Abstract
The function of androgen receptor (AR)/microRNA-21 (miR-21) axis in tumor development was well investigated. However, the roles of the axis performed in hypoxia/reoxygenation (H/R)-induced apoptosis of mouse renal tubular epithelial cells (RTECs) is not known. In this study, H/R-induced apoptosis of RTECs was established to evaluate the role of miR-21-AR axis. The protocol of 8-h hypoxia and 24-h reoxygenation were selected to produce H/R injury. Our data showed that H/R increased miR-21 and caspase-3 expression, reduced the expression AR and programmed cell death protein 4 (PDCD4). By contrast, AR-siRNA increased H/R-induced apoptosis, and promoted caspase-3 expression, but reduced PDCD4 expression (vs. H/R group). pre-miR-21 reduced, while antagomiR-21 promoted apoptosis and PDCD4 expression in H/R-induced RTECs. Moreover, pre-miR-21 promoted, while antagomiR-21 reduced caspase-3 expression in H/R-induced RTECs. Together, H/R increased miRNA-21 and reduced AR expression, then regulating PDCD4- and caspase-3-dependent apoptosis. AR/miR-21 axis could be a potential therapeutic target for the kidney ischemia injury.
Keywords: Kidney, androgen receptor/miR-21 axis, renal hypoxia/reoxygenation
Introduction
Ischemia injury in cerebrum, heart and kidney, etc. is the main cause of death and disability [1]. Ischemia-related renal injury involves a series of complex pathophysiological processes, including energy exhaustion, oxidative stress and inflammatory reaction [2,3]. Hypoxia is the primary initiator of the ischemic injury [4]. In renal ischemia-reperfusion, blood flow is blocked, followed by blood reperfusion and acute inflammation, including production of a large number of pro-inflammatory factors and release of chemokines, increased expression of adhesion molecules, activation of neutrophils and monocytes and tissue infiltration [5]. Thus, it is of particular interest to investigate the potential mechanisms or signaling pathway involved in hypoxia/reoxygenation (H/R)-induced renal injury.
MicroRNAs (miRNAs) are small endogenous non-coding RNAs of about 21-22 nucleotides, which are ubiquitous in animals, plants, viruses and other organisms [6]. The function of miRNAs is related to the regulation of gene expression [7]. miRNAs have been reported to modulate ischemia-reperfusion injury. For examples, miR-320 level was reduced by ischemia-reperfusion injury in heart [5]. After acute myocardial infarction, the expression of miR-127 was up-regulated, and blockage of the expression of miR-127 could reduce the occurrence of arrhythmia after myocardial infarction [8]. miR-21 is a extensively-researched miRNA, which has been detected in renal ischemia-reperfusion injury [9]. Therefore, whether miR-21 is involved in H/R-induced renal injury and the potential mechanisms still deserve investigation.
Androgen receptor (AR) is a key macromolecule mediating androgen, and can be detected in kidney [10]. Androgen has been reported to affect the nitric oxide synthase (NOS) activity in kidney by binding with androgen receptor, thus further affecting renal hemodynamics and various physiological functions [11]. Interestingly, miR-21 and AR formed an axis to regulate each other’s expression, resulting in a positive feedback loop to regulate biological activities, such as antitumor [12]. In this study, a mouse kidney epithelial cell line (TCMK-1) was challenged by hypoxia-reoxygenation and the roles of AR/miR-21 axis performed in the ischemia injury of renal cells were investigated by regulating the expression of miR-21 and/or AR.
Materials and methods
Cell culture
TCMK-1 cells were purchased from the Cell Bank of Chinese Academy of Sciences and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; cat. no. 04-007-1A; Biological Industries) and 100 U/ml penicillin and 100 µg/ml streptomycin (Beijing Solarbio Science & Technology Co., Ltd.) in 5% CO2 at 37°C. H/R was produced using D-Hanks solution and normal medium (DMEM medium containing 10% newborn bovine serum). D-Hanks solution was balanced in an airtight container with N2 for more than 1 h to produce anaerobic solution. The normal medium was sucked out and added with the anaerobic solution. Then, the culture bottle was incubated in an airtight container with 95% N2-5% CO2 (V/V) at 37°C. Then, the medium was replaced with normal medium and put back into incubator for reoxygenation. H/R regimes included: reoxygenation 1 h after 45 min of hypoxia; reoxygenation 3 h after 1 h of hypoxia; reoxygenation 6 h after 2 h of hypoxia; reoxygenation 12 h after 4 h of hypoxia; reoxygenation 24 h after 8 h of hypoxia.
Cell transfection
Cells at 80% confluence were transfected with AR-siRNA (GenePharma Co. Ltd., Shanghai, China) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 6 h, the medium was replaced with fresh DMEM medium containing 10% FBS and cultured in an 5% CO2 incubator at 37°C for 24 h. 48 h after transfection, subsequent experiments were performed. The expression of AR was verified using reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The sequences of the siRNA-AR were listed in Table 2.
Table 2.
Sequences of the siRNAs
| Genes | Sequence (5’-3’) | |
|---|---|---|
| AR-siRNA-1 | F | CUCCAAGGAUAGUUACCUA |
| R | UAGGUAACUAUCCUUGGAG | |
| AR-siRNA-2 | F | GGUCCUUCACUAAUGUCAA |
| R | UUGACAUUAGUGAAGGACC | |
| AR-siRNA-3 | F | CGACAGUGACUAGAAGAAA |
| R | UUUCUUCUAGUCACUGUCG | |
Experimental groups
The experimental groups included: a control group; a AR negative control group (siRNA-NC); a AR-siRNA group (AR-siRNA); a H/R group (H/R); a H/R + AR negative control group (H/R + siRNA-NC); a H/R + AR-siRNA group (H/R + AR-siRNA); a miRNA negative control group (miR-21-NC); a pre-miR-21 group (pre-miR-21); a H/R + miR-21-NC; a H/R + pre-miR-21; a H/R + antagomiR-21. After transfection, hypoxia and reoxygenation were performed 2 h later. Flow cytometry, quantitative fluorescence PCR and western blot were performed at 12 h, 24 h and 48 h after reoxygenation.
ELISA
TNF-α level was measured following the instruction of the TNF-α assay kit (m1002095, Shanghai Mlbio, Shanghai, China) as previously described [13].
Flow cytometry
Flow cytometry was used to detect cell apoptosis. 3×106 cells were collected and centrifuged with 1 ml PBS at 1500 rpm for 3 min. The cells were washed twice. 3 μl Annexin V-FITC and 5 μl PI were added into each tube. After slightly mixing, the cells were incubated at room temperature at dark for 10 min, followed by measurement with flow cytometer (NovoCyte 2060R, ACEABIO, China).
Fluorescence quantitative polymerase chain reaction (FQPCR)
mRNA levels in the different treatment groups was extracted using a TRIzol assay kit (Baosheng Science & Technology Innovation Co. Ltd., Shanghai, China). mRNA was transcribed into cDNA using a reverse transcription kit (cat. no. 639522, Takara Biotechnology Co., Ltd., Dalian, China) at 37°C following the protocol (25°C 10 min, 37°C 120 min and 85°C 5 min ) and qPCR was used to detect the expression level of the target genes by using SYBR Green (HY-K0501, MedChemExpress, Shanghai, China). The thermocycling conditions were as follows: Initial denaturation at 95°C for 10 min, followed by 40 cycles of a PCR at 95°C for 10 sec, 60.3°C for 30 s and 72°C for 30 s. The 2-ΔΔCq method was used to quantify the results as previously described [14,15]. The primers (5’-3’) used were listed in Table 1.
Table 1.
Sequences of the primers
| Genes | Sequence (5’-3’) | Primer length (bp) | Product length (bp) | Annealing temperature (°C) |
|---|---|---|---|---|
| PDCD4 F | TCTTTCACATCCACCTCTTCCACAT | 25 | 377 | 61.8 |
| PDCD4 R | GACCCTGACAATTTAAGCGACTCTC | 25 | ||
| Caspase-12 F | GGATAGCCACTGCTGATA | 18 | 256 | 60.3 |
| Caspase-12 R | AGTTCACCTGGGACCTCA | 18 | ||
| GAPDH F | TCAACGGCACAGTCAAGG | 18 | 357 | 60.3 |
| GAPDH R | TGAGCCCTTCCACGATG | 17 | ||
| miR-21 F | ACGTTGTGTAGCTTATCAGACTG | 23 | - | 58.4 |
| miR-21 R | AATGGTTGTTCTCCACACTCTC | 22 | ||
| U6 F | CTCGCTTCGGCAGCACA | 17 | 94 | 60.00 |
| U6 R | AACGCTTCACGAATTTGCGT | 24 |
Western blot
Protein was extracted from the cells by a protein isolation kit (28-9425-44, ReadyPrep; GE Healthcare Life Sciences) as previously described [16]. Protein levels were quantified with a bicinchoninic acid protein assay kit. Protein (25 μg/lane) was separated via 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked in 5% skim milk for 2 h at room temperature and incubated with the following primary antibodies overnight at 4°C: rabbit polyclonal anti-AR (ab45172, Abcam, 1/5000), mouse monoclonal anti-β-actin (TA-09, ZSBio, 1/2000), rabbit polyclonal anti-PDCD4 (ab79405, Abcam, 1/5000), rabbit polyclonal anti-caspase-3 (ab32499, Abcam, 1/10000). After incubation with the second antibodies at room temperature for 2 h, enhanced chemiluminescence exposure liquid droplet (cat. no. RPN2133; GE Healthcare Life Sciences, Chalfont, UK) was added to the membranes. The membranes were visualized using a gel imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Densitometry was performed using Quantity One version 1.4.6 (Bio-Rad Laboratories, Inc.). Experiments were repeated three times.
Statistical analysis
Data were presented as the mean ± standard error of the mean and analyzed using SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA). Significant differences were determined using one-way analysis of variance followed by the Student-Newman-Keuls post-hoc test. P < 0.05 was considered to indicate a statistically significant difference.
Results
Hypoxia/reoxygenation promotes miRNA-21 and caspase-3, and reduces PDCD4
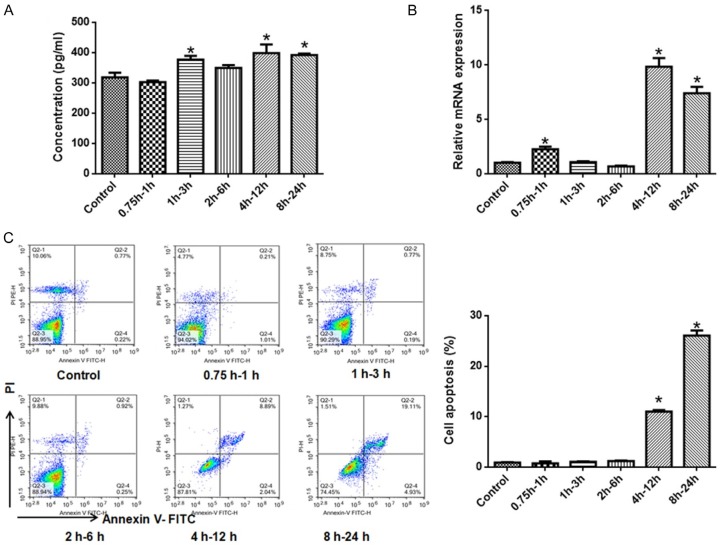
Initially, we screened the optimal time of hypoxia/reoxygenation in TCMK-1 cells. Compared with control group, TNF-α level increased significantly in groups of hypoxia for 1 h and reoxygenation for 3 h, hypoxia for 4 h and reoxygenation for 12 h, and hypoxia for 8 h and reoxygenation for 24 h (Figure 1A). The expression of caspase-12 at mRNA level increased significantly in groups of hypoxia for 0.75 h followed by reoxygenation for 1 h, hypoxia for 4 h followed by reoxygenation for 12 h, and hypoxia for 8 h followed by reoxygenation for 24 h (Figure 1B). The apoptosis increased significantly in groups of hypoxia for 4 h followed by reoxygenation for 12 h, and hypoxia for 8 h followed by reoxygenation for 24 h. The apoptotic rate in the cells receiving a regime of hypoxia for 8 h and reoxygenation for 24 h was the highest (about 20%) (Figure 1C). As inflammation and apoptosis are critical features for ischemia injury [17], the regime of hypoxia for 8 h and reoxygenation for 24 h was selected in the subsequent experiment to evaluate the role of AR/miR-21 axis performed in the H/R-induced renal injury.
Figure 1.
Hypoxia/reoxygenation promotes TNF-α and caspase-12 expression, and induces apoptosis of TCMK-1 cells. A. TNF-α level was promoted by different hypoxia/reoxygenation regimes, especially 4 h-12 h and 8 h-24 h; B. Caspase-12 expression at mRNA level was up-regulated by hypoxia/reoxygenation regimes, especially 4 h-12 h and 8 h-24 h; C. Apoptosis detected by flow cytometry. Left: representative images of flow cytometry; Right: quantification data. Apoptosis was induced by hypoxia/reoxygenation regimes, especially 8 h-24 h. N=6 in each group, *P < 0.05 vs. control (One-way ANOVA followed by the Student-Newman-Keuls).
We detected miR-21, PDCD4 and caspase-3 expression in the regime of hypoxia for 8 h and reoxygenation for 24 h. Compared with control group, the expression of miR-21 and caspase-3 in H/R group was significantly higher, while the expression of PDCD4 was significantly lower (P < 0.05) (Figure 2).
Figure 2.
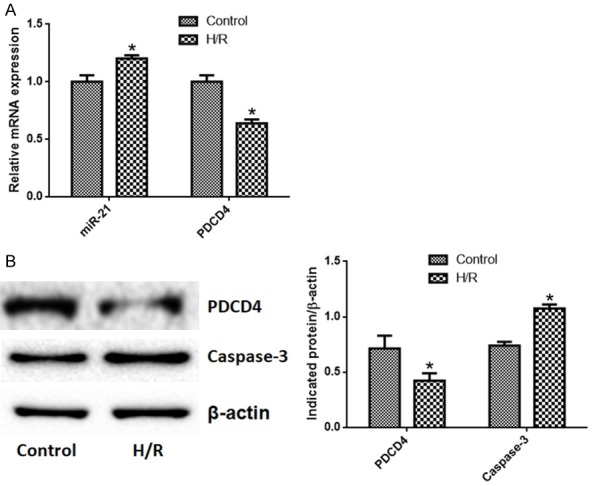
Hypoxia/reoxygenation promotes miRNA-21 and caspase-3 expression, and reduces PDCD4 expression in TCMK-1 cells. A. miR-21 expression was increased by hypoxia/reoxygenation and PDCD4 expression at the mRNA level was reduced by hypoxia/reoxygenation; B. PDCD4 was reduced while caspase-3 expression was promoted by hypoxia/reoxygenation. N=6 in each group, *P < 0.05 vs. control (Unpaired student t test).
AR-siRNA increases H/R-induced apoptosis, promotes caspase-3 expression, and reduces PDCD4 expression
To test the role of AR performed in H/R-induced apoptosis, we silenced AR expression. As shown in Figure 3A, 3B, AR expression in AR-siRNA1 group was significantly lower than that in control group (P < 0.05). To test the function of miR-21, we designed pre-microRNA-21 and antagomiR-21. As shown in Figure 3C, the expression of miR-21 in pre-microRNA-21 group was significantly higher than that in control group, and the expression of miR-21 in antagomiR-21 group was significantly lower than that in control group (P < 0.05).
Figure 3.
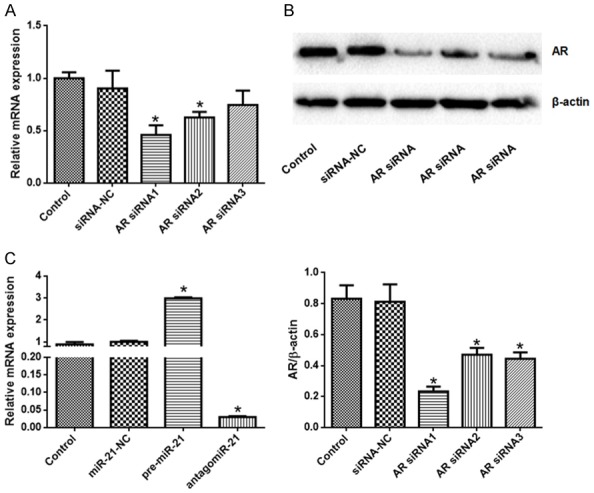
AR-siRNAs reduced AR expression and miR-21 was regulated by pre-miR-21 and antagomiR-21. A. AR expression at the mRNA level was reduced by AR-siRNAs, especially AR-siRNA1; B. AR expression at the protein level was reduced by AR-siRNAs, especially AR-siRNA1; C. miR-21 expression was promoted by pre-miR-21 and inhibited by antagomiR-21. N=6 in each group, *P < 0.05 vs. control (One-way ANOVA followed by the Student-Newman-Keuls).
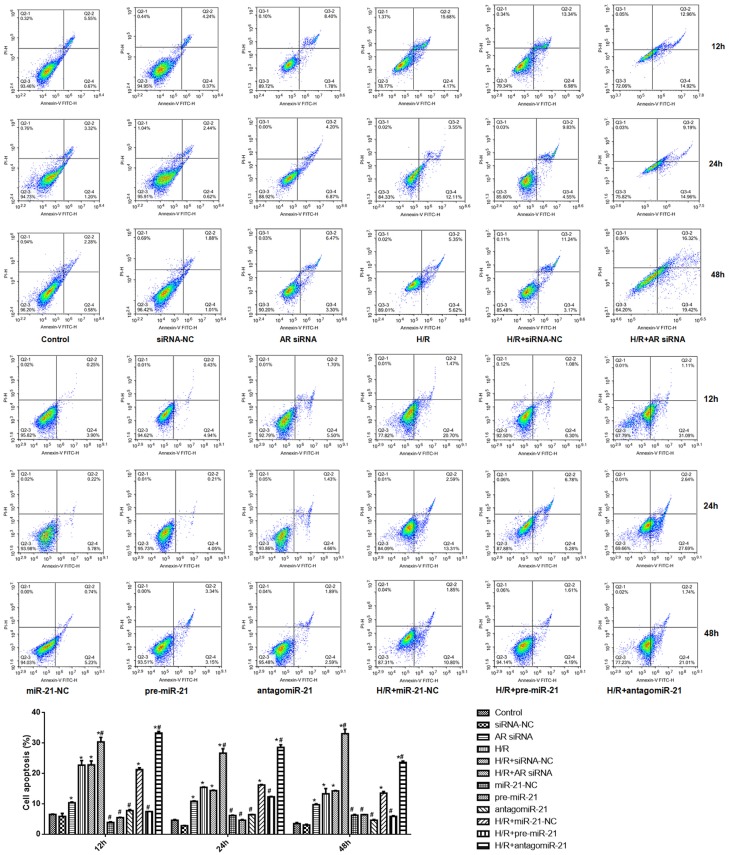
Compared with control group, the apoptotic rates of AR-siRNA, H/R, H/R + siRNA-NC, H/R + AR-siRNA, H/R + RNA-21-NC and H/R + antagomiR-21 groups increased significantly at 12 h, 24 h and 48 h. Compared with H/R group, the apoptotic rates of H/R + AR-siRNA group and H/R + antagomiR-21 group increased significantly at 12 h, 24 h and 48 h, respectively (Figure 4).
Figure 4.
AR-siRNA and antgomiR-21 promotes, while pre-miR-21 antagonizes hypoxia/reoxygenation-induced apoptosis. Upper panel: representative images of flow cytometry; Down panel: quantification data. *P < 0.05 vs. control; #P < 0.05 vs. H/R. N=6 in each group (One-way ANOVA followed by the Student-Newman-Keuls).
AR-siRNA reduces AR expression, and pre-miR-21 and antagomiR-21 regulate miRNA-21 expression in H/R model
As shown in Figure 5A, the expression of miR-21 in H/R, pre-microRNA-21, H/R + miR-21-NC and H/R + pre-microRNA-21 increased significantly at 12 h, 24 h and 48 h, while the expression of miR-21 in antagomiR-21 and antagomiR-21 + H/R groups decreased significantly compared with control group. Compared with H/R group, the expression of miR-21 in H/R + pre-microRNA-21 groups increased significantly, and decreased significantly in H/R + antagomiR-21 group.
Figure 5.

pre-miR-21 promotes miR-21 expression and AR-siRNA reduces AR expression in hypoxia/reoxygenation model. A. Pre-miR-21 promotes miR-21 expression; B. AR-siRNA reduced AR expression at mRNA level. *P < 0.05 vs. control; #P < 0.05 vs. H/R. N=6 in each group (One-way ANOVA followed by the Student-Newman-Keuls).
Compared with control group, AR expression at mRNA level in AR-siRNA group, H/R group, H/R + AR siRNA-NC group and H/R + AR-siRNA group decreased significantly (P < 0.05) (Figure 5B). Compared with H/R group, AR expression in H/R + AR-siRNA group decreased significantly (P < 0.05).
AR-siRNA and pre-miR-21 reduces, while antagomiR-21 promotes PDCD4 expression
The expression of PDCD4 in each group was shown in Figure 6. Compared with control group, the expression of PDCD4 at mRNA level in H/R, H/R + siRNA-NC, H/R + AR-siRNA, pre-miR-21, and H/R + pre-miR-21 groups at 12 h, 24 h and 48 h decreased significantly, while the expression of PDCD4 in antagomiR-21 group increased significantly. Compared with H/R group, the expression of PDCD4 H/R + pre-miR-21 groups at 12 h, 24 h and 48 h decreased significantly, and the expression of PDCD4 in antagomiR-21 + H/R group increased significantly. We also obtained a consistent result of PDCD4 expression at protein level.
Figure 6.

pre-miR-21 reduces, while AR siRNA and antagomiR-21 promotes pre-miR-21 PDCD4 expression in hypoxia/reoxygenation model. The expression of PDCD4 at mRNA level (A) and protein level (B) in H/R, H/R + siRNA-NC, H/R + AR-siRNA, pre-miR-21, and H/R + pre-miR-21 groups at 12 h, 24 h and 48 h decreased significantly, while the expression of PDCD4 in antagomiR-21 group increased significantly. The expression of PDCD4 H/R + pre-miR-21 groups at 12 h, 24 h and 48 h decreased significantly, and the expression of PDCD4 in antagomiR-21 + H/R group increased significantly. N=6 in each group (One-way ANOVA followed by the Student-Newman-Keuls).
AR-siRNA and antagomiR-21 increases, while pre-miRNA-21 reduces caspase-3 expression
The expression of caspase-3 protein in each group was shown in Figure 7. Compared with control group, the expression of caspase-3 in AR-siRNA group, H/R group, H/R + siRNA-NC group, H/R + AR-siRNA group, H/R + microRNA-21-NC group and H/R + antagomiR-21 group increased significantly at 12 h, 24 h and 48 h. Compared with H/R group, the expression of caspase-3 in H/R + AR-siRNA group and H/R + antagomiR-21 group increased significantly at 12 h, 24 h and 48 h, while the expression of caspase-3 in 12 h, 24 h and 48 h H/R + pre-microRNA-21 group decreased significantly (P < 0.05).
Figure 7.
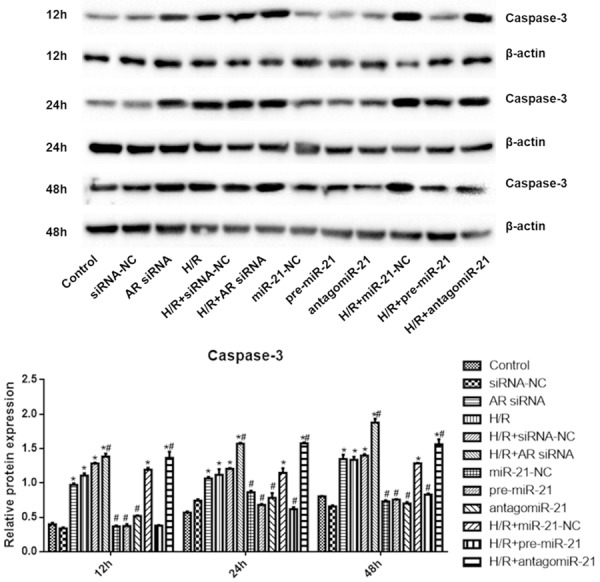
pre-miR-21 reduces, while AR siRNA and antagomiR-21 promotes caspase-3 expression in hypoxia/reoxygenation model. Compared with control group, the expression of caspase-3 in AR-siRNA group, H/R group, H/R + siRNA-NC group, H/R + AR-siRNA group, H/R + microRNA-21-NC group and H/R + antagomiR-21 group increased significantly at 12 h, 24 h and 48 h. Compared with H/R group, the expression of caspase-3 in H/R + AR-siRNA group and H/R + antagomiR-21 group increased significantly at 12 h, 24 h and 48 h, while the expression of caspase-3 in 12 h, 24 h and 48 h H/R + pre-microRNA-21 group decreased significantly. *P < 0.05 vs. control; #P < 0.05 vs. H/R. N=6 in each group (One-way ANOVA followed by the Student-Newman-Keuls).
Discussion
In this study, we provided data to classify the potential mechanisms involved in H/R-induced apoptosis of mouse renal tubular epithelial cells through regulating AR/miR-21 axis. Moreover, H/R reduced PDCD4 and promoted caspase-3-dependent apoptosis of renal cells. This study would implicate potential therapeutic targets for renal ischemia injury.
Renal ischemia injury involves complex pathophysiological processes such as energy exhaustion, oxidative stress and inflammatory reaction [2]. Inflammation, as one of the main response of renal ischemia-reperfusion injury, is important for the pathological process of renal ischemia-reperfusion injury [18]. TNF-α is a cytokine produced by activated macrophages that inhibits osteoblasts and stimulates osteoclasts, and is associated with inflammation [19]. Apoptosis is another important cause of renal injury induced by ischemia-reperfusion [20]. In this study, we initially determined the regime of H/R based upon both of inflammation and apoptosis. Apoptosis is a process of programmed cell death, an autonomous and orderly process of cell death [21]. Caspase-12 located at the adventitia of endoplasmic reticulum. Once endoplasmic reticulum is stressed, caspase-12 and caspase-7 are cleaved; caspase-9 is further activated by activated caspase-12, which triggers caspase-9-dependent apoptotic cascade [22,23]. In this study, the optimal hypoxia-reoxygenation regime was determined based on the expression of TNF-α, apoptosis and caspase-12. Our data revealed that the regime of reoxygenation for 24 h and hypoxia for 8 h triggered obvious inflammation and apoptosis. Therefore, this regime was selected in the subsequent experiment.
In myocardial ischemia-reperfusion injury, it has been found that protective effect of ischemia preconditioning is closely related to the increased expression of miR-21 [24]. Overexpression of miR-21 can decrease the apoptotic rate of renal tubular epithelial cells caused by ischemia-reperfusion injury [6]. miR-21 negatively regulates PDCD4 gene and thus inhibits apoptosis of tissues [25]. In the study of myocardium, we found that miR-21 can inhibit apoptosis of cardiomyocytes by inhibiting PDCD4 and downstream transcription activator [26]. In the study of renal ischemia-reperfusion injury, we also found that miR-21 can reduce apoptosis by inhibiting the expression of PDCD4 [27].
Caspases are inactive protease precursors with high inertia and conservativeness before being cleaved by hydrolysis [28]. Caspase-3 plays a key role in apoptotic pathway [29], likely through Fas and TNFRI [30]. The results of this study implicated that the expression of miR-21 increased after hypoxia, while the expression of PDCD4 decreased, which was consistent with the above results. Our results also suggest that hypoxia can promote the expression of miR-21 and negatively regulate the PDCD4 expression. Therefore, we further synthesized pre-miR-21 and antagomiR-21 to test the function of miR-21 in the process. Our data revealed that pre-miR-21 could increase miR-21, while antagomiR-21 decreased the expression of miR-21. We also revealed that caspase-3 expression was promoted after hypoxia.
Androgen diffuses freely into cells, binding to AR in target cells, forming hormone-receptor complex, then enters into the nuclei, and regulates basic transcription [31]. Studies have shown that androgen has protective effects on ischemia-reperfusion injury of heart and brain [32,33]. Androgen could promote Bcl-2, decrease Bax expression to inhibit neuronal apoptosis, thereby alleviating the damage of nervous system caused by hypoxic-ischemia [34]. However, endogenous androgens may also have pro-inflammatory and pro-apoptotic effects after ischemia-reperfusion [35]. Chaves et al. found that exogenous androgens can block the cardioprotective effect of exercise and increase the cardiac damage caused by ischemia-reperfusion [36]. Kim et al. reported that inhibition of androgen could alleviate oxidative stress-induced kidney injury [37]. Our results revealed that silencing AR expression could promote apoptosis, and overexpression of miR-21 after H/R could inhibit apoptosis, while inhibiting the expression of miR-21 could promote apoptosis. These results revealed that high expression of AR had a protective effect, but after silencing its expression, the protective effect will lose, thus inducing apoptosis. Our results also revealed that the cell damage caused by miR-21 after H/R had a certain protective effect.
The expression of PDCD4 decreased when pre-miR-21 was used, and increased when antagomiR-21 was used. It was also found that pre-mir-21 after H/R decreased the expression of caspase-3 and antagomiR-21 after H/R increased the expression of caspase-3. These results point that the expression of PDCD4 is negatively regulated by miR-21, which further down-regulated caspase-3 and ultimately inhibited apoptosis. The results showed that silencing AR could also increase the expression of caspase-3, thus promoting the apoptosis of renal cells induced by H/R.
In conclusion, H/R can regulate AR/miR-21 axis through increasing the expression of miR-21 and decreasing the expression of AR, which trigger PDCD4 and caspase-3-dependent apoptosis. AR/miR-21 axis is likely a potential therapeutic target for H/R-induced renal injury.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (81660119).
Disclosure of conflict of interest
None.
References
- 1.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson KE, Nomiya M, Yamaguchi O. Chronic pelvic ischemia: contribution to the pathogenesis of lower urinary tract symptoms (LUTS): a new target for pharmacological treatment? Low Urin Tract Symptoms. 2015;7:1–8. doi: 10.1111/luts.12084. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Siraj S, Zhang R, Chen Q. Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and protects the heart from I/R injury. Autophagy. 2017;13:1080–1081. doi: 10.1080/15548627.2017.1300224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang L, Wang J, Yuan Y, Zhang Y, Liu H, Wu C, Yan Y. MicRNA-320 facilitates the brain parenchyma injury via regulating IGF-1 during cerebral I/R injury in mice. Biomed Pharmacother. 2018;102:86–93. doi: 10.1016/j.biopha.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci U S A. 2010;107:14339–14344. doi: 10.1073/pnas.0912701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conde E, Gimenez-Moyano S, Martin-Gomez L, Rodriguez M, Ramos ME, Aguado-Fraile E, Blanco-Sanchez I, Saiz A, Garcia-Bermejo ML. HIF-1alpha induction during reperfusion avoids maladaptive repair after renal ischemia/reperfusion involving miR127-3p. Sci Rep. 2017;7:41099. doi: 10.1038/srep41099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Guo JX, Shao ZQ. miR-21 targets and inhibits tumor suppressor gene PTEN to promote prostate cancer cell proliferation and invasion: an experimental study. Asian Pac J Trop Med. 2017;10:87–91. doi: 10.1016/j.apjtm.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier G. Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol. 2000;15:1261–1270. doi: 10.14670/HH-15.1261. [DOI] [PubMed] [Google Scholar]
- 11.Neugarten J, Ding Q, Friedman A, Lei J, Silbiger S. Sex hormones and renal nitric oxide synthases. J Am Soc Nephrol. 1997;8:1240–1246. doi: 10.1681/ASN.V881240. [DOI] [PubMed] [Google Scholar]
- 12.Mishra S, Deng JJ, Gowda PS, Rao MK, Lin CL, Chen CL, Huang T, Sun LZ. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene. 2014;33:4097–4106. doi: 10.1038/onc.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang SJ, Song ZJ, Wang XC, Zhang ZR, Wu SB, Zhu GQ. Curculigoside facilitates fear extinction and prevents depression-like behaviors in a mouse learned helplessness model through increasing hippocampal BDNF. Acta Pharmacol Sin. 2019 doi: 10.1038/s41401-019-0238-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Cao J, Zhou Y, Wang L, Zhu G. GPR30 activation improves memory and facilitates DHPG-induced LTD in the hippocampal CA3 of middle-aged mice. Neurobiol Learn Mem. 2018;149:10–19. doi: 10.1016/j.nlm.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Zhu G, Yang S, Xie Z, Wan X. Synaptic modification by L-theanine, a natural constituent in green tea, rescues the impairment of hippocampal long-term potentiation and memory in AD mice. Neuropharmacology. 2018;138:331–340. doi: 10.1016/j.neuropharm.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Anselmi A, Abbate A, Girola F, Nasso G, Biondi-Zoccai GG, Possati G, Gaudino M. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: a review of evidence. Eur J Cardiothorac Surg. 2004;25:304–11. doi: 10.1016/j.ejcts.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Edwards JK. Acute kidney injury: new antagonist prevents I/R injury. Nat Rev Nephrol. 2015;11:631. doi: 10.1038/nrneph.2015.161. [DOI] [PubMed] [Google Scholar]
- 19.Liu CW, Sung HC, Lin SR, Wu CW, Lee CW, Lee IT, Yang YF, Yu IS, Lin SW, Chiang MH, Liang CJ, Chen YL. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-alpha-treated endothelial cells: evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-kappaB pathway. Sci Rep. 2017;7:44689. doi: 10.1038/srep44689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Schmeichel AM, Iida H, Schmelzer JD, Low PA. Ischemia-reperfusion injury causes oxidative stress and apoptosis of Schwann cell in acute and chronic experimental diabetic neuropathy. Antioxid Redox Signal. 2005;7:1513–1520. doi: 10.1089/ars.2005.7.1513. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Yang S, Zhu G. Postnatal calpain inhibition elicits cerebellar cell death and motor dysfunction. Oncotarget. 2017;8:87997–88007. doi: 10.18632/oncotarget.21324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Liu J, Chen S, Liu J, Liu L, Liu G, Wang F, Jiang W, Zhang C, Wang S, Yuan X. Caspase-12 is involved in stretch-induced apoptosis mediated endoplasmic reticulum stress. Apoptosis. 2016;21:432–442. doi: 10.1007/s10495-016-1217-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhu G, Wang X, Wu S, Li X, Li Q. Neuroprotective effects of puerarin on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s disease model in mice. Phytother Res. 2014;28:179–186. doi: 10.1002/ptr.4975. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Zhu Y, Zhang L, Wu T, Wu T, Zhang W, Decker AM, He J, Liu J, Wu Y, Jiang X, Zhang Z, Liang C, Zou D. Human stem cells overexpressing miR-21 promote angiogenesis in critical limb ischemia by targeting CHIP to enhance HIF-1alpha activity. Stem Cells. 2016;34:924–934. doi: 10.1002/stem.2321. [DOI] [PubMed] [Google Scholar]
- 25.Young MR, Santhanam AN, Yoshikawa N, Colburn NH. Have tumor suppressor PDCD4 and its counteragent oncogenic miR-21 gone rogue? Mol Interv. 2010;10:76–79. doi: 10.1124/mi.10.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan H, Rao J, Yuan J, Gao L, Huang W, Zhao L, Ren J. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate ischemic neuronal death by targeting miR-21/PDCD4 signaling pathway. Cell Death Dis. 2017;8:3211. doi: 10.1038/s41419-017-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu G, Wang X, Wu S, Li Q. Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell death. Neurochem Int. 2012;60:400–408. doi: 10.1016/j.neuint.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Wu S, Zhou Y, Yang G, Tian H, Geng Y, Hu Y, Lin K, Wu W. Sulforaphane-cysteine induces apoptosis by sustained activation of ERK1/2 and caspase 3 in human glioblastoma U373MG and U87MG cells. Oncol Rep. 2017;37:2829–2838. doi: 10.3892/or.2017.5562. [DOI] [PubMed] [Google Scholar]
- 30.Qian QZ, Cao XK, Liu HY, Zheng GY, Qian QQ, Shen FH. TNFR/TNF-alpha signaling pathway regulates apoptosis of alveolar macrophages in coal workers’ pneumoconiosis. Oncotarget. 2018;9:1302–1310. doi: 10.18632/oncotarget.18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao L, Yang L, Li R, Rodrigues DN, Crespo M, Hsieh JT, Tilley WD, de Bono J, Selth LA, Raj GV. Disrupting androgen receptor signaling induces snail-mediated epithelial-mesenchymal plasticity in prostate cancer. Cancer Res. 2017;77:3101–3112. doi: 10.1158/0008-5472.CAN-16-2169. [DOI] [PubMed] [Google Scholar]
- 32.Seara FAC, Barbosa RAQ, de Oliveira DF, Gran da Silva DLS, Carvalho AB, Freitas Ferreira AC, Matheus Nascimento JH, Olivares EL. Administration of anabolic steroid during adolescence induces long-term cardiac hypertrophy and increases susceptibility to ischemia/reperfusion injury in adult Wistar rats. J Steroid Biochem Mol Biol. 2017;171:34–42. doi: 10.1016/j.jsbmb.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Kelicen Ugur P, Lule S, Cincioglu M, Pekiner C, Gursoy-Ozdemir Y. Megestrol acetate inhibits the expression of cytoplasmic aromatase through nuclear C/EBPbeta in reperfusion injury-induced ischemic rat hippocampus. Eur J Pharmacol. 2011;654:217–225. doi: 10.1016/j.ejphar.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Hadoke PW, Takov K, Korczak A, Denvir MA, Smith LB. Influence of androgen receptor in vascular cells on reperfusion following hindlimb ischaemia. PLoS One. 2016;11:e0154987. doi: 10.1371/journal.pone.0154987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Tsai BM, Kher A, Baker LB, Wairiuko GM, Meldrum DR. Role of endogenous testosterone in myocardial proinflammatory and proapoptotic signaling after acute ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H221–226. doi: 10.1152/ajpheart.00784.2004. [DOI] [PubMed] [Google Scholar]
- 36.Chaves EA, Pereira-Junior PP, Fortunato RS, Masuda MO, de Carvalho AC, de Carvalho DP, Oliveira MF, Nascimento JH. Nandrolone decanoate impairs exercise-induced cardioprotection: role of antioxidant enzymes. J Steroid Biochem Mol Biol. 2006;99:223–230. doi: 10.1016/j.jsbmb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Kil IS, Seok YM, Yang ES, Kim DK, Lim DG, Park JW, Bonventre JV, Park KM. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J Biol Chem. 2006;281:20349–20356. doi: 10.1074/jbc.M512740200. [DOI] [PubMed] [Google Scholar]