentration increase when compared with MCAO group. However, p38 expression had no changed in each group (Figure 5A). These results showed that NOB could protect cerebral ischemic-reperfusion injury via p38/MAPKAP-2 signaling pathway.
Figure 5.
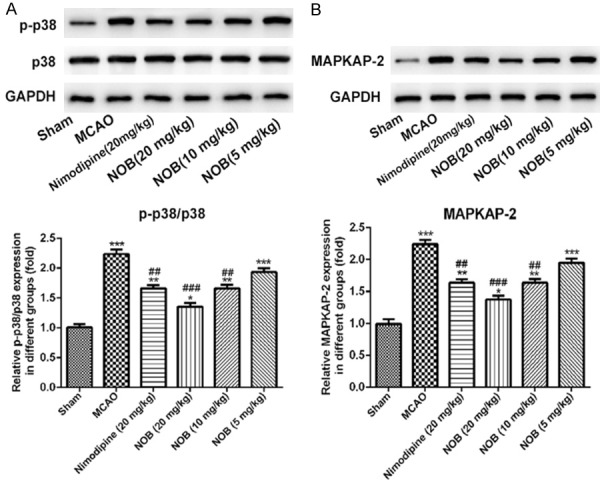
Effects of nobiletin on the expression of p38, p-p38 and MAPKAP-2 in brain tissue. Western blot analysis of p38 and p-p38 (A) and MAPKAP-2 (B) from each group (n = 10 rats per group). Data are represented as mean ± S.D. of 10 rats of each group. *P<0.05, **P<0.01, ***P<0.001 vs. Sham; ##P<0.01, ###P<0.001 vs. MCAO. NOB, nobiletin.
Discussion
Natural products can be used to regulate cytokine-activity in the treatment of diseases [6]. NOB had been emphasized that NOB exerts pleiotropic biological activities, including the anti-agglutination, anti-thrombosis, anti-inflammation, anti-tumor and cardiovascular-protections. Due to a serious hypoxia of brain tissue of MCAO rats, microcirculation blood flow barriers, eventually leading to blood brain edema, namely the brain water content increased, thus increase the brain index. Here, NOB administration can dramatically reduce the brain index and brain edema. Additionally, NOB improved the neurological function and decreased cerebral infarction area in brain tissues of MCAO rats. These data prompted that NOB may have a neuroprotective effect against I/R injury during stroke therapy.
Currently, tissue plasminogen activator (tPA), a thrombolytic agent, was used to the treatment of ischemic stroke to restore cerebral blood flow and promote the production of ROS [30,31]. We found that the level of MDA was significantly increased in the NOB group compared with the MCAO group (Figure 3). Furthermore, the significantly decreased levels of SOD, LDH were observed in the NOB group compared with MCAO group with dose dependent effect. These results indicated that NOB reduced production of ROS in MCAO rats. Overproduction of ROS induces oxidative stress, which then activates the nuclear factor kappa-beta (NF-κB) signaling pathway to synthesize pro-inflammatory cytokines such the TNF-α and IL-6, which promotes neuronal inflammation. Inflammation in the brain causes ischemic stroke, which occurs in ~80% patients with stroke and causes the release of free radicals, leading to oxidative damage of brain tissue [32,33]. In this study, the levels of pro-inflammatory mediators TNF-α, IL-6 were reduced and level of the anti-inflammatory cytokine IL-10 was increased with NOB treatment compared with MCAO rats.
Apoptosis is an essential mechanism for cell death following many types of chemotherapy. In the present study, cell apoptosis was determined by TUNEL staining. The results showed that following NOB treatment significantly inhibited the cell apoptosis compared with the MCAO group. The mitochondrial (or intrinsic) apoptosis signaling pathways were mainly regulated by the interaction among the Bcl-2 protein family members [34]. The upregulation of Bax expression could lead to an increase of mitochondrial outer membrane permeability, thereby releasing cytochrome C from the mitochondria into the cytoplasm, followed by the activation of caspases and apoptotic complexes [35]. Next, we explored that the expression levels of apoptosis-related proteins. Furthermore, Bcl2 was increased, but Bax and cleaved-caspase3 were notably decreased in the NOB group compared with MCAO group in a dose-dependent manner. These results indicated that NOB inhibited the cell apoptosis in MCAO rats.
I/R injury-induced oxidative stress and inflammation also triggers multiple-cell apoptotic pathways responsible for cell death by necrosis or apoptosis [36,37]. The MAPK signaling participated in the occurrence of drug damage and tumor-related drug resistance [38]. Evidence demonstrated that p38 MAPK was a major downstream player in cerebral ischemic-reperfusion injury [39]. When extracellular stimuli occurs, p38 can be phosphorylated, followed by p-p38 entering the nucleus, activating transcription factors and ultimately promoting apoptosis. Previous studies have showed that pretreatment with the p38 MAPK inhibitor (SB203580) obviously enhanced cell apoptosis through the Bax upregulation, Bcl-2 downregulation, and caspase-3 activation [40-42]. In the present study, we found that the expression of p-p38 and MAPKAP-2 were significantly increased in MCAO group compared with sham group and reduced in the NOB group compared with MCAO group as the concentration increase. The expression of p38 was no changed in each group. These results indicated that NOB might protect cerebral ischemic-reperfusion injury via p38/MAPKAP-2 signal pathway.
Conclusion
In summary, the present study demonstrated that NOB has a protective effect on cerebral neurons in I/R injury. NOB possessed potent anti-inflammation and anti-oxidant activities. Treatment of NOB effectively attenuated brain infarct region, improved neurological deficit function and reduced cell apoptosis. The molecular mechanisms underlying these effects may be associated with the p38/MAPKAP-2 signal pathways. These novel findings provide the pharmacological foundation for the further development of NOB for the treatment of ischemic stroke. Furthermore, NOB may be a promising neuroprotective candidate, and requires further laboratory and clinical investigation.
Acknowledgements
All animal experiment protocols were approved by the Institutional Animal Care Committee of Wuxi People’s Hospital.
Disclosure of conflict of interest
None.
Abbreviations
- NOB
Nobiletin
- I/R
Ischemia/reperfusion
- MCAO
Middle cerebral artery occlusion
- ECA
External carotid artery
- ICA
Internal carotid artery
- TTC
2,3,5-triphenyltetrazolium chloride
- MAPK
Mitogen activated protein kinase
- ROS
Reactive oxygen species
- TNF-α
Tumor necrosis factor-alpha
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- SOD
Superoxide dismutase
- MDA
Malondialdehyde
- LDH
Lactate dehydrogenase
- TUNEL
Terminal Transferase-Mediated dUTP Nick-End Labeling
- DAPI
4’, 6-diamidino-2-phenylindole
- PVDF
Polyvinylidene difluoride
References
- 1.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004;22:281–299. [PubMed] [Google Scholar]
- 2.Zevallos J, Santiago F, Gonzalez J, Rodriguez A, Pericchi L, Rodriguez-Mercado R, Nobo U. Burden of stroke in Puerto Rico. Int J Stroke. 2015;10:117–119. doi: 10.1111/ijs.12350. [DOI] [PubMed] [Google Scholar]
- 3.Writing Group Members; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M, Brott TG. Emerging therapies for acute ischemic stroke: new therapies on trial. Stroke. 2003;34:359–361. doi: 10.1161/01.str.0000054627.69159.c2. [DOI] [PubMed] [Google Scholar]
- 5.Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W, Jia LS. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem Biophys Res Commun. 2011;404:941–945. doi: 10.1016/j.bbrc.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 6.Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B. 2015;5:8–24. doi: 10.1016/j.apsb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Thompson JW, Narayanan SV, Koronowski KB, Morris-Blanco K, Dave KR, Perez-Pinzon MA. Signaling pathways leading to ischemic mitochondrial neuroprotection. J Bioenerg Biomembr. 2015;47:101–110. doi: 10.1007/s10863-014-9574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan Y, Wang N, Xia P, Wang E, Guo Q, Ye Z. Inhibition of Rac1 ameliorates neuronal oxidative stress damage via reducing Bcl-2/Rac1 complex formation in mitochondria through PI3K/Akt/mTOR pathway. Exp Neurol. 2018;300:149–166. doi: 10.1016/j.expneurol.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, Deng H, Xu S, Zhang J. MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. Int J Mol Sci. 2015;16:24895–24917. doi: 10.3390/ijms161024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallucci GM, Tong M, Chen X, Stonestreet BS, Lin A, de la Monte SM. Rapid alterations in cerebral white matter lipid profiles after ischemic-reperfusion brain injury in fetal sheep as demonstrated by MALDI-mass spectrometry. Pediatr Dev Pathol. 2019;22:344–355. doi: 10.1177/1093526619826721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu L, Chen W, Tian F, Yuan C, Wang H, Yue H. Neuroprotective role of fucoxanthin against cerebral ischemic/reperfusion injury through activation of Nrf2/HO-1 signaling. Biomed Pharmacother. 2018;106:1484–1489. doi: 10.1016/j.biopha.2018.07.088. [DOI] [PubMed] [Google Scholar]
- 14.Xue X, Qu XJ, Yang Y, Sheng XH, Cheng F, Jiang EN, Wang JH, Bu W, Liu ZP. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor kappaB p65 activation. Biochem Biophys Res Commun. 2010;403:398–404. doi: 10.1016/j.bbrc.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi M, Hino M, Yoshimura M, Amakura Y, Nomoto H. Identification of sinensetin and nobiletin as major antitrypanosomal factors in a citrus cultivar. Exp Parasitol. 2019;200:24–29. doi: 10.1016/j.exppara.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Yang SY, Denning SM, Mizuno S, Dupont B, Haynes BF. A novel activation pathway for mature thymocytes. Costimulation of CD2 (T,p50) and CD28 (T,p44) induces autocrine interleukin 2/interleukin 2 receptor-mediated cell proliferation. J Exp Med. 1988;168:1457–1468. doi: 10.1084/jem.168.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He PP, Shen QQ, Wen M, Zou JQ, Wang Y, Yang JX, Hu LZ, Zheng XL, Chen YS, Su H, Liu J, Ouyang XP, Tang CK. Nobiletin reduces LPL-mediated lipid accumulation and pro-in fl ammatory cytokine secretion through upregulation of miR-590 expression. Biochem Biophys Res Commun. 2019;508:97–101. doi: 10.1016/j.bbrc.2018.11.075. [DOI] [PubMed] [Google Scholar]
- 18.Sp N, Kang DY, Joung YH, Park JH, Kim WS, Lee HK, Song KD, Park YM, Yang YM. Nobiletin inhibits angiogenesis by regulating Src/FAK/STAT3-mediated signaling through PXN in ER(+) breast cancer cells. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18050935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang G, Lin CC, Yang Y, Yuan L, Wang P, Wen X, Pan MH, Zhao H, Ho CT, Li S. Nobiletin prevents trimethylamine oxide-induced vascular inflammation via inhibition of the NF-kappaB/MAPK pathways. J Agric Food Chem. 2019;67:6169–6176. doi: 10.1021/acs.jafc.9b01270. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Zhang W, Li M, Cao D, Yang X, Gong J. Nobiletin ameliorates ischemia-reperfusion injury by suppressing the function of Kupffer cells after liver transplantation in rats. Biomed Pharmacother. 2017;89:732–741. doi: 10.1016/j.biopha.2017.02.087. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Zhao H, Zhang X, Chen L, Zhao X, Bai X, Zhang J. Nobiletin protects against cerebral ischemia via activating the p-Akt, p-CREB, BDNF and Bcl-2 pathway and ameliorating BBB permeability in rat. Brain Res Bull. 2013;96:45–53. doi: 10.1016/j.brainresbull.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda N, Ishii T, Oyama D, Fukuta T, Agato Y, Sato A, Shimizu K, Asai T, Asakawa T, Kan T, Yamada S, Ohizumi Y, Oku N. Neuroprotective effect of nobiletin on cerebral ischemia-reperfusion injury in transient middle cerebral artery-occluded rats. Brain Res. 2014;1559:46–54. doi: 10.1016/j.brainres.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Bu J, Yu L, Chen J, Liu H. Nobiletin improves propofol-induced neuroprotection via regulating Akt/mTOR and TLR 4/NF-kappaB signaling in ischemic brain injury in rats. Biomed Pharmacother. 2017;91:494–503. doi: 10.1016/j.biopha.2017.04.048. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Wang S, Tian S, He Y, Lou H, Yang Z, Kong Y, Cao X. Nobiletin inhibits breast cancer via p38 mitogen-activated protein kinase, nuclear transcription factor-kB, and nuclear factor erythroid 2-related factor 2 pathways in MCF-7 cells. Food Nutr Res. 2018:62. doi: 10.29219/fnr.v62.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang G, Li S, Yuan L, Yang Y, Pan MH. Effect of nobiletin on the MAPK/NF-kB signaling pathway in the synovial membrane of rats with arthritis induced by collagen. Food Funct. 2017;8:4668–4674. doi: 10.1039/c7fo01311f. [DOI] [PubMed] [Google Scholar]
- 26.Lien LM, Wang MJ, Chen RJ, Chiu HC, Wu JL, Shen MY, Chou DS, Sheu JR, Lin KH, Lu WJ. Nobiletin, a polymethoxylated flavone, inhibits glioma cell growth and migration via arresting cell cycle and suppressing MAPK and Akt pathways. Phytother Res. 2016;30:214–221. doi: 10.1002/ptr.5517. [DOI] [PubMed] [Google Scholar]
- 27.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 28.Wei X, Liu H, Sun X, Fu F, Zhang X, Wang J, An J, Ding H. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci Lett. 2005;386:58–62. doi: 10.1016/j.neulet.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 29.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 30.Lott C, Hennes HJ, Dick W. Stroke--a medical emergency. J Accid Emerg Med. 1999;16:2–7. doi: 10.1136/emj.16.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haslund-Vinding J, McBean G, Jaquet V, Vilhardt F. NADPH oxidases in oxidant production by microglia: activating receptors, pharmacology and association with disease. Br J Pharmacol. 2017;174:1733–1749. doi: 10.1111/bph.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park EK, Choo MK, Oh JK, Ryu JH, Kim DH. Ginsenoside Rh2 reduces ischemic brain injury in rats. Biol Pharm Bull. 2004;27:433–436. doi: 10.1248/bpb.27.433. [DOI] [PubMed] [Google Scholar]
- 33.Huang XP, Qiu YY, Wang B, Ding H, Tang YH, Zeng R, Deng CQ. Effects of Astragaloside IV combined with the active components of Panax notoginseng on oxidative stress injury and nuclear factor-erythroid 2-related factor 2/heme oxygenase-1 signaling pathway after cerebral ischemia-reperfusion in mice. Pharmacogn Mag. 2014;10:402–409. doi: 10.4103/0973-1296.141765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Liu X, Rong F. PUMA mediates the apoptotic signal of hypoxia/reoxygenation in cardiomyocytes through mitochondrial pathway. Shock. 2011;35:579–584. doi: 10.1097/SHK.0b013e318211601a. [DOI] [PubMed] [Google Scholar]
- 36.Hu GQ, Du X, Li YJ, Gao XQ, Chen BQ, Yu L. Inhibition of cerebral ischemia/reperfusion injury-induced apoptosis: nicotiflorin and JAK2/STAT3 pathway. Neural Regen Res. 2017;12:96–102. doi: 10.4103/1673-5374.198992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90:1281–1289. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- 38.Gotlieb WH, Saumet J, Beauchamp MC, Gu J, Lau S, Pollak MN, Bruchim I. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–250. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Xiong XX, Pan F, Chen RQ, Hu DX, Qiu XY, Li CY, Xie XQ, Tian B, Chen XQ. Neuroglobin boosts axon regeneration during ischemic reperfusion via p38 binding and activation depending on oxygen signal. Cell Death Dis. 2018;9:163. doi: 10.1038/s41419-017-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang N, Wang MM, Wang YH, Zhang ZN, Cao HR, Lv YH, Yang Y, Fan PH, Qiu F, Gao XM. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem Toxicol. 2014;67:193–200. doi: 10.1016/j.fct.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Xiao YT, Yan WH, Cao Y, Yan JK, Cai W. P38 MAPK pharmacological inhibitor SB203580 alleviates total parenteral nutrition-induced loss of intestinal barrier function but promotes hepatocyte lipoapoptosis. Cell Physiol Biochem. 2017;41:623–634. doi: 10.1159/000457933. [DOI] [PubMed] [Google Scholar]
- 42.Yun SM, Kim YS, Kim KH, Hur DY. Ampelopsin induces DR5-mediated apoptotic cell death in EBV-infected cells through the p38 pathway. Nutr Cancer. 2019:1–6. doi: 10.1080/01635581.2019.1639778. [DOI] [PubMed] [Google Scholar]