Abstract
The proliferation of technology enthuses clinicians, researchers, and entrepreneurs to revolutionize health care and care delivery. Intersecting in the field of digital health, academic-industry collaboration (AIC) play a critical role in advancing evidence-based innovations into real world application. AIC models vary, but historically have not included the strong emphasis on rapid research and discovery that the digital health field demands. Due to the voluminous availability of real time patient and client data, academic health centers offer a rich interdisciplinary environment to develop, pilot and evaluate innovations in pragmatic settings. Despite the opportunity between academic health centers and industry to advance digital health innovation through rapid research, limited evidence exists of such collaboration. The purpose of this case report is to examine an AIC facilitating research of new health technologies within an academic health center. This paper presents a case report involving collaboration between diverse technology industry partners and an academic health center that encompasses a university health system (UCHealth), a university technology transfer office (CU Innovations), an innovation center (CARE Innovation Center), and research collaborators (mHealth Impact Laboratory). Case assertions discuss the lessons learned and recommendations when implementing such collaboration in practice. The principal finding is that academic health centers offer an innovative environment for AIC in digital health. Collaborations between academia and industry provide much promise in ensuring health innovations are scientifically sound while meeting the needs of a rapidly evolving technical climate.
Keywords: Digital health, academic-industry collaboration (AIC), academic medical centers, innovation, academic health centers
Introduction
The ubiquitous use of technology galvanizes both academic researchers and entrepreneurs in population health. Intersecting in the field of digital health, academic-industry collaborations (AICs) play a critical role in advancing science into real world application (1,2). Examples of successful AICs include traditional biotechnology (biotech) innovation models, academic entrepreneurship efforts, and government-sponsored technology partnership programs (3-8). Such partnerships seek to balance both scientific rigor and commercial viability, yielding sustainable evidence-based digital health products (9). Collaboration models vary but often exclude an emphasis on iterative rapid research, an approach the pace of technology demands (3-8).
Various barriers limit the ability to establish digital health AICs that promote responsive evaluation. While not explicitly unique to the field of digital health, scarce research dollars, limited recruitment pools, and strict institutional infrastructure affect collaboration success (10-13). Additionally, conflicting priorities and timelines exacerbate the challenges between academic and industry partners (12,14-16).
University-integrated health systems offer a fruitful environment to foster AICs, thus advancing innovation and science in digital health (10,17-19). Academic Health Centers (AHCs) encompassing health profession schools (e.g., medicine, public health, pharmacy, and nursing) and an affiliated health system offer the access and support required for translating validated innovations into practice (20). Proposed as “catalysts” to digital health success (10), AHCs leverage interdisciplinary expertise, patient data, and clinical trial infrastructure to advance novel digital health products. AHC-based AICs (AHC-AICs) offer promise in tackling the barriers described, yielding evidence-based technologies without thwarting innovation (1,14,21-23).
At least 70 university digital health centers exist within AHCs in the United States (24), but digital health AICs in this context are unexplored in the literature (20,23). The purpose of this paper describes a nascent academic-industry partnership designed to rapidly implement and test digital health products. This report specifically examines the research partnership between multiple industry technology partners and an AHC that includes: the UCHealth health delivery system, the CARE Innovation Center (CARE), CU Innovations (technology transfer office), and the mHealth Impact Lab. The case report synthesizes findings based on initiated (n=9) and completed (n=2) digital health projects conducted through the CARE and supported by the mHealth Impact Lab in 2018. Projects spanned a variety of digital modalities including but not limited to sensors, wearables, and mobile applications. The setting of all proposed research was within the UCHealth system and included 4 different industry technology partners. Based on qualitative feedback obtained by AHC-AIC stakeholders, this report presents lessons learned and future considerations with digital health AHC-AICs in practice.
Case presentation
Key partners
As seen in Figure 1, the AHC partnership includes five key partners: (I) a university health system, (II) innovation technology transfer office, (III) industry-AHC convener, (IV) research support collaborators, and (V) multiple technology industry partners.
Figure 1.
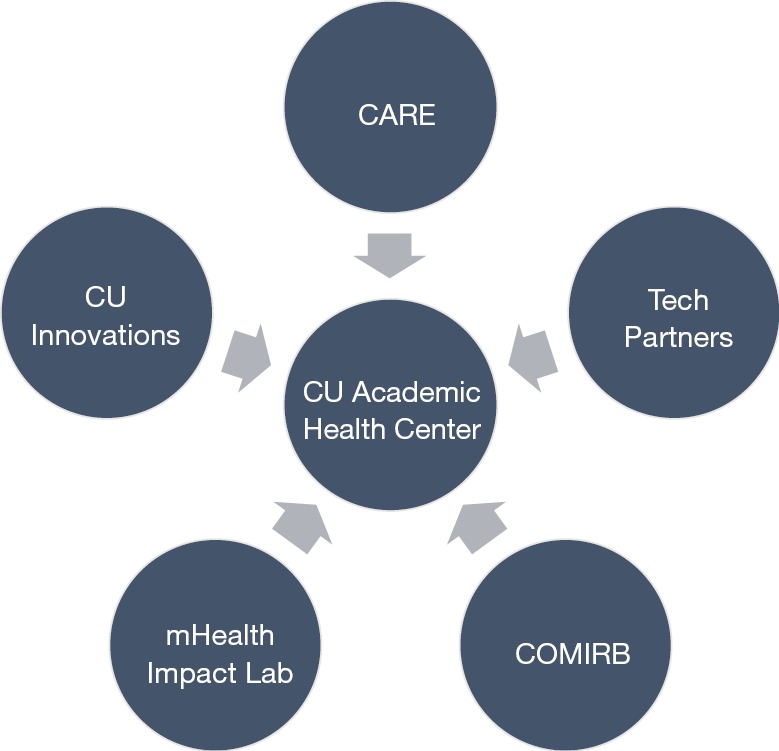
University of Colorado Academic Health Center: Academic-Industry Partnership.
The University of Colorado Anschutz Medical Campus (AMC) is an academic health center that includes multiple health profession schools, centers, and institutes. AMC houses two university teaching hospitals, treating roughly 2 million patients per year (25). The UCHealth System encompasses 2,000 providers, 150 clinics, and 10 hospitals in Colorado with affiliated hospitals in Wyoming and Nebraska.
CARE situates within UCHealth, and has financial support from CU Innovations, the technology transfer office of the University of Colorado. Both CU Innovations and CARE convene industry partners, both startup and established, to implement and test emerging health technologies within the health system infrastructure.
Located at AMC, the mHealth Impact Lab is an electronic research organization (eRO) that operates through the Colorado School of Public Health. The mHealth Impact Lab works with academic and industry partners to create and curate high quality technologies aimed to improve health promotion, disease prevention, and health care. For this specific partnership, the mHealth Impact Lab assists with proposal consulting and research implementation support, and serves as the liaison between CARE and the Colorado Multiple Institutional Review Board (COMIRB).
Lastly, the AHC-AIC facilitates projects with diverse types of technology enterprises from small to large industry partners. These include: smaller digital health startups looking to pilot test or accelerate products through clinical validation; enterprises with advanced research and data science expertise, seeking larger scale partnership; technology developers, seeking to facilitate academic ideas through software programming; and larger established technology companies to clinically validate suites of digital health solutions through established AHC infrastructure.
Innovation workflow
While academic innovation occurs in a variety of ways across US institutions (22), CU Innovations and the CARE Innovation Center prioritize clinical validation of digital health tools within the AHC. This approach expands on the traditional innovation tech transfer process (26) to include research and evaluation in addition to education, vetting, incubation, acceleration, and launch of a particular digital health product to market.
When a potential technology industry partner emerges, CU Innovations and CARE stakeholders convene the Innovation Council (iCouncil) to assess the AHC-AIC opportunity. The iCouncil consists of multi-disciplinary stakeholders and subject matter experts for vetting. This innovation workflow reflects the importance of healthcare as a complex adaptive system (27) when testing digital and mHealth tools in practice. The clinical validation process is evolving depending on the context of the industry technology partner.
Implementation of partnership and clinical validation process
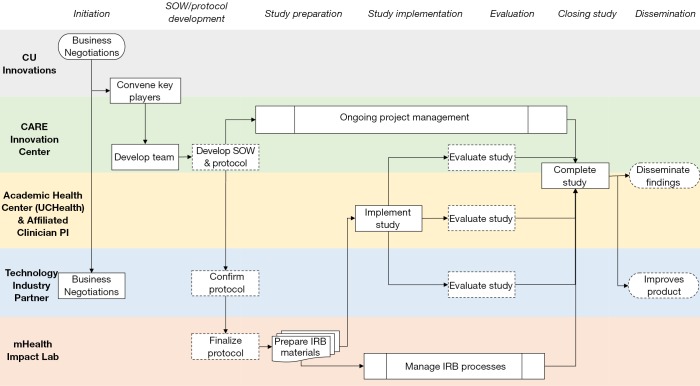
The summary for the clinical validation process and partnership implementation can be found in Figure 2. Detailed steps throughout the clinical validation process are found in Table 1.
Figure 2.
Summary of clinical validation process and partnership implementation.
Table 1. Process steps.
| Step | Description |
|---|---|
| 1. Business Negotiations | When an industry start-up or established technology company wishes to test out an innovation in the health care delivery environment, they approach CU Innovations as a first step. CU Innovations is aware of the CARE priorities for improvements in patient care, and will forward relevant opportunities to the CARE team for consideration. If the innovation or proposed solution meets a CARE priority and there is a clinician researcher within the UC Health system who could serve as a lead investigator of a clinical trial to investigate the effects of the innovation, CARE convenes the key players to begin the clinical validation process |
| 2. Convening key players | At this point, CARE will involve mHealth Impact and schedule a conference call between the CARE team, clinical subject matter experts, and mHealth Impact personnel to specify the scope of work and identify the relevant IRB considerations. For each project, the group must consider who will assume responsibility for identification, recruitment, informed consent and enrollment of patient participants in research. They also consider who will assume responsibility for collection, management and analysis of study data, and discuss data access and ownership issues. UCHealth has internal clinical research teams with capacity to perform all these research functions, but they are not always available for research implementation; in such cases, mHealth Impact will step in to provide all or some of the research implementation activities. During this initial partnership-building phase, intellectual property (IP) and non-disclosure agreements (NDA) are also discussed. Typically, IP rests with the technology partner and all parties participate in a non-disclosure agreement; however, some partnerships may involve co-development and co-ownership agreements as appropriate |
| 3. Protocol development | Many times, the technology partner articulates what they would like to learn from their digital health product and how they can support the work within the AHC. The academic research partner (mHealth Impact) in concert with the internal CARE team and UCHealth clinical research team as they are available then proposes methods and protocols for articulating those claims with appropriate rigor. As noted above, each project includes at least one UCHealth clinician researcher who serves as a subject matter expert. These conversations are facilitated by CARE project management personnel. mHealth Impact will interface with clinician researchers and the CARE team to develop or refine the research protocol and submit it for review to the University institutional review board (IRB) with additional materials as needed to fully address protections for human subjects engaged in research. The focus and scope of the research proposed will determine if a research study is classified as ‘exempt’ from IRB approval or if it represents minimal or greater than minimal risk requiring more extensive oversight |
| 4. IRB processes | Technology partners may be unfamiliar with human subjects research and the associated regulatory compliance requirements and approvals required by and managed through an IRB. The academic research partner provides critical insight into ethical approaches to scientific clinical trials and into compliance with federal regulations for the protection of human subjects in research. mHealth Impact usually manages the IRB application process using established digital health protocol templates to facilitate application creation. These templates significantly shorten the timelines for review of protocols by the IRB, and facilitate more rapid implementation of research on technology innovation in the care setting |
| 5. Research team development | Clinical research teams embedded within the UCHealth system and/or teams through mHealth Impact may be engaged to conduct the proposed work. This includes teams with capacity to recruit, obtain informed consent and enroll participants within the AHC such as a research coordinator or team of research assistants to support the appointed clinician principal investigator |
| 6. Implementation and analysis of study | The implementation of the study depends on the scope of work between the AHC-AIP. In general, these projects require support at the clinic level, study champions at the leadership level, an investigative team, and a project manager to help navigate all aspects of a complex adaptive system. Depending on the scope of work, the analysis of clinical trial data may be the responsibility of the technology partner, mHealth Impact, or an internal group on the AMC campus that specializes in biostatistics and data analytics |
| 7. Study completion and dissemination | Upon completion or cancellation of the study, two forms of dissemination of knowledge may occur, which are highly dependent on the IP/NDAs established in the early stages of the AHC-AIP. (1) Academic publications help advance the evidence base, but it is essential to do so without revealing and proprietary information that may affect the solution’s development and commercialization potential. (2) Additionally, insights are retained within the technology industry partner to better inform user experience and user interface (UX/UI) design, and future iterations of digital health product |
Case assertions
The AHC sees opportunity in improving patient care and saving costs, but waiting for technologies to become commercially available can be inefficient. Additionally, commercially available options have usually come to market without a rigorous analysis of their clinical validity, limiting their appeal to clinicians. Technology companies have products but lack large sample populations for implementation and testing, especially at early stages. Research teams have expertise in the design and ethics of conducting clinical research, but do not always have access to patient populations or emergent technologies. This partnership integrates these five partners to maximize the rapid, rigorous investigation of technologies that could potentially benefit patients and improve both the quality and cost of care.
Lessons learned
Leverage strengths of each partner
The literature proposes the potential for AHCs to leverage academic and industry expertise to advance digital health, and that potential was realized in this AHC-AIC (14,21). UCHealth offered the partnership a variety of strengths to advance digital and mobile health research. Such strengths included the available infrastructure and access to patient populations to include as research participants, clinician researchers who wanted to improve patient care, and administrators who were motivated to cut costs in care delivery without compromising care (10,18,28). Research partners like the mHealth Impact Lab offered skills in conducting rigorous human subjects research related to technology with a strong understanding of the field of digital health. This expertise in the design and delivery of clinical research, both in the design and delivery of research, facilitated the work in a productive ethical way. Industry technology partners thrive in ‘fail fast’ mentality and business processes (29,30). Such culture expedited study timelines and offered momentum for academic partners. Depending on the entry point of industry, these partners had capacity to quickly iterate on new technology innovations that may have the potential to impact health, care delivery, and health outcomes. A focus on commercialization demands designing for dissemination principles, an area where many academic interventionists fall short.
Required skillsets for AHC-AIC in practice
As the partnership evolved, specific skills were identified as needed and distributed across partners to alleviate various barriers. Maintaining the validity of a study can be challenging, thus clinical buy-in remains critical for study execution. A key facilitator for this rapid research was strong clinical support and clinical leadership to champion the work within the academic medical center (10,31). Such relationships executed the trials in a way that maximized access to patient populations and improved study implementation. Additionally, strong scientific investigative skills were needed to rigorously validate and project management skills to maintain strict timelines that navigate all aspects of complex adaptive systems (32,33).
The critical role of the IRB
IRBs must operate independently and according to strict federal regulations, thus they may not always have capacity to respond quickly to review innovation protocols on rapid, industry-familiar timelines. Under this initiative, the CARE developed agreements with COMIRB to dedicate resources to facilitate priority review of CARE protocols submitted by the mHealth Impact Lab. Additional pre-review and communication processes were established to ensure minimal required changes and timely notification of review-related events. The team also developed a strong understanding of the breadth of technologies cycling through the system and provided substantial supporting documentation for each, helping the IRB make determinations quickly.
Partnerships alone are not enough to overcome barriers
Balancing scientific rigor while considering commercial viability proves challenging (12,14-16). After a significant learning curve, the partners established role clarity and timeline expectations. This helped maneuver through institutional bureaucracy that rapidly worked with COMIRB to submit and implement studies. The scope of work for each project helped refine this process, however pragmatic barriers persisted that were primarily out of the partnership’s control. Such examples included technology device changes during studies, study site clinic complexity and competing projects underway in similar patient populations, limited bandwidth for IRB reviewers, and other regulatory factors that resulted in review timeframes inconsistent with industry partners’ more rapid development timeline-based expectations While this avenue of collaboration shows potential, establishing partnerships alone is not enough to overcome all logistical and ethical barriers among institutions; clear and ongoing communication is essential.
Recommendations
Cross-disciplinary flexibility
The partnerships thus far have worked with various technology products, addressed multiple health topics, and targeted diverse patient populations. This diversity required flexibility where protocols are similar among projects but with substantive changes in detail depending on terms agreed in a memorandum of understanding (MOU). Adapting to each study’s particular needs demanded an iterative process to ensure research objectives were pursued in a timely and ethical way that is appropriate to answer research questions with minimal burden placed on patients and providers.
Established relationship with IRB and research administration
The mHealth Impact Lab maintained regular, ongoing communication with COMIRB and with clinical research administration leadership at UCHealth. A team continually reviewed and discussed process improvement initiatives to ensure that protocol modifications related to changes with technology device, patient recruitment, or adaptive trial designs were minimized. This positive relationship allowed the partnership to test digital health solutions in a structured and rapid way.
Improving IRB understanding by industry
Upon initial partnership, collaborators identified the lack of awareness of IRB processes among technology industry partners as a barrier to successful implementation. While technology partners were collecting data and quality improvement information regularly for their products, these enterprises were unaware of federal and state regulations related to human subjects research and the ethical approaches of conducting formal scientific studies. Without this understanding, there was limited appreciation for the role of the IRB, the importance of IRB review, and the associated elongation of planning timelines in order to obtain IRB approval. For this AHC-AIC, the mHealth Impact Lab acted as an expert resource and IRB liaison, providing raised awareness of IRB processes and helping to manage timeline expectations and improve project planning. This allowed the partnership to improve project planning and execution without requiring each industry partner to develop a robust in-house understanding of the regulations, which would be impractical and inefficient.
Empathize with and explicitly address diverse values and priorities
Partners held goals in common, but also had conflicting value propositions due to their diverse business needs. Understanding and balancing these differing priorities was key to content partners and to successful partnership. Identifying disparate priorities early helped with a more rapid implementation of projects. This partnership welcomed conversations around intellectual property in the initial stages, allowing for explicit scopes of work and MOUs. Revisiting these discussions regularly was critical for the success of all partners: scholarship helped advance the evidence base, but it is important to do that without revealing confidential information that affects the industry partner’s development and commercialization potential.
Discussion
Researchers, clinicians, and entrepreneurs do not typically collaborate in the design, development, evaluation and dissemination of mHealth solutions, and are often found to be operating in parallel rather than collaboratively (14). Products such as wearables, mobile applications, telemedicine technologies, web-based platforms, and health information websites are developed at a rapid pace within the technology industry, but are not routinely evaluated using scientific methods in partnership with academic researchers (34-36).
The assertions of this case report highlight an approach for AICs within AHCs to aid an innovation environment that promotes advancing evidence-based technologies intended to improve health outcomes. While there are many strengths and limitations to consider with these partnerships, the focus of this case report highlights rapid and responsive research within the AHC. An AHC-AIC may help gain access to populations, build capacity to research digital health products, rapidly, ensure clinical expertise is engaged, promote ethical human subjects handling, and access innovative products from the technology industry (10,14,31). Strengths of AICs present the potential to expand funding possibilities, foster rapid development and research cycles, and design for dissemination, thus yielding sustainable products proven to enhance health outcomes.
This case report offers a high-level snapshot to describe an AHC-AIC. These partnerships are dynamic in nature and evolve by project and collaboration thus challenging generalization. This limitation offers promise in future research in learning from AICs in digital health. Many AICs exist (3-8); while this is just one model, additional research is needed to develop a formal consensus. This case is one that offers a model for rapid, rigorous research, allowing technology companies a platform to test and iterate their minimum viable products to generate both a greater value proposition and offer hospital systems ways to explore potential improvements in patient care. Currently, there lacks consensus of an AIC structure for ethical, robust research that can be responsive to the fast paced, quickly evolving mHealth technology environment. A formal review is needed to further understand academic and industry partnerships, models of collaboration, and best practices to advance evidence-based digital and mHealth solutions.
Conclusions
This case report contributes to the scarce body of literature on how academic industry collaborations in AHCs facilitate relevant digital health research. This report illustrates one of many ways AICs occur, bringing health systems, academia, and technology industries together to advance high quality digital and mobile health solutions. AHCs provide promise for rapidly testing technologies in real world settings, offering an intersection to implement AICs in a way to focus on rapid clinical validation. While more understanding is needed of best practices, AHCs offer a promising environment that considers strengths of each disciplines and intersects required skills and resources to advance the work. As the opportunity for mHealth and digital technologies increases, the demand for rigorously evaluated solutions produced by academic and industry is inevitable. Productive collaboration between fields is required to ensure digital health products are evidence-based, theory informed, and reach communities to positively influence health outcomes.
Acknowledgments
The authors wish to thank the University of Colorado Anschutz Medical Campus and CU Innovations for their contributions to the academic health center partnership, specifically Kimberly A. Muller, Esq. (CU Innovations Managing Director).
Funding: Dr. Portz’s is funded by a career development award from the National Institution on Aging (K76AG059934).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Fort DG, Herr TM, Shaw PL, et al. Mapping the evolving definitions of translational research. J Clin Transl Sci 2017;1:60-6. 10.1017/cts.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownson RC, Colditz GA, Proctor EK. Dissemination and implementation research in health: translating science to practice. Oxford University Press; 2017. [Google Scholar]
- 3.Gnyawali DR, Park BJ. Co-opetition and Technological Innovation in Small and Medium-Sized Enterprises: A Multilevel Conceptual Model. J Small Bus Manag 2009;47:308-30. 10.1111/j.1540-627X.2009.00273.x [DOI] [Google Scholar]
- 4.Iyawa GE, Herselman M, Botha A. Digital Health Innovation Ecosystems: From Systematic Literature Review to Conceptual Framework. Procedia Comput Sci 2016;100:244-52. 10.1016/j.procs.2016.09.149 [DOI] [Google Scholar]
- 5.Mention A-L. Co-operation and co-opetition as open innovation practices in the service sector: Which influence on innovation novelty? Technovation 2011;31:44-53. 10.1016/j.technovation.2010.08.002 [DOI] [Google Scholar]
- 6.Ritala P, Sainio L-M. Coopetition for radical innovation: technology, market and business-model perspectives. Technol Anal Strateg Manag 2014;26:155-69. 10.1080/09537325.2013.850476 [DOI] [Google Scholar]
- 7.Flood J, Minkler M, Hennessey Lavery S, et al. The Collective Impact Model and Its Potential for Health Promotion: Overview and Case Study of a Healthy Retail Initiative in San Francisco. Health Educ Behav 2015;42:654-68. 10.1177/1090198115577372 [DOI] [PubMed] [Google Scholar]
- 8.Etzkowitz H, Zhou C. The triple helix: University–industry–government innovation and entrepreneurship. Routledge; 2017. [Google Scholar]
- 9.Ankrah S, Al-Tabbaa O., Universities–industry collaboration : A systematic review. Scand J Manag 2015;31:387-408. 10.1016/j.scaman.2015.02.003 [DOI] [Google Scholar]
- 10.Depasse JW, Chen CE, Sawyer A, et al. Academic Medical Centers as digital health catalysts. Healthcare 2014;2:173-6. 10.1016/j.hjdsi.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 11.Patrick K, Hekler EB, Estrin D, et al. The Pace of Technologic Change: Implications for Digital Health Behavior Intervention Research. Am J Prev Med 2016;51:816-24. 10.1016/j.amepre.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 12.Yardley L, Choudhury T, Patrick K, et al. Current issues and future directions for research into digital behavior change interventions. Am J Prev Med 2016;51:814-5. 10.1016/j.amepre.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 13.Grundy QH, Wang Z, Bero LA. Challenges in Assessing Mobile Health App Quality: A Systematic Review of Prevalent and Innovative Methods: A Systematic Review of Prevalent and Innovative Methods. Am J Prev Med 2016;51:1051-9. 10.1016/j.amepre.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 14.Hingle M, Patrick H, Sacher PM, et al. The Intersection of Behavioral Science and Digital Health: The Case for Academic–Industry Partnerships. Health Educ Behav 2019;46:5-9. 10.1177/1090198118788600 [DOI] [PubMed] [Google Scholar]
- 15.Moore C, Werner L, BenDor AP, et al. Accelerating Harmonization in Digital Health. World Health Popul 2017;17:43-54. 10.12927/whp.2017.25306 [DOI] [PubMed] [Google Scholar]
- 16.Lim SY, Anderson EG, editors. Institutional Barriers Against Innovation Diffusion: From the Perspective of Digital Health Startups. 2016 49th Hawaii International Conference on System Sciences (HICSS); 2016: IEEE. [Google Scholar]
- 17.Kohn LT, Institute of Medicine. Committee on the Roles of Academic Health Centers in the 21st C. Academic health centers: leading change in the 21st century. Washington, DC: Washington, National Academies Press; 2004. [PubMed] [Google Scholar]
- 18.Toner M, Tompkins RG. Invention, innovation, entrepreneurship in academic medical centers. Surgery 2008;143:168-71. 10.1016/j.surg.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostrovsky A, Barnett M, editors. Accelerating change: fostering innovation in healthcare delivery at academic medical centers. Healthcare; 2014: Elsevier. [DOI] [PubMed] [Google Scholar]
- 20.Tseng J, Samagh S, Fraser D, et al., editors. Catalyzing healthcare transformation with digital health: Performance indicators and lessons learned from a Digital Health Innovation Group. Healthcare; 2018: Elsevier. [DOI] [PubMed] [Google Scholar]
- 21.Abroms LC, Allegrante JP, Auld ME, et al. Toward a Common Agenda for the Public and Private Sectors to Advance Digital Health Communication. Am J Public Health 2019;109:221-3. 10.2105/AJPH.2018.304806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellner AL, Stout S, Sullivan EE, et al. Health systems innovation at academic health centers: leading in a new era of health care delivery. Acad Med 2015;90:872-80. 10.1097/ACM.0000000000000679 [DOI] [PubMed] [Google Scholar]
- 23.Sharma A, Harrington RA, McClellan MB, et al. Using digital health technology to better generate evidence and deliver evidence-based care. J Am Coll Cardiol 2018;71:2680-90. 10.1016/j.jacc.2018.03.523 [DOI] [PubMed] [Google Scholar]
- 24.Hostetter M, Klein S, McCarthy D, et al. Findings from a survey of health care delivery innovation centers. The Commonwealth Fund [Internet] 2015. Available online: https://www.commonwealthfund.org/publications/publication/2015/apr/findings-survey-health-care-delivery-innovation-centers
- 25.University of Colorado. CU Facts and Figures 2017-2018. Accessed on September 2019. Available online: https://www.cu.edu/cu-facts-and-figures
- 26.Mowery DC, Nelson RR, Sampat BN, et al. Ivory tower and industrial innovation: University-industry technology transfer before and after the Bayh-Dole Act. Stanford University Press; 2015. [Google Scholar]
- 27.Lipsitz LA. Understanding health care as a complex system: the foundation for unintended consequences. JAMA 2012;308:243-4. 10.1001/jama.2012.7551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dzau VJ, Ackerly DC, Sutton-Wallace P, et al. The role of academic health science systems in the transformation of medicine. Lancet 2010;375:949-53. 10.1016/S0140-6736(09)61082-5 [DOI] [PubMed] [Google Scholar]
- 29.Khanna R, Guler I, Nerkar A. Fail often, fail big, and fail fast? Learning from small failures and R&D performance in the pharmaceutical industry. Acad Manag J 2016;59:436-59. 10.5465/amj.2013.1109 [DOI] [Google Scholar]
- 30.Schaller RRJIs Moore's law: past, present and future. IEEE Spectrum 1997;34:52-9. 10.1109/6.591665 [DOI] [Google Scholar]
- 31.Mann DM, Chokshi SK, Lebwohl R, et al. Building digital innovation capacity at a large academic medical center. NPJ Digit Med 2019;2:13. 10.1038/s41746-019-0088-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Begun JW, Zimmerman B, Dooley K. Health care organizations as complex adaptive systems. In: Mick SM, Wyttenbach M, editors. Advances in Health Care Organization Theory. San Francisco: Jossey-Bass, 2003;253-88. [Google Scholar]
- 33.Rouse WB. Health care as a complex adaptive system: implications for design and management. Bridge (Wash D C) 2008;38:17. [Google Scholar]
- 34.Ioannidis JPA. Stealth Research: Is Biomedical Innovation Happening Outside the Peer-Reviewed Literature? JAMA 2015;313:663-4. 10.1001/jama.2014.17662 [DOI] [PubMed] [Google Scholar]
- 35.Bull S. Beyond acceptability and feasibility: moving mHealth into impact. mHealth 2016;2:45. 10.21037/mhealth.2016.12.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Informatics IIfH. Patient adoption of mHealth: use, evidence and remaining barriers to mainstream acceptance. IMS Institute for Healthcare Informatics Parsipanny, NJ; 2015. [Google Scholar]