Abstract
Neurotrophic keratitis is an underdiagnosed degenerative condition induced by impairment to the corneal nerves which may lead to persistent epithelial defects and corneal blindness. Current medical and surgical treatments are only supportive and poorly tackle the underlying problem of corneal anesthesia; hence, fail to provide a permanent cure. Cenegermin is a newly introduced recombinant human nerve growth factor (rhNGF) that may address this issue. Preliminary clinical trials have demonstrated the safety and efficacy of topical cenegermin in patients with moderate to severe neurotrophic keratitis; however, the clinical experience with this drug is still limited. This review summarizes the pathogenesis and management of neurotrophic keratitis as well as the mechanism of action, uses, and limitations of cenegermin eye drops in the treatment of neurotrophic keratitis.
Keywords: cenegermin, corneal nerves, neurotrophic keratitis, nerve growth factors, persistent epithelial defect
Background
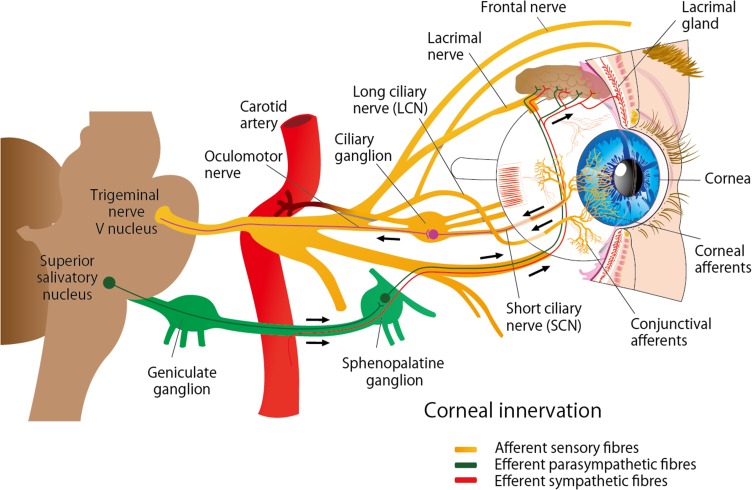
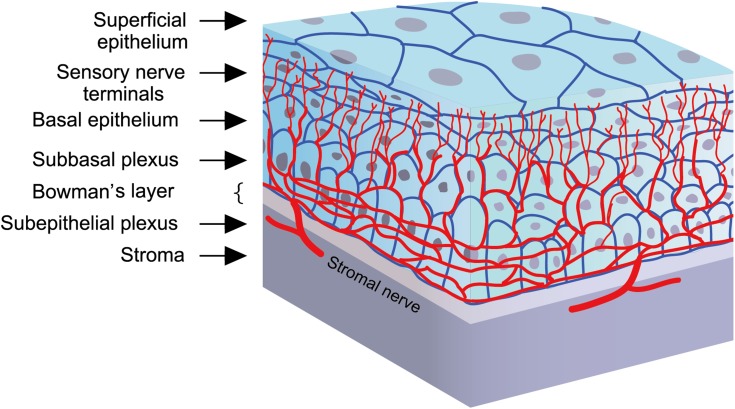
The cornea is the most densely innervated tissue in the human body and is supplied by the ophthalmic branch of the trigeminal nerve and autonomic nerves (Figure 1). Nerve fibers penetrate the corneal periphery at the limbus and approach toward the central cornea at the level of the anterior stroma while penetrating the Bowman’s membrane to create the sub-basal nerve plexus, beneath the basement membrane of the epithelium. Terminal branches from the sub-basal plexus pass anteriorly into the epithelial cell layers, terminating within or in between epithelial cells. (Figure 2).1–3 These nerves are essential for maintaining the integrity and clarity of the cornea including the limbal stem cell niche by mediating protective blinking and tearing reflexes as well as other trophic functions.1,4,5 Corneal nerves release neuropeptides, such as substance P and calcitonin gene-related peptide, that promote epithelial cell proliferation, migration, adhesion, and differentiation. In turn, corneal epithelial cells release neurotrophic factors, such as nerve growth factor (NGF) and epidermal growth factor, which promote neuronal extension and survival.1,6 This balance is vital for corneal healing and maintenance. Corneal nerve damage results in loss of corneal sensation and trophic functions which consequently leads to epithelial breakdown and poor healing; a condition known as neurotrophic keratitis or neurotrophic keratopathy.6–8 Cenegermin is a newly introduced recombinant human nerve growth factor (rhNGF) to promote healing in neurotrophic keratitis. Preliminary clinical trials have demonstrated the safety and efficacy of topical cenegermin in patients with moderate to severe neurotrophic keratitis; however, the clinical experience with this drug is still limited. This review summarizes the pathogenesis and management of neurotrophic keratitis as well as the mechanism of action, uses, and limitations of cenegermin eye drops in the treatment of neurotrophic keratitis.
Figure 1.
Nerve supply of the cornea. The cornea is innervated by the ophthalmic branch of the trigeminal nerve (V1) and by sympathetic and parasympathetic autonomic nerves.
Figure 2.
Corneal nerves distribution: Nerve fibers penetrate the corneal periphery at the limbus and approach toward the central cornea at the level of the anterior stroma while penetrating the Bowman’s membrane to create the sub-basal nerve plexus, at the level of the basal epithelial cells and basement membrane of the epithelium. Terminal branches from the sub-basal plexus pass anteriorly into the epithelial cell layers, terminating within or in between epithelial cells.
Figure 3.
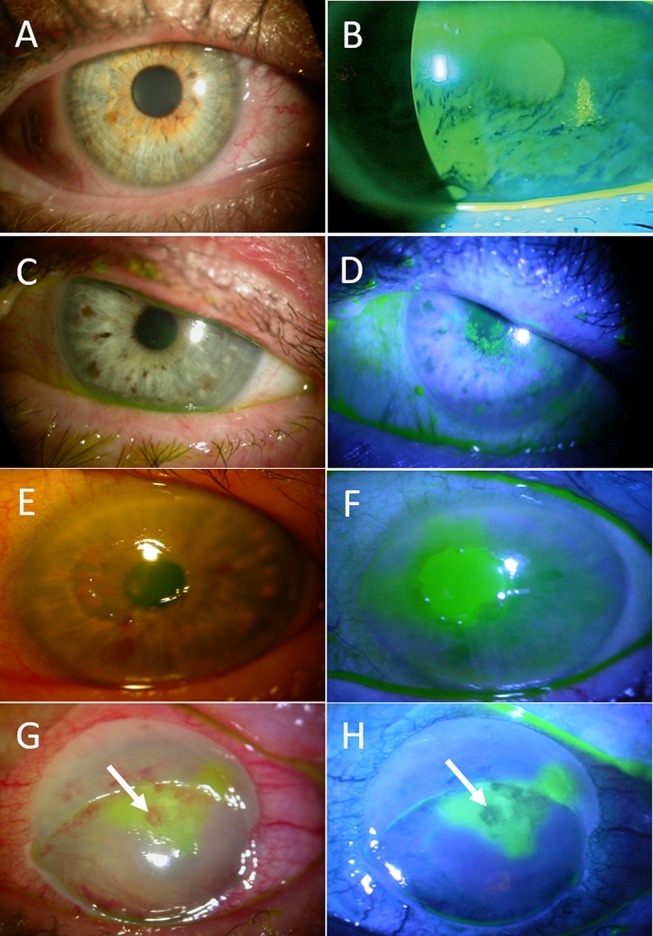
Stages of neurotrophic keratitis: Impaired cornea sensitivity and lack of trophic support trigger nonspecific epithelial irregularity and tear film changes (A, B). Stage 1- mild (C, D) is characterized by corneal punctate keratopathy due to focal epithelial damage and loss of tight junctions. It is associated with mild stromal edema with or without corneal neovascularization. Stage 2 – moderate (E, F) is distinguished by the presence of central persistent epithelial defect, surrounded by edematous, cloudy, and poorly adherent epithelium. Stage 3- severe (G, H) is characterized by stromal ulceration and thinning that may progress to melting and perforation (arrows).
Etiology Of Neurotrophic Keratitis
The most common causes of corneal nerves damage include herpetic keratitis, chemical burns, physical injuries, corneal surgery, long-term use of contact lenses, and prolonged use of topical medications.7–9 Intracranial mass such as neuroma, meningioma, and aneurysms may compress the trigeminal nerve or ganglion and produce impairment of corneal sensitivity.10 Systemic diseases such as diabetes, and multiple sclerosis may damage sensory fibers leading to corneal hypoesthesia.11 More recently, it has been determined dry eye can also cause corneal nerve damage, as well as corneal degenerative disorders such as keratoconus and dystrophies.
Stages Of Neurotrophic Keratitis
Based on the severity, neurotrophic keratitis is classified into three overlapping stages (Figure 3): epithelial alterations (stage 1), persistent epithelial defects (stage 2), and corneal ulcers (stage 3).6–9,12 Due to decreased corneal sensation, patients rarely complain of ocular symptoms, and there is a significant discrepancy between clinical findings and subjective symptoms.9 Therefore, disease progression is often asymptomatic and may lead to profound vision loss resulting from scarring and corneal perforation.
Diagnosis Of Neurotrophic Keratitis
The diagnosis of neurotrophic keratitis is mainly based on clinical history and clinical signs such as presence of persistent epithelial defects or ulcers and decreased corneal sensitivity. According to the literature, the estimated prevalence of neurotrophic keratitis is less than 5/10,000 individuals, being classified as a rare orphan disease (ORPHA137596).12 With the evolution of the diagnostic imaging and the quantitative analysis of corneal innervation,13–16 the prevalence and classification should be reevaluated to include the correlations between corneal innervation, clinical findings, and patients’ symptoms. Coexisting ocular surface disorders such as dry eye and exposure keratitis should also be considered.
Management Of Neurotrophic Keratitis
Management of neurotrophic keratitis can be divided into medical management, non-surgical intervention, and surgical management.6 The objective of treatment is to arrest progression and reverse neurotrophic keratitis changes that have occurred at the time of presentation. Conventional therapy for stage 1 aims to prevent epithelial breakdown, generally by administering lubricating agents such as preservative-free artificial tears, autologous serum drops, and discontinuing toxic topical medications specifically unnecessary use of antibiotics and anti-inflammatory agents. However, they all provide nonspecific symptomatic relief, which may be temporary. Stage 2 and 3 therapies aim to facilitate corneal healing and prevent corneal melting and perforation; these include procedures such as tarsorrhaphy, botulinum-induced ptosis, conjunctival flap, and amniotic membrane transplantation to restore ocular surface integrity. However, these procedures are usually performed late and therefore carry the risk of corneal scarring and poor vision. Collectively, current medical and surgical treatments poorly tackle the essential problem of corneal anesthesia and hence fail to provide a permanent cure.
Substantial evidence supports the use of neurotrophic factors to fulfill the unmet need in the treatment of neurotrophic keratitis. Specifically, the role of NGF in maintaining corneal homeostasis in vitro, ex vivo, and in animal models.17,18 Pilot studies also demonstrated the efficacy and reproducibility of using murine NGF (mNGF) for the treatment of corneal neurotrophic ulcers.19–21 However, the development of mNGF has been mired by its complex tertiary structure, which complicates the manufacturing of recombinant human NGF (rhNGF) suitable for clinical use. Recently, an Escherichia coli–derived rhNGF formulation for topical ophthalmic use has been introduced and demonstrated safety and efficacy in treating moderate-to-severe neurotrophic keratitis, as will be discussed in detail below.22,23
Cenegermin Eye Drops
Cenegermin is an rhNGF produced in Escherichia Coli as a pro-peptide, with a molecular formula of “C583H908N166O173S8”. After topical instillation, cenegermin is cleaved to mature NGF, which is a small (13kDa) protein composed of 118 amino acids with 3 disulfide bonds that forms a cysteine knot structure. Like the neurotrophin family, NGF binds to cell receptors TrkA and p75 receptors to regulate growth, survival, and differentiation of neuronal cells.24
Cenegermin under the tradename (OXERVATETM) was approved for the treatment of neurotrophic keratitis in the United States on August 22, 2018, and for the treatment of moderate to severe neurotrophic keratitis in the European Union on July 20, 2017. Due to the assumed rarity of this disease, cenegermin granted orphan drug designation in the United States and the European Union in 2014 and 2015, respectively. Cenegermin eye drops is a sterile, preservative-free ophthalmic solution containing 0.002% (0.02mg/mL) of the active ingredient, cenegermin. It is packaged in a carton of 7 multi-dose vials (1.0ml) intended to be used 6 times a day at 2 hr intervals for eight weeks. Only one vial is used per day before being discarded. The remaining vials in the carton are refrigerated between 2°C to 8°C for up to 14 days or until time of use. The other inactive ingredients in the product include disodium hydrogen phosphate anhydrous, hydroxypropylmethylcellulose, L-methionine, mannitol, polyethylene glycol 6000, sodium dihydrogen phosphate dihydrate, trehalose dihydrate, water, and hydrochloric acid and/or sodium hydroxide to adjust pH to 7.1–7.3.
Mechanism Of Action Of Cenegermin
Neurologist Dr. Rita Levi-Montalcini of Italy first discovered NGF in the 1950s, and this work won her the Nobel prize in 1986. As stated earlier, NGF is known to support corneal integrity via many mechanisms, although its exact role in treating neurotrophic keratitis is not entirely clear. NGF acts directly on corneal epithelial cells to stimulate their growth and survival, maintains limbal epithelial stem cell potential, binds receptors on lacrimal glands to promote tear production and has been experimentally shown to support corneal re-innervation.17,25–27 These mechanisms are essential to overcome the degenerative cycle of neurotrophic keratitis including impaired corneal innervation, loss of corneal sensitivity, reduced reflex blinking and tear production, and corneal epithelial breakdown.28
NGF is typically released in the aqueous humor and tear film and binds to NGF receptors expressed on anterior segments of the eye including iris, ciliary body, lens, cornea, and conjunctiva,29,30 by the lacrimal gland,31,32 and by all the intraocular tissues.29 NGF binds with two transmembrane receptors: the low-affinity receptor P75NTR and high-affinity tropomyosin receptor kinase A (TrkA). NGF binding to TrkA leads to intracellular activation of Ras, Cdc42/Rac/Rho, MAPK, PI3K, and PLC-γ.33 The biological activity and potency of cenegermin have been evaluated in vitro using a TF-1 human bone marrow erythroblast cell line that expresses the TrkA receptor. The quantified amount of TF-1 cells proliferation was similar after exposure to either NGF or cenegermin.
Safety And Efficacy Of Cenegermin For The Treatment Of Neurotrophic Keratitis
The safety and efficacy of cenegermin eye drops for the treatment of neurotrophic keratitis have been evaluated in several clinical trials (Table 1). First, an open-label uncontrolled study as performed in 12 patients (14 eyes) with severe corneal neurotrophic ulcers that had been present for 45±24 days. After treatment with murine NGF (200 μg/mL) 10 times/day for two days followed by 6 times/day, complete corneal healing with scarring was achieved between 10 days to 6 weeks (mean 34 days). During an average follow-up of 6.6 months, scarring disappeared, none of the patients had systemic or ocular side effects, and corneal integrity, visual acuity, and sensitivity were maintained without disease relapse.19
Table 1.
Summary Of Clinical Studies Evaluating Safety And Efficacy Of Recombinant Human Nerve Growth Factor (rhngf) Eye Drops
| Study | Design | Subjects | Study Groups | Treatment Regimen | Outcome Measures | Results |
|---|---|---|---|---|---|---|
| NGF0112 (Phase 1) NCT01744704 Ferrari et al 201420 |
RCT, double masked, dose ascending in Switzerland and UK | 74 healthy volunteers | 0.5–5 µg/mL NGF 20 µg/mL NGF 60–180 µg/mL NGF Vehicle (control) (1:1:1:1) |
One drop Up to 3 times/day for 1–5 days |
S (AEs) and PK | All doses were well tolerated Mild discomfort at highest dose No significant systemic effect |
| NGF0212 (Phase 1) NCT01756456 Bonini et al 201821 |
Multicenter, RCT, double-masked, vehicle-controlled in Europe |
18 with unilateral NK | 10 µg/mL rhNGF 20 µg/mL rhNGF Vehicle (1:1:1) |
One drop 6 times/day for 8 weeks; 48 or 56 weeks follow up period |
S, PK and E | Ocular pain (28%) No significant systemic effect Corneal Healing at week 4 in 25% (control), 42% (both doses) and at week 8 in 50% (control), 67% (10 µg) and 85% (20 µg). |
| NGF0212 (Phase 2) NCT01756456 Bonini et al 201833 |
Multicenter, RCT, double-masked, vehicle-controlled in Europe |
156 with unilateral NK | 10 µg/mL rhNGF 20 µg/mL rhNGF Vehicle (1:1:1) |
One drop 6 times/day for 8 weeks; 48 or 56 weeks follow up period |
S and E | Well tolerated, Mild transient AEs No significant systemic effect Corneal Healing at week 4 in 20% (control), 55% (10 µg) and 58% (20 µg). At week 8 in 43% (control), 75% (10 µg) and 74% (20 µg). 96% no recurrence |
| NGF0214 (Phase 2) NCT02227147 |
Multicenter, RCT, double-masked, parallel group study in US | 48 with uni- or bilateral NK | 20 µg/mL NGF (n= 24) Vehicle (n=24) (1:1) |
One drop 6 times/day for 8 weeks | S and E | Corneal healing in 65% of NGF group and 17% of control |
| NGF0213 (Phase 2a) NCT02101281 Sacchetti et al 201820 |
Single-center, open-label, two dose study in Austria | 40 with moderate to severe dry eye | 20µg/mL rhNGF 4µg/mL NGF |
One drop BID for 28 days |
S and E | Both doses were well tolerated, safe and effective in improving symptoms and signs of DED. |
| NGF0116 (Phase 2) NCT03035864 |
Single center RCT, double-masked, Parallel group study in Italy | 120 with post cataract and refractive surgery | 20µg/mL NGF Vehicle (2:1) |
One drop 6 times/day for 8 weeks |
S and E | Not available |
| NGF0216 (Phase 2) NCT03019627 |
Single center RCT, double-masked, Parallel group study in the US | 150 with dry eye disease | 20µg/mL NGF Vehicle (2:1) |
One drop 6 times/day for 8 weeks |
S and E | Not available |
Abbreviations: RCT, Randomized clinical trial; S, Safety; E, Efficacy; AEs, Adverse Events; PK, Pharmacokinetics.
The same research group subsequently performed a prospective, noncomparative, interventional case series in 45 eyes of 43 patients with stage 2–3 neurotrophic keratitis unresponsive to other treatments within 6 weeks. Patients received murine NGF (200µg/mL) every 2 hrs for 2 days followed by six times/day. After 12 days to 6 weeks of treatment (mean ~1 month), all patients had complete healing with significant improvement of corneal sensitivity and visual acuity. Hyperemia (100% of cases), photophobia (85%), and ocular and periocular pain (70%) were side effects reported during the first 2 to 7 days. During a mean follow-up of 15.8 months, recurrence of PED has observed in 3 cases with trigeminal nerve resection, and 28.6% of cases had superficial or deep new corneal blood vessels.20
Following these studies conducted with murine NGF, regulatory studies commenced with rhNGF. To support regulatory approval, one Phase I study (NGF0112)22 in healthy volunteers, and two randomized, controlled, double-masked Phase II studies (NGF0212 and NGF0214) in neurotrophic keratitis patients were conducted.23,34 Data from additional studies in other conditions, including moderate to severe dry eye disease35 were also provided to support the safety database.
In the two-Phase II studies, the safety and efficacy of cenegermin eye drops were evaluated in patients with neurotrophic keratitis for eight weeks. In the first study,34 patients were eligible if they had unilateral stage 2 or 3 neurotrophic keratitis refractory to conventional non-surgical treatments for at least 2 weeks and had decreased corneal sensitivity (<40 mm by Cochet-Bonnet aesthesiometer). The study subjects (n=156) had a mean age of 61 years, with majority female (61%) and white (91%). Patients were randomized to receive 20µg/mL rhNGF, 10µg/mL rhNGF, or placebo 6 times daily for 8 weeks. After the treatment period, patients entered a 48-week follow-up period. The primary endpoint was the percentage of eyes achieving corneal healing (defined as completely staining free cornea with no residual fluorescein staining in the area of the corneal lesion). Results showed cenegermin group had a significantly higher proportion of patients with complete healing compared to placebo. At 4 weeks, complete healing was observed in 55% of patients in 10µg/mL rhNGF, 58% in 20µg/mL rhNGF, and 20% in the vehicle group. At 8 weeks, complete healing increased to 75% of patients in 10µg/mL NGF, 74% in 20µg/mL NGF, and 43% in the vehicle group. Interestingly, there were no statistically significant differences in improvement in corneal sensitivity in patients of the treatment groups, who achieved complete corneal clearing at week 4, 6, or 8. Additionally, the recurrence of PED or corneal ulcer during follow-up was more frequent in the cenegermin groups. Of the completely healed patients at 8 weeks, 17% of 10µg/mL NGF, 20% of 20µg/mL NGF, and 10% of vehicle patients had recurrences.
In the second study (clinical trial #NCT02227147), the eligibility criteria were similar to the first study, aside from both unilateral and bilateral patients were eligible. Patients (n=48) were randomized to receive 20µg/mL Oxervate or placebo 6 times daily for 8 weeks. The mean patient age was approximately 65, and the majority of patients were female. The primary endpoint was the percentage of patients achieving complete resolution of corneal fluorescein staining (0 mm lesion size and no residual staining) at week 8. Results showed that 65.2% of NGF patients achieved closure at week 8, compared to 16.7% in the placebo (p<0.001).
Overall, complete corneal healing was shown in 70% of patients treated with cenegermin compared to 28% of patients treated with placebo. Cenegermin eye drops worked similarly in men and women, and in patients above and below age 65 years. No differences in response among races could be determined as the majority of patients (90%) were White.
Regarding adverse events, 64% of NGF patients experienced an adverse event compared to 50% in the placebo group. In patients that received NGF, the most common adverse event was eye pain reported in 16% of patients (compared to 7.9% reported in the placebo group). Other adverse reactions occurring more frequently than in the placebo group included visual acuity reduction (10.7%), corneal deposits (4%), cataract (4%), foreign body sensation (2.7%), ocular hyperemia (6.7%), inflammation (5.3%) and tearing (5.3%). Based on the conducted studies, ocular NGF at treatment doses had no immunogenic systemic potential. The ocular metabolizing enzymes do not metabolize NGF except for tissue proteases that could degrade it to the corresponding amino acids. After ocular administration, the majority passes through the nasolacrimal duct reaching the nasal and then the oropharyngeal cavity and is then degraded by proteases. However, long-term safety has not been established. Further studies should also be performed to evaluate changes in corneal nerves in terms of morphology and function.
When To Use Cenegermin For Neurotrophic Keratitis
As stated earlier, until now, treatment options for neurotrophic keratitis have been limited to palliative treatments aimed at reducing symptoms and not directly targeting the underlying pathology. Cenegermin eye drops (Oxervate ™ Dompé US Inc., Boston, MA) represents the first-ever topical biologic medication approved in ophthalmology and is the first-ever application of a human NGF as drug or treatment. It may be used as a first-line treatment for patients with Stage 2 or 3 neurotrophic keratitis that have not responded to other conventional non-surgical treatments for ~2 weeks. The recommended dose is one drop in the affected eye(s), 6 times per day at 2 hr intervals, for eight weeks. If a dose is missed, treatment should be continued as normal, at the next scheduled administration. If more than one topical ophthalmic product is being used, administer the eye drops at least 15 mins apart to avoid diluting the drug.
Side Effects And Limitations
As reported in the preliminary clinical trials, the most common adverse reaction is eye pain following instillation, which was reported in approximately 16% of patients. Other adverse reactions occurring in 1-10% of patients included corneal deposits, foreign body sensation, ocular hyperemia. Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies may not reflect the rates observed in practice.
Like other temperature-sensitive medications, the storage and handling of cenegermin remain a significant concern. Cenegermin is stored in a freezer, and it usually takes about 30 mins to be thawed at room temperature before use. The multiple steps used to withdraw the eye drops and the multi-dose vial carry the risk of contamination. Also, the treatment cost may be prohibitive to some patients. In Europe, eight-week course of cenegermin treatment typically costs £14,500.
Conclusion
Neurotrophic keratitis is an underdiagnosed degenerative condition induced by impairment of corneal nerves and may lead to corneal blindness. Cenegermin has been demonstrated to be safe and effective in the treatment of neurotrophic keratitis based on conducted regulatory trials. However, the clinical experience with this drug is still limited. Further studies are needed to evaluate corneal nerve morphology and function. A single-dose generic medication with a controlled delivery and an affordable cost is necessary.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542. doi: 10.1016/s0014-4835(03)00050-2 [DOI] [PubMed] [Google Scholar]
- 2.Al-Aqaba MA, Fares U, Suleman H, Lowe J, Dua HS. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2010;94(6):784–789. doi: 10.1136/bjo.2009.173799 [DOI] [PubMed] [Google Scholar]
- 3.Stepp MA, Tadvalkar G, Hakh R, Pal-Ghosh S. Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia. 2017;65(6):851–863. doi: 10.1002/glia.23102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Versura P, Giannaccare G, Pellegrini M, Sebastiani S, Campos EC. Neurotrophic keratitis: current challenges and future prospects. Eye Brain. 2018;10:37–45. doi: 10.2147/EB.S117261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Aqaba MA, Anis FS, Mohammed I, Dua HS. Nerve terminals at the human corneoscleral limbus. Br J Ophthalmol. 2018;102(4):556–561. doi: 10.1136/bjophthalmol-2017-311146 [DOI] [PubMed] [Google Scholar]
- 6.Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107–131. doi: 10.1016/j.preteyeres.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 7.Lambiase A, Rama P, Aloe L, Bonini S. Management of neurotrophic keratopathy. Curr Opin Ophthalmol. 1999;10(4):270–276. [DOI] [PubMed] [Google Scholar]
- 8.Semeraro F, Forbice E, Romano V, et al. Neurotrophic keratitis. Ophthalmologica. 2014;231(4):191–197. doi: 10.1159/000354380 [DOI] [PubMed] [Google Scholar]
- 9.Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–579. doi: 10.2147/OPTH.S45921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puca A, Meglio M, Vari R, Tamburrini G, Tancredi A. Evaluation of fifth nerve dysfunction in 136 patients with middle and posterior cranial fossae tumors. Eur Neurol. 1995;35(1):33–37. doi: 10.1159/000117086 [DOI] [PubMed] [Google Scholar]
- 11.Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S. Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol. 1977;95(12):2193–2196. doi: 10.1001/archopht.1977.04450120099012 [DOI] [PubMed] [Google Scholar]
- 12.Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol. 2017;232(4):717–724. doi: 10.1002/jcp.25623 [DOI] [PubMed] [Google Scholar]
- 13.Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf. 2017;15(1):15–47. doi: 10.1016/j.jtos.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.dell’Omo R, Cifariello F, De Turris S, et al. Confocal microscopy of corneal nerve plexus as an early marker of eye involvement in patients with type 2 diabetes. Diabetes Res Clin Pract. 2018;142:393–400. doi: 10.1016/j.diabres.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 15.Moein HR, Kheirkhah A, Muller RT, Cruzat AC, Pavan-Langston D, Hamrah P. Corneal nerve regeneration after herpes simplex keratitis: a longitudinal in vivo confocal microscopy study. Ocul Surf. 2018;16(2):218–225. doi: 10.1016/j.jtos.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagali NS, Allgeier S, Guimaraes P, et al. Reduced corneal Nerve fiber density in type 2 diabetes by wide-area mosaic analysis. Investigative Ophthalmology & Visual Science. 2017;58(14):6318–6327. doi: 10.1167/iovs.17-22257 [DOI] [PubMed] [Google Scholar]
- 17.Lambiase A, Sacchetti M, Bonini S. Nerve growth factor therapy for corneal disease. Curr Opin Ophthalmol. 2012;23(4):296–302. doi: 10.1097/ICU.0b013e3283543b61 [DOI] [PubMed] [Google Scholar]
- 18.Lambiase A, Mantelli F, Sacchetti M, Rossi S, Aloe L, Bonini S. Clinical applications of NGF in ocular diseases. Archives Italiennes De Biologie. 2011;149(2):283–292. doi: 10.4449/aib.v149i2.1363 [DOI] [PubMed] [Google Scholar]
- 19.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. NEngJMed. 1998;338:1174–1180. doi: 10.1056/NEJM199804233381702 [DOI] [PubMed] [Google Scholar]
- 20.Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107(7):1347–1351; discussion 1351–1342. doi: 10.1016/s0161-6420(00)00163-9 [DOI] [PubMed] [Google Scholar]
- 21.Smith RE, Sadun AA. Clearing the cornea with nerve growth factor. N Engl J Med. 1998;338(17):1222–1223. doi: 10.1056/NEJM199804233381710 [DOI] [PubMed] [Google Scholar]
- 22.Ferrari MP, Mantelli F, Sacchetti M, et al. Safety and pharmacokinetics of escalating doses of human recombinant nerve growth factor eye drops in a double-masked, randomized clinical trial. BioDrugs. 2014;28(3):275–283. doi: 10.1007/s40259-013-0079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonini S, Lambiase A, Rama P, et al. Phase I trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1468–1471. doi: 10.1016/j.ophtha.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 24.Bradshaw RA, Pundavela J, Biarc J, Chalkley RJ, Burlingame AL, Hondermarck H. NGF and ProNGF: regulation of neuronal and neoplastic responses through receptor signaling. Advances in Biological Regulation. 2015;58:16–27. doi: 10.1016/j.jbior.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacchetti M, Bruscolini A, Lambiase A. Cenegermin for the treatment of neurotrophic keratitis. Drugs of Today (barcelona, Spain : 1998). 2017;53(11):585–595. doi: 10.1358/dot.2017.53.11.2722395 [DOI] [PubMed] [Google Scholar]
- 26.Nishida T, Yanai R. Advances in treatment for neurotrophic keratopathy. Current Opinion in Ophthalmology. 2009;20:276–281. [DOI] [PubMed] [Google Scholar]
- 27.Kruse FE, Tseng SC. Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Investigative Ophthalmology & Visual Science. 1993;34(6):1963–1976. [PubMed] [Google Scholar]
- 28.Reichard M, Hovakimyan M, Guthoff RF, Stachs O. In vivo visualisation of murine corneal nerve fibre regeneration in response to ciliary neurotrophic factor. Exp Eye Res. 2014;120:20–27. doi: 10.1016/j.exer.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 29.Lambiase A, Bonini S, Manni L, et al. Intraocular production and release of nerve growth factor after iridectomy. Investigative Ophthalmology & Visual Science. 2002;43(7):2334–2340. [PubMed] [Google Scholar]
- 30.Qi H, Chuang EY, Yoon KC, et al. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Mol Vis. 2007;13:1934–1941. [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen DH, Beuerman RW, Thompson HW, DiLoreto DA. Growth factor and neurotrophic factor mRNA in human lacrimal gland. Cornea. 1997;16(2):192–199. [PubMed] [Google Scholar]
- 32.Ghinelli E, Johansson J, Rios JD, et al. Presence and localization of neurotrophins and neurotrophin receptors in rat lacrimal gland. Investigative Ophthalmology & Visual Science. 2003;44(8):3352–3357. doi: 10.1167/iovs.03-0037 [DOI] [PubMed] [Google Scholar]
- 33.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Current Opinion in Neurobiology. 2001;11(3):272–280. doi: 10.1016/s0959-4388(00)00208-7 [DOI] [PubMed] [Google Scholar]
- 34.Bonini S, Lambiase A, Rama P, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1332–1343. doi: 10.1016/j.ophtha.2018.02.022 [DOI] [PubMed] [Google Scholar]
- 35.Sacchetti M, Lambiase A, Schmidl D, et al. Effect of recombinant human nerve growth factor eye drops in patients with dry eye: a phase IIa, open label, multiple-dose study. Br J Ophthalmol. 2019. doi: 10.1136/bjophthalmol-2018-312470 [DOI] [PMC free article] [PubMed] [Google Scholar]